Abstract
Peripheral arterial disease (PAD) is a major health problem affecting millions of patients worldwide. Many will suffer from intermittent claudication (IC), which leads to marked impairment of quality of life (QoL). Besides surgical and endovascular interventions to improve limb-specific outcomes, pharmacotherapy is an effective tool in the treatment of IC. Cilostazol, a Federal Drug Administration-approved medication for the treatment of IC, has demonstrated consistent efficacy in improving exercise capacity and overall health-related QoL. This manuscript will review the pharmacokinetics, safety, and efficacy of cilostazol in the treatment of patients with IC as well as compare this agent with other proven non-invasive therapies for PAD.
Introduction
Peripheral arterial disease (PAD) is most commonly due to atherosclerosis, affects millions of patients worldwide, and is associated with significant morbidity and mortality (CitationHiatt 2001). PAD is defined as an obstruction of the infra-renal abdominal aorta and lower extremity arteries that reduces arterial flow during exercise and/or at rest. Intermittent claudication (IC) is the most common symptom in patients with PAD and is associated with exercise-induced discomfort in the muscles, relieved with rest; this may lead to marked impairment of quality of life (QoL) and daily activities. However, IC is one symptom. Many patients have limited walking distance (atypical claudication) but do deny IC, ie, fatigue or tired or aching legs. Investigators have found that peak walking distance, peak walking time, and peak heart rate are all significantly reduced in PAD patients versus healthy controls (CitationHiatt et al 1992).
Following the general principle that more intense activities require greater oxygen consumption, the requirement for oxygen consumption in a healthy person increases from 4 mL/kg/min at rest to approximately 7 mL/kg/min for self-care, 9 mL/kg/min for house-cleaning, 13 mL/kg/min for dancing, 14 mL/kg/min for golf, 24 mL/kg/min for jogging, and 31 mL/kg/min for marathon running (CitationAinsworth et al 2000). Considering that the peak oxygen capacity for an individual with PAD rarely exceeds 14.8 ± 0.8 mL/kg/min (CitationHiatt et al 1992), it appears that even when working at maximum capacity, many PAD patients may lack the ability to complete the activities of daily life. Subjects with IC exhibited a mean exercise duration of 10.6 minutes, which was approximately half that demonstrated by age-matched controls (p < 0.05) (CitationHiatt et al 1987). In addition, the PAD-associated deficits in exercise performance were accompanied by a roughly 50% decline in oxygen capacity, indicating that the level of impairment in PAD is comparable to that associated with class III congestive heart failure (CitationHiatt 2001).
A number of drugs () have been tested for IC therapy, with mostly disappointing results. Among the multitude of failed pharmacotherapies, prostaglandins appeared to have promising potential more than a decade ago but more recently have been shown to have only modest efficacy (CitationHiatt 2001). Antiplatelet medications, serotonin blockers, and vasodilators have also been studied, but thus far none have demonstrated significant benefits for patients with IC (CitationHiatt 2002). In the US, two agents have been approved for such an indication (cilostazol and pentoxifylline), but only cilostazol has demonstrated consistent efficacy in both extending exercise capacity and improving QoL (CitationBeebe et al 1999; CitationDawson et al 2000; CitationMoney et al 1998). As a result, cilostazol may be the most clinically effective pharmacologic option for IC in US patients. This manuscript will provide an overview of the pharmacology, metabolism, safety, and efficacy of cilostazol in the treatment of patients with typical IC and will compare this agent to other proven, non-invasive therapies for PAD.
Table 1 Pharmacotherapies tested for intermittent claudication therapy
Pharmacology
Cilostazol () was approved by the Federal Drug Administration (FDA) in 1999 for the treatment of IC (CitationKumar and Bhattacharya 2007). It became available in generic form in 2006. It is a reversible selective inhibitor of phosphodiesterase (PDE) type III. Similar to other members of its class, one of the primary effects of cilostazol is an increase in cyclic adenosine monophosphate (cAMP) in platelets, vascular smooth muscle, endothelial cells, and other PDE-III-rich cells, which may lead to a number of beneficial outcomes (). Among these potential benefits, cilostazol has been shown to inhibit platelet activation/aggregation, reduce thrombosis, enhance vasodilation (CitationChapman and Goa 2003), and induce nitric oxide (NO) production (CitationHashimoto et al 2006) as well as inhibit smooth muscle cell proliferation (CitationTakahashi et al 1992; CitationHayashi et al 2000), increase limb blood flow (CitationElam et al 1998), increase plasma high-density lipoprotein-cholesterol (HDL-C) (CitationLee et al 2001) and reduce plasma triglyceride levels (CitationElam et al 1998), potentiate angiogenesis (CitationLee et al 2001), and reduce inflammation (CitationAgrawal et al 2007). Although the exact mechanism for cilostazol’s benefits in IC remains unknown, any of these properties could potentially contribute to the symptomatic efficacy associated with this pharmacologic agent.
Table 2 Beneficial effects of cilostazol
In vitro and/or ex vivo studies using platelets from healthy volunteers, in patients with atherosclerosis and in patients with cerebrovascular disease, have shown that cilostazol reversibly inhibits the primary and secondary stages of platelet aggregation (CitationChapman and Goa 2003). Cilostazol has also been found to be superior to aspirin in suppressing platelet aggregation ex vivo and to have some effects on platelet function more potent than the antiplatelet drug ticlopidine (CitationIkeda et al 1987). Cilostazol has been shown to reduce platelet aggregation without prolonging bleeding time (CitationTamai et al 1999). While selectively inhibiting the function of human platelets, cilostazol does not affect the production of prostacyclin and does not inhibit the function of vascular endothelial cells (CitationTani et al 1992). Cilostazol also produces vasodilatation by inhibiting smooth muscle cell contraction secondary to the elevation of cAMP levels and the blocking of calcium ion release (CitationChapman and Goa 2003). Cilostazol has also been shown to induce NO production by activating endothelial nitric oxide synthase (eNOS) (CitationHashimoto et al 2006).
Cilostazol has been shown to inhibit neointimal hyperplasia and restore endothelial function after balloon injury to the carotid artery of rats by inhibiting vascular smooth muscle cell growth, thus stimulating the production of hepatocyte growth factor in rapidly regenerating endothelial cells (CitationAoki et al 2001).
Cilostazol also has beneficial effects on lipids. After 12 weeks of therapy with cilostazol, a 10% increase in HDL-C and a 15% decrease in plasma triglycerides have been noted (CitationElam et al 1998; CitationLee et al 2001). These beneficial changes with cilostazol were evident at 2 weeks for HDL-C and 4 weeks for triglycerides and persisted throughout the 12 weeks of therapy. There were no significant effects on low-density lipoprotein-cholesterol (LDL-C) or total cholesterol, but there was an increase of 6% in apolipoprotein A levels (CitationElam et al 1998, CitationLee et al 2001). More interestingly, in animal models of myocardial infarction (MI), cilostazol in combination with the potent statin atorvastatin had synergistic effects on nitric oxide production and reduction of MI size (CitationManickavasagam et al 2007). Cilostazol may potentiate angiogenesis and has been shown to increase vascular endothelial growth factors levels, which were significantly related to improved levels of exercise tolerance (CitationLee et al 2001).
Additionally, in hypertensive patients with type 2 diabetes mellitus, inflammatory markers – such as highly-sensitive C reactive protein (hs-CRP), erythrocyte sedimentation rate (ESR), total leukocyte count, and plasma malondialdehyde – have been shown to be elevated, suggesting high levels of inflammation and oxidative stress. Cilostazol has been shown to reduce these inflammatory markers (CitationAgrawal et al 2007). In a randomized, open, add-on preventive controlled trial specifically assessing the effects of cilostazol on inflammatory markers, 60 hypertensive patients ≥45 years of age with diabetes mellitus were recruited. Cilostazol 100 mg 2×/day was given to 30 patients in addition to their standard medical treatment. At the 1-month follow-up, the cilostazol group showed significant (p < 0.001) reductions in hs-CRP (24%), ESR (39%), total leukocyte count (13%), and plasma malondialdehyde (18%).
Metabolism
Cilostazol is taken orally, and maximal plasma concentration of the drug is observed at 2.7 hours after the final administration, reaching its steady state within 96 hours (CitationBramer et al 1999). Cilostazol is highly bound to plasma proteins, primarily albumin, and has a free fraction of up to 5% in patients with IC (CitationBramer et al 1999). It is metabolized by the hepatic enzyme CYP3A4 and to a lesser extent CYP2C19, primarily by oxidative metabolism to numerous metabolites (CitationBramer et al 1997). The main metabolites identified include dehydrocilostazol and monohydroxycilostazol, which have inhibitory effects on platelet aggregation (CitationBramer et al 1997).
Safety
A black box warning indicates that cilostazol should be avoided in patients with congestive heart failure. The warning does not specify the heart failure classification or the degree of severity that should be avoided. This warning was imposed by the FDA because of the prior experience of chronic oral milrinone therapy (another PDE-III inhibitor) in patients with heart failure (CitationPacker et al 1991). Regarding cardiovascular events and mortality, the risks associated with cilostazol seem to be numerically comparable to placebo. In an analysis that included the entire cilostazol safety database in addition to 4 trials conducted outside the US (CitationPratt 2001), no significant differences were found between cilostazol- and placebo-treated groups in rates of MI, stroke, or cardiovascular death during follow-up (CitationPratt 2001). Moreover, in two trials the distribution of total cardiovascular morbidity and mortality was actually higher in the placebo group (8%) than among patients receiving either 50 mg 2×/day (6%) or 100 mg 2×/day (7%) doses of cilostazol (CitationPratt 2001). Thus, cilostazol seems to be a relatively safe alternative for IC therapy, offering an acceptable risk-benefit profile for patients with this condition. However, because of the existing black box warning against giving cilostazol to patients with congestive heart failure, the authors do not recommend prescribing cilostazol to such patients with IC.
Similar to other drugs in its class, cilostazol is associated with a fairly high frequency of non-life threatening adverse effects, particularly headaches, which may occur as a result of its vasodilatory properties. Headaches occurred in 443 of 1374 patients (32%) treated with cilostazol compared with 40 of 355 (11%) treated with the other approved therapy for IC, pentoxifylline, and 127 of 973 (13%) treated with placebo (CitationPratt 2001). In addition, more patients in the cilostazol group than in the pentoxifylline and placebo groups reported diarrhea (17% versus 8% and 7%, respectively), abnormal stools (14% versus 5% and 4%, respectively), peripheral edema (7% versus 4% and 4%, respectively), and palpitations (9% versus 2% and 1%, respectively).
A large (n = 1439) ongoing, long-term, post-marketing clinical study conducted in the US, Japan and other Asian countries, South America, and the UK from 1999 through 2003 recently concluded (CitationHiatt 2006). Patients exposed to marketed formulations of cilostazol were evaluated to measure spontaneously reported adverse events. At the end of the study, 872 treatment-emergent adverse events were reported. A total of 773 events were considered serious and 99 non-serious according to FDA definitions. Of these, 61 events in 41 cases were considered drug related by the study investigators. Treatment-related serious adverse events occurring with the greatest frequency included congestive heart failure (14 cases; 2%), gastrointestinal hemorrhage (6 cases; 1%), atrial fibrillation (4 cases; <1%), diarrhea (3 cases; <1%), and dyspnea (3 cases; <1%). At total of 572 events were considered unexpected. Of the 872 serious and non-serious events observed, 181 required the discontinuation of cilostazol due to adverse events. The most frequent adverse events requiring discontinuation were congestive heart failure (26 cases; 2%), diarrhea (15 cases; 1%), and headaches (14 cases; 1%).
Efficacy
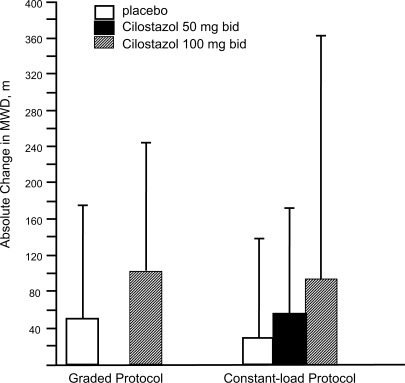
Once the goals of risk factor modification for patients with PAD have been met, the focus of PAD management shifts from general cardio-protection to treatment strategies specific for the clinical manifestations of PAD such as IC. There are consistent data supporting the efficacy of cilostazol for IC. In one the of most important studies included in the agent’s New Drug Application, 698 patients with moderate to severe IC were randomized to a standard dose of cilostazol 100 mg 2×/day, a standard dose of pentoxifylline (400 mg 3×/day), or placebo (3×/day) for a period of 24 weeks. By the end of the study, patients included in the cilostazol group had shown a 54% improvement in maximal walking distance from the baseline compared with increases of 30% in the pentoxifylline group (p < 0.05) and 34% in the placebo group (p < 0.05) () (CitationDawson et al 2000). Earlier trials have demonstrated a dose-response relationship with cilostazol. In one study (CitationBeebe et al 1999), IC patients who received cilostazol 100 mg 2×/day for 24 weeks increased their maximal walking distance by 51% over the baseline, whereas those who received a 50 mg 2×/day dose showed an increase of only 38% compared to baseline values (p < 0.001 for both doses versus placebo) (). In a second trial, the net improvement in maximal walking distance over placebo was 21% with cilostazol 100 mg 2×/day compared to 7% for cilostazol 50 mg 2×/day. Notably, patients in both the 50 mg and 100 mg cilostazol groups showed improvements in global measures of physical functioning compared to placebo-treated patients (p < 0.05) (CitationStrandness et al 1998). Collectively, more than 2,700 IC patients were investigated in eight clinical trials with variable results, but there was a consistent trend toward improvement with cilostazol over placebo. CitationDawson et al (1998) and CitationMoney et al (1998) have shown a significant improvement in maximal walking distance in those who received cilostazol 100 mg 2×/day compared with those who received placebo. Recently, a meta-analysis from six phase III cilostazol trials (CitationRegensteiner et al 2002) with study durations of 12 to 24 weeks found a net benefit of the drug in both the constant workload treadmill and graded treadmill tests. Among the 749 patients who had been evaluated with a constant workload treadmill test, cilostazol 100 mg 2×/day was associated with a 76% improvement in maximal walking distance from the baseline compared with a 20% improvement for placebo (p < 0.0001). Similarly, 895 patients evaluated with a graded treadmill test showed a 40% maximal walking distance improvement with cilostazol 100 mg 2×/day compared with a 20% increase with placebo (p < 0.05) (). When data for both types of treadmill protocols were combined, cilostazol 100 mg 2×/day demonstrated superiority over placebo in both maximal walking distance (p < 0.05) and pain-free walking distance values (p < 0.05). Furthermore, cilostazol demonstrated efficacy on several pain and physical dimensions on the Short Form-36 physical functioning scores and Walking Impairment Questionnaire, indicating that the drug’s beneficial effects on exercise capacity are indeed accompanied by meaningful improvements in QoL. The beneficial effects of cilostazol were statistically superior to placebo as early as 4 weeks following treatment onset. However, the therapeutic effects of cilostazol were seen to improve progressively as study duration increased. In clinical practice, 12 to 24 weeks should be expected as a typical waiting period for improvement to occur.
Figure 2 Mean percent change in maximal walking distance over time among intermittent claudication patients randomized to cilostazol 100 mg 2×/day (n = 227), pentoxifylline 400 mg 3×/day (n = 232), or placebo (n = 239) for 24 weeks. MWD = maximal walking distance. Reprinted from CitationDawson, DL, Cutler BS, Hiatt WR, et al 2000. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med, 109:523–30. Copyright © 2000, with permission from Elsevier.
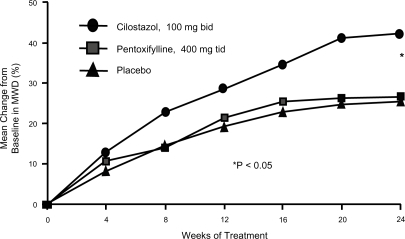
Figure 3 Mean percent change in maximal walking distance (MWD) among intermittent claudication patients receiving cilostazol 100 mg 2×/day (n = 140), cilostazol 50 mg 2×/day (n = 139), or placebo (n = 140) for 24 weeks. Reprinted from CitationBeebe, HG, Dawson DL, Cutler BS, et al 1999. A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial. Arch Intern Med, 159:2041–50. Copyright © 1999 with permission from American Medical Association.
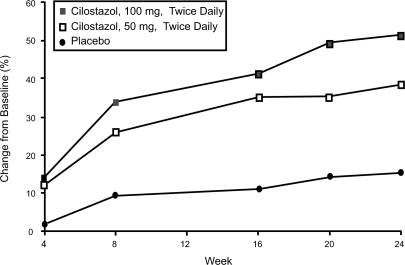
Figure 4 Mean absolute change in maximal walking distance (MWD), as measured by graded or constant-load protocols, among intermittent claudication patients receiving cilostazol 100 mg 2×/day, cilostazol 50 mg 2×/day, or placebo in six randomized controlled trials. Reprinted from CitationRegensteiner JG, Ware JE Jr, McCarthy WJ, et al 2002. Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials. J Am Geriatr Soc, 50:1939–46. Copyright © 2002 with permission from Blackwell Publishing.
In a randomized, placebo-controlled study, patients receiving either cilostazol 100 mg 2×/day, pentoxifylline 400 mg 3×/day, or placebo were monitored for 24 weeks with cross-over to placebo after that period. When these patients were followed up for an additional 6 weeks, those in the cilostazol treatment group demonstrated a significant loss of treatment benefit after the change (p < 0.05) (CitationCleanthis et al 2005), whereas there was no significant change following pentoxifylline therapy.
Interestingly, cilostazol has also been shown to provide long-term vessel patency after percutaneous transluminal angioplasty (PTA) in hemodialysis patients with PAD (CitationIshii et al 2008). In a recent Japanese study assessing lower extremity arterial patency rates 5 years after initial PTA, 193 hemodialysis patients with 372 consecutive lesions underwent successful PTA and were randomized to receive either cilostazol 100 mg 2×/day in conjunction with standard medical treatment (71 patients with 130 lesions) or standard medical therapy without cilostazol (122 patients with 242 lesions). After 5 years, the patency rate was 52% in the cilostazol group and 33% in the control group (p < 0.05). Moreover, in the Cilostazol for Restenosis Trial (CitationDouglas et al 2005), 750 patients with successful coronary bare metal stent implantation were randomized to receive either cilostazol 100 mg 2×/day or placebo for 6 months in additional to aspirin and clopidogrel 75 mg daily. At 6 months, the cilostazol group had a 36% relative risk reduction in the restenosis rate compared with those who received placebo, as determined by quantitative coronary angiography. Furthermore, in the Cilostazol for Diabetic Patients in Drug-Eluting Stent Trial (CitationAhn et al 2008), 280 patients successfully underwent coronary stenting and were randomized to receive either aspirin and cilostazol or aspirin and clopidogrel. After a mean follow-up of 7.1 months, 237 patients underwent repeat coronary angiography; those randomized to receive aspirin and cilostazol had a 50% decrease in the incidence of restenosis compared to those who received aspirin and clopidogrel. The trial concluded that combination therapy with aspirin and cilostazol for the prevention of restenosis is comparable and may even be superior to that of aspirin and clopidogrel in diabetic patients who undergo drug-eluting stent implantation.
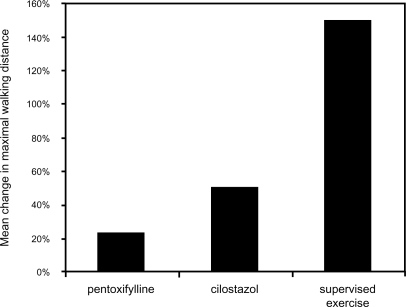
Supervised exercise rehabilitation is an effective and safe intervention that has been demonstrated to improve the walking distance of patients with IC. Multiple controlled trials have demonstrated that both walking distance until the onset of claudication and absolute claudication distance improve in patients who receive supervised exercise rehabilitation. One meta-analysis of 21 studies found that patients who were treated with supervised exercise rehabilitation had a 180% increase in distance walked until the onset of claudication compared with a 40% increase in control group individuals. Similarly, the absolute distance walked increased by 130% in patients who received supervised exercise rehabilitation compared with a 30% increase among those who did not (CitationGardner and Poehlman 1995). Another meta-analysis of 10 randomized trials comprising 250 patients with IC found that initial maximal walking time increased by approximately 150% in patients treated with exercise rehabilitation compared with control group individuals (CitationLeng et al 2000). Overall, supervised exercise therapy has been shown to be very effective in improving maximal treadmill walking distance (CitationMilani and Lavie 2007) ().
Conclusions
In summary, cilostazol remains the only drug thus far proven to demonstrate consistent benefits in clinical trials in patients with IC. In addition to limb-specific outcomes, cilostazol has also been shown to provide pleotropic effects; these may provide additional clinical benefits, but clinical trials are needed to validate such effects. Finally, studies are also needed to compare the effects of cilostazol and supervised exercise training in patients with IC to either therapy by itself.
Disclosures
None of the authors report conflicts of interest.
References
- AgrawalNKMaitiRDashD2007Cilostazol reduces inflammatory burden and oxidative stress in hypertensive type 2 diabetes mellitus patientsPharmacol Res561182317548203
- AhnYJeongMHJeongJW2008Randomized comparison of cilostazol vs clopidogrel after drug-eluting stenting in diabetic patients – clilostazol for diabetic patients in drug-eluting stent (CIDES) trialCirc J7235918159096
- AinsworthBEHaskellWLWhittMC2000Compendium of physical activities: an update of activity codes and MET intensitiesMed Sci Sports Exerc32SupplS49850410993420
- AokiMMorishitaRHayashiS2001Inhibition of neointimal formation after balloon injury by cilostazol, accompanied by improvement of endothelial dysfunction and induction of hepatocyte growth factor in rat diabetes modelDiabetologia4410344211484082
- BeebeHGDawsonDLCutlerBS1999A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trialArch Intern Med15920415010510990
- BramerSLForbesWPMallikaarjunS1999Cilostazol pharmacokinetics after single and multiple oral doses in healthy males and patients with intermittent claudication resulting from peripheral arterial diseaseClin Pharmacokinet37Suppl 211110702882
- BramerSLTataPNVMallikaarjunS1997Disposition of 14 C-cilostazol after single dose administration to healthy human subjectsPhar Res1411 SupplS612
- ChapmanTMGoaKL2003Cilostazol: a review of its use in intermittent claudicationAm J Cardiovasc Drugs31173814727939
- CleanthisMBhattacharyaVSmoutJ2005Combined aspirin and cilostazol treatment is associated with reduced platelet aggregation and prevention of exercise induced platelet activationYearb Soc Acad Res Surg39
- DawsonDLCutlerBSMeissnerMH1998Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trialCirculation98678869715861
- DawsonDLCutlerBSHiattWR2000A comparison of cilostazol and pentoxifylline for treating intermittent claudicationAm J Med1095233011063952
- DouglasJSJrHolmesDRJrKereiakesDJ2005Cilostazol for Restenosis Trial (CREST) Investigators. Coronary stent restenosis in patients treated with cilostazolCirculation11228263216246948
- ElamMBHeckmanJCrouseJR1998Effect of the novel antiplatelet agent cilostazol on plasma lipoproteins in patients with intermittent claudicationArterioscler Thromb Vasc Biol18194279848888
- GardnerAWPoehlmanET1995Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysisJAMA274975807674529
- HashimotoAMiyakodaGHiroseY2006Activation of endothelial nitric oxide synthase by cilostazol via a cAMP/protein kinase A- and phosphatidylinositol 3-kinase/Akt-dependent mechanismAtherosclerosis189350716545819
- HayashiSMorishitaRMatsushitaH2000Cyclic AMP inhibited proliferation of human aortic vascular smooth muscle cells, accompanied by induction of p53 and p21Hypertension352374310642304
- HiattWRWolfelEERegensteinerJG1992Skeletal muscle carnitine metabolism in patients with unilateral peripheral arterial diseaseJ Appl Physiol73346531506390
- HiattWR2001Medical treatment of peripheral arterial disease and claudicationN Engl J Med34416082111372014
- HiattWR2002Pharmacologic therapy for peripheral arterial disease and claudicationJ Vasc Surg3612839112469066
- HiattWR2006The US experience with cilostazol in treating intermittent claudicationAtheroscler Suppl6213116275166
- HiattWRNawazDBrassEP1987Carnitine metabolism during exercise in patients with peripheral vascular diseaseJ Appl Physiol62238373610932
- IkedaYKikuchiMMurakamiH1987Comparison of the inhibitory effects of cilostazol, acetylsalicylic acid and ticlopidine on platelet functions ex vivo. Randomized, double-blind cross-over studyArzneimittelforschung3756362956957
- IshiiHKumadaYToriyamaT2008Cilostazol improves long-term patency after percutaneous transluminal angioplasty in hemodialysis patients with peripheral artery diseaseClin J Am Soc Nephrol310344018322041
- KumarMBhattacharyaV2007Cilostazol: a new drug in the treatment intermittent claudicationRecent Patents Cardiovasc Drug Discov21815
- LeeTMSuSFHwangJJ2001Differential lipogenic effects of cilostazol and pentoxifylline in patients with intermittent claudication: potential role for interleukin-6Atherosclerosis158471611583728
- LengGCFowlerBErnstEExercise for intermittent claudication. 2000Cochrane Database Syst Rev2CD00099010796572
- ManickavasagamSYeYLinY2007The cardioprotective effect of a statin and cilostazol combination: relationship to Akt and endothelial nitric oxide synthase activationCardiovasc Drugs Ther213213017620005
- MilaniRVLavieCJ2007The role of exercise training in peripheral arterial diseaseVasc Med12351818048473
- MoneySRHerdJAIsaacsohnJL1998Effect of cilostazol on walking distances in patients with intermittent claudication caused by peripheral vascular diseaseJ Vasc Surg2726774discussion 274–5.9510281
- PackerMCarverJRRodehefferRJ1991Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research GroupN Engl J Med3251468751944425
- PrattCM2001Analysis of the cilostazol safety databaseAm J Cardiol8728D33D11137829
- RegensteinerJGWareJEJrMcCarthyWJ2002Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trialsJ Am Geriatr Soc5019394612473004
- StrandnessDEDalmanRPanianS1998Two doses of cilostazol versus placebo in the treatment of claudication: results of a randomized, multicenter trialCirculation9817 Suppl 11129665051
- TakahashiSOidaKFujiwaraR1992Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in cultureJ Cardiovasc Pharmacol2090061282592
- TamaiYTakamiHNakahataR1999Comparison of the effects of acetylsalicylic acid, ticlopidine and cilostazol on primary hemostasis using a quantitative bleeding time test apparatusHaemostasis292697610754379
- TaniTSakuraiKKimuraY1992Pharmacological manipulation of tissue cyclic AMP by inhibitors. Effects of phosphodiesterase inhibitors on the functions of platelets and vascular endothelial cellsAdv Second Messenger Phosphoprotein Res25215271372812