Abstract
Endothelial dysfunction is the common link between cardiovascular disease risk factors and the earliest event in the cascade of incidents that results in target organ damage. Angiotensin II, the terminal pressor effector arm of the renin-angiotensin-aldosterone system, increases blood pressure (BP) by vasoconstriction and sodium and fluid retention, and has a pro-oxidative action that induces endothelial dysfunction and contributes to vascular remodeling. Angiotensin receptor blockers (ARBs) reduce BP and morbidity and mortality in patients with hypertension, ventricular hypertrophy, diabetes mellitus, and renal disease. Olmesartan medoxomil is a long-acting, well-tolerated, effective ARB that prevents or reverses endothelial dysfunction in animal models of atherosclerosis, hypertension, diabetes, nephropathy, and retinopathy. Olmesartan medoxomil, a prodrug of olmesartan approved for the treatment of hypertension, has been shown to ameliorate endothelial dysfunction in patients with hypertension or diabetes. In randomized studies, the drug reduces vascular inflammation and the volume of large atherosclerotic plaques, increases the number of regenerative endothelial progenitor cells in the peripheral circulation, improves endothelium-dependent relaxation, and restores the normal resistance vessel morphology. Importantly, the impact of olmesartan medoxomil on endothelial dysfunction is thought to be independent of BP lowering.
Introduction
Major advances in the treatment of cardiovascular disease (CVD) have been accompanied by the development of effective prevention strategies.Citation1,Citation2 In spite of these advances, CVD continues to be the leading cause of death in most countries in the world.Citation3 The ongoing epidemic of obesity and increasing prevalence of diabetes mellitus and chronic kidney disease will further contribute to keeping diseases of the heart and blood vessels as the predominant cause of cardiovascular mortality.Citation4–Citation6
The common link between CVD risk factors such as hypertension, diabetes, and smoking is now recognized to be vascular endothelial dysfunction.Citation7 Under normal circumstances, the vascular endothelium influences vessel tone and modulates structure, vessel permeability, adhesion and migration of inflammatory cells, and hemostasis. In addition, the healthy vascular endothelium has a role in the prevention of oxidative damage. In contrast, endothelial dysfunction may be one of the earliest events initiating the cascade of maladaptive changes that contribute to target organ damage.Citation8
Endothelial dysfunction of the coronary arteries is associated with an increased CVD risk.Citation9 In addition to the conventional cardiovascular risk factors (coronary artery disease [CAD], age, and body mass index), markers of endothelial dysfunction were identified as independent predictors of adverse events during a mean of 46 months of follow-up in a prospective study in patients undergoing cardiac catheterization.Citation9 Markers of endothelial dysfunction that predicted adverse outcomes included the change in coronary vascular resistance (ΔCVR) after intracoronary administration of acetylcholine and epicardial constriction with acetylcholine. Survival was improved in patients with the best microvascular responses after a 2-minute intracoronary infusion of acetylcholine.Citation9
Carotid artery intima-media thickness (IMT) is linearly correlated with the risk of myocardial infarction (MI) and stroke.Citation10 A recent meta-analysis of eight studies showed that the risk of MI increased by approximately 25% and the risk of stroke increased by approximately 15% for each increase of one standard deviation in carotid artery IMT.Citation10 Other studies have demonstrated reduced endothelial vasodilator capacity in siblings of hypertensive subjects.Citation11,Citation12 The common factor responsible for this phenomenon may be a novel polymorphism in the principal arginine transporter that accounts, at least in part, for the link between endothelial dysfunction, L-arginine, nitric oxide metabolism, and essential hypertension.Citation13
Natural history of endothelial dysfunction
The development and progression of atherosclerosis is a result of inflammatory processes including expression of markers of endothelial activation.Citation14 For example, vascular cell adhesion molecule-1 facilitates the binding of monocytes and T-lymphocytes to vessel walls. A variety of cytokines and growth factors, such as macrophage colony-stimulating factor-1 (M-CSF-1) and transforming growth factor (TGF)-α and TGF-β, are released from inflammatory lesions, recruit additional leucocytes, and stimulate proliferation of vascular smooth muscle cells.
As discussed elsewhere, the presence of monocytes in the intima is a characteristic of the early stages of plaque formation.Citation15–Citation18 Chemoattractant substances such as monocyte chemoattractant protein-1 (MCP-1) are generated by vascular inflammation and promote infiltration of monocytes into the intima. After infiltrating the vascular endothelium, monocytes are transformed into tissue macrophages, which engulf lipid particles and become “foam cells”. Accumulation of foam cells gives rise to a visible fatty streak on the luminal surface of arteries. Work by the author and his associates first established an obligatory role of angiotensin II (Ang II) in mediating the cascade of initiating events leading to the appearance of foam cells.Citation15–Citation18
Endothelial dysfunction involves accumulation of lipids, inflammation, and proliferation of vascular smooth muscle cells. Recruitment of inflammatory cells, proliferation of vascular smooth muscle, and accumulation of lipids by foam cells all contribute to thickening of the intima and the creation of an atherosclerotic plaque. Atherosclerosis occurs preferentially in areas of turbulent blood flow and low shear stress.Citation19
Role of the renin-angiotensin-aldosterone system (RAAS) in endothelial dysfunction
The renin-angiotensin-aldosterone system (RAAS) has a well-established role in the regulation of sodium levels, fluid balance, and blood pressure (BP). Emerging evidence suggests that this system is also involved in the pathophysiology of type 2 diabetes mellitus and obesity.Citation20–Citation26
The classical view of the RAAS holds that the octapeptide Ang II is the terminal effector in this endocrine signaling pathway, and that upon binding to Ang II type 1 (AT1) receptors, vasoconstriction and sodium and water retention occur, along with mitogenic and proliferative effects on vascular endothelial and smooth muscle cells.Citation27 There is evidence that Ang II is involved in pathologic inflammation, and that it increases oxidative stress by regulating nicotinamide adenine dinucleotide phosphate (NADPH) levels, the principal source of reactive oxygen species (ROS) in the vasculature. Ang II also accelerates senescence of endothelial progenitor cells (EPCs).Citation28 Senescence of EPCs is accelerated in patients with essential hypertension, and patients with CAD have fewer EPCs in peripheral blood.Citation29
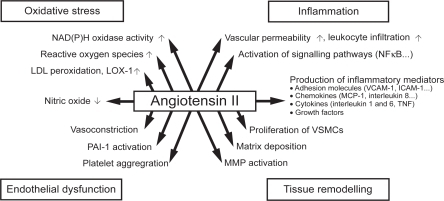
A seminal study assessing the role of Ang II in the development of atherosclerosis in primates first documented a critical contribution of the renin-angiotensin system in the origin of fatty streak formation. In this study,Citation30 the administration of the angiotensin receptor blocker (ARB), losartan, prevented the development of aortic fatty streak formation by a mechanism independent of BP. The central role of Ang II in endothelial dysfunction and atherosclerotic plaque formation is illustrated in . Later studies in knockout mice confirm that the AT1 receptor is involved in atherosclerosis. Apolipoprotein E knockout mice (ApoE−/−) have impaired endothelium-dependent vasodilation, significantly higher superoxide radical release rates, and develop marked atherosclerotic lesions when fed a high cholesterol diet. In contrast, AT1−/− mice have greatly reduced vascular oxidative stress, endothelial dysfunction, and atherosclerotic lesion formation irrespective of BP and plasma cholesterol levels. When ApoE−/− mice were crossbred with AT1−/− mice, the resulting progeny (ApoE−/−, AT1−/−) had significantly lower BP levels and superoxide release rates than ApoE−/− mice and did not develop atherosclerosis. These studies suggest a fundamental role of AT1 receptor activation in atherogenesis.Citation31 In the pursuit of the mechanism accounting for the actions of Ang II in atherogenesis, we found that early activation of monocytes occurred through upregulation of the bone marrow renin-angiotensin system, whereby hypercholesterolemia stimulates the expression of bone marrow CD11b(+) receptors in monocytes.Citation32–Citation34
Figure 1 Atherosclerotic plaque formation in relationship to Ang II. Reprinted from the Lancet, 369, Schmeider RE, Hilgers KF, Schlaich MP. Renin-angiotensin system and cardiovascular risk, 1208–1219.Citation109 Copyright © 2007, with permission from Elsevier.
Over the last decade, it has become clear that the classical view of the RAAS is in need of revision. An additional RAAS effector that counterbalances the biological actions of Ang II has been isolated and characterized. This additional effector, the heptapeptide Ang-(1–7), is a fragment produced by hydrolysis of Ang I by several tissue-specific endopeptidases (prolyl oligopeptidase and thimet oligopeptidase in vascular endothelial and smooth muscle cells, and neprilysin in kidney cells) that bind to the G-protein-coupled mas receptor.Citation35–Citation38 More recently, it has been shown that Ang-(1–7) is also produced by a homolog of angiotensin-converting enzyme (ACE), known as angiotensin-converting enzyme 2 (ACE2). ACE2 is a membrane-bound metallopeptidase that has a high specificity for Ang II and acts to regulate the balance between Ang II and Ang-(1–7) in tissues via a mitogen-activated protein kinase phosphatase.Citation27,Citation39 Like Ang II, Ang-(1–7) is pleiotropic; however, in contrast to the effects of Ang II, Ang-(1–7) acts as a vasodilator and growth inhibitor and has been shown to counter-regulate the effects of Ang II in human endothelial cells.Citation40 The role of Ang-(1–7) in CVD and hypertension is shown in .
Table 1 Ang-(1–7) in cardiovascular disease and hypertension
Disruption of ACE2 resulted in marked increases in Ang II levels and cardiac contractility defects in rat models of hypertension.Citation41 In contrast, ablation of ACE in ACE2-deficient mice restored the normal cardiac phenotype.Citation41 These data demonstrate that a counter-regulatory balance exists between the two arms of the RAAS, as illustrated in .
Figure 2 The renin-angiotensin-aldosterone system. Reproduced with permission from Trask AJ, Ferrario CM. Angiotensin-(1–7): pharmacology and new perspectives in cardiovascular treatments. Cardiovasc Drug Rev. 2007;25(2):162–174.Citation60 Copyright © 2007. Blackwell Publishing.
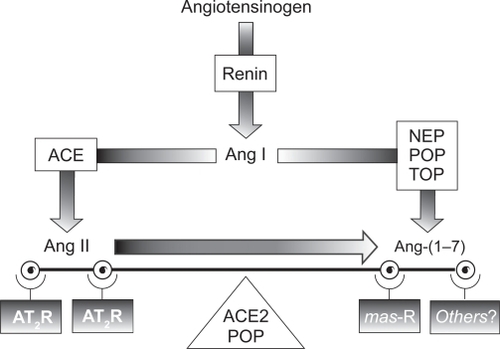
As proposed by Ferrario,Citation42 under normal physiological conditions, both the ACE/Ang II and the ACE2/Ang(1–7) axes of the RAAS strike a balance between proliferative and antiproliferative effects. However, when a physiological imbalance occurs as a result of a pathological process, such as increased activity of the ACE/Ang II/AT1 receptor axis, the failure of compensation by the opposing axis favors hypertension and consequent cardiovascular remodeling.
Drugs that target and block the effects of Ang II would be expected to prevent or reverse endothelial dysfunction and improve long-term morbidity and mortality outcomes in a broad spectrum of patients. This is, indeed, the case. Three classes of agents, ACE inhibitors, ARBs, and, most recently, direct renin inhibitors (DRIs), have been developed to specifically target the RAAS. Most experience has been gained with ACE inhibitors and ARBs, which are widely approved and used for the treatment of hypertension and prevention of CVD, although a recent study showed that aliskiren resulted in striking reductions of atherosclerosis in fat-fed low-density lipoprotein (LDL) receptor-deficient (LDLr−/−) mice.Citation43
ARBs selectively target the AT1 receptor and have a well-established role in the management of hypertension. Drugs in this class have been shown to improve outcomes in patients with hypertension, ventricular hypertrophy, diabetes mellitus, renal disease, and congestive heart failure.Citation44–Citation51
Within the ARB class, olmesartan medoxomil is a long-acting Ang II antagonist approved for the treatment of mild to severe hypertension, alone or in combination with other agents. In addition to producing sustained reductions in BP, the drug corrects the underlying defects that lead to endothelial dysfunction in animal models of CVD, and in patients with CVD or hypertension. Moreover, the beneficial effects of the drug on endothelial dysfunction have been shown to complement those of the 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors. Intriguing evidence suggests that olmesartan upregulates cardiac and vascular expression of ACE2, which leads to greater conversion of Ang II into the vasodilator and anti-proliferative peptide Ang-(1–7).Citation27,Citation30,Citation39,Citation42,Citation52–Citation62 The purpose of this paper is to review the extensive literature on the effects of olmesartan medoxomil on endothelial dysfunction, both in humans and animal models.
Studies in humans
Olmesartan medoxomil produces broad-based improvements in endothelial dysfunction in patients with hypertension. The drug reduces vascular micro-inflammation, decreases the volume of large atherosclerotic plaques, improves endothelium-dependent relaxation, and restores normal resistance vessel morphology. An overview of beneficial effects of olmesartan medoxomil on endothelial function in humans is provided in .
Table 2 Overview of beneficial effects of olmesartan medoxomil on endothelial function in humans
Effect of olmesartan medoxomil on markers of vascular inflammation: the EUTOPIA study
Olmesartan medoxomil significantly reduced vascular inflammation in patients with hypertension in the European Trial on Olmesartan and Pravastatin in Inflammation and Atherosclerosis (EUTOPIA) study. EUTOPIA was a randomized, placebo-controlled, multicenter study in 199 patients with hypertension and vascular micro-inflammation. Vascular micro-inflammation was defined as the presence of any diagnosed atherosclerotic disease (eg, coronary or peripheral arterial disease), type 2 diabetes mellitus, and/or an LDL-cholesterol level between 3.89 and 6.48 mmol/L (152–253 mg/dL).Citation63 In addition to hypertension (seated diastolic BP [SeDBP] of 95 to 110 mmHg), eligible patients were required to have a high-sensitivity C-reactive protein (hsCRP) concentration >3 mg/L but ≤20 mg/L, and detectable serum levels of interleukin-6 (IL-6) and intercellular adhesion molecule-1 (ICAM-1).Citation63 Patients were randomized to 12 weeks of treatment with olmesartan medoxomil 20 mg/day or placebo. Hydrochlorothiazide 12.5 or 25 mg/day could be added for patients whose SeDBP remained ≥90 mmHg. After 6 weeks of double-blind treatment, all patients started treatment with the HMG-CoA reductase inhibitor pravastatin 20 mg/day and continued treatment for the remainder of the study.Citation63 The final full analysis set comprised 100 patients in the olmesartan medoxomil and 99 patients in the placebo treatment groups.
The primary outcome was the impact of olmesartan medoxomil (alone or in combination with pravastatin) on a panel of anti-inflammatory measures: hsCRP, high sensitivity tumor necrosis factor-α (hsTNF-α), IL-6, ICAM-1, and MCP-1.Citation63 Olmesartan medoxomil produced significant reductions from baseline in hsCRP, hsTNF-α, IL-6, and MCP-1 (all comparisons p < 0.05 vs baseline), but not ICAM-1, after 6 and 12 weeks of treatment. In contrast, there were no statistically significant reductions from baseline in any inflammatory markers in patients treated with placebo, with the exception of IL-6 at Week 6 (but not at Week 12).Citation63 The difference in hsTNF-α between olmesartan medoxomil and placebo recipients at Week 12 was statistically significant.
There were no significant changes in the serum lipid profile between baseline and Week 6 in recipients of olmesartan medoxomil or placebo. As expected, the addition of pravastatin produced significant reductions in total cholesterol and LDL cholesterol between Week 6 and Week 12 of treatment in both groups.Citation63 Systolic BP (SBP)/DBP decreased significantly compared with baseline in both treatment groups, with significantly greater reductions achieved with olmesartan medoxomil than placebo at all time points with the exception of SBP at Week 12.Citation63
Effect of olmesartan medoxomil on EPCs in patients with type 2 diabetes mellitus
ARBs increase the number of regenerative EPCs in peripheral blood of patients with type 2 diabetes mellitus, a condition associated with endothelial dysfunction.Citation64 In a randomized, double-blind, placebo-controlled study, before treatment with olmesartan medoxomil, the number of EPCs was significantly lower in 18 patients with type 2 diabetes mellitus and hypertension compared with 38 age-matched healthy volunteers.Citation64 After 12 weeks of treatment with olmesartan medoxomil 40 mg/day (n = 9), the number of circulating EPCs increased significantly in patients with type 2 diabetes mellitus (from 231 to 465 per high power field width, p < 0.05 vs placebo). There was no change in the number of CD34+ hematopoietic progenitor cells after treatment with olmesartan medoxomil.
Effect of olmesartan medoxomil on atherosclerotic lesions: the MORE study
The results of the Multicentre, Olmesartan, atherosclerosis Regression Evaluation (MORE) study suggest that olmesartan medoxomil reduces the volume of larger atherosclerotic plaques in patients with hypertension, independent of BP reduction.Citation65 After 2 years of treatment, there was no statistically significant difference between treatment groups in the IMT of the common carotid artery (CC-IMT), as determined by 2-dimensional ultrasound, the primary efficacy outcome in the trial. Among patients randomized to olmesartan medoxomil (20–40 mg/day, n = 78), the mean change in CC-IMT was −0.090 mm, and among those randomized to atenolol (50–100 mg/day, n = 77), the mean reduction in CC-IMT was −0.082 mm. The overall mean change in plaque volume, as determined by 3-dimensional ultrasound, was −4.4 μL in patients randomized to olmesartan medoxomil and +0.1 μL in patients randomized to atenolol, although the difference was not statistically significant. However, the reduction in plaque volume was significantly greater in recipients of olmesartan medoxomil (−11.5 μL) (p = 0.023 vs atenolol) than atenolol (+0.6 μL) when the analysis was restricted to patients with a baseline plaque volume greater than or equal to the median value (33.7 μL). There was no statistically significant difference in mean SBP/DBP at baseline (158/96 vs 157/96 mmHg, respectively) or in the mean reduction in SBP/DBP after 2 years of treatment (−25/−15 vs −22/−14 mmHg, respectively) between patients randomized to olmesartan medoxomil or atenolol. There was a higher proportion of men (73% vs 50% in the olmesartan medoxomil group), more current smokers (38% vs 31% in the olmesartan medoxomil group), and more patients with a history of CVD (14% vs 9% in the olmesartan medoxomil group) among those randomized to atenolol.Citation65 It is possible that technical limitations in terms of resolving differences in the volume of small plaques by 3-dimensional ultrasound sonography accounted for the failure of observing differences in plaque regression.
Effect of olmesartan medoxomil on endothelium-dependent vasodilation
Olmesartan medoxomil improved endothelium-dependent coronary artery dilation in patients with hypertension in a randomized study in 26 patients with untreated hypertension (BP > 140/90 mmHg). After 12 weeks, the mean decrease in SBP was similar in patients randomized to olmesartan medoxomil (n = 13; −28.7 mmHg) or amlodipine (n = 13; −26.7 mmHg).Citation66 However, there was a significant increase of the delta change in corrected myocardial blood flow (before vs after treatment p < 0.001) and a significant decrease in the ΔCVR (before vs after treatment p < 0.01) from rest after a cold pressor test as measured by positron emission tomography among patients treated with olmesartan medoxomil.Citation66 In contrast, 12 weeks of treatment with amlodipine had no effect on either parameter.Citation66
Among patients treated with olmesartan medoxomil, there was also a significant negative correlation between changes in serum superoxide dismutase (SOD) activity and CVR.Citation58
The study by Naya et alCitation66 provides evidence that treatment with an ARB (olmesartan medoxomil) but not a calcium channel blocker (amlodipine) has a salutary effect on the coronary microcirculation and improves endothelium-dependent coronary dilation independent of BP lowering.Citation66
Effect of olmesartan medoxomil on vessel morphology: the VIOS studyCitation67,Citation68
The objective of VIOS (Vascular Improvement with Olmesartan medoxomil Study) was to examine the impact of olmesartan medoxomil on vascular remodeling in nondiabetic patients with stage 1 hypertension as evaluated in the office setting. A total of 100 patients were randomized to 12 months of treatment with either olmesartan medoxomil 20 mg/day or atenolol 50 mg/day. The dosage of either agent was doubled to achieve optimal BP control, and hydrochlorothiazide, amlodipine, or hydralazine could be added to ongoing therapy.Citation67 As a result of this study design, the mean reduction in BP was not statistically different between treatment groups at Week 12, 28, or 52 of treatment.
The mean augmentation index (augmentation pressure/pulse pressure), a surrogate measure of vascular compliance, decreased significantly between baseline and the end of treatment in olmesartan medoxomil recipients, but was unchanged among atenolol recipients. Significant decreases in central aortic pressure were also documented, with no differences between the two treatment groups.Citation67 On the other hand, prior to initiation of the treatment period, measures of central aortic pressure in the overall group of patients averaged 131 ± 16 mmHg, a value that is within the range established for prehypertension by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) classification.
Before initiation of treatment, the wall width (WW), media cross sectional area (MCSA), and wall-to-lumen (W/L) ratio of resistance arteries in gluteal subcutaneous biopsies were all significantly higher in a subgroup of 55 patients with hypertension than in a parallel group of 11 normotensive volunteers.Citation67 At baseline, these dimensions were similar in hypertensive patients randomized to olmesartan medoxomil (n = 27) or atenolol (n = 22); however, after 12 months of treatment with olmesartan medoxomil, but not with atenolol, there was a significant decrease in all of these arteriolar dimensions (p < 0.01 vs baseline). The mean W/L ratio in arteries from patients receiving olmesartan medoxomil therapy was significantly reduced after 1 year of treatment compared with baseline (11.1% ± 0.5 vs 14.9% ± 0.8, respectively; p < 0.01), whereas the mean W/L ratio in arteries from atenolol-treated patients was not significantly decreased (15.5% vs 16.0%, respectively; p > 0.05). The mean W/L ratio in olmesartan medoxomil recipients (11.1%) was similar to that in normotensive controls (11.0%) after 1 year of treatment. The VIOS trial demonstrated that 1 year of treatment with olmesartan medoxomil restored the normal resistance vessel morphology in stage 1 hypertension independent of BP lowering.Citation67
Studies in animals
Olmesartan has been shown to reduce oxidative stress and endothelial inflammation, and to prevent or reverse the formation of atherosclerotic lesions and vascular remodeling processes in a variety of animal models of atherosclerosis. The drug has also been shown to have renoprotective effects in animal models of kidney disease and retinoprotective effects in an animal model of retinopathy.
Diabetes, insulin resistance, and metabolic syndrome animal models
Mice fed a high fat diet have increased oxidative stress in cardiac, vascular, and hepatic tissue.Citation69 Olmesartan 5 mg/kg/day markedly suppressed cardiac inflammation and fibrosis, ameliorated endothelial dysfunction, and retarded vascular remodeling in an animal model of diabetes.Citation69 The drug also prevented hepatic steatosis, halted progression of hepatic fibrosis, and suppressed the disruption in vascular endothelial NO synthase (eNOS) dimer. The beneficial effects of olmesartan were associated with a decrease in apoptosis signal regulating kinase-1 (ASK-1). ROS activate ASK-1, which, in turn, has been shown to be involved in Ang II-induced cardiac hypertrophy and remodeling.Citation70 Interestingly, hydralazine lowered BP in the same strain of diabetic mice, but, unlike olmesartan, had no effect on cardiac remodeling, vascular endothelial function, or hepatic fibrosis.Citation69 Moreover, olmesartan had no effect on any of these parameters in ASK-1-deficient mice. The results of the study suggest that Ang II plays a central role in cardiovascular remodeling and endothelial dysfunction in the setting of diabetes, and that olmesartan prevents vascular remodeling, in part, by attenuating the generation of ROS.Citation69
Models of hypertension
Treatment with olmesartan 15 mg/kg/day and enalapril 25 mg/kg/day from age 8 weeks to age 12 weeks significantly decreased the left ventricular weight:body weight ratio and normalized cardiac collagen content and the media:lumen ratio in spontaneously hypertensive rats (SHR). Significant improvements in aortic collagen content were also obtained with a lower dose of olmesartan (1 mg/kg/day) but not enalapril (2 mg/kg/day).Citation71
In another study in SHR that served as the rationale for the VIOS study (see aboveCitation67), olmesartan 10 mg/kg/day produced a greater reduction in the arteriolar W/L ratio than atenolol after 8 weeks of treatment, despite similar reductions in BP.Citation57 Treatment with olmesartan significantly increased lumen diameter and significantly decreased WW and the W/L ratio of mesenteric arteries compared with control SHR. The improvement in W/L ratio was also significantly greater than that achieved with atenolol. Treatment with atenolol also decreased WW and the W/L ratio but did not significantly alter the lumen diameter. The lumen diameter was significantly greater in olmesartan-treated animals than in control rats when exposed to luminal pressures ranging from 20 mmHg to 100 mmHg. Atenolol-treated animals did not differ from control animals in this respect. The heart weight:body weight ratio, a measure of cardiac hypertrophy, was significantly decreased in animals treated with olmesartan, but not with atenolol. The results of this study are consistent with those of the 1-year-long VIOS trial in humans and demonstrate that treatment with olmesartan can restore resistance vessel morphology after pathological changes have occurred.
Olmesartan reduced the production of ROS, suppressed tissue infiltration of macrophages, and prevented ventricular hypertrophy and fibrosis in hypertensive Dahl salt-sensitive rats with advanced heart failure who were administered olmesartan from age 17 weeks to age 20 weeks.Citation72 The addition of olmesartan 0.3 mg/kg/day to ongoing treatment with an ACE inhibitor (temocapril 0.2 mg/kg/day) produced further reductions in 4-hydroxy-2-nonenal-modified protein levels, a lipid peroxidation product and marker of ROS generation, in addition to those produced by the ACE inhibitor alone.Citation72 The beneficial effects of olmesartan in these models may be attributable, in part, to upregulation of ACE2, which results in increased Ang-(1–7) levels.Citation53,Citation61 For example, Yokoyama et alCitation57 detected elevated concentrations of Ang I, Ang II, and Ang-(1–7) in SHR treated with olmesartan, but not atenolol. In another study in SHR, treatment with olmesartan at a dose of 10 mg/kg/day increased ACE2 and Ang-(1–7) production and was associated with improved vascular remodeling of the aorta.Citation55 In this study, olmesartan produced a five-fold increase in ACE2 mRNA in the thoracic aorta. In contrast, neither atenolol nor hydralazine had an impact on ACE2 mRNA expression. Olmesartan selectively reduced the media:lumen ratio and media thickness of the thoracic aorta but not the carotid arteries in this model. These data demonstrate that BP-independent vascular remodeling is regulated by AT1 receptors and can be modulated by treatment with an ARB.Citation55
In a subsequent experiment, treatment with olmesartan 10 mg/kg/day for 14 days produced a 61% reduction in the cross-sectional area of the neointima of balloon-injured carotid arteries in SHR.Citation62 The intensity of ACE2 immunostaining in tissue taken from the injured artery was significantly greater in olmesartan-treated animals compared with vehicle-treated controls. There was no change in neointima thickness or immunostaining intensity in the uninjured contralateral carotid artery, which suggests that changes in ACE2 expression are regulated by a factor other than BP.Citation62
Coronary artery ligation-induced MI in normotensive rats results in left ventricular dysfunction and left ventricular hypertrophy, and is associated with increased plasma Ang I, Ang II, angiotensin-(1–7), elevated serum aldosterone, and reduced AT-1a receptor mRNA.Citation53 Blockade of AT-1a receptors with an ARB (losartan or olmesartan) for 28 days attenuated cardiac hypertrophy, reduced aldosterone levels, and was associated with further increases in Ang levels. Olmesartan improved contractility; losartan did not. Both ARBs produced a three-fold increase in ACE2 mRNA levels and downregulated AT-1a receptor expression in non-infarcted ventricular tissue.Citation53 The beneficial effects of ARB treatment in this model were independent of effects of BP and infarct size. The results suggest that upregulation of ACE2 and increased conversion of Ang II to Ang-(1–7) counterbalances the vasopressor effects resulting from the ACE-mediated conversion of Ang I to Ang II.
Animal models of atherosclerosis
Hypercholesterolemia-induced atherosclerosis in cynomolgus monkeys (Macaca fascicularis) is similar to that in humans. When maintained on a high cholesterol diet, monkeys develop fatty aortic streaks that can be detected by intravascular ultrasonography; increased levels of TGF-β1, ICAM-1, and M-CSF; and accumulations of macrophages in the intima, as evidenced by increased binding of specific antibodies in aortic cross sections.Citation30,Citation73,Citation74 Treatment with olmesartan 10 mg/kg/day maintained TGF-β1, ICAM-1, and M-CSF levels similar to those in control monkeys fed a normal diet; significantly reduced the accumulation of macrophages in the intima; significantly reduced the extent of lipid deposition in the aorta; and significantly reduced the atherosclerotic intima area, and intima area:media area ratio of the aorta.
In a subsequent 12-month study in the same monkey model of atherosclerosis,Citation74 treatment with olmesartan 3 mg/kg/day significantly reduced serum levels of MCP-1, an inflammatory marker, compared with cholesterol-fed monkeys; significantly reduced the intima volume:total volume ratio of the thoracic aorta; and produced significant regression in the size of existing atherosclerotic lesions as indicated by the atherosclerotic area:total surface area of the aorta. Importantly, all of the effects attributable to olmesartan in these studies occurred without significant effects on BP or total cholesterol, LDL-cholesterol, or high-density lipoprotein-cholesterol levels.Citation73,Citation74
In Watanabe heritable hyperlipidemic rabbits, the combination of olmesartan and pravastatin had complementary inhibitory effects on atherosclerotic lesions. Olmesartan 0.5 mg/kg/day and pravastatin 25 mg/kg/day produced significant reductions in BP and blood cholesterol levels that were accompanied by significant reductions in the surface area and thickness of atherosclerotic lesions and in aortic cholesterol content. The anti-atherosclerotic effects of the two drugs in combination were significantly greater than those produced by monotherapy with either agent.Citation75 Immunohistochemical staining of cross sections of aortic tissue showed that both drugs inhibited macrophage infiltration, that pravastatin inhibited lipid deposition, and that olmesartan reduced MCP-1 expression and formation of Nɛ–(carboxymethyl)lysine protein adducts, a marker of oxidative stress.Citation75
The combination of olmesartan and pravastatin produced additive improvements in left ventricular remodeling in rats with MI induced by ligation of the left anterior descending coronary artery. When administered alone, both drugs decreased cardiomyocyte size; however, the magnitude of the improvement was greater when the two drugs were co-administered. Treatment with the two drugs also downregulated left ventricular atrial natriuretic peptide mRNA. The effects on cardiomyocyte hypertrophy were dose-dependent.Citation75
The combination of olmesartan and pravastatin also significantly reduced the progression of atherosclerosis in ApoE*3Leiden transgenic mice, which develop a human-like lipoprotein profile when fed a cholesterol-rich diet. The number and extent of atherogenic lesions and the number of macrophages and T lymphocytes per cross section was significantly reduced in mice treated with the combination compared with controls. When administered alone or in combination with pravastatin, olmesartan reduced the quantity of macrophages in lesions compared with control.Citation76
The combination of olmesartan and pravastatin improved endothelial function in salt-loaded Dahl salt-sensitive hypertensive rats.Citation77 Vascular endothelium-dependent relaxation to acetylcholine, coronary arterial remodeling, and eNOS activity were all significantly improved after 4 weeks of treatment with olmesartan 0.5 mg/kg/day plus pravastatin 100 mg/kg/day. Olmesartan prevented disruption of vascular eNOS dimers and downregulation of dihydrofolate reductase to a greater extent than pravastatin; conversely, Akt phosphorylation was enhanced by pravastatin, but not by olmesartan medoxomil.
Studies in ApoE knockout mice
ApoE knockout mice are predisposed to develop atherosclerosis. When fed a high cholesterol diet, these mice develop marked atherosclerotic lesions in the proximal aorta and exhibit signs of oxidative stress (increased staining for NADPH oxidase, an enzyme that produces ROS).Citation78 At a dose of 3 mg/kg/day, olmesartan significantly inhibited NADPH oxidase activity, decreased superoxide production, reduced the formation of atherosclerotic lesions, and inhibited lipid deposition.Citation78 In another study in which apoE knockout mice were fed either a high cholesterol or normal diet for 25 weeks, treatment with olmesartan 10 mg/kg/day produced significant reductions in the surface area of aortic lesions and the cross-sectional area of aortic valves in both groups of mice.Citation79
Renoprotective effects
Olmesartan has been shown to significantly improve urinary protein and β2-microglobulin excretion, ameliorate glomerular sclerosis and tubulointerstitial injury, and to significantly reduce staining for TGF-β, vascular endothelial growth factor, and type IV collagen in glomeruli of diabetic rats.Citation80 SHR have enhanced intrarenal angiotensinogen production that contributes to increased Ang II levels and leads to hypertension and renal injury. Olmesartan reduced the urinary excretion rate of total protein, prevented glomerular sclerosis, interstitial expansion, and reduced the numbers of monocytes/macrophages in the interstitium or glomeruli of SHR. The drug also reduced angiotensinogen mRNA and protein levels in the kidney cortex, as measured by real-time PCR.Citation81 In corpulent SHR, treatment with the drug significantly reduced BP and kidney pentosidine content (which is correlated with proteinuria) and reduced histologic renal damage and proteinuria.Citation82 In another study in SHR, olmesartan, but not nifedipine or atenolol, significantly reduced proteinuria and prevented glomerular and tubulointerstitial damage (mesangial activation, podocyte injury, tubulointerstitial injury, and inflammatory cell infiltration). The drug also selectively prevented abnormal iron deposition in the interstitium, corrected chronic hypoxia, reduced expression of heme oxygenase and p47phox (a subunit of NADPH oxidase), and inhibited pentosidine formation in this animal model. The unique renoprotective properties of olmesartan were independent of BP reductions and appeared to be attributable to decreased oxidative stress, correction of chronic hypoxia, and inhibition of advanced glycation end product formation, and of abnormal iron deposition.Citation83
Retinoprotective effects
Olmesartan has been shown to have beneficial effects in several animal models of early and late stage retinopathy. The drug prevented elongation of oscillatory potential peaks in a dose-dependent manner in diabetic stroke-prone SHR. In mice with oxygen-induced retinopathy, the drug significantly prevented retinal neovascularization at a dosage of 1 mg/kg/day. Plasma concentrations of olmesartan in these experiments were comparable to the in vitro IC50 value of the AT1 receptor.Citation84
Miscellaneous effects of olmesartan
Olmesartan has been shown to have antiproliferative effects in animal models of CVD. Olmesartan significantly reduced endothelial inflammatory events in a mouse model of vascular injury that involves the placement of a polyethylene cuff around the femoral artery.Citation85 At a dose of 3 mg/kg/day, the drug significantly reduced TNF-α and MCP-1 levels, which were accompanied by decreased neointima formation and vascular smooth muscle proliferation. In another study that used the same model of endothelial inflammation, olmesartan reduced the number of bromodeoxyuridine-positive vascular smooth muscle cells present in the media and neointima, and prevented phosphorylation of extracellular signal-regulated kinase (ERK, an enzyme associated with progression of fibrosis and cell proliferation) and signal transducer and activator of transcription, all of which indicate that cell division in inflammatory lesions is attenuated by olmesartan.Citation86 Treatment with the drug also significantly reduced the rate of DNA synthesis in vascular smooth muscle cells in vitro, as indicated by incorporation of [3H]-thymidine, and blocked activation of ERK.Citation87
Endothelium-dependent relaxation of the aorta of 12-month-old Wistar-Kyoto rats is markedly impaired compared with those of 3-month-old rats.Citation88 Thus, aged rats serve as a model for age-related endothelial dysfunction. Tiron, a superoxide scavenger, partially improved endothelium-dependent relaxation in this model, suggesting involvement of superoxide. Treatment of aged rats with olmesartan 5 mg/kg/day for 2 weeks significantly reduced superoxide production. Endothelium-dependent relaxation also improved in aged rats after long-term treatment with olmesartan or temocapril, but not cerivastatin or hydralazine. Indomethacin also improved endothelium-dependent relaxation when administered alone, but not after treatment with olmesartan or temocapril. Cyclooxygenase (COX)-2 protein expression and superoxide production were increased in the aortas of aged rats, but were attenuated by olmesartan and temocapril. These results suggest that inhibition of the RAAS corrects age-related endothelial dysfunction, in part, by inhibiting synthesis of COX-2-derived vasoconstricting factors and superoxide anions.Citation88
Summary and conclusions
Endothelial dysfunction is the common link between CVD risk factors and is one of the earliest events in the cascade of changes that results in target organ damage in patients with hypertension. It has been shown that coronary artery endothelial dysfunction is associated with increased CVD risk, and increased carotid artery IMT is predictive of MI and stroke. The large arteries in hypertensive patients are thicker and stiffer than those in normotensive individuals; thus, reversal of the underlying processes that cause these structural changes and normalization of vessel wall dimensions should be a goal of antihypertensive therapy.
Ang II is a potent vasoconstrictor, which promotes sodium and fluid retention and has mitogenic and proliferative effects on vascular endothelial and smooth muscle cells. Under normal physiological conditions, these effects are counterbalanced by Ang-(1–7). In contrast, under pathological conditions, the effects of Ang II become predominant and endothelial dysfunction results. ARBs correct the pathological imbalance in the RAAS, in part, by augmenting the activity of the ACE2/Ang-(1–7) axis.Citation42,Citation54
While it would be expected that all Ang II receptor blockers share similar characteristics in terms of the mechanisms of action, both pastCitation89 and emerging data suggest that this may not be the case. A systematic review, performed to identify the factors that determine the antihypertensive activity of the ARBs using 24-hour ambulatory BP monitoring data from 35 studies, showed the greatest reductions in both SBP and DBP being seen with olmesartan medoxomil.Citation90 Studies of the pharmacological characteristics of olmesartan provide support for the clinical findings. In vitro studies of guinea pig aorta showed that olmesartan inhibited Ang II-induced contraction with a potency 160, 3.4, and 1.2 times greater than losartan, EXP-3174, and candesartan, respectively.Citation91,Citation92 Since the effects of olmesartan lasted for more than 90 minutes after the drug was removed, these data showed that olmesartan acts as a specific and insurmountable antagonist of Ang II-induced vessel contraction. This means that even in the presence of high quantities of Ang II, the physiological response to this mediator shall remain low, whereas a surmountable antagonism (fast reversible binding, short-lasting inhibition) would lead to a full physiological response, even if in the presence of higher doses of the agonist peptide. Additional comparative studies of the pharmacokinetic characteristics of olmesartan showed that the IC50 values for olmesartan receptor binding is eight times greater than telmisartan and 474 times more potent than losartan.Citation93 Another interesting feature of olmesartan is its ability to inhibit ACE. This evidence comes from the observation that treatment with olmesartan in stroke-prone SHR decreases plasma Ang II levels while concomitantly elevating plasma Ang-(1–7) levels through increase in ACE2 activity.Citation58 The experimental finding is in keeping with a previous report showing no increases in plasma Ang II levels in patients treated with olmesartan for 2 years.Citation94 This novel finding contrasts with the known effects of ARBs in increasing plasma levels of Ang II.Citation95 Altogether, the data suggest that the molecular characteristics of olmesartan medoxomil confer this drug with unique properties that translate into a greater specificity and actions.
Studies in a wide range of animal models have demonstrated that treatment with olmesartan significantly reduces inflammation, prevents formation of new lesions, and promotes regression of pre-existing atherosclerotic lesions. Improvements have been demonstrated in animal models of atherosclerosis, hypertension, diabetes mellitus, nephropathy, and retinopathy. Studies in animals have also shown that the combination of olmesartan and pravastatin has complementary pleiotropic effects on the progression of atherosclerosis. Olmesartan reduces inflammation, while pravastatin inhibits progression of pre-existing lesions.
Olmesartan medoxomil produces long-lasting and clinically significant reductions in BP in patients with hypertension. It is now clear that broad-based improvements in endothelial dysfunction are also obtained in patients with hypertension that are independent of the BP-lowering effects of the drug. In the randomized, placebo-controlled EUTOPIA trial, olmesartan medoxomil significantly reduced vascular inflammation in patients with hypertension. The drug did not significantly reduce the CC-IMT compared with atenolol after 2 years of continuous treatment in the large randomized MORE study. However, olmesartan medoxomil did produce significant reductions in the volume of large atherosclerotic plaques compared with atenolol in patients enrolled in this trial. In the VIOS study, 1 year of treatment with olmesartan medoxomil restored the normal morphology of resistance vessels in patients with hypertension. WW, MCSA, and the W/L ratio of arteries decreased significantly in recipients of olmesartan medoxomil, but not atenolol. Moreover, the W/L ratio in olmesartan medoxomil-treated patients was restored to the same dimensions as that in normotensive controls at the end of the study. Clinical studies in patients have also shown that olmesartan medoxomil significantly increased the number of regenerative EPCs in the peripheral circulation and improves endothelium-dependent relaxation. Importantly, the positive benefits of olmesartan medoxomil on improving endothelial dysfunction appear to be independent of BP lowering.
Acknowledgements
The preparation of this article was supported by Daiichi Sankyo, Inc. I thank Blair Jarvis, MSc, and Alan J Klopp, PhD, for providing editorial assistance in the preparation of this review.
Disclosures
The author declares no conflicts of interest.
References
- HardoonSLWhincupPHLennonLTWannametheeSGCapewellSMorrisRWHow much of the recent decline in the incidence of myocardial infarction in British men can be explained by changes in cardiovascular risk factors? Evidence from a prospective population-based studyCirculation2008117559860418212284
- KahnRRobertsonRMSmithREddyDThe impact of prevention on reducing the burden of cardiovascular diseaseCirculation2008118557658518606915
- PerkovicVHuxleyRWuYPrabhakaranDMacMahonSThe burden of blood pressure-related disease: a neglected priority for global healthHypertension200750699199717954719
- CoreshJSelvinEStevensLAPrevalence of chronic kidney disease in the United StatesJAMA2007298172038204717986697
- FoxCSCoadySSorliePDIncreasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart StudyCirculation2007115121544155017353438
- KestenbaumBRudserKDde BoerIHDifferences in kidney function and incident hypertension: The Multi-Ethnic Study of AtherosclerosisAnn Intern Med2008148750150818378946
- MunzelTSinningCPostFWarnholtzASchulzEPathophysiology, diagnosis and prognostic implications of endothelial dysfunctionAnn Med200840318019618382884
- PahkalaKHeinonenOJLagstromHVascular endothelial function and leisure-time physical activity in adolescentsCirculation2008118232353235919015403
- HalcoxJPSchenkeWHZalosGPrognostic value of coronary vascular endothelial dysfunctionCirculation2002106665365812163423
- LorenzMWMarkusHSBotsMLRosvallMSitzerMPrediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysisCirculation2007115445946717242284
- McAllisterASAtkinsonABJohnstonGDHaddenDRBellPMMcCanceDRBasal nitric oxide production is impaired in offspring of patients with essential hypertensionClin Sci (Lond)199997214114710409468
- SchlaichMPParnellMMAhlersBAImpaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjectsCirculation2004110243680368615569830
- YangZVenardosKJonesEMorrisBJChin-DustingJKayeDMIdentification of a novel polymorphism in the 3’UTR of the L-arginine transporter gene SLC7A1: contribution to hypertension and endothelial dysfunctionCirculation2007115101269127417325243
- ZhangCThe role of inflammatory cytokines in endothelial dysfunctionBasic Res Cardiol2008103539840618600364
- FerrarioCMRichmondRSSmithRLevyPStrawnWBKivlighnSRenin-angiotensin system as a therapeutic target in managing atherosclerosisAm J Ther2004111445314704595
- FerrarioCMStrawnWBRole of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular diseaseAm J Cardiol200698112112816784934
- StrawnWBDeanRHFerrarioCMNovel mechanisms linking angiotensin II and early atherogenesisJ Renin Angiotensin Aldosterone Syst200011111711967786
- StrawnWBFerrarioCMMechanisms linking angiotensin II and atherogenesisCurr Opin Lipidol200213550551212352014
- HumphreyJDMechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stressHypertension200852219520018541735
- AbuissaHJonesPGMarsoSPO’KeefeJHJrAngiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trialsJ Am Coll Cardiol200546582182616139131
- SchuppMClemenzMGinesteRMolecular characterization of new selective peroxisome proliferator-activated receptor gamma modulators with angiotensin receptor blocking activityDiabetes200554123442345216306360
- SchuppMLeeLDFrostNRegulation of peroxisome proliferator-activated receptor gamma activity by losartan metabolitesHypertension200647358658916365190
- EngeliSBohnkeJGorzelniakKWeight loss and the renin-angiotensin-aldosterone systemHypertension200545335636215630041
- EngeliSSchlingPGorzelniakKThe adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome?Int J Biochem Cell Biol200335680782512676168
- SharmaAMIs there a rationale for angiotensin blockade in the management of obesity hypertension?Hypertension2004441121915173127
- SharmaAMEngeliSThe role of renin-angiotensin system blockade in the management of hypertension associated with the cardiometabolic syndromeJ Cardiometab Syndr200611293517675902
- VaragicJTraskAJJessupJAChappellMCFerrarioCMNew angiotensinsJ Mol Med200886666367118437333
- ImanishiTHanoTNishioIAngiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stressJ Hypertens20052319710415643130
- VasaMFichtlschererSAicherANumber and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery diseaseCirc Res2001891E1E711440984
- StrawnWBChappellMCDeanRHKivlighnSFerrarioCMInhibition of early atherogenesis by losartan in monkeys with diet-induced hypercholesterolemiaCirculation2000101131586159310747353
- WassmannSCzechTvan EickelsMFlemingIBohmMNickenigGInhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1A receptor double-knockout miceCirculation2004110193062306715277329
- RichmondRSTallantEAGallagherPEFerrarioCMStrawnWBAngiotensin II stimulates arachidonic acid release from bone marrow stromal cellsJ Renin Angiotensin Aldosterone Syst20045417618215803435
- StrawnWBRichmondRSAnn TallantEGallagherPEFerrarioCMRenin-angiotensin system expression in rat bone marrow haematopoietic and stromal cellsBr J Haematol2004126112012615198742
- StrawnWBFerrarioCMAngiotensin II AT1 receptor blockade normalizes CD11b+ monocyte production in bone marrow of hypercholesterolemic monkeysAtherosclerosis2008196262463217692319
- SantosRASimoes e SilvaACMaricCAngiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor MasProc Natl Acad Sci U S A2003100148258826312829792
- SantosRACastroCHGavaEImpairment of in vitro and in vivo heart function in angiotensin-(1–7) receptor MAS knockout miceHypertension2006475996100216567589
- CastroCHSantosRAFerreiraAJBaderMAleninaNAlmeidaAPEvidence for a functional interaction of the angiotensin-(1–7) receptor Mas with AT1 and AT2 receptors in the mouse heartHypertension200546493794216157793
- Dias-PeixotoMFSantosRAGomesERMolecular mechanisms involved in the angiotensin-(1–7)/Mas signaling pathway in cardiomyocytesHypertension200852354254818695148
- GallagherPEFerrarioCMTallantEAMAP Kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptidesAm J Physiol Cell Physiol20082955C1169C117418768926
- SampaioWOHenrique de CastroCSantosRASchiffrinELTouyzRMAngiotensin-(1–7) counterregulates angiotensin II signaling in human endothelial cellsHypertension20075061093109817984366
- CrackowerMASaraoROuditGYAngiotensin-converting enzyme 2 is an essential regulator of heart functionNature2002417689182282812075344
- FerrarioCMAngiotensin-converting enzyme 2 and angiotensin-(1–7): an evolving story in cardiovascular regulationHypertension200647351552116365192
- LuHRateriDLFeldmanDLRenin inhibition reduces hypercholesterolemia-induced atherosclerosis in miceJ Clin Invest2008118398499318274671
- BrennerBMCooperMEde ZeeuwDEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyN Engl J Med20013451286186911565518
- CohnJNTognoniGA randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failureN Engl J Med2001345231667167511759645
- DicksteinKKjekshusJEffects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist LosartanLancet2002360933575276012241832
- LewisEJHunsickerLGClarkeWRRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med20013451285186011565517
- ParvingHHLehnertHBrochner-MortensenJGomisRAndersenSArnerPThe effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetesN Engl J Med20013451287087811565519
- PfefferMAMcMurrayJJVelazquezEJValsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or bothN Engl J Med2003349201893190614610160
- PfefferMASwedbergKGrangerCBEffects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programmeLancet2003362938675976613678868
- The_ONTARGET_Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular eventsN Engl J Med20083581547155918378520
- StrawnWBFerrarioCMTallantEAAngiotensin-(1–7) reduces smooth muscle growth after vascular injuryHypertension199933(1 Pt 2):2072119931106
- IshiyamaYGallagherPEAverillDBTallantEABrosnihanKBFerrarioCMUpregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptorsHypertension200443597097615007027
- FerrarioCMTraskAJJessupJAAdvances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular functionAm J Physiol Heart Circ Physiol20052896H2281H229016055515
- IgaseMStrawnWBGallagherPEGearyRLFerrarioCMAngiotensin II AT1 receptors regulate ACE2 and angiotensin-(1–7) expression in the aorta of spontaneously hypertensive ratsAm J Physiol Heart Circ Physiol20052893H1013H101915833808
- TallantEAFerrarioCMGallagherPEAngiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptorAm J Physiol Heart Circ Physiol20052894H1560H156615951342
- YokoyamaHAverillDBBrosnihanKBSmithRDSchiffrinELFerrarioCMRole of blood pressure reduction in prevention of cardiac and vascular hypertrophyAm J Hypertens200518792292916053988
- AgataJUraNYoshidaHOlmesartan is an angiotensin II receptor blocker with an inhibitory effect on angiotensin-converting enzymeHypertens Res2006291186587417345786
- TraskAJAverillDBGantenDChappellMCFerrarioCMPrimary role of angiotensin-converting enzyme-2 in cardiac production of angiotensin-(1–7) in transgenic Ren-2 hypertensive ratsAm J Physiol Heart Circ Physiol20072926H3019H302417308000
- TraskAJFerrarioCMAngiotensin-(1–7): pharmacology and new perspectives in cardiovascular treatmentsCardiovasc Drug Rev200725216217417614938
- GallagherPEFerrarioCMTallantEARegulation of ACE2 in cardiac myocytes and fibroblastsAm J Physiol Heart Circ Physiol20082956H2373H237918849338
- IgaseMKoharaKNagaiTMikiTFerrarioCMIncreased expression of angiotensin converting enzyme 2 in conjunction with reduction of neointima by angiotensin II type 1 receptor blockadeHypertens Res200831355355918497476
- FliserDBuchholzKHallerHAntiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammationCirculation200411091103110715313950
- BahlmannFHde GrootKMuellerOHertelBHallerHFliserDStimulation of endothelial progenitor cells: a new putative therapeutic effect of angiotensin II receptor antagonistsHypertension200545452652915767470
- StumpeKAgabiti-RoseiEZielinskiTCarotid intima-media thickness and plaque volume changes following 2-year angiotensin II-receptor blockade. The multicentre olmesartan atherosclerosis regresssion evaluation (MORE) studyTher Adv Cardiovasc Dis2007129710619124398
- NayaMTsukamotoTMoritaKOlmesartan, but not amlodipine, improves endothelium-dependent coronary dilation in hypertensive patientsJ Am Coll Cardiol200750121144114917868805
- SmithRYokoyamaHAverillDBSchiffrinELFerrarioCMReversal of vascular hypertrophy in hypertensive patients through blockade of angiotensin II receptorsJASH200823165172
- SmithRDYokoyamaHAverillDBThe protective effects of angiotensin II blockade with olmesartan medoxomil on resistance vessel remodeling (The VIOS study): rationale and baseline characteristicsAm J Cardiovasc Drugs20066533534217083268
- YamamotoEDongYFKataokaKOlmesartan prevents cardiovascular injury and hepatic steatosis in obesity and diabetes, accompanied by apoptosis signal regulating kinase-1 inhibitionHypertension200852357358018678790
- IzumiyaYKimSIzumiYApoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodelingCirc Res200393987488314551246
- PorteriERodellaLRizzoniDEffects of olmesartan and enalapril at low or high doses on cardiac, renal and vascular interstitial matrix in spontaneously hypertensive ratsBlood Press200514318419216036499
- YoshidaJYamamotoKManoTAT1 receptor blocker added to ACE inhibitor provides benefits at advanced stage of hypertensive diastolic heart failureHypertension200443368669114757777
- MiyazakiMTakaiSAnti-atherosclerotic efficacy of olmesartanJ Hum Hypertens200216Suppl 2S7S1211967727
- TakaiSJinDSakaguchiMMuramatsuMMiyazakiMThe regressive effect of an angiotensin II receptor blocker on formed fatty streaks in monkeys fed a high-cholesterol dietJ Hypertens200523101879188616148612
- KatoMSadaTMizunoMKitayamaKInabaTKoikeHEffect of combined treatment with an angiotensin II receptor antagonist and an HMG-CoA reductase inhibitor on atherosclerosis in genetically hyperlipidemic rabbitsJ Cardiovasc Pharmacol200546455656216160612
- van der HoornJWKleemannRHavekesLMKooistraTPrincenHMJukemaJWOlmesartan and pravastatin additively reduce development of atherosclerosis in APOE*3Leiden transgenic miceJ Hypertens200725122454246217984667
- YamamotoEYamashitaTTanakaTPravastatin enhances beneficial effects of olmesartan on vascular injury of salt-sensitive hypertensive rats, via pleiotropic effectsArterioscler Thromb Vasc Biol200727355656317170375
- TsudaMIwaiMLiJMInhibitory effects of AT1 receptor blocker, olmesartan, and estrogen on atherosclerosis via anti-oxidative stressHypertension200545454555115723967
- KatoMSadaTChumaHSeverity of hyperlipidemia does not affect antiatherosclerotic effect of an angiotensin II receptor antagonist in apolipoprotein E-deficient miceJ Cardiovasc Pharmacol200647676476916810077
- KogaKYamagishiSTakeuchiMCS-886, a new angiotensin II type 1 receptor antagonist, ameliorates glomerular anionic site loss and prevents progression of diabetic nephropathy in Otsuka Long-Evans Tokushima fatty ratsMol Med200281059159912477969
- KoboriHOzawaYSuzakiYNishiyamaAEnhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive ratsJ Am Soc Nephrol20051672073208015888567
- NangakuMMiyataTSadaTAnti-hypertensive agents inhibit in vivo the formation of advanced glycation end products and improve renal damage in a type 2 diabetic nephropathy rat modelJ Am Soc Nephrol20031451212122212707391
- IzuharaYNangakuMInagiRRenoprotective properties of angiotensin receptor blockers beyond blood pressure loweringJ Am Soc Nephrol200516123631364116236804
- NakamuraHInoueTArakawaNPharmacological and pharmacokinetic study of olmesartan medoxomil in animal diabetic retinopathy modelsEur J Pharmacol200551223239246
- JinnoTIwaiMLiZCalcium channel blocker azelnidipine enhances vascular protective effects of AT1 receptor blocker olmesartanHypertension200443226326914707152
- LiuHWIwaiMTakeda-MatsubaraYEffect of estrogen and AT1 receptor blocker on neointima formationHypertension200240445145712364346
- MinLJMogiMLiJMIwanamiJIwaiMHoriuchiMAldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cellsCirc Res200597543444216081869
- MukaiYShimokawaHHigashiMInhibition of renin-angiotensin system ameliorates endothelial dysfunction associated with aging in ratsArterioscler Thromb Vasc Biol20022291445145012231564
- FerrarioCMThe role of angiotensin antagonism in stroke prevention in patients with hypertension: focus on losartanCurr Med Res Opin200420111797180415537480
- FabiaMJAbdillaNOltraRFernandezCRedonJAntihypertensive activity of angiotensin II AT1 receptor antagonists: a systematic review of studies with 24 h ambulatory blood pressure monitoringJ Hypertens20072571327133617563549
- KoikeHSadaTMizunoMIn vitro and in vivo pharmacology of olmesartan medoxomil, an angiotensin II type AT1 receptor antagonistJ Hypertens Suppl2001191S3S1411451212
- YanagisawaHAmemiyaYKanazakiTNonpeptide angiotensin II receptor antagonists: synthesis, biological activities, and structure-activity relationships of imidazole-5-carboxylic acids bearing alkyl, alkenyl, and hydroxyalkyl substituents at the 4-position and their related compoundsJ Med Chem19963913233388568823
- LeMTPugsleyMKVauquelinGVan LiefdeIMolecular characterisation of the interactions between olmesartan and telmisartan and the human angiotensin II AT1 receptorBr J Pharmacol2007151795296217572702
- IchikawaSTakayamaYLong-term effects of olmesartan, an Ang II receptor antagonist, on blood pressure and the renin-angiotensin-aldosterone system in hypertensive patientsHypertens Res200124664164611768722
- SchindlerCBrosnihanKBFerrarioCMComparison of inhibitory effects of irbesartan and atorvastatin treatment on the renin angiotensin system (RAS) in veins: a randomized double-blind crossover trial in healthy subjectsJ Clin Pharmacol200747111212017192509
- IyerSNAverillDBChappellMCYamadaKAllredAJFerrarioCMContribution of angiotensin-(1–7) to blood pressure regulation in salt-depleted hypertensive ratsHypertension200036341742210988275
- MoriguchiATallantEAMatsumuraKOpposing actions of angiotensin-(1–7) and angiotensin II in the brain of transgenic hypertensive ratsHypertension1995256126012657768571
- Campagnole-SantosMJHeringerSBBatistaENKhoslaMCSantosRADifferential baroreceptor reflex modulation by centrally infused angiotensin peptidesAm J Physiol1992263(1 Pt 2):R89R941636797
- GrobeJLMeccaAPLingisMPrevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7)Am J Physiol Heart Circ Physiol20072922H736H74217098828
- FerreiraAJSantosRAAlmeidaAPAngiotensin-(1–7): cardioprotective effect in myocardial ischemia/reperfusionHypertension200138(3 Pt 2): 66566811566952
- FerreiraAJSantosRAAlmeidaAPAngiotensin-(1–7) improves the post-ischemic function in isolated perfused rat heartsBraz J Med Biol Res20023591083109012219180
- DelliPizziAMHilcheySDBell-QuilleyCPNatriuretic action of angiotensin(1–7)Br J Pharmacol19941111138012686
- HellerJKramerHJMalyJCervenkaLHoracekVEffect of intrarenal infusion of angiotensin-(1–7) in the dogKidney Blood Press Res2000232899410765110
- VallonVHeyneNRichterKKhoslaMCFechterK[7-D-ALA]-angiotensin 1–7 blocks renal actions of angiotensin 1–7 in the anesthetized ratJ Cardiovasc Pharmacol19983211641679676737
- LootAERoksAJHenningRHAngiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in ratsCirculation2002105131548155011927520
- FerrarioCMMartellNYunisCCharacterization of angiotensin-(1–7) in the urine of normal and essential hypertensive subjectsAm J Hypertens19981121371469524041
- LuqueMMartinPMartellNFernandezCBrosnihanKBFerrarioCMEffects of captopril related to increased levels of prostacyclin and angiotensin-(1–7) in essential hypertensionJ Hypertens19961467998058793704
- FerrarioCMSmithRDBrosnihanBEffects of omapatrilat on the renin-angiotensin system in salt-sensitive hypertensionAm J Hypertens200215655756412074359
- SchmeiderREHilgersKFSchlaichMPRenin-angiotensin system and cardiovascular riskLancet20073691208121917416265