Abstract
Previously, indirect thrombin inhibitors such as unfractionated heparin or low-molecular-weight heparin were used as a standard anticoagulation during percutaneous coronary intervention to prevent procedural thrombotic complications but at a risk of hemorrhagic complications. More recently, bivalirudin, a member of the direct thrombin inhibitor class, has been shown to have 1) predictable pharmacokinetics, 2) ability to inhibit free- and clot-bound thrombin, 3) no properties of platelet activation, 4) avoidance of heparin-induced thrombocytopenia, and 5) a significant reduction of bleeding without a reduction in thrombotic or ischemic endpoints compared to heparin and glycoprotein IIbIIIa inhibitors when used in patients presenting with acute coronary syndrome who are planned for an invasive treatment strategy.
Introduction
Currently, coronary artery disease manifesting as an acute coronary syndrome (ACS) is extremely common, resulting in over 1.5 million hospitalizations in the United States in 2004 (CitationRosamond et al 2007). With the advancements provided by medical research and medical technology, many of these patients are being evaluated and treated more quickly and more invasively with cardiac catheterization and subsequent percutaneous coronary intervention (PCI). In addition, continued attempts are focused on reducing negative peri-procedural clinical events including ischemia, bleeding, and mortality. Previously, unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) were used as standard anticoagulation therapy during PCI to prevent procedural thrombotic complications. More recently, direct thrombin inhibitors (DTI) have been evaluated as an anticoagulant in this clinical setting. Bivalirudin (Angiomax®, The Medicines Company, NJ) (previously known as Hirulog®), a member of the DTI class, has been shown to have 1) predictable pharmacokinetics, 2) ability to inhibit free- and clot-bound thrombin, 3) avoidance of platelet activation, 4) avoidance of heparin-induced thrombocytopenia (HIT), and 5) a significant reduction of bleeding without a reduction in thrombotic or ischemic endpoints compared to heparin and glycoprotein IIbIIIa inhibitors (GPIIaIIIb inhibitors) when used in patients presenting with ACS.
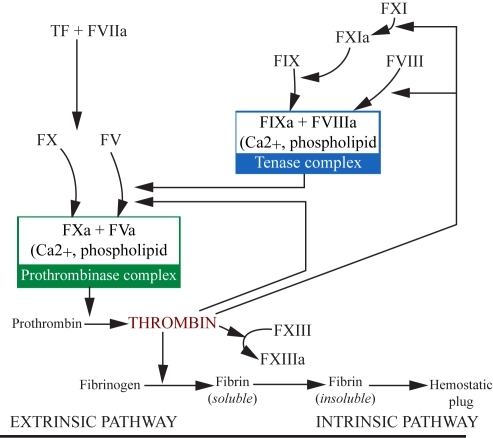
Figure 1 The coagulation cascade in atherothrombosis. Reprinted with permission from CitationArora UK, Dhir M. 2005. Direct thrombin inhibitors part 1 of 2. J Invasive Cardiol, 17:34–8. Copyright © 2005 HMP Communications.
Abbreviations: F, factor; T, tissue.
Pathophysiology of acute coronary syndromes
The diagnosis of ACS describes a clinical presentation of symptoms that are compatible with acute myocardial ischemia. By definition, ACS includes unstable angina (USA), non-ST-elevation myocardial infarction (NSTEMI), and ST-elevation myocardial infarction (STEMI) (CitationAnderson et al 2007). Most patients who present with ACS are hypothesized to be a result of a sudden luminal thrombosis in a epicardial coronary artery (CitationBurke et al 1997, Citation1998; CitationVirmani et al 2000). This thrombotic process might occur secondary to three differing pathologies: plaque rupture, plaque erosion, or a calcified nodule. The majority of ACS cases are due to plaque rupture (CitationVirmani et al 2006) and therefore will be the focus of the remainder of this review.
The predisposing lesion to plaque rupture is a thin cap fibroatheroma (TCFA) or “vulnerable plaque” which is characterized by a necrotic core, an overlying thin fibrous cap (< 65 μm) which contains a dense concentration of macrophages (>25/hpf) and few smooth muscle cells (CitationSchaar et al 2004). As a result of external (eg, shear stress) and internal forces (ie, enzymatic and degradation active processes within the plaque), the fibrous cap ruptures allowing direct contact between the highly thrombogenic necrotic core and circulating platelets and monocytes. These circulating platelets adhere to and become activated by the exposed subendothelial components, most notably collagen and von Willebrand factor (vWF). Following activation, these platelets release chemoattractants (eg, adenosine diphosphate and thromboxane A2) which promote further platelet adhesion to the site of endothelial injury. In addition, activated macrophages and smooth muscle cells from the exposed necrotic core release tissue factor (TF) leading to activation of the coagulation cascade (CitationArora and Dhir 2005) and thrombin production (CitationSciulli and Mauro 2002). Thrombin binds to fibrin particles and continues to strongly activate platelets (CitationKumar et al 1995); activate Factors V, VIII, and XIII (which further stimulate thrombin production and stabilize the fibrin-bound thrombin) (CitationKumar et al 1994; CitationSciulli and Mauro 2002); convert fibrinogen to fibrin (CitationWeitz et al 1990); and activate carboxypeptidase B, a known fibrinolysis inhibitor (CitationSakharov et al 1997; CitationFenton et al 1998). The fibrin-bound thrombin (or fibrin split product-bound thrombin) remains enzymatically active and is protected from degradation by fluid-phase inhibitors (CitationWeitz et al 1990, Citation1998).
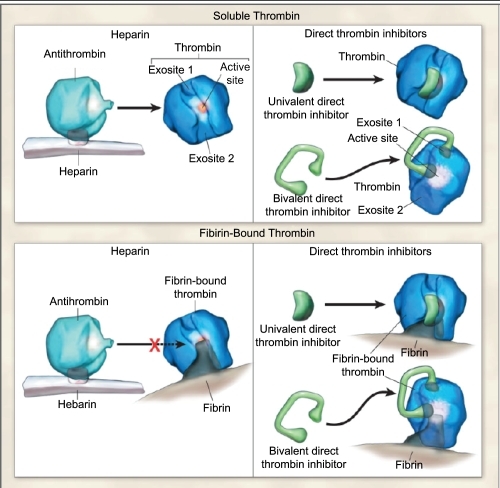
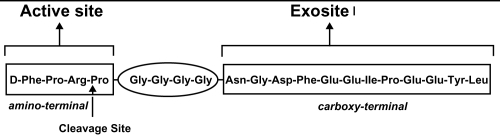
Previous research has suggested that there are three receptor sites on thrombin: one active catalytic and two exosites (CitationHogg and Bock 1997; CitationBecker et al 1999) (). The catalytic site plays a role in the conversion of fibrinogen to fibrin and further activation of platelets and clotting factors (CitationSciulli and Mauro 2002). Exosites 1 and 2 are positively charged and therefore bind negatively charged compounds: fibrinogen and heparin-antithrombin complex, respectively. Cleavage of fibrinogen occurs over the catalytic site after the fibrinogen particle aligns itself with exosite 1. Following cleavage, although the fibrin monomer may remain attached to exosite 1, the thrombin’s active site remains free to initiate further catalytic processes (CitationHogg and Bock 1997; CitationFenton et al 1998). In contrast, when a heparin-antithrombin complex nears free thrombin, it is theorized that the heparin subunit binds to exosite 2 and the antithrombin (AT) subunit attaches to the active site resulting in deactivation of the free-thrombin. However, when a heparin-antithrombin complex nears fibrin-bound thrombin and the heparin subunit binds to exosite 2, there is a conformational change that occurs at thrombin’s active site resulting in a decreased ability for the AT subunit to bind and inactivate the active site (therefore, leaving thrombin enzymatically active) (CitationHogg and Bock 1997 CitationFenton et al 1998).
Figure 2 Bivalirudin’s action on thrombin. Reprinted with permission from CitationDi Nisio M, Middeldorp S, Buller HR. 2005. Direct thrombin inhibitors. N Engl J Med, 353:1028–40. Copyright © 2005 Massachusetts Medical Society. All rights reserved.
Anticoagulation therapy for ACS
In patients presenting with ACS, it is imperative to modify the underlying pathophysiology of plaque rupture and thrombosis in attempt to reduce the likelihood of myocardial infarction (MI) and death. This is most effectively achieved with a combination of antiplatelet (eg, aspirin or GPIIbIIIa inhibitors) and anticoagulant medications (CitationAnderson et al 2007). Because platelets are a prominent mediator in thrombus formation after plaque rupture, it is not surprising that studies regarding patients presenting with ACS and comparing aspirin with placebo have consistently shown a dramatic benefit in reduction of MI, stroke, death, and other major cardiac endpoints (CitationLewis et al 1983; CitationCairns et al 1985; CitationISIS-2 1988; CitationTheroux et al 1988; CitationRISC 1990). Other forms of antiplatelet medications such as ADP receptor (P2Y12) antagonists (eg, clopidogrel) have been shown to reduce similar adverse outcomes when used alone or especially when used in conjunction with aspirin (CitationSchror 1993; CitationCAPRIE 1996; CitationMehta et al 2001; CitationYusuf et al 2001).
Also, anticoagulants are necessary to prevent thrombus propagation or even possibly lyse existing thrombus. Several randomized trials comparing aspirin (ASA) alone versus UFH with ASA revealed an approximate 33%–50% reduction in short-term (but not long-term) rates of MI or death (CitationTelford and Wilson 1981; CitationWilliams et al 1986; CitationTheroux et al 1988, Citation1993; CitationCohen et al 1994; CitationOler et al 1996; CitationYusuf et al 2001). Studies evaluating the addition of LMWH (eg, nadroparin, dalteparin, enoxaparin) to ASA therapy in ACS patients report significant reductions in MI, stroke, and need for re-revascularization procedures (CitationGurfinkel et al 1995; CitationFRISC 1996), but possibly at a cost of higher rates of major and minor bleeding in those patients treated with an early invasive strategy (CitationFerguson et al 2004).
Until recently, heparin has been nearly universally used as the systemic anticoagulant of choice in the treatment of ACS and during PCI. Heparin acts via binding and catalyzing the activity of AT. The heparin-AT complex in turn equally inhibits the activity of Factor Xa and IIa (thrombin) (CitationHirsh 1991; CitationSciulli and Mauro 2002). In addition, UFH inhibits thrombin by simultaneously binding to AT and keeping these two molecules in proximity of one another (CitationHirsh 1991). It is important to note that heparins do not inhibit thrombin bound to fibrin, thrombin bound to fibrin degradation products, or Factor Xa bound to platelets (CitationMirshahi et al 1989; CitationFurie and Furie 1992; CitationHogg and Bock, 1997). In addition, heparin binds to endothelial cells and a number of plasma proteins which limits its availability to interact with AT and therefore reduces its potential anticoagulant effect (CitationDa 1989; Citationde Romeuf and Mazurier 1993). In slight contrast, LMWH chains are not long enough to bridge thrombin to AT, which therefore leads to more Factor Xa inhibition than thrombin inhibition (CitationWeitz 1997). UFH and LMWH can be inactivated by platelet factor 4 (PF4) (CitationEitzman et al 1994) and the subsequent UFH(LMWH)-PF4 complex may act as a source of immunoglobin G mediated HIT (HIT Type II) (CitationChong 2003; CitationLehman and Chew 2006). Therefore, because of its non-specific binding to endothelium and plasma proteins, heparin’s half-life is dose dependent and anticoagulant’s bioavailability and effects have high intra- and inter-patient variability.
These obvious limitations of these heparin compounds have driven the research and development of other antithrombotic agents. One newer class of anticoagulants, Factor Xa inhibitors, were investigated in the Organization for the Assessment of Strategies for Ischemic Syndromes (OASIS) 5 and Trials. These trials compared fondaparinux with standard UFH or LMWH strategies in patients presenting with USA/NSTEMI. Short-term combined outcomes of death, MI, or refractory ischemia was similar in the two groups (5.8% vs 5.7%, p = NS) with significantly less bleeding in the fondaparinux arm (2.2% vs 4.1%, p ≤ 0.001). However, at 180 days, fondaparinux was associated with significant reductions in death, MI and stroke, but with an increased risk of catheter-associated thrombus at the time of PCI (0.9% vs 0.3%). Therefore, it has been recommended that patients who received fondaparinux prior to PCI also receive another anti-Factor IIa (eg, UFH) to support PCI (CitationYusuf et al 2006a, Citationb; CitationAnderson et al 2007).
Another class of anticoagulants for the treatment of arterial thrombosis is direct thrombin inhibitors. The class of DTIs include hirudin and its synthetic derivative (eg, bivalirudin), molecules that react with the active-site of thrombin (eg, Phe-Pro-Arg-chloromethylketone [PPACK], argatroban, melagatran, and ximelagatran), and thrombin-binding DNA aptamers. The biologic activity of DTIs, unlike those of heparin, is independent of the presence of antithrombin as they act directly on the thrombin molecule. Bivalent DTIs (hirudin, lepirudin, desirudin, and bivalirudin) interact with the active site and exosite 1 of thrombin, whereas univalent DTIs (argatroban) bind only to the active site (CitationDi Nisio et al 2005). Although these three classes work with differing mechanisms, they all present advantages over heparin including: 1) the ability to inactivate fibrin-bound thrombin, 2) they do not bind to endothelial cells or plasma proteins, and 3) they have improved predictability of pharmacokinetics (CitationArora and Dhir 2005).
For the purposes of this review, only bivalirudin will be discussed in detail. Of note when compared to hirudin, bivalirudin has a wider therapeutic window (allowing less laboratory monitoring) and wider safety margin (allowing the administration of higher doses which may result in greater availability/inhibition of thrombin and thrombomodulin) (CitationBittl 1995).
Pharmacology of bivalirudin
Mechanism of action
Bivalirudin is a semi-synthetic 20 amino-acid polypeptide derived from native hirudin (CitationMaraganore et al 1990) (). This compound is specific for thrombin, binds at a 1:1 ratio with thrombin, is active against unbound and bound thrombin, is not inactivated by PF4, and does not require any cofactors for activity (CitationBates and Weitz 1998; CitationSciulli and Mauro 2002). The amino-terminal segment has a high affinity and specificity for binding to thrombin’s active site and the carboxy-terminal segment binds to exosite 1. Bivalirudin acts by direct competitive inhibition with fibrinogen to exosite 1 and the active site which results in complete inhibition of fibrin formation and catalytic function (CitationMaraganore et al 1990). The binding is reversible as thrombin slowly cleaves bivalirudin near the amino-terminal end resulting in separation of the amino-terminal from the active site (CitationParry et al 1994). As a result of this detachment, the carboxy-terminal affinity to exosite 1 is weakened and bivalirudin may be displaced altogether from the thrombin by a fibrinogen particle (CitationParry et al 1994) ().
Figure 3 Structure of bivalirudin. Reprinted with permission from CitationSciulli TM, Mauro VF. 2002. Pharmacology and clinical use of bivalirudin. Ann Pharmacother, 36:1028–41. Copright © 2002 Harvey Whitney Books Co.
Pharmokinetics and pharmacodynamics
Previous research has shown that bivalirudin appears to have very predictable, linear pharmacokinetic properties. Peak plasma concentrations following bolus infusions occurred within 5 minutes (CitationFox et al 1993) and steady-state concentrations during continuous infusions were directly related to dose (CitationLidon et al 1993; Angiomax Product Information 2000). Bivalirudin has been shown to have linear pharmacokinetics with rapid plasma clearance (3 mL/min/kg) and a small volume of distribution (0.2 L/kg) (CitationBates 2004). Although the anticoagulant effects of bivalirudin may slightly vary between patients, there is very little variability once a response to a dose is given. Specifically, previous studies have shown that the anticoagulant effects of bivalirudin are directly related to dose: prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time (TT), and activated clotting time (ACT) all rise linearly with an increase in dose (CitationCannon et al 1993; CitationFox et al 1993; CitationLidon et al 1993; CitationTopol et al 1993; CitationSharma et al 1993). These anticoagulant properties occur within minutes of an intravenous bolus injection and return to baseline approximately 1 hour after infusion is discontinued in patients with normal healthy patients (CitationFox et al 1993; Angiomax Product Information 2000). In addition, there is no evidence that bivalirudin binds to non-thrombin plasma proteins, and it is cleared by (predominantly) renal elimination and intravascular proteolysis (Angiomax Product Information 2000; CitationRobson 2000; CitationReed and Bell 2002). It has been shown that compared to normal healthy patients, bivalirudin clearance is approximately half in hospitalized patients with normal renal function (creatinine clearance greater than 60 mL/min), nearly 40% in patients with creatinine clearance between 10 and 60 mL/min, and 10% in patients requiring hemodialysis (Angiomax Product Information 2000; CitationRobson 2000). As a result, the plasma half-life of bivalirudin is 22 minutes, 34 minutes, and 210 minutes in patients with normal renal function (creatinine clearance greater than 60 mL/min), moderate renal dysfunction, and on hemodialysis, respectively (Angiomax Product Information 2000; CitationWhite 2001). Therefore, in clinical scenarios involving severe renal dysfunction, the recommendation is to maintain the standard bolus dosing and reduce the infusion rate to 1 mg/kg/hour if the creatinine clearance less than 30 mL/minute or 0.25 mg/kg/hour if the patient is on hemodialysis. Currently, there is no antidote to counteract the anticoagulants effects (CitationBates 2004). Current dosage recommendations have been made ().
Table 1 Current dosage recommendations for bivalirudin for various patient subpopulations
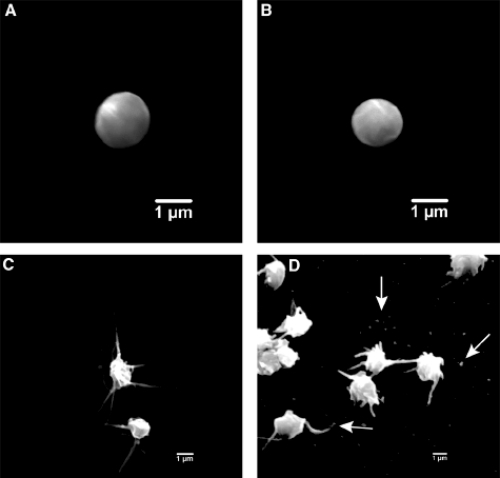
Bivalirudin’s anticoagulant effects are not affected by concurrent aspirin use (CitationFox et al 1993). Importantly, research evaluating the direct platelet effects of therapeutic concentrations of bivalirudin (compared to UFH or LMWH) have shown bivalirudin did not activate platelets (see ), decreased platelet surface coverage, attenuated platelet attachment to injured endothelium, and reduced sCD40L (a marker of inflammation) in healthy and in patients undergoing PCI (CitationTopol et al 1993; CitationShen et al 1997; CitationAnand et al 2007). In addition, there is a dose-dependent relationship between bivalirudin and a decrease in plasma concentrations of fibrinopeptide A (a marker of fibrinogen conversion) (CitationTopol et al 1993) and inhibition of plasminogen activator inhibitor activity (CitationShen et al 1997). Therefore, bivalirudin has a more predictable anticoagulation response among treated patient population. Of note, lepirudin’s binding to thrombin is irreversible and argatroban binds only reversibly to thrombin’s active site (CitationBates and Weitz 1998).
Figure 4 Scanning electron photomicrographs representing differences in platelet activation between anticoagulants. (A) normal platelet at rest, (B) platelet response to bivalirudin therapy, (C) platelet response and activation with UFH therapy, (D) platelet response (release of microparticles) after UFH therapy. Reprinted with permission from CitationAnand SX, Kim MC, Kamran M, et al. 2007. Comparison of platelet function and morphology in patients undergoing percutaneous coronary intervention receiving bivalirudin versus unfractionated heparin versus clopidogrel pretreatment and bivalirudin. Am J Cardiol, 100:417–24. Copyright © 2007 Elsevier.
According to initial trials, the most common reported adverse effects of bivalirudin were back pain, nausea, headache, and hypotension. Less frequently reported reactions (< 10% of cases) include insomnia, hypertension, vomiting, anxiety, dyspepsia, bradycardia, abdominal pain, fever, nervousness, and pelvic pain (CitationTopol et al 1993). These complaints did not occur at a higher rate when compared to patients receiving heparin therapy. There has been no data that suggests there are any effects on reproductive parameters, teratogenicity, mutagenicity, or antigenic/immunogenic response (CitationBates 2004). Any explained drop in blood pressure or hematocrit should be assessed with an expedited work-up for bleeding or hemorrhage. There has been no reported associated with bivalirudin and thrombocytopenia. In addition, bivalirudin is contraindicated in patients with active major bleeding or hypersensitivity to the drug or its components (Angiomax Product Information 2000).
Clinical trials
There has been an extensive amount of in vitro and clinical research investigating the safety and efficacy of bivalirudin which has led to its current clinical indications for use in ACS patients ().
Table 2 Guideline-based indications for the use of bivalirudin in ACS patients (CitationAnderson et al 2007; CitationKing et al 2007)
Unstable angina or NSTEMI patients
The initial study to assess the efficacy and appropriate dosing with a DTI (Hirulog) as a sole anticoagulant in patients undergoing coronary angioplasty (POBA) was reported in 1993. This multi-center trial enrolled 291 patients undergoing POBA who received an intravenous bolus followed by continuous infusion of Hirulog for 4 hours following the procedure. Several dosing strategies were used and coagulation and hematologic variables were measured throughout the therapy. The primary endpoint of abrupt vessel closure occurred in 6.2% of patients; no significant bleeding complications were noted in any patients; and a more predictable, dose-response curve of both ACTs and aPTTs was seen. This study was the first to report that it was possible to safely perform POBA with an anti-coagulant other than heparin in aspirin-pretreated patients (CitationTopol et al 1993).
The first randomized comparisons of bivalirudin versus heparin in patients undergoing coronary intervention were reported in HAS Trial and the Bivalirudin Angioplasty Trial (BAT) – these two trials used the same patient database but differing methods of analysis. These studies randomized 4,312 patients to heparin or bivalirudin immediately before POBA for unstable or post-infarction angina. Investigators stated the primary endpoint was combined death in the hospital, abrupt vessel closure, or “rapid clinical deterioration of cardiac origin” requiring emergent coronary artery bypass surgery (CABG), intra-aortic balloon counterpulsation (IABP), or repeated POBA. The results indicated that there was no significant difference between the two groups in combined primary endpoint (11.4% bivalirudin vs 12.2% heparin-arm), but did result in a lower incidence of bleeding (3.8% vs 9.8%, p < 0.001). More specifically, in the patients undergoing POBA for post-infarction angina, the use of bivalirudin resulted in a lower incidence of combined primary endpoint (9.1% vs 14.2%, p = 0.04) and a lower incidence of bleeding (3% vs 11%, p < 0.001) (CitationBittl et al 1995). Following the BAT analysis, a FDA-endorsed re-analysis based on an intention-to-treat analysis confirmed these findings. Specifically, bivalirudin was at least as effective in preventing death, MI, or need for revascularization at 180 days (23.0% vs 24.7%, p = 0.153), but with fewer major bleeding complications (3.7% vs 9.3%, p =< 0.001). These findings were consistent among patients undergoing angioplasty for either unstable angina or post-infarction angina (CitationBittl et al 2001).
Another study, TIMI-8, attempted to compare the efficacy and safety of low-dose bivalirudin versus UFH in patients presenting with USA/NSTEMI. 133 patients were randomized (prior to early termination of the trial), and results suggested a trend towards less combined nonfatal MI or death or major hemorrhage at 14 days in patients receiving bivalirudin (2.9% vs 9.2%, 0% vs 4.6%, respectively) (CitationTIMI-8 2002).
With the introduction of coronary stents, clopidogrel, and GPIIb/IIIa inhibitors use in PCI, bivalirudin was tested in the Comparison of Abciximab Complications with Hirulog for Ischemic Events Trial (CACHET) and the Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events Trials (REPLACE-1 and REPLACE-2). First, CACHET, a pilot trial of 268 patients undergoing PCI, compared the use of bivalirudin (at varying doses) with and without abciximab versus standard UFH therapy with abciximab. The results of CACHET suggested that bivalirudin with planned or provisional abciximab appeared to be as safe and effective as standard UFH plus abciximab in these patients undergoing PCI (CitationLincoff et al 2002).
Subsequently, the REPLACE trials were conducted among patients undergoing urgent or elective PCI with nearly 85% of patients receiving clopidogrel before the procedure and stent implantation during the procedure. REPLACE-1 was a pilot-study that showed that bivalirudin use in contemporary PCI trended to less ischemic and bleeding outcomes (CitationLincoff et al 2004). This allowed further investigation in REPLACE-2, which randomized 6,010 patients to receive UFH (65 U/kg) plus abciximab (Reopro®, Centocor Inc., PA, USA) or eptifibatide (Integrillin®, Millenium Pharmaceuticals, Inc, MA, USA) for 12–18 hours or bivalirudin (0.75 mg/kg bolus with 1.75 mg/kg/hour infusion during the procedure) with provisional GPIIb/IIIa inhibitors use available for “bail-out” purposes (defined as abrupt vessel closure, obstructive dissection, new or suspected thrombus, slow coronary flow, distal embolization, persistent residual stenosis, unplanned stent placement, prolonged ischemia, or other clinical instability). The primary endpoint of composite death, MI, severe ischemia requiring repeat revascularization, or in-hospital major bleeding within 30 days of randomization revealed no significant difference between the two patients groups (9.2% bivalirudin vs 10% GPIIb/IIIa inhibitors, p = 0.32). However, there was less major bleeding (2.4% vs 4.1%, p = 0.001) and thrombocytopenia (0.7% vs 1.7%, p = 0.001) in those patients initially treated with bivalirudin. Although there was no significant difference in mortality at 12 month follow-up, there were patient subgroups that seemed to have a strong trend towards a reduction in mortality: elderly over 75 years of age, diabetics, renal insufficiency (as defined as a creatinine clearance less than 60 mL/min), unstable angina, or any previous heparin therapy (CitationLincoff et al 2003).
In 2006, the PROTECT-TIMI-30 study reported on its investigation comparing eptifibatide plus UFH or LMWH versus bivalirudin alone in 857 patients presenting with ACS and undergoing PCI. This trial randomized patients into three treatment arms: bivalirudin alone, eptifibatide plus reduced-dose UFH or eptifibatide plus reduced-dose LMWH. Although the null hypothesis was to show that coronary flow reserve after PCI was better with eptifibatide, the results actually showed that bivalirudin was associated with higher coronary flow reserve following PCI. Secondary endpoint analysis suggested that patients undergoing PCI who were randomized to eptifibatide experienced improved Thrombolysis In Myocardial Infarction (TIMI) myocardial perfusion grade following PCI, shorter duration of ischemia on continuous Holter monitoring after PCI, more minor bleeding events, a higher transfusion rate, and no difference in biomarkers for myonecrosis, inflammation, and thrombin generation (CitationGibson et al 2006).
More recently, guidelines have recommended an early invasive strategy for patients presenting with moderate- or high-risk ACS in conjunction with aggressive antiplatelet, antithrombotic medications (CitationAnderson et al 2007). In these patients, the use of bivalirudin as an anticoagulant was extensively evaluated in the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) Trial. This prospective, randomized trial compared UFH plus GPIIbIIIa inhibitors, bivalirudin plus GPIIbIIIa inhibitors, and bivalirudin alone in over 13,000 patients. Outcomes at 30 days revealed non-inferior rates of composite ischemic endpoint (death, MI, unplanned revascularization) (7.3% vs 7.8%, respectively, p = NS), but a significant reduction in major bleeding and net clinical outcomes endpoint (defined as combined composite ischemia and major bleeding) (3.0% vs 5.7%, p < 0.001, and 10.1% vs 11.7%, p = 0.02) in patients who received bivalirudin alone compared to UFH plus GPIIbIIIa inhibitors. Similarly, outcomes at 1 year reported no significant difference in the composite ischemic endpoint (15.4% vs 16.2%, p = 0.29) or overall mortality (3.8% vs 3.9%, p = 0.62) between the same two groups. Further multivariate analysis revealed that predictors of mortality at 1 year were major bleeding event(s) at 30 days (HR 2.89, CI 2.24–3.72, p < 0.0001) and/or MI (HR 2.47, CI 1.87–3.27, p < 0.0001) (CitationStone et al 2006, Citation2007a).
Subsequent analysis investigated the 7,789 patients from the ACUITY trial who underwent PCI. Thirty-day endpoints of composite ischemia or net clinical outcomes were not different between the groups; however there was still significantly less bleeding in the bivalirudin alone group compared to patients receiving UFH plus GPIIbIIIa inhibitors (3.5% vs 6.8%, p < 0.0001). Of note, in patients who were troponin-positive at the time of PCI, there were no significant difference in rates of ischemic complications in the between the treatment groups (but an absolute 1% increase in ischemic endpoints in the bivalirudin-alone group). In addition, in patients who had received clopidogrel loading prior to their PCI, rates of 30-day ischemic events and overall reduction in combined net clinical outcomes were similar between the bivalirudin-alone and UFH plus GPIIbIIIa inhibitors groups (8.1% vs 8.4%, p = NS and 11.1% vs 13.8%, p = NS) (CitationStone et al 2007b). These robust data resulted in a Class I indication for the use of bivalirudin in patients presenting with USA/NSTEMI (CitationAnderson et al 2007).
Of note, one study reported the safety and efficacy of using bivalirudin during PCI requiring rotational atherectomy (RA) in patients presenting for elective or emergent revascularization. This smaller, single-center study reviewed 253 cases, 56 of whom were treated with bivalirudin during their RA procedure, and reported no difference in the incidence of any myonecrosis as measured by elevated CK-MB post-procedure (CitationGurm et al 2007).
HIT/HITTS patients
Up to 5% of patients given heparin develop HIT or HIT with thrombotic syndrome (HITTS). Initial case reports of the safety and efficacy of using hirolug in the setting of POBA in patients with HIT/HITTS were published in 1995 (CitationChamberlin et al 1995). Since that time, further investigation (ATBAT trial) was conducted in a prospective, single-arm study evaluating the safety and efficacy of bivalirudin in patients undergoing PCI with newly diagnosed or previous HIT/HITTS. In this trial, 52 patients were identified and received bivalirudin during and up to 4 hours following the PCI procedure. The primary endpoint was major bleeding prior to discharge. The investigators reported no major bleeding events in any patient undergoing PCI (one major bleeding event in a patient who underwent elective CABG) or need for transfusion. More importantly, there was no significant thrombocytopenia observed in the 52 patients following PCI after administration of bivalirudin. These data supported bivalirudin’s Class I indication for use in patients with or at high-risk of HIT/HITTS during treatment for ACS or undergoing PCI (CitationMahaffey et al 2003; CitationAnderson et al 2007).
STEMI patients
Initial investigation for the use of bivalirudin for patients presenting with STEMI and undergoing PCI was reported in the HERO-2 Trial. This study evaluated the efficacy of using bivalirudin in conjunction with standard thrombolytic (streptokinase) therapy (versus standard UFH+streptokinase) in over 17,000 STEMI patients. At 30 days, comparison between the two groups revealed no significant difference in overall mortality, but patients randomized to bivalirudin therapy experienced significantly less reinfarction within 96 hours of presentation (RR 0.70, CI 0.56–0.87, p = 0.001) (CitationWhite 2001).
Further investigation for the use of bivalirudin in patients presenting with STEMI undergoing PCI was reported in the BiAMI Trial. Presented in 2006, this prospective, single-arm study treated patients presenting with STEMI to standard-dosing bivalirudin infusion for the duration of the procedure, with abciximab used only if TIMI 3 flow was not established post-stenting. Event rates at 1 month reported that bivalirudin provided comparable ischemic protection with fewer bleeding complications (compared to previously reported UFH plus inhibitors IIb/IIIa data) (CitationStella et al 2004, Citation2006).
In 2007, the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS AMI) was presented. This trial randomized over 3,600 patients presenting with STEMI to UFH plus GPIIb/IIIa inhibitors or to bivalirudin monotherapy with provisional GPIIb/IIIa inhibitors for large thrombus or refractory no-flow. At 30 days, there was a significant 24% reduction in net clinical events (defined as combined death, MI, ischemia requiring repeat-revascularization, stroke, or major bleeding) and a 40% reduction in major bleeding. In addition, patients in the bivalirudin monotherapy arm had lower rates of cardiac mortality (1.8% vs 2.9%, p = 0.035), but higher rates of acute stent thrombosis in the first 24 hours post-PCI (0.3% vs 1.3%, p = 0.0009) (CitationStone 2007).
It is important to note that for several of these studies there was post-hoc analysis investigating whether a hazard existed if UFH or LMWH was administered prior to the study medication (bivalirudin). The SWITCH trial, in which ACS patients were treated with LMWH and switched (at varying time interval prior to the procedure) to bivalirudin for PCI, showed that switching from LWMH to bivalirudin was not associated with an increase in major bleeding regardless of the time from last dose of LMWH (CitationWaksman et al 2006). Later, analysis from REPLACE-2 showed that there was no significant difference in major or minor bleeding in patients who were randomized to bivalirudin after receiving UFH or LMWH when compared to those patients who were not treated with any heparinoid medication prior to randomization. In contrast, patients who were randomized to receive UFH plus GPIIbIIIa inhibitors after being treated with a heparin experienced a significant increase in all bleeding events and transfusions (CitationGibson et al 2007).
Other patient subgroups
Although beyond the scope of this review, the successful use of bivalirudin in CABG in patients with either HIT/HITTS or a contraindication to protamine use has been reported (CitationJabr et al 2004; CitationMerry 2004; CitationMerry et al 2004). More specifically, two randomized, multicenter trials compared UFH with protamine reversal to bivalirudin in patients undergoing CABG with or without cardiopulmonary bypass. At 12 weeks follow-up, there were no significant differences in procedural success, mortality, 24-hour blood loss, overall incidence of transfusions, and duration of surgery between the two treatment groups (CitationDyke et al 2006; CitationSmedira et al 2006).
In addition, the use of bivalirudin during percutaneous peripheral revascularization has been investigated (CitationEres 2006). A recent small, prospective study has reported acceptable in-hospital and 30-day ischemic and bleeding outcomes in this patient population and clinical setting (APPROVE Trial) (CitationAPPROVE 2004).
Impact of anemia, bleeding, and transfusions in ACS
The presence of anemia in ACS patients may not only be a predictor of an increase the risk of major hemorrhagic complications during PCI but also may be linked to an increase in mortality (CitationRao et al 2005; CitationEikelboom et al 2006). Previous studies have shown that anemic patients undergoing PCI were more likely to have in-hospital death (CitationMcKechnie et al 2004), composite major adverse cardiac events (CitationLee et al 2004; CitationMcKechnie et al 2004), and higher post-procedure cardiac biomarker release (CitationLee et al 2004). The occurrence of these hemorrhagic complications was investigated in an analysis over 9,900 patients who underwent PCI. Investigators followed these patients for the incidence of ischemic and bleeding complications by activated clotting time (ACT) quartile. Ischemic endpoints (defined as death, MI, or revascularization at 48 hours) were not correlated with maximal procedural ACT, but higher doses of UFH (> 5000 U, or up to 90 U/kg) were independently associated with higher rates of events. There was a significant linear relationship between ACT quartile and occurrence of an overall bleeding event (CitationBrener et al 2004).
In regards to use with bivalirudin, evaluation of anemic versus non-anemic patients from another trial suggests that anemic patients undergoing PCI were more likely to be older, non-Caucasian, female gender, of lower body weight, of worse renal function, have previous MI, have prior revascularization (PCI or CABG), and/or present with ACS. In addition, anemic patients were found to have an increased risk of 1-year mortality, major bleeding, and transfusion rate (4.3% vs 1.5%, 4.9% vs 2.8%, 3.6% vs 0.7%, respectively). However, there was no increase in rates of ischemic events in the anemic patients (7.6% vs 7.3%, p = NS). When causes of death were investigated in these patients, there was not a preponderance of cardiovascular related (versus non-cardiovascular-related) deaths in the anemic group (CitationVoeltz et al 2007).
In addition, a post-hoc analysis of a more recent larger trial examined the predictors of major bleeding and its impact on 30-day outcomes, including mortality. The study’s results showed that patients who were randomized to UFH plus GPIIbIIIa inhibitors had higher major bleeding rates compared to those patients that received bivalirudin alone (5.7% vs 3.0%, p < 0.001). Furthermore, patients with major bleeding had higher 30-day rates of mortality (7.3% vs 1.2%, p < 0.0001), composite ischemia (23.1% vs 6.8%, p < 0.0001), and stent thrombosis (3.4% vs 0.6%, p < 0.0001) compared to those patients who did not. Of note, patients who had a major bleeding event following PCI had a > 7-fold times mortality rate than those patients who did not (OR 7.55, CI 4.68–2.18, p < 0.0001), which was a stronger predictor of mortality than peri-procedural MI (CitationManoukian et al 2007).
Based on these data, baseline anemia and peri-procedural major bleeding events following PCI are a powerful independent predictor of (short-term) mortality in ACS patients who are managed invasively. Therefore, it seems imperative that improvements in treatment modalities or antithrombotic medications (eg, DTIs) reduce bleeding rates during and following PCI.
Summary
In patients presenting with ACS and selected for an early, invasive treatment strategy with cardiac catheterization and subsequent PCI, aggressive antiplatelet and antithrombotic medications are required to avoid worsened ischemic outcomes. But, these potent anticoagulants pose risks of increased bleeding events. Currently, with a number of available anticoagulant medications but inadequate comparative data, there is no recommendation for one preferred regimen in treating ACS patients. However, bivalirudin has several pharmacologic and pharmacodynamic properties that appear advantageous over indirect thrombin inhibitors. Numerous trials regarding USA/NSTEMI patients undergoing PCI have shown that these properties have translated to excellent clinical efficacy including similar rates of ischemic events and lower rates of bleeding when compared to therapy including a heparin plus GPIIbIIIa inhibitors. Therefore, bivalirudin is a safe and effective anticoagulant for use in ACS with PCI. Further (long-term) data are still needed to assess bivalirudin’s role in STEMI patients.
Acknowledgements
The authors would like to thank Dr. John F. Moran, M.D. for his review of this manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
References
- AnandSXKimMCKamranMComparison of platelet function and morphology in patients undergoing percutaneous coronary intervention receiving bivalirudin versus unfractionated heparin versus clopidogrel pretreatment and bivalirudinAm J Cardiol20071004172417659921
- AndersonJLAdamsCDAntmanEMACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction developed in collaboration with the American College of Emergency Physicians the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency MedicineJ Am Coll Cardiol200750e1e15717692738
- APPROVEThe Angiomax Peripheral Procedure Registry of Vascular Events Trial (APPROVE): in-hospital and 30-day resultsJ Invasive Cardiol200416651615550739
- AroraUKDhirMDirect thrombin inhibitors part 1 of 2J Invasive Cardiol20051734815640538
- BatesERBivalirudin: an anticoagulant option for percutaneous coronary interventionExpert Rev Cardiovasc Ther200421536215151464
- BatesSMWeitzJIDirect thrombin inhibitors for treatment of arterial thrombosis: potential differences between bivalirudin and hirudinAm J Cardiol19988212P18P
- BeckerDLFredenburghJCStaffordARExosites 1 and 2 are essential for protection of fibrin-bound thrombin from heparin-catalyzed inhibition by antithrombin and heparin cofactor IIJ Biol Chem199927462263310037709
- BittlJAComparative safety profiles of hirulog and heparin in patients undergoing coronary angioplasty. The Hirulog Angioplasty Study InvestigatorsAm Heart J1995130658657668214
- BittlJAChaitmanBRFeitFBivalirudin versus heparin during coronary angioplasty for unstable or postinfarction angina: Final report reanalysis of the Bivalirudin Angioplasty StudyAm Heart J2001142952911717596
- BittlJAStronyJBrinkerJATreatment with bivalirudin Hirulog. as compared with heparin during coronary angioplasty for unstable or postinfarction angina Hirulog Angioplasty Study InvestigatorsN Engl J Med199533376497643883
- BrenerSJMoliternoDJLincoffAMRelationship between activated clotting time and ischemic or hemorrhagic complications: analysis of 4 recent randomized clinical trials of percutaneous coronary interventionCirculation2004110994815302778
- BurkeAPFarbAMalcomGTEffect of risk factors on the mechanism of acute thrombosis and sudden coronary death in womenCirculation199897211069626170
- BurkeAPFarbAMalcomGTCoronary risk factors and plaque morphology in men with coronary disease who died suddenlyN Engl J Med19973361276829113930
- CairnsJAGentMSingerJAspirin, sulfinpyrazone, or both in unstable angina. Results of a Canadian multicenter trialN Engl J Med19853131369753903504
- CannonCPMaraganoreJMLoscalzoJAnticoagulant effects of hirulog a novel thrombin inhibitor in patients with coronary artery diseaseAm J Cardiol199371778828456753
- CAPRIEA randomized blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events CAPRIE. CAPRIE Steering CommitteeLancet19963481329398918275
- ChamberlinJRLewisBLeyaFSuccessful treatment of heparin-associated thrombocytopenia and thrombosis using HirulogCan J Cardiol19951151147780873
- ChongBHHeparin-induced thrombocytopeniaJ Thromb Haemost200311471812871282
- CohenMAdamsPCParryGCombination antithrombotic therapy in unstable rest angina and non-Q-wave infarction in nonprior aspirin users Primary end points analysis from the ATACS trial. Antithrombotic Therapy in Acute Coronary Syndromes Research GroupCirculation1994898188281698
- DaLHeparin binding and neutralizing proteins1989Boca RatonCRC Press
- De RomeufCMazurierCHeparin binding assay of von Willebrand factor vWF in plasma milieu – evidence of the importance of the multimerization degree of vWFThromb Haemost199369436408322266
- Di NisioMMiddeldorpSBullerHRDirect thrombin inhibitorsN Engl J Med200535310284016148288
- DykeCMSmediraNGKosterAA comparison of bivalirudin to heparin with protamine reversal in patients undergoing cardiac surgery with cardiopulmonary bypass: the EVOLUTION-ON studyJ Thorac Cardiovasc Surg2006131533916515902
- EikelboomJWMehtaSRAnandSSAdverse impact of bleeding on prognosis in patients with acute coronary syndromesCirculation20061147748216908769
- EitzmanDTChiLSagginLHeparin neutralization by platelet-rich thrombi. Role of platelet factor 4Circulation199489152398149517
- EresAUse of bivalirudin as the foundation anticoagulant during percutaneous peripheral interventionsJ Invasive Cardiol200618125816598112
- FentonJW2ndOfosuFABrezniakDVThrombin and antithromboticsSemin Thromb Hemost1998248791
- FergusonJJCaliffRMAntmanEMEnoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trialJAMA2004292455415238590
- FoxIDawsonALoyndsPAnticoagulant activity of Hirulog a direct thrombin inhibitor in humansThromb Haemost199369157638456428
- FRISCLow-molecular-weight heparin during instability in coronary artery disease Fragmin during Instability in Coronary Artery Disease FRISC study groupLancet199634756188596317
- FurieBFurieBCMolecular and cellular biology of blood coagulationN Engl J Med199232680061538724
- GibsonCMMorrowDAMurphySAA randomized trial to evaluate the relative protection against post-percutaneous coronary intervention microvascular dysfunction ischemia, and inflammation among antiplatelet and antithrombotic agents: the PROTECT-TIMI-30 trialJ Am Coll Cardiol20064723647316781360
- GibsonCMTenYMurphySAAssociation of prerandomization anticoagulant switching with bleeding in the setting of percutaneous coronary intervention A REPLACE-2 analysisAm J Cardiol20079916879017560876
- GurfinkelEPManosEJMejailRILow molecular weight heparin versus regular heparin or aspirin in the treatment of unstable angina and silent ischemiaJ Am Coll Cardiol19952631387608429
- GurmHSRajagopalVBhattDLThe safety of a bivalirudin-based approach in patients undergoing rotational atherectomyJ Invasive Cardiol200719225817476038
- HirshJHeparinN Engl J Med19913241565742027360
- HoggPJBockPEModulation of thrombin and heparin activities by fibrinThromb Haemost199777424339065987
- ISIS-2Randomised trial of intravenous streptokinase oral aspirin both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative GroupLancet19882349602899772
- JabrKJohnsonJMcDonaldMHPlasma-modified ACT can be used to monitor bivalirudin Angiomax anticoagulation for on-pump cardiopulmonary bypass surgery in a patient with heparin-induced thrombocytopeniaJournal of Extra-Corporeal Technology200436174715334761
- KingSB3rdSmithSCJrHirshfeldJWJr2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention A Report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesCirculation2007
- KumarRBeguinSHemkerHCThe influence of fibrinogen and fibrin on thrombin generation – evidence for feedback activation of the clotting system by clot bound thrombinThromb Haemost199472713217900079
- KumarRBeguinSHemkerHCThe effect of fibrin clots and clot-bound thrombin on the development of platelet procoagulant activityThromb Haemost19957496288571330
- LeePCKiniASAhsanCAnemia is an independent predictor of mortality after percutaneous coronary interventionJ Am Coll Cardiol200444541615358017
- LehmanSJChewDPBivalirudin in percutaneous coronary interventionVasc Health Risk Manag200623576317323589
- LewisHDJrDavisJWArchibaldDGProtective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative StudyN Engl J Med19833093964036135989
- LidonRMTherouxPJuneauMInitial experience with a direct antithrombin Hirulog, in unstable angina Anticoagulant, antithrombotic, and clinical effectsCirculation19938814955018403297
- LincoffAMBittlJAHarringtonRABivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trialJAMA200328985363
- LincoffAMBittlJAKleimanNSComparison of bivalirudin versus heparin during percutaneous coronary intervention the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trialAm J Cardiol2004931092615110198
- LincoffAMKleimanNSKottke-MarchantKBivalirudin with planned or provisional abciximab versus low-dose heparin and abciximab during percutaneous coronary revascularization: results of the Comparison of Abciximab Complications with Hirulog for Ischemic Events Trial CACHETAm Heart J20021438475312040347
- MahaffeyKWLewisBEWildermannNMThe anticoagulant therapy with bivalirudin to assist in the performance of percutaneous coronary intervention in patients with heparin-induced thrombocytopenia ATBAT study: main resultsJ Invasive Cardiol200315611614608128
- ManoukianSVFeitFMehranRImpact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY TrialJ Am Coll Cardiol2007491362817394970
- MaraganoreJMBourdonPJablonskiJDesign and characterization of hirulogs: a novel class of bivalent peptide inhibitors of thrombinBiochemistry19902970951012223763
- McKechnieRSSmithDMontoyeCPrognostic implication of anemia on in-hospital outcomes after percutaneous coronary interventionCirculation2004110271715226214
- MehtaSRYusufSPetersRJEffects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE studyLancet20013585273311520521
- MerryAFBivalirudin, blood loss and graft patency in coronary artery bypass surgerySemin Thromb Hemost2004303374615282656
- MerryAFRaudkiviPJMiddletonNGBivalirudin versus heparin and protamine in off-pump coronary artery bypass surgeryAnn Thorac Surg20047792531 discussion 93114992900
- MirshahiMSoriaJSoriaCEvaluation of the inhibition by heparin and hirudin of coagulation activation during r-tPA-induced thrombolysisBlood1989741025302502207
- OlerAWhooleyMAOlerJAdding heparin to aspirin reduces the incidence of myocardial infarction and death in patients with unstable angina. A meta-analysisJAMA199627681158769591
- ParryMAMaraganoreJMStoneSRKinetic mechanism for the interaction of Hirulog with thrombinBiochemistry19943314807147993908
- RaoSVO’GradyKPieperKSImpact of bleeding severity on clinical outcomes among patients with acute coronary syndromesAm J Cardiol2005961200616253582
- ReedMDBellDClinical pharmacology of bivalirudinPharmacotherapy200222105S111S12064567
- RISCRisk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease The RISC GroupLancet1990336827301976875
- RobsonRThe use of bivalirudin in patients with renal impairmentJ Invasive Cardiol200012Suppl F33F6
- RosamondWFlegalKFridayGHeart disease and stroke statistics – 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics SubcommitteeCirculation2007115e6917117194875
- SakharovDVPlowEFRijkenDCOn the mechanism of the antifibrinolytic activity of plasma carboxypeptidase BJ Biol Chem199727214477829162090
- SchaarJAMullerJEFalkETerminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque June 17 and 18, 2003, Santorini, GreeceEur Heart J20042510778215191780
- SchrorKThe basic pharmacology of ticlopidine and clopidogrelPlatelets1993425261
- SciulliTMMauroVFPharmacology and clinical use of bivalirudinAnn Pharmacother20023610284112022907
- SharmaGVLapsleyDVitaJAUsefulness and tolerability of hirulog a direct thrombin-inhibitor, in unstable angina pectorisAm J Cardiol1993721357608256726
- ShenGXXMFentonJWEffect of hirulog-1 on fibrinolysis and platelet deposition abstractAtherosclerosis1997134195
- SmediraNGDykeCMKosterAAnticoagulation with bivalirudin for off-pump coronary artery bypass grafting: the results of the EVOLUTION-OFF studyJ Thorac Cardiovasc Surg20061316869216515924
- StellaJStellaDIaffaldanoRThe bivalirudin in the management of patients with ST-segment elevation acute myocardial infarction undergoing primary PCI BIAMI Trial. Society of Cardiovascular Angiography and Interventions2006
- StellaJFStellaREIaffaldanoRAAnticoagulation with bivalirudin during percutaneous coronary intervention for ST-segment elevation myocardial infarctionJ Invasive Cardiol200416451415353822
- StoneGWHORIZONS AMI: Bivalirudin reduces bleeding, adverse clinical events in STEMI2007Washington, D.CTCT
- StoneGWMcLaurinBTCoxDABivalirudin for patients with acute coronary syndromesN Engl J Med200635522031617124018
- StoneGWWareJHBertrandMEAntithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trialJAMA2007a298249750618056903
- StoneGWWhiteHDOhmanEMBivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy ACUITY trialLancet2007b3699071917368152
- TelfordAMWilsonCTrial of heparin versus atenolol in prevention of myocardial infarction in intermediate coronary syndromeLancet19811122586112564
- TherouxPOuimetHMcCansJAspirin, heparin, or both to treat acute unstable anginaN Engl J Med19883191105113050522
- TherouxPWatersDQiuSAspirin versus heparin to prevent myocardial infarction during the acute phase of unstable anginaCirculation199388204588222097
- TIMI-8Bivalirudin as a replacement for unfractionated heparin in unstable angina/non-ST-elevation myocardial infarction: observations from the TIMI 8 trial. The Thrombolysis in Myocardial InfarctionAm Heart J20021432293411835024
- TopolEJBonanRJewittDUse of a direct antithrombin hirulog, in place of heparin during coronary angioplastyCirculation199387162298491018
- VirmaniRBurkeAPFarbAPathology of the vulnerable plaqueJ Am Coll Cardiol200647C13816631505
- VirmaniRKolodgieFDBurkeAPLessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesionsArterioscler Thromb Vasc Biol20002012627510807742
- VoeltzMDPatelADFeitFEffect of anemia on hemorrhagic complications and mortality following percutaneous coronary interventionAm J Cardiol2007991513717531572
- WaksmanRWolframRMTorgusonRLSwitching from Enoxaparin to Bivalirudin in Patients with Acute Coronary Syndromes without ST-segment Elevation who Undergo Percutaneous Coronary Intervention. Results from SWITCH – a multicenter clinical trialJ Invasive Cardiol200618370516877786
- WeitzJILow-molecular-weight heparinsN Engl J Med1997337688989278467
- WeitzJIHudobaMMasselDClot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitorsJ Clin Invest199086385912384594
- WeitzJILeslieBHudobaMThrombin binds to soluble fibrin degradation products where it is protected from inhibition by heparin-antithrombin but susceptible to inactivation by antithrombin-independent inhibitorsCirculation199897544529494024
- WhiteHThrombin-specific anticoagulation with bivalirudin versus heparin in patients receiving fibrinolytic therapy for acute myocardial infarction: the HERO-2 randomised trialLancet200135818556311741625
- WilliamsDOKirbyMGMcPhersonKAnticoagulant treatment of unstable anginaBr J Clin Pract19864011463518780
- YusufSMehtaSRChrolaviciusSComparison of fondaparinux and enoxaparin in acute coronary syndromesN Engl J Med2006a35414647616537663
- YusufSMehtaSRChrolaviciusSEffects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trialJAMA2006b29515193016537725
- YusufSZhaoFMehtaSREffects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevationN Engl J Med200134549450211519503