Abstract
Sitagliptin (Januvia®, Merck Pharmaceuticals) is a dipeptidyl-peptidase inhibitor (DPP-4 inhibitor) that has recently been approved for the therapy of type 2 diabetes. Like other DPP-4 inhibitors its action is mediated by increasing levels of the incretin hormones glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP). Sitagliptin is effective in lowering HbA1c, and fasting as well as postprandial glucose in monotherapy and in combination with other oral antidiabetic agents. It stimulates insulin secretion when hyperglycemia is present and inhibits glucagon secretion. In clinical studies it is weight neutral. This article gives an overview of the mechanism of action, the pharmacology, and the clinical efficacy and safety of sitagliptin in type 2 diabetes therapy.
Utilizing the therapeutic potential of GLP-1 in type 2 diabetes
Since glucagon-like peptide-1 (GLP-1) itself is not feasible for type 2 diabetes therapy due to its very short biological half-life, two major strategies have been developed to utilize the beneficial effects of GLP-1 (CitationDrucker 2006). On the one hand, long-acting, dipeptidyl-peptidase ihibitor (DPP-4 inhibitor)-resistant peptides with a high similarity to the native GLP-1 can be used as injectable therapeutic agents (incretin mimetics or GLP-1 analogues). Exendin-4, or exenatide in the recombinant form, is such a peptide originally found in the saliva of the gila monster. Exenatide has a very high amino acid sequence similarity with GLP-1 and is a GLP-1 receptor agonist. It has been approved for type 2 diabetes therapy for patients having insufficient glucose control under a therapy with metformin, sulfonylureas, or a combination of both under the trade name Byetta® (Eli Lilly Pharmaceuticals, Indianapolis, IN, USA and Amylin Pharmaceuticals, San Diego, CA, USA) (CitationBray 2006). Liraglutide is a GLP-1 analogue under development by Novo Nordisk Pharmaceuticals (Copenhagen, Denmark) and is being evaluated for efficacy and safety in type 2 diabetes in clinical studies in phase III (CitationFeinglos et al 2005).
The other way to utilize GLP-1 effects in type 2 diabetes is the direct inhibition of DPP-4 by orally active substances (CitationDeacon and Holst 2002). Sitagliptin (Januvia®, Merck Pharmaceuticals, Whitehouse Station NJ, USA) is a highly selective DPP-4 inhibitor that has been approved for type 2 diabetes therapy. Other DPP-4 inhibitors are also in development or close to approval, such as vildagliptin (Galvus®, Novartis Pharmaceuticals, Basel, Switzerland).
DPP-4 inhibition in type 2 diabetes
The incretin effect is diminished in type 2 diabetes. Raising concentrations of intact GLP-1 can lower or even normalize plasma glucose in type 2 diabetic patients (Gallwitz 2006). GLP-1 is degraded very rapidly by DPP-4, an enzyme that is localized vastly in the endothelium and can also be measured in the circulation. DPP-4 cleaves peptides with an N-terminal alanine or proline amino acid residue (CitationMentlein et al 1993). The two GLP-1 fragments resulting from DPP-4 activity are both biologically inactive; the fragment GLP-1(9-36) amide has even been described as having GLP-1 antagonistic properties in some studies (CitationKnudsen and Pridal 1996). DPP-4 inhibition raises intact GLP-1 plasma concentrations to levels observed in the stimulated state after a meal. Besides GLP-1, which has a very high affinity towards DPP-4 as a substrate, other peptides, such as glucose-dependent insulinotropic peptide (GIP), pituitary adenylate cyclase-activating polypeptide (PACAP), and gastrin-releasing peptide (GRP) (see ) are also inactivated by DPP-4 by enzymatic cleavage. Some of these peptides also play a role in glucose homeostasis (CitationNauck and El-Ouaghlidi 2005). DPP-4 is also expressed on T-lymphocytes where it has also been described as CD-26 receptor. The effect of pharmacological inhibition of this receptor with DPP-4 inhibitors has not been completely clarified yet, but so far, the DPP-4 inhibitors in development for the treatment of type 2 diabetes have not shown any immunological adverse effects in this respect. Besides DPP-4, other dipeptidyl peptidases such as DPP-8 or DPP-9 also degrade peptide hormones. In the development of DPP-4 inhibitors it was important to have a high specificity for DPP-4 and no inhibitory activity towards the other DPPs (CitationDemuth et al 2005).
Table 1 Hormones and regulatory peptides as substrates for DPP-4 (modified according to Mentlein (1999)
The pharmacological profile of sitagliptin
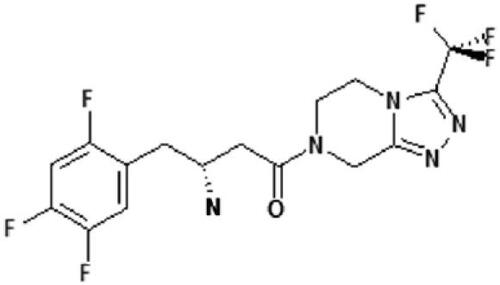
Sitagliptin (MK-0431), chemically (2R)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine (see ) has a very high selectivity towards DPP-4, with an IC(50) of 18 nM. There is no affinity towards other DDP enzymes (DPP-8 and DPP-9). It has been approved for the treatment of type 2 diabetes in the USA and Europe and is registered by the name Januvia® (Merck Pharmaceuticals, Whitehouse Station, NJ, USA) (CitationKim et al 2005). In healthy volunteers and in patients with type 2 diabetes of different ethnic background, the tolerability of different doses given once or twice daily is good. The main pharmacokinetic parameters (Tmax, Cmax and t1/2) measured in studies were similar at baseline and in the steady state after longer administration. Steady-state plasma concentrations of sitaglitptin are reached after 3 days. Given once daily, the accumulation rate is modest (AUC accumulation ratio [day 10/day 1] range, 1.05–1.29) (CitationBergman et al 2006; CitationMiller and St Onge 2006). The elimination and excretion is mainly renal (75% of an oral dose is found in the urine as unchanged drug), and the rest is metabolized via the cytochromes CYP 3A4 and CYP 2C8. Drug–drug interactions were not observed under sitagliptin therapy in clinical studies, and especially no such interactions were found with other antihyperglyemic agents in type 2 diabetic patients (CitationHerman et al 2006a). The elimination half-time is 12–14 hours (CitationHerman et al 2006b). Doses of 50–200 mg/d sitagliptin administered once daily lead to a ≥80% inhibition of DPP-4 over 24 hours and sitagliptin plasma levels of >100 nM. As a result, the concentrations of biologically active, intact GLP-1 are increased 2–3-fold in the postprandial state. In all the studies performed the safety data are very good and hypoglycemic episodes or other adverse events did not differ significantly from those observed in the control groups. In monotherapy or in studies investigating a combination with metformin or thiazolidinediones (TZDs), sitagliptin did not cause hypoglycemia (CitationBergman et al 2006; CitationMiller and St Onge 2006).
Animal studies with sitagliptin
Animal studies have been performed to investigate the effect of sitagliptin on beta- and alpha-cells in the pancreatic islets. Furthermore, the effect of sitagliptin on islet cell mass has been investigated. The latter studies were performed with des-fluoro-sitagliptin in a rodent model with diabetes (high-fat-diet [HFD] treated, streptozotocin-induced [STZ] diabetic mice). The treatment with the sitagliptin analogue over a period of 2–3 months improved glycemic and lipid parameters in these mice (HbA1c, fasting and postprandial glucose, triglycerides, and free fatty acids). A histological evaluation of the islets in treated animals at the end of the study showed an increase in insulin-positive cells and a normalization of the beta- to alpha-cell ratio (see ) (CitationMu et al 2006). The insulin secretion of isolated islets from sitagliptin-treated animals was significantly higher than that of sulfonylurea-treated control animal islets, indicating that the effect was independent of ameliorating glucose toxicity. Sitagliptin might improve beta-cell function and beta-cell mass in type 2 diabetes according to these animal data (CitationMu et al 2006). In favor of this hypothesis are studies in isolated islets from mice treated with vildagliptin for 9 weeks that show that glucose-stimulated insulin secretion was markedly enhanced (CitationAhren et al 2005). The beta-cell protective effect of DDP-4 inhibition is most likely GLP-1 mediated. Incubation of INS-1 cells from an insulinoma cell line in vitro with GLP-1 show a dose-dependent inhibition of STZ-induced apoptosis of these cells. In animal studies in vivo, the administration of DPP-4 inhibitors significantly increased endogenous GLP-1 concentrations along with the improvement in beta-cell function and mass (CitationPospisilik et al 2003). Other gut hormones like GIP, GRP, and PACAP might also contribute to this effect, as other animal studies including studies with a double receptor “knock-out” (DIRKO-mice) show. Further results in support come from mice deficient in DPP-4/CD26 (CD26-/-; DPP-4-“knock-out” mice). These animals are resistant to STZ-induced diabetes and have elevated circulating plasma concentrations of intact GLP-1 and other incretin hormones (CitationMarguet et al 2000). Nevertheless, further studies are needed to completely clarify the role of DPP-4 inhibitors on beta- and alpha-cell function and islet cell mass in type 2 diabetes.
Figure 2 Morphometric analysis of islet cell composition in STZ-induced diabetic mice after MK0431 (des-fluoro-sitagliptin) treatment. Digital images of immunohistochemically stained pancreas sections were captured to quantify the percentage of insulin-positive area in whole pancreas sections as beta-cell volume (A) (#p < 0.03 vs vehicle-treated group; n = 4 animals), and ratios of insulin positive beta-cell to total islet area (B).*p < 0.001 vs vehicle-treated group. Reproduced with permission from CitationMu J,Woods J, Zhou YP, et al. 2006. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes, 55:1695–704. Copyright © 2006 The American Diabetes Association.
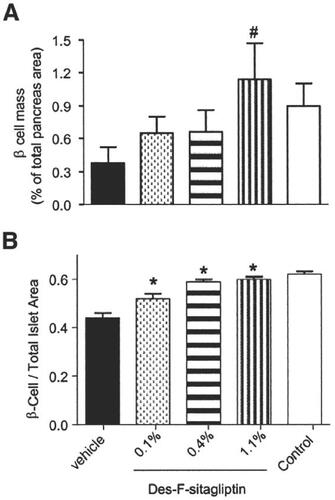
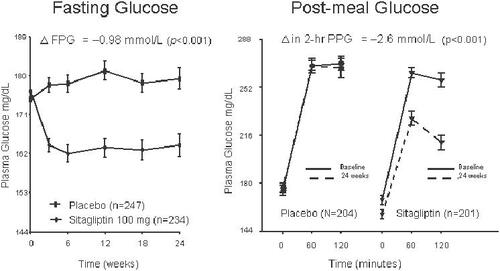
Figure 3 Fasting plasma glucose (FPG, left panel) and postprandial glucose (PPG, right panel) in sitagliptin (100 mg/d) monotherapy over 24 weeks in formerly drug-naïve patients with type 2 diabetes. From data of CitationAschner et al (2006a).
Sitagliptin in clinical studies in type 2 diabetes
Sitagliptin improved the glycemic parameters HbA1c, fasting glucose, and postprandial glucose in clinical studies in patients with type 2 diabetes in monotherapy in doses of 100 mg and 200 mg given once daily in a 24-week study. HbA1c was dose-dependently reduced by 0.79% (100 mg/d) and 0.94% (200 mg/d) as well as fasting glucose (17.1 mg/dL and 21.3 mg/dL, respectively) (CitationAschner et al 2006c). The postprandial glucose was significantly reduced in a standardized meal-tolerance test (2 h postprandial glucose 46.7 mg/dL and 54.1 mg/dL, respectively). Beta-cell function as determined by HOMA-B, the postprandial insulin- and C-peptide responses, as well as the proinsulin/insulin ratio also improved in type 2 diabetic patients. In other monotherapy studies with durations of 12 or 18 weeks, glycemic parameters were also improved in a comparable manner (). In all monotherapy studies, sitagliptin was weight neutral, the 200 mg/d doses even led to a weight reduction of 1.1 kg in the study patients. While the 200 mg dose was more potent than the 100 mg dose in the 24-week study by Ashner (CitationAschner et al 2006c), this slight difference in the potencies of 100 mg and 200 mg were not observed in the shorter 18-week study by Raz (CitationRaz et al 2006c). This might be due to the differences in study durations as well as the different study populations. The maximal approved dose for sitagliptin is 100 mg daily. The sitagliptin therapy was well tolerated, and the incidence of hypoglycemia or other adverse events was not increased (CitationAschner et al 2006a, Citation2006b; CitationRaz et al 2006a, Citation2006b).
Figure 4 Mean (±SEM) HbA1c (a) and fasting plasma glucose (b) over time for placebo (open circles), once-daily sitagliptin 100 mg (solid diamonds) and once-daily sitagliptin 200 mg (solid squares) groups. Reproduced with permission from Raz I, Hanefeld M, Xu L, et al. 2006c. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia, 49:2564–71. Copyright © 2006 Springer Science and Business Media.
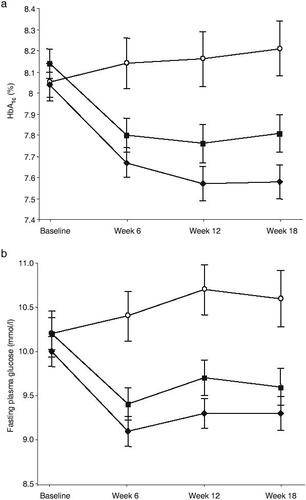
Figure 5 HbA1c development in combination therapies of sitagliptin as add on to either metformin or pioglitazone.The results of the combination studies on HbA1c development are shown for the 24-week study with metformin (left panel) (from data of CitationKarasik et al 2006) and for the study with pioglitazone (right panel) (from data of CitationRosenstock et al 2006a).
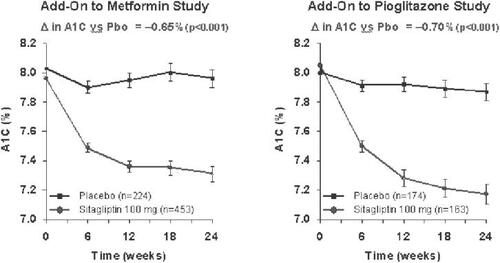
Figure 6 Percentage of patients reaching the HbA1c goal of <7% in mono or combination therapy studies with sitagliptin. Studies of 24 weeks’ duration are shown: sitagliptin monotherapy (left panel) (from data of CitationAschner et al 2006b), combination with metformin (middle panel) (from data of CitationKarasik et al 2006), combination with pioglitazone (right panel) (from data of CitationRosenstock et al 2006a).
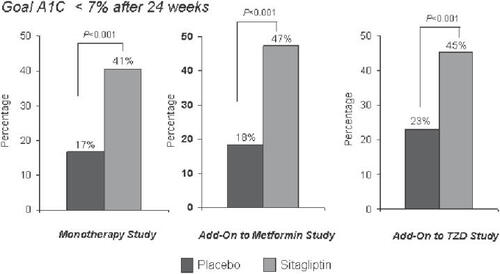
In combination therapies as add on therapy to already existing oral antidiabetic treatment, the addition of sitagliptin (100 mg/d) to metformin led to a significant reduction of HbA1c (0.65%), fasting plasma glucose (25.4 mg/dL), and postprandial glucose (2 h) (50.6 mg/dL) after 24 weeks. The baseline HbA1c in this study was 8.0%. As in the monotherapy studies, beta-cell function measured by the above parameters improved. The addition of sitagliptin to the ongoing metformin therapy was also weight neutral, the combination therapy was well tolerated and the gastrointestinal side-effects as well as hypoglycemic episodes were not increased (CitationCharbonnel et al 2006; CitationKarasik et al 2006).
Comparable results were observed in a study with similar design with an add-on combination of sitagliptin to an existing pioglitazone therapy. As in the metformin combination study, the glycemic parameters HbA1c, and fasting and 2-h postprandial glucose improved. From a baseline HbA1c of 7.9%, a significantly higher percentage of patients reached a target HbA1c <7.0% (45%) compared with the group continuing on pioglitazone monotherapy (23%). Sitagliptin was again weight neutral for the development of the body weight in the patients with pioglitazone treatment. The incidence of side-effects was similar in the combination therapy and pioglitazone monotherapy groups, the incidence of hypoglycemias was not higher with the combination of sitagliptin and pioglitazone (CitationRosenstock et al 2006a, Citation2006b).
In two studies investigating sitagliptin monotherapy and one study investigating the combination of sitagliptin with metformin, beta-cell function was estimated using a frequent-sampling meal-tolerance test in a subgroup of patients. In these studies basal and stimulated insulin responses were improved as well as the disposition index. The sensitivity of the beta-cells to glucose was enhanced, whereas the incidence of hypoglycemic episodes remained very low, suggesting a glucose-dependent mechanism for beta-cell responsiveness (CitationXu et al 2006a, Citation2006b).
Sitagliptin was also generally well tolerated and effective in patients with impaired renal function. In this study, a dose of 25 mg/d was chosen for patients with a creatinine clearance of <30 mL/min or end stage renal disease, and a dose of 50 mg/d was given to patients with a creatinine clearance between 30 and 50 mL/min (CitationScott et al 2006).
Perspectives
Sitagliptin has been shown to be effective, well tolerated, and safe in the treatment of type 2 diabetes in monotherapy or in the combination with metformin or thiazolidinediones. It reduces the glycemic parameters HbA1c, and fasting and postprandial glucose and improves beta-cell function. The reduction of HbA1c observed in the studies was at least as good as that seen with other oral antihyperglycemic agents. In this respect, it has to be noted, however, that the potency of HbA1c reduction in type 2 diabetes by oral agents is also dependent on the baseline HbA1c; in studies with higher baseline HbA1c values, usually higher reductions of HbA1c are observed.
Sitagliptin is weight neutral and does not increase the incidence of hypoglycemic episodes or the occurrence of adverse events. The studies published so far contain data from up to 24 weeks; longer studies will be published soon. In man, long-term data on immunological effects mediated by CD-26 are unknown yet, but to date, no alterations in immune functions have been detected in clinical studies or long-term animal studies.
Sitagliptin and DPP-4 inhibitors in general address a novel multimodal principle of action in type 2 diabetes. By preserving stimulated circulating plasma levels of incretin hormones, insulin secretion is stimulated under hyperglycemic conditions and glucagon secretion is suppressed. Therefore, not only insulin secretion and insulin resistance are altered, as by the previously used oral antihyperglycemic agents, but also unmet needs of type 2 diabetes therapy are covered by this novel therapeutic principle (CitationAhren 2005; CitationGallwitz 2006).
In case the effects observed on beta-cell function and beta-cell mass in preclinical studies also apply to human studies, sitagliptin could also have the potential to be useful in pre-diabetic stages and early stages of type 2 diabetes to retard or prevent the disease progression (CitationGallwitz 2006).
References
- AhrenBWhat mediates the benefits associated with dipeptidyl peptidase-IV inhibition?Diabetologia200548605715770467
- AhrenBSorhede WinzellMBurkeyBBeta-cell expression of a dominant-negative HNF-1alpha compromises the ability of inhibition of dipeptidyl peptidase-4 to elicit a long-term augmentation of insulin secretion in miceEur J Pharmacol2005521164816171801
- AschnerPKipnesMLuncefordJSitagliptin monotherapy improved glycemic control in the fasting and postprandial states and beta-cell function after 24 weeks in patients with type 2 diabetes (T2DM)Diabetes2006a55Suppl 1A462
- AschnerPKipnesMLuncefordJSitagliptin monotherapy improved glycemic control in patients with type 2 diabetesDiabetologia2006b49Supl 15
- AschnerPKipnesMSLuncefordJKEffect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetesDiabetes Care2006c292632717130196
- BergmanAJStevensCZhouYPharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteersClin Ther200628557216490580
- BrayGMExenatideAm J Health Syst Pharm200663411816484515
- CharbonnelBKarasikALiuJEfficacy and safety of sitagliptin added to ongoing metformin therapy in type 2 diabetes patients who were inadequately controlled on metformin aloneDiabetologia200649Suppl 15
- DeaconCFHolstJJDipeptidyl peptidase IV inhibition as an approach to the treatment and prevention of type 2 diabetes: a historical perspectiveBiochem Biophys Res Commun20022941412054731
- DemuthHUMcIntoshCHPedersonRAType 2 diabetes—therapy with dipeptidyl peptidase IV inhibitorsBiochim Biophys Acta20051751334415978877
- DruckerDJThe biology of incretin hormonesCell Metab2006315365
- FeinglosMNSaadMFPi-SunyerFXEffects of liraglutide (NN2211), a long-acting GLP-1 analogue, on glycaemic control and bodyweight in subjects with Type 2 diabetesDiabet Med20052210162316026367
- GallwitzBTherapies for the treatment of type 2 diabetes mellitus based on incretin actionMinerva Endocrinol2006311334716682937
- HermanGABergmanAYiBTolerability and pharmacokinetics of metformin and the dipeptidyl peptidase–4 inhibitor sitagliptin when co-administered in patients with type 2 diabetesCurr Med Res Opin2006a2219394717022853
- HermanGABergmannJWagnerJASitagliptin, a DPP–4 inhibitor: an overview of the pharmacokinetic (PK) profile and the propensity for drug–drug interactions (DDI)Diabetologia2006b49Suppl 1481
- KarasikACharbonnelBLiuJSitagliptin added to ongoing metformin therapy enhanced glycemic control and beta-cell function in patients with type 2 diabetesDiabetes200655Suppl 1A 119
- KimDWangLBeconiM(2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl) butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetesJ Med Chem2005481415115634008
- KnudsenLBPridalLGlucagon-like peptide-1-(9-36) amide is a major metabolite of glucagon-like peptide-1-(7-36) amide after in vivo administration to dogs, and it acts as an antagonist on the pancreatic receptorEur J Pharmacol1996318429359016935
- MarguetDBaggioLKobayashiTEnhanced insulin secretion and improved glucose tolerance in mice lacking CD26Proc Natl Acad Sci USA2000976874910823914
- MentleinRDipeptidyl-peptidase IV (CD26)—role in the inactivation of regulatory peptidesRegul Pept19998592410588446
- MentleinRGallwitzBSchmidtWEDipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serumEur J Biochem1993214829358100523
- MillerSSt OngeELSitagliptin: a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetesAnn Pharmacother20064013364316868220
- MuJWoodsJZhouYPChronic inhibition of dipeptidyl peptidase–4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetesDiabetes200655169570416731832
- NauckMAEl-OuaghlidiAThe therapeutic actions of DPP-IV inhibition are not mediated by glucagon-like peptide-1Diabetologia2005486081115761719
- PospisilikJAMartinJDotyTDipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic ratsDiabetes2003527415012606516
- RazIHanefeldMXuLSitagliptin monotherapy improved glycemic control and beta-cell function after 18 weeks in patients with type 2 diabetes (T2DM)Diabetes2006a55Suppl 1A 462
- RazIHanefeldMXuLEfficacy and safety of sitagliptin over 18 weeks in patients with type 2 diabetesDiabetologia2006b49Suppl 1481
- RazIHanefeldMXuLEfficacy and safety of the dipeptidyl peptidase–4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitusDiabetologia2006c4925647117001471
- RosenstockJBrazgRAndryukPJAddition of sitagliptin to pioglitazone improved glycemecic control with neutral weight effect over 24 weeks in inadequately controlled type 2 diabetesDiabetologia2006a49Suppl 126
- RosenstockJBrazgRAndryukPJAddition of sitagliptin to pioglitazone improved glycemic control with neutral weight effect over 24 weeks in inadequately controlled type 2 diabetes (T2DM)Diabetes2006b55Suppl 1A 132A 133
- ScottRHartleyPLuoEUse of sitagliptin in patients with type 2 diabetes (T2DM) and renal insufficiency (RI)Diabetes200655Suppl 1A 462
- XuLManCDCobelliCSitagliptin improved beta-cell function in patients with type 2 diabetes: a model-based analysisDiabetologia2006a49Suppl 1396
- XuLManCDCobelliCSitagliptin improved beta-cell function in patients with type 2 diabetes (T2DM): a model-based analysisDiabetes2006b55Suppl 1A 466