Abstract
Type 2 diabetes has become a major burden to the health care systems worldwide. Among the drugs approved for this indication, glimepiride and rosiglitazone have gained substantial importance in routine use. While glimepiride stimulates β-cell secretion and leads to reduction of blood glucose values, rosiglitazone activates PPARγ and improves insulin resistance, at the vascular and metabolically active cells. Therefore, the combination of the two drugs may be an interesting approach to improve glycemic control and lower cardiovascular risk. A fixed combination of both drugs has been approved for clinical use in the US and EU. The combination of glimepiride and rosiglitazone is generally well tolerated and the use of a fixed combination may lead to improved adherence of the patients to their therapy. The purpose of this review is to evaluate the clinical data that have been published on this combination, appearing to represent a convenient way to obtain therapeutic targets in patients with type 2 diabetes mellitus.
Introduction
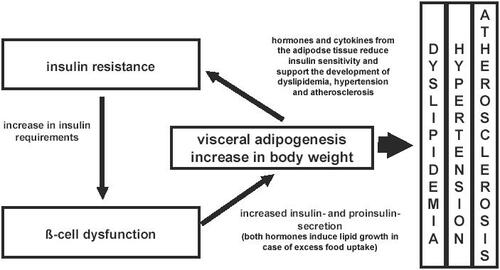
Type 2 diabetes is a leading cause of morbidity and mortality in many countries and the number of cases is currently approaching pandemic proportions (CitationZimmet et al 2001). Patients with both types of diabetes mellitus have an increased risk of fatal cardiovascular events. About 75% patients with type 2 diabetes die from macrovascular complications, but only 35% of the patients with type 1 diabetes. This significant difference is linked to insulin resistance and β-cell dysfunction, the underlying disorders in type 2 diabetes (CitationPickup and Williams 2002). Insulin resistance leads to increased β-cell activity, and the impairment of β-cell function is followed by a deterioration of the β-cell secretion product, leading to secretion of the insulin precursor proinsulin. While proinsulin has only about 10%–20% of the blood-glucose-lowering activity of insulin, it has comparable effects on the induction of adipogenesis (CitationPfützner et al 2006b). The consecutive growth of adipose tissue, however, is accompanied by a hormonal secretion pattern that impairs insulin resistance (). With advancing disease progression, even more proinsulin is secreted, which is known to contribute to the increased cardiovascular risk by inducing plasminogen activator inhibitor type-I (PAI-I) secretion, consecutively leading to an impairment of fibrinolysis (CitationSchneider et al 1992; CitationPfützner et al 2004).
Figure 1 The relation of insulin resistance, β-cell dysfunction, obesity, and their associated complications.
Different treatment moieties are available to address these pathophysiological components of type 2 diabetes. The first attempt in primary care is usually to treat the patients with a combined approach of increased physical activity and dietary recommendations, which should be accompanied by patient training about the disease in order to increase the adherence to the required lifestyle changes. However in daily routine, lifestyle modifications are not consequently followed and a progressive deterioration of blood glucose metabolism leading to increased hemoglobin A1c (HbA1c) values requires the introduction of oral anti-diabetic agents. At this stage, several therapeutic options are available including metformin, sulfonylurea drugs (SU), thiazolidinediones, alpha-glucosidase inhibitors, and injectable or pulmonary insulin. While it would directly address β-cell dysfunction, insulin is not frequently used for therapy initiation because patients do not want to inject and the therapy is also not recommended as first-line approach for economic reasons in many countries. The price of the drugs may also be the reason for the more hesitant use of thiazolidinediones (TZD) in initial diabetes mono-therapy. The currently most frequently prescribed drugs for first-line treatment are the SUs and metformin. In many therapeutic guidelines, metformin is recommended for obese patients while use of SUs is suggested in patients with normal or slightly increased body weight (CitationAmerican Diabetes Association 2006).
With the currently predominantly used therapies, type 2 diabetes appears to be a constantly progressing disease and mono-therapy may last for approximately 5–10 years before a further increase in HbA1c indicates the requirement of more intensive treatment regimens. At this stage, a second oral anti-diabetic drug will be introduced to increase the efficacy of the therapeutic approach. One approach may be the combination of SU and TZD in order to benefit from the synergistic therapeutic actions of both drug classes.
Rationale for the combination
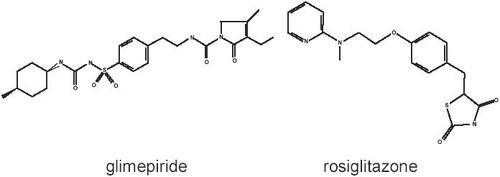
Glimepiride [1-p-[[2-(3-ethyl-4-methyl-2-oxo-3-pyrro-line-1-carboxamido) ethyl] phenyl] sulphonyl]-3-(trans-4-methylcyclohexyl) urea] is a sulfonylurea drug that stimulates β-cell secretion by binding to a 65 kDa β-cell receptor leading to a decrease in gluco/hexokinase binding to porin proteins and an increase in the expression of glukokinase mRNA. The chemical structure is shown in . The largest effects appear during the first 4 hours after uptake and doses of 1–8 mg are usually given before or with breakfast. The extra-pancreatic effects seem to be similar to those of other SUs (CitationMcCall AL 2001). The unfavorable cardiovascular effects of SUs, eg, increase in diazoxide-induced KATP-channel opening, ST segment changes, and blood pressure increase, are less pronounced with glimepiride than with glibenclamide (CitationLangtry and Balfour 1998). By increasing β-cell output, glimepiride lowers blood glucose levels and HbA1c, the major treatment targets in the management of type 2 diabetes. Recent investigations describe an additional PPARγ-stimulating effect of glimepiride and an induction of endothelial NO synthesis, which makes glimepiride the most interesting SU candidate for a combination with TZDs (CitationFukuen et al 2005; CitationUeba et al 2005).
A lifestyle change with regular performance of physical exercise and weight loss would be a normal and physiological way to improve insulin resistance, the second component of the underlying pathophysiology. The only drug class effectively addressing this condition is the class of thiazolidinediones or PPARγ-agonists, with two currently commercially available drugs, pioglitazone and rosiglitazone. The chemical structure of rosiglitazone [(±)-5-[[4-[2-(metyl-2-pyridinylamino) ethoxy] phenyl] methyl]-2, 4-thiazolidenedione, (Z)-2-butenedioate (1:1)] is also shown in . TZDs activate the nuclear peroxisome proliferator-activated receptor (PPAR)γ, which is expressed predominately in adipose tissue and regulates the gene transcription involved in adipocyte differentiation and glucose and lipid metabolism (CitationKersten et al 2000; CitationSchoonjans et al 2000; Debril et al 2001). The metabolic effects of PPARγ activation by rosiglitazone comprise an increase in peripheral insulin sensitivity in muscle, liver, and adipose tissue (CitationHallsten et al 2002; CitationWagstaff et al 2002), improvement of postprandial and fasting glucose concentrations as well as long-term glucose control, improvement of adipogenesis leading to an increase in HDL cholesterol (CitationWagstaff et al 2002), reduction of vascular inflammation (CitationNatali et al 2004), improvement of arterial elasticity (Shargorodsky et al 2003), and a reduction of laboratory markers for cardiovascular risk (CitationHaffner et al 2002; CitationMarx et al 2003a, Citationb; CitationMohanty et al 2004; CitationPfützner et al 2006a).
The rationale for the fixed combination is the synergistic effect of both substances via different modes of action on elevated blood glucose levels and the potential that the observed anti-inflammatory effects of rosiglitazone at the vascular level may correct the expected negative influence of a SU on the chronic vascular inflammation.
Efficacy and tolerability of the fixed combination of glimepiride and rosiglitazone
The fixed combination of rosiglitazone has only recently been approved for clinical use. The idea of manufacturing a fixed combination of rosiglitazone with a sulfonylurea drug came from the results of past clinical trials performed with the drugs given as separate tablets. An overview of the important published studies on this topic is provided in .
Table 1 Overview on the clinical studies applying a combination of rosiglitazone and a SU drug in comparison with uptitration of the SU in patients failing on low dose SU therapy
Studies comparing the rosiglitazone/SU combination vs SU mono-therapy
While the benefit of adding rosiglitazone to sulfonylurea drugs was initially shown several years ago vs continuation of the pre-existing therapy in European, American, and Asian patients (CitationWolffenbüttel et al 2000; CitationVongthavaravat et al 2002; CitationYang et al 2003; CitationZhu et al 2003), a series of controlled clinical trials compared the addition of rosiglitazone to different sulfonylureas at a low dose (glibenclamide, gliclazide, glipizide, and glimepiride) with the uptitration of the respective SU drugs in patients at early disease stages (see below).
CitationKerenyi et al (2004) compared the efficacy of a daily combination of 8 mg of rosiglitazone with 7.5 mg glibenclamide vs the uptitration of the SU, in a total of 340 patients with inadequately controlled type 2 diabetes (FPG ≥7.0 and ≤15.0 mmol/L) on glibenclamide 7.5 mg/day. They were randomized to either additional treatment with rosiglitazone 8 mg/day or uptitration of the glibenclamide dose (maximum dose = 15 mg/day) for an observation period of 26 weeks. Treatment with the rosiglitazone/SU combination reduced HbA1c by 0.91% and FPG by 2.4 mmol/L (intensified glibenclamide mono-therapy: HbA1c: –0.14%, FPG: +0.2 mmol/L, both p < 0.001 compared with the combination). With the rosiglitazone/SU combination, an increase in HDL cholesterol by 15.8% and a decrease in free fatty acids by 15.3% and triglycerides by 5.8% could be observed. Both treatments were well tolerated and had predictable safety profiles, which led to the final conclusion that addition of rosiglitazone provided significantly improved glycemic control compared with uptitration of glibenclamide (CitationKerenyi et al 2004).
A second study investigated the TZD/SU combination with gliclazide as the SU component. A total of 471 patients with type 2 diabetes who were inadequately controlled on a half-maximal dose of gliclazide (160 mg/day) were randomized to receive either the addition of rosiglitazone (4 mg bid) or to have their gliclazide uptitrated to a maximum of 320 mg/day during a 26-week treatment period. A reduction in HbA(1c) of 1.3% (p < 0.001) was observed in the combination treatment group compared with the uptitrated gliclazide at endpoint. The proportion of patients who achieved an HbA1c value <7% was also greater in the combination group (48% vs 22%). FPG was reduced by 3.0 mmol/L (p < 0.001) in the rosiglitazone/SU group compared with the uptitrated gliclazide group after 26 weeks. The observed side-effects included an increased incidence of signs or symptoms suggestive of hypoglycemia with rosiglitazone/SU compared with uptitrating the gliclazide dose (6% vs 2%). Only 1% of patients reported severe hypoglycemia. The combination treatment led to increases in plasma lipoproteins, and more patients experienced edema (11% vs 3%). A significant increase in body weight was observed in patients receiving rosiglitazone plus gliclazide vs uptitrated gliclazide (3.4 kg; p < 0.001). The authors concluded that the addition of rosiglitazone (4 mg bid) to gliclazide (160 mg/day) was well tolerated, and significantly more effective in improving glycemia than uptitrating gliclazide to 320 mg/day (CitationBaksi et al 2004).
The aim of a recently published study (CitationRosenstock et al 2005) in older patients with type 2 diabetes was to compare the efficacy, safety, and tolerability of adding rosiglitazone vs glipizide dose escalation when the patients were inadequately controlled on low dose SU therapy. A total of 227 patients (age: >60 years) were randomized to receive rosiglitazone (4 mg) or placebo once daily in combination with glipizide 10 mg twice daily for 2 years in a doub-le-blind, parallel-group study design. Treatment options were individualized, and escalation of study medication was specifically defined. Disease progression, defined as the time to reach confirmed FPG >10 mmol/L while on maximum doses of both glipizide and study medication or placebo, was reported in 28.7% of patients uptitrating glipizide plus placebo compared with only 2.0% taking rosiglitazone and glipizide combination (p < 0.001). The combination significantly decreased HbA1c, FPG, insulin resistance, and plasma free fatty acids. The authors concluded that the addition of rosiglitazone to SU in older patients with type 2 diabetes significantly improved glycemic control and reduced disease progression compared with uptitrated glipizide alone and without increasing hypoglycemia. These benefits were associated with increased patient treatment satisfaction and reduced medical care utilization in terms of emergency room visits and length of hospitalization (CitationHerman et al 2005; CitationRosenstock et al 2005).
The purpose of a fourth clinical trial (CitationRosenstock et al 2006b), a 24-week, randomized, double-blind, controlled study, was to demonstrate that the early addition of rosiglitazone to submaximal therapeutic doses of glimepiride leads to greater glycemic improvement than uptitration of glimepiride to maximal dose. Prior to study entry, subjects were treated with a single oral agent or low-dose oral combination therapy. Mean duration of diabetes was approximately 5 years in the two treatment groups. All subjects received low-dose glimepiride (2 mg) during a 6-week run-in period. At randomization, subjects either added 4 mg of rosiglitazone to the glimepiride dose (add-on group) or added placebo to an increased glimepiride dose (4 mg, uptitration group) once daily. After 8 weeks, glimepiride was increased to 4 mg in the rosiglitazone add-on group (n = 180) or to 8 mg in the glimepiride uptitration group (n = 181), if fasting blood glucose was ≥110 mg/dL. While no change from baseline in HbA1c or FPG was observed in the uptitration group (−0.08%; –0.6 mg/dL, not significant in both cases), a significant reduction in both observation parameters was seen in the rosiglitazone add-on group (−0.68%; –27.7 mg/dL; p < 0.05 in both cases and between groups). In this study, the addition of rosiglitazone to low-dose glimepiride led to clinically and statistically significant decreases in HbA1c and FPG levels compared with uptitration of glimepiride alone, and more subjects reached the ADA goal of HbA1c <7% (63.9% vs 39.8%, p < 0.05). Addition of rosiglitazone also showed significant improvements in HOMA β-cell function (18%) and insulin sensitivity (15%) estimates relative to glimepiride alone (p < 0.05). Both therapies were well tolerated. The incidence of hypoglycemia with blood glucose ≤50 mg/dL was low and similar between the groups. The authors concluded that their study supported the paradigm that early introduction of oral combination therapy is more effective in achieving glycemic control than increasing doses of SU mono-therapy, without increasing the risk of confirmed hypoglycemia (CitationRosenstock et al 2006b).
In another randomized double-blind parallel study with patients with type 2 diabetes, 3 mg of glimepiride was used as a baseline therapy in all treatment groups. Based on the randomization scheme, the patients received additional treatment with placebo (group 0), 4 mg (group 4), or 8 mg (group 8) of rosiglitazone for 4 months. The investigation was performed to assess efficacy and safety of the rosiglitazone/glimepiride combination therapy (HbA1c, FPG, hypoglycemia, and adverse events). The combination of 4 mg or 8 mg of rosiglitazone with glimepiride 3 mg significantly improved glycemic control compared with glimepiride alone. HbA1c levels were significantly reduced from baseline in group 4 by –0.63% and group 8 by –1.17%, but not in group 0 (−0.08%, p < 0.001 vs both other groups). FPG was significantly reduced in group 8 vs group 0 (p < 0.001) and a greater proportion of patients treated with the combination achieved target levels of HbA1c (ADA goal of HbA1c <7%: group 4: 43%; group 8: 68% vs group 0: 32%). Mean body weight increased in group 8 by 1.7 kg (p < 0.01 vs baseline), whereas the weight gain with the lower rosiglitazone dose was not significantly different from that with the glimepiride mono-therapy arm. All treatments were generally well tolerated with no significant differences in the incidence or profile of adverse events between the treatment groups. There were no withdrawals due to hypoglycemia, hepatotoxicity, or edema. In particular, combination treatment did not increase the incidence of hypoglycemia compared with glimepride alone (CitationHamman et al 2003).
For a subgroup of 102 patients (group 0: n = 30; group 4: n = 31; group 8: n = 41; 46 women, 56 men, mean age: 62.8 ± 9.1 years, BMI 28.7 ± 4.5 kg/m2, diabetes duration 6.4 ± 4.8 years, HbA1c 8.1 ± 1.5%), additional samples were available from this study for assessment of HOMAIR score, insulin, intact proinsulin, and adiponectin after 0 and 16 week of treatment. Insulin resistance was defined by elevated intact proinsulin values or HOMAIR >2. All parameters were comparable in the three groups at baseline. While no changes were seen for any of the observation parameters except an increase in adiponectin from 8.4 ± 5.1 mg/L to 11.9 ± 6.2 mg/L (+42%, p < 0.001) with glimepiride alone, substantial and significant dose-dependent improvements were observed after addition of rosiglitazone for fasting glucose (group 0: –9 ± 48 mg/dL; group 4: –38 ± 47 mg/dL; group 8: –46 ± 53 mg/dL), HbA1c (−0.1 ± 0.7%; −1.1 ± 1.2%; –1.3 ± 1.2%), insulin (+1.4 ± 6.2 µU/mL; –1.2 ± 5.3 µU/mL; –3.7 ± 9.9 µU/mL), and intact proinsulin (+1.6 ± 7.1 pmol/L; –2.0 ± 4.6 pmol/L; –3.1 ± 6.1 pmol/L). After adjustment for changes in body weight, a significant additional contribution of PPARγ activation (p < 0.001) to the adiponectin increase was detected, while glimepiride alone did not induce a comparable effect (−0.5 ± 5.8 mg/L; +8.8 ± 22.9 mg/L; +14.3 ± 19.9 mg/L). The number of insulin-resistant patients decreased in both rosiglitazone treatment groups, while no change was seen with glimepiride alone. Next to the reported effects on glucose control, rosiglitazone provided an additional beneficial effect on insulin resistance and β-cell function leading to lower HOMAIR scores, lower values for insulin, and intact proinsulin, and a more pronounced increase in adiponectin values. These results supported the clinical rationale of combining rosiglitazone with sulfonylurea drugs in patients with type 2 diabetes (CitationPfützner et al 2006a).
In accordance with the current treatment guidelines, rosiglitazone was added to a SU drug in all aforementioned trials, but CitationMcCluskey et al (2004) investigated the converse situation. A total of 40 patients who failed on rosiglitazone mono-therapy were treated with additional glimepiride vs placebo for 26 weeks. The outcomes were greater reductions for the glimepiride vs the placebo combination in HbA1c (mean [SE], –12% [0.1%] vs –3% [2%]; p < 0.001) and FPG (mean [SE], –24.4 [6.0] mg/dL vs 5.9 [8.0] mg/dL; p < 0.01). More patients in the glimepiride group achieved the HbA1c target of ≤7% (60% vs 14%; p < 0.01). There were no significant differences in the rate or type of adverse events between groups, and no episodes of severe hypoglycemia occurred with either treatment (CitationMcCluskey et al 2004). It needs to be pointed out, however, that the inclusion criteria of the patients in this trial may have potentially resulted in selection of pharmacological non-responders to rosiglitazone therapy, but unfortunately no pharmacogenetic characterization is provided for the trial participants in this manuscript.
Addition of pioglitazone (15 mg) or rosiglitazone (4 mg) to glimepiride in patients with type 2 diabetes and metabolic syndrome for 12 months in a double-blind randomized parallel trial resulted in a comparable reduction in HbA1c and FPG with both drugs, comparable improvement of insulin resistance and the prothrombotic state, but with better lipid values resulting from the pioglitazone/glimepiride combination. In general, however, the overall efficacy and side-effect profiles of both TZD/SU combinations were considered to be beneficial for the metabolic control of the patients (CitationDerosa et al 2004 and Citation2005a).
In summary, all these dual combination trials have demonstrated that the use of rosiglitazone together with sulfonylurea drugs provides a better glycemic control compared with further intensifying the SU mono-therapy. This result was accompanied by an improvement in the cardiovascular risk profile of the patients. The metabolic improvements were associated with an equal or increased number of hypoglycemic events. However, this increase in hypoglycemia needs to be analyzed in the context of the observed parallel HbA1c improvements. Other observed side-effects of the TZD/SU combination vs SU alone were increases in body weight and an increased incidence of mild edema, which are established side-effects of rosiglitazone therapy. The combination was well tolerated and no indications of hepatotoxicity were observed in any of the trials.
Studies of rosiglitazone/SU in other treatment combinations
In the past, the usual oral combination to be prescribed after failure of SU or metformin mono-therapy was the combination of SU with metformin. Some studies have investigated the combination of rosiglitazone with metformin vs metformin/SU (eg, CitationDerosa et al 2005b, Citation2006), but no investigation is available comparing rosiglitazone/glimepiride with the metformin/SU combination. In comparison with metformin, rosiglitazone decreases liver fat and increases peripheral glucose uptake. The decrease in liver fat is associated with an increase in serum adiponectin concentrations (CitationTiikainen et al 2004). Rosiglitazone positively affects CV risk markers, reducing plasmatic concentration of C-reactive protein (PCR-), matrix metallo-proteinases (MMP-9), tumor necrosis factor-alpha (TNF-alpha), serum amyloid, and soluble CD40L in type 2 diabetic patients with and without coronary disease (CitationWellington 2005). These effects seem to be conserved when rosiglitazone is used in combination with glimepiride (CitationPfützner et al 2006a) and both drugs are, therefore, interesting candidates also for multiple component therapies, especially when a fixed-dose combination of rosiglitazone and glimepiride may help to reduce the number of daily tablets.
CitationOrbay et al (2004) investigated the addition of rosiglitazone to a combination of glimepiride and metformin therapy in patients with insufficiently controlled type 2 diabetes over 26 weeks. Thirty patients were taking glimepiride (3 mg) and metformin (850 mg) two times per day and added rosiglitazone (4 mg) before breakfast. Mean HbA1c levels decreased significantly from 7.54 ± 0.9% to 6.57 ± 0.7% (p < 0.001) and FPG levels fell from 169 ± 38 mg/dL to 136 ± 28 mg/dL (p < 0.001), respectively. Insulin levels decreased from 19.6 ± 9.8 U/L to 14.7 ± 11.6 U/L (p < 0.05) at endpoint. No elevations of alanine aminotransferase or aspartate aminotransferase levels greater than 2.5 times the upper limit of the reference range were observed. This study confirmed that the addition of rosiglitazone (4 mg/day) to SU and metformin treatment may provide a promising approach to achieve target levels of glycemia (CitationOrbay et al 2004).
A different study by CitationRoberts et al (2005) evaluated the efficacy and tolerability of glimepiride in patients with type 2 diabetes mellitus that were inadequately controlled with a combination of immediate- or extended-release metformin and a thiazolidinedione. In this multicenter, randomized, double-blind, placebo-controlled, parallel-group, 2-arm study consisting of a 4-week stabilization and eligibility period and a 26-week treatment period, 170 patients with type 2 diabetes received glimepiride (uptitration possible) or placebo in combination with an established regimen of immediate- or extended-release metformin and rosiglitazone or pioglitazone. Demographic variables were similar at baseline between the glimepiride and placebo groups. HbA1c was significantly improved at endpoint with glimepiride combination therapy compared with placebo (mean [SE], –1.31% [0.08] vs –0.33% [0.08], respectively; p < 0.001). Most patients (62.2%) who received glimepiride achieved an HbA1c value of ≤7%, compared with 26.0% of patients receiving placebo (p < 0.001 between groups). Patients on glimepiride therapy had a higher BMI at endpoint (adjusted change from baseline to endpoint 1.26 ± 0.16 kg/m2 with glimepiride and 0.17 ± 0.16 kg/m2 with placebo (p < 0.001). There were no significant differences in lipid levels between groups. Clinically significant adverse events, laboratory abnormalities, and rates of severe hypoglycemia were similar between treatment groups. The overall incidence of hypoglycemia, however, was 51.2% in the glimepiride group and 8.3% in the placebo group (p < 0.001). This study showed that in these patients with type 2 diabetes not adequately controlled by dual combination therapy with metformin and a thiazolidinedione, the addition of glimepiride improved glycemic control compared with placebo with an acceptable tolerability profile. Although there were significantly more episodes of hypoglycemia with triple therapy than with dual therapy and placebo, the risk for severe hypoglycemia was low. This study again supports the use of glimepiride in conjunction with TZD combination therapies (CitationRoberts et al 2005).
A controlled study investigating the effects of adding rosiglitazone in comparison to a long acting insulin analogue on metabolic control in patients inadequately controlled with a metformin/SU combination therapy was recently published by CitationRosenstock et al (2006b). In this 24-week multicenter, randomized, open-label, parallel trial, 217 patients (HbA1c: 7.5%–11%, BMI >25 kg/m²) on ≥50% of maximal-dose SU and metformin received add-on insulin glargine 10 units/day or rosiglitazone 4 mg/day. Insulin glargine was forced-titrated to target FPG (≤100–120 mg/dL), and rosiglitazone was increased to 8 mg/day any time after 6 weeks, if FPG was >5.5 mmol/L. A similar improvement in HbA1c was observed in both groups (−1.7% vs –1.5% for insulin glargine vs rosiglitazone, respectively). When baseline HbA1c was >9.5%, the reduction with insulin glargine was greater than with rosiglitazone (p < 0.05). Also, insulin glargine yielded better FPG values than rosiglitazone (−3.6 ± 0.23 mmol/L vs –2.6 ± 0.22 mmol/l; p < 0.001). The final daily insulin glargine dose was 38 ± 26 IU vs 7.1 ± 2 mg for rosiglitazone. Confirmed hypoglycemic events at plasma glucose <3.9 mmol/L (<70 mg/dL) were slightly greater for the insulin glargine group (n = 57) than for the rosiglitazone group (n = 47) (p = 0.0528). More patients in the insulin glargine group had confirmed nocturnal hypoglycemia of <3.9 mmol/L (p < 0.05) and <2.8 mmol/L (p < 0.05) than in the rosiglitazone group. The effects on total and LDL cholesterol, and triglyceride levels with insulin glargine contrasted with those of rosiglitazone (−4.4%, –1.4%, and –19.0% vs +10.1%, +13.1%, and +4.6%, respectively; p < 0.005). HDL cholesterol was unchanged with insulin glargine but increased with rosiglitazone by 4.4%, p < 0.05). Insulin glargine led to less weight gain than rosiglitazone (1.6 ± 0.4 kg vs 3.0 ± 0.4 kg; p < 0.05), fewer adverse events (7% vs 29%; p < 0.001), and no peripheral edema (0 vs 12.5%). In conclusion, both triple therapies achieved comparable improvements in HbA1c. Rosiglitazone was associated with less hypoglycemia and more weight gain (CitationRosenstock et al 2006a).
As shown above, many studies suggest the use of a rosi-glitazone/SU combination in different stages of type 2 diabetes. Further studies supporting the concept and efficacy of the fixed dose combination of rosiglitazone with glimepiride are currently under way and their results will soon become publicly available.
Recent findings from the ADOPT trial, a monotherapy outcome study comparing rosiglitazone, metformin, and glyburide over four years (CitationKahn et al 2006), showed the typical side-effects of each drug class. Gastrointestinal problems were most frequently observed with metformin, sulfonylureas led to hypoglycaemia, and weight gain and edema were most frequently seen with rosiglitazone. In addition, there was a small but significant number of leg and forearm fractures in postmenopausal women with rosiglitazone. As it is known that PPARγ activation may influence bone metabolism, further research is required to elucidate the impact of these findings on the risk/benefit analysis of the rosiglitazone/glimepiride combination.
Quality of life and patient satisfaction
The fixed combination of glimepiride with rosiglitazone has only been recently approved. Therefore, no report focussing on patient satisfaction with this fixed combination has been published yet. Improved patient satisfaction with the combination of rosiglitazone and glipizide given as separate tablets was already reported from the RESULT study (CitationRosenstock et al 2005; CitationHerman et al 2005). It can, therefore, be expected that the additional reduction of the daily number of tablets to be taken may lead to an even better treatment satisfaction and adherence of the patients to their therapy.
Conclusion
The combination of rosiglitazone with glimepiride has been investigated in multiple trials, some of which especially addressed the benefit of adding a TZD in early stages to low-dose SU therapy vs increasing the SU dose or a head-to-head comparison with the SU/metformin combination. Introducing the synergistic effects of both drug classes at these stages improved overall glycemic control without necessarily increasing the risk for hypoglycemic episodes, and with a safety profile comparable with rosiglitazone mono-therapy. In all studies, more patients on the combination therapy reached the HbA1c treatment target compared with the respective comparator arms. While these trials provide the scientific rationale for combining TZDs with SUs, the introduction of a fixed-dose combination of rosiglitazone and glimepiride may be regarded as a helpful tool to increase patients convenience, since it reduces the number of daily tablets. It may, therefore, support patients’ adherence to the essential anti-diabetic therapy. The available pharmacological formulations, allowing for up- or downtitration of the glimepiride doses, may be welcomed by physicians as a means of responding to a potentially increased number of hypoglycemic events without losing metabolic efficacy.
Place in therapy
Based on published investigations, the place for the fixed-dose combination of rosiglitazone and glimepiride may be as a second-line therapeutic approach in patients failing on low- to mid-dose SU mono-therapy and in the triple combination with metformin. In this treatment segment, this fixed combination has to compete with the fixed combination of pioglitazone with glimepriride, which has also been approved recently in the US and Europe.
As mentioned above, the fixed combination of rosiglitazone with glimepiride is available as single tablet formulation containing 4 mg of rosiglitazone with either 1 mg, 2 mg, or 4 mg of glimepiride. It should be given once daily with the first meal of the day and dosage should be adapted to individual needs in order to obtain the optimal glycemic control with the lowest incidence of adverse events, particularly severe hypoglycemia and heart failure. The maximum recommended dose is 8 mg of rosiglitazone and 4 mg of glimepiride. The recommended starting dose for patients inadequately controlled with SU alone or who had initially responded to rosiglitazone and require a better glycemic control, is 4 mg/1 mg. This starting dose is also recommended in elderly and debilitated patients and in case of renal, hepatic, or adrenal insufficiency. When switching from a combination therapy of rosiglitazone plus glimepiride as separate tablets, the corresponding fixed-dose formulation should be taken and sufficient time should be given to assess adequacy of therapeutic response. If hypoglycemia occurs during the titration, the dose of glimepiride may be reduced (CitationGlaxoSmithKline 2006).
Disclosures
AP and TF have received research support, travel support, speaker and consulting fees from Glaxo-Smith-Kline (rosiglitazone), Takeda (pioglitazone), and Daichi-Sankyo (rivoglitazone). BW has no conflict of interest.
Abbreviations
FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL, high density lipoprotein; HOMA, homeostatic model assessment; LDL, low density lipoprotein; SU, sulfonylurea; TZD, thiazolidinedione.
References
- American Diabetes AssociationStandards of Medical Care in DiabetesDiabetes Care200629S44216373931
- BaksiAJamesREZhouBComparison of uptitration of gliclazide with the addition of rosiglitazone to gliclazide in patients with type 2 diabetes inadequately controlled on half-maximal doses of a sulphonylureaActa Diabetol20044163915224207
- DebrilMBRenaudJPFajasLThe pleiotropic functions of peroxisome proliferator-activated receptor γJ Mol Med200279304711327101
- DerosaGCiceroAFGaddiAVMetabolic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and metabolic syndrome treated with glimepiride: a twelve month, multicenter, randomized, controlled, parallel group trialClin Ther2004267445415220018
- DerosaGCiceroAFGaddiAVA comparison of the effects of pioglitazone and rosiglitazone combined with glimepiride on pro-thrombotic state in type 2 diabetic patients with the metabolic syndromeDiabetes Res Clin Pract2005a6951315955382
- DerosaGCiceroAFGaddiAVLong-term effects of glimepiride or rosiglitazone in combination with metformin on blood pressure control in type 2 diabetic patients affected by the metabolic syndrome: a 12 month, double-blind, randomized clinical trialClin Ther2005b2713839116291411
- DerosaGGaddiAVPiccinniMNDifferential effect of glimepiride and rosiglitazone on metabolic control of type 2 diabetic patients treated with metformin: a randomized, double-blind, clinical trialDiabetes Obes Metab2006819720516448524
- FukuenSIwakiMYasuiASulfonylurea agents exhibit peroxisome proliferator-activated receptor gamma agonistic activityJ Biol Chem200528023653915764598
- GlaxoSmithKline2006 URL: http://US.gsk.com/product/assets/us_fixedcombinationofglimepirideandrosiglitazone.pdf
- HaffnerSMGreenbergASWestonWMEffect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitusCirculation20021066798412163427
- HallstenJKVirtanenKALonnqvistFRosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetesDiabetes20025134798512453903
- HamannAMatthaeiSMauersbergerHEfficacy and Safety of Rosiglitazone in Combination with GlimepirideDiabetes Metab200329Suppl 22288
- HermanWHDiraniRGHorblyukRReduction in use of health-care services with combination sulfonylurea and rosiglitazone: findings from the Rosiglitazone Early vs SULfonylurea Titration (RESULT) studyAm J Manag Care200511273815839187
- KahnSEHaffnerSMHeiseMAGlycemic durability of rosiglitazone, metformin, or glyburide monotherapyN Engl J Med200635524274317145742
- KerenyiZSamerHJamesRCombination therapy with rosiglitazone and glibenclamide compared with upward titration of glibenclamide alone in patients wit type 2 diabetes mellitusDiabetes Res Clin Pract2004632132314757293
- KerstenSDesvergneBWahliWRoles of PPARs in health and diseaseNature2000405421410839530
- LangtryHDBalfourJAGlimiperide. A Review of its use in the management of type 2 diabetes mellitusDrugs199855563849561345
- MarxNFroehlichJSiamLAntidiabetic PPAR gamma-activator rosiglitazone reduces MMP-9 serum levels in type 2 diabetic patients with coronary artery diseaseArterioscler Thromb Vasc Biol2003a23283812588772
- MarxNImhofAFroehlichJEffect of rosiglitazone treatment on soluble CD40L in patients with type 2 diabetes and coronary artery diseaseCirculation2003b1071954712695287
- McCallALClinical review of glimepirideExpert Opin Pharmacother2001269971311336617
- McCluskeyDTougerMSMelisRResults of a randomized, double-blind, placebo-controlled study administering glimepiride to patients with type 2 diabetes mellitus inadequately controlled with rosiglitazone monotherapyClin Ther20042617839015639690
- MohantyPAljadaAGhanimHEvidence for a potent antiinflammatory effect of rosiglitazoneJ Clin Endocrinol Metab20048927283515181049
- NataliABaldewegSToschiEVascular effects of improving metabolic control with metformin or rosiglitazone in type 2 diabetesDiabetes Care20042713495715161787
- OrbayESarginMSarginHAddition of rosiglitazone to glimepiride and metformin combination therapy in type 2 diabetesEndocr J200451521715644569
- PfütznerAKannPPfütznerAHIntact and total proinsulin: new aspects for diagnosis and treatment of type 2 diabetesClin Lab2004505677315481632
- PfütznerASchöndorfTSeidelDImpact of rosiglitazone on β-cell function, insulin resistance and adiponektin concentrations— Results from a double blind oral combination study with glimepirideMetabolism2006a55205
- PfütznerAPanskyAMaiwormAMesenchymal stem cell differentiation into adipocytes is equally induced by insulin and proinsulin in vitroDiabetologia2006b49Suppl 1P707
- PickupJWilliamsGTextbook of Diabetes20023rd edOxfordBlackwell Publishing
- RobertsVLStewartJIssaMTriple therapy with glimepiride in patients with type 2 diabetes mellitus inadequately controlled by metformin and a thiazolidinedione: results from a 30-week, randomized, double-blind, placebo-controlled, parallel-group studyClin Ther20052715354716330290
- RosenstockJGoldsteinBJVinikAIEffect of early addition of rosiglitazone to sulphonylurea therapy in older patients with type 2 diabetes (>60 years): the Rosiglitazone Early vs SULfonylurea Titration (RESULT) studyDiabetes Obes Metab20058495716367882
- RosenstockJSugimotoDStrangePTriple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patientsDiabetes Care2006a29554916505505
- RosenstockJFerreira-CornwellCWestonWMRosiglitazone added early to glimepiride provides superior glycaemic control than uptitration of glimepiride alone in type 2 diabetes (T2DM)Diabetes2006b55Suppl,1P549
- SchneiderDJNordtTKSobelBEStimulation by proinsulin of expression of plasminogen activator inhibitor type-I in endothelial cellsDiabetes19924189051612205
- SchoonjansKAuwerxJThiazolidinediones: an updateLancet200035510081010768450
- ShargorodskyMWainsteinJGavishDTreatment with rosiglitazone reduces hyperinsulinemia and improves arterial elasticity in patients with type 2 diabetes mellitusAm J Hypertens2002166172212878365
- TiikkainenMHakkinenAMKorsheninnikovaEEffects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetesDiabetes20045321697615277403
- UebaHKurokiMHashimotoSGlimepiride induces nitric oxide production in human coronary artery endothelial cells via a PI3-kinase-Akt dependent pathwayAtherosclerosis200518335916216590
- VongthavaravatVWajchenbergBLWaitmanJNAn international study of the effects of rosiglitazone plus sulphonylurea in patients with type 2 diabetesCurr Med Res Opin2002184566112564655
- WagstaffAJGoaKLRosiglitazone: a review of its use in the management of type 2 diabetes mellitusDrugs20026218053712149047
- WellingtonKRosiglitazone/MetforminDrugs20056515819216033298
- WolffenbüttelBHGomisRSquatritoSAddition of low-dose rosiglitazone to sulphonylurea therapy improves glycaemic control in Type 2 diabetic patientsDiab Med200017407
- YangJDiFHeREfffect of addition of low-dose rosiglitazone to sulphonylurea therapy on glycaemic control in type 2 diabetic patientsChin Med J (Engl)2003116785712875702
- ZhuXXPanCYLiGWAddition of rosiglitazone to existing sulfonylurea treatment in chinese patients with type 2 diabetes and exposure to hepatitis B or CDiabetes Technol Ther20035334212725705
- ZimmetPAlbertiKGShawJGlobal and societal implications of the diabetes epidemicNature2001407782711742409