Abstract
Background
A randomized, controlled trial was conducted in an outpatient setting to examine the effect of beta-blocker dosing frequency on patient compliance, clinical outcome, and health-related quality of life in patients with stable angina pectoris.
Methods
One hundred and twelve beta-blockers-naive outpatients with stable angina pectoris were randomized to receive betaxolol, 20 mg once daily or metoprolol tartrate, 50 mg twice daily for 8 weeks. The principal outcome measure was overall compliance measured electronically, whereas secondary outcome measures were drug effectiveness and health-related quality of life.
Results
The overall compliance was 86.5 ± 21.3% in the betaxolol group versus 76.1 ± 26.3% in the metoprolol group (p < 0.01), and the correct number of doses was taken on 84.4 ± 21.6% and 64.0 ± 31.7% of treatment days, respectively (p < 0.0001). The percentage of missed doses was 14.5 ± 21.5% in the once-daily group and 24.8 ± 26.4% in the twice-daily group (p < 0.01). The percentage of doses taken in the correct time window (58.6% vs 42.0%, p = 0.01), correct interdose intervals (77.4% v 53.1%, p < 0.0001), and therapeutic coverage (85.6% vs 73.7%, p < 0.001) were significantly higher in the once-daily group. Both studied drugs had similar antianginal effectiveness. Health-related quality of life improved in both groups, but this increase was more pronounced in the betaxolol arm in some dimensions.
Conclusions
The study demonstrates that patient compliance with once-daily betaxolol is significantly better than with twice daily metoprolol. Similarly, this treatment provides better quality of life. These results demonstrate possible therapeutic advantages of once-daily over twice-daily beta-blockers in the treatment of stable angina pectoris.
Introduction
Non-compliance is a frequent phenomenon in outpatient care. It is estimated that, in general, at least 50% of patients fail to receive full treatment benefit due to inadequate compliance (CitationRoter et al 1998). This, in turn, leads to profound consequences. Non-compliance is a cause of additional procedures or treatment, and it contributes to unnecessary hospital admissions: up to 10% hospitalizations are attributed to this misbehavior (CitationSullivan and Kreling 1990; CitationMalhotra et al 2001; CitationHope et al 2004). With its enormous cost burden, non-compliance is not only a serious medical problem, but also a social problem.
Low compliance is a well-known phenomenon in asymptomatic diseases of which hypertension is the most frequently studied one. In contrast to this condition, ischemic heart disease is a classical example of symptomatic disease. Thus, one may assume that chest pain episodes should motivate patients to follow doctors’ instructions. Nevertheless, even anti-anginal treatment with symptom-releasing drugs such as nitrates is not fully executed by patients (CitationKardas 2004).
Beta-blockers are the drugs of choice in the therapy for chronic stable angina. In their case, non-compliance seems to be present as well, despite possible consequences. A recent study based on self-reports found as many as 56% of coronary artery disease patients non-compliant with this class of drugs (CitationNewby 2006). However, unlike the other antianginal treatment, which with non-compliance became just ineffective, the consequences of non-compliance with beta-blockers are much more profound and include increased risk of incident coronary heart disease and death (CitationHorwitz et al 1990; CitationPsaty et al 1990). Thus, the problem of patient compliance during beta-blocker treatment is of highest clinical importance.
A number of factors such as intelligence, memory, age, education, and a number of drugs a patient takes does not seem to influence the level of adherence (CitationCramer 2002). On the contrary, existing experience points to the number of daily doses as an important predictor of patient compliance (CitationClaxton et al 2001), although the benefit of once-daily over twice-daily dosing is still not fully proved in a number of clinical conditions. Two decades ago, this concept was tested in the field of beta-blockers, revealing better compliance with once-daily formulation (CitationBaird et al 1984). However, the methodology of that study, ie, the assessment of compliance by means of simple pill count, did not allow any insight in dose timing history and until now little is known about patient compliance with beta-blockers. Recently, new possibilities have come with the use of electronic monitoring, which is the most precise method of compliance assessment. This methodology has been shown to be more accurate in revealing non-compliance than refill data, pill count, provider assessment, or patient self-reporting (CitationVrijens and Urquhart 2005). Therefore, the BETTER trial (once-daily betaxolol vs twice-daily metoprolol: the effect on patient compliance and clinical outcome) aimed to precisely assess the impact of different dosing regimens of beta-blockers on compliance and clinical outcome to find out whether once-daily beta-blocker regimen ensures real clinical advantage.
Materials and methods
Patients and study design
This was an open, randomized, single-center, parallel-group study, investigating compliance in patients treated with betaxolol or metoprolol. The study protocol has been a priori approved by the Ethical Committee of the Medical University of Lodz.
The inclusion criteria were ischemic heart disease outpatients CCS class I-II, aged 40–75 years, beta-blockers-naive, whose mental state enabled conscious participation in the study and who had signed a conscious consent form.
The main exclusion criteria were unstable angina pectoris, NYHA class III and IV heart failure, heart rate <60/min, II° or III° atrio-ventricular block, systolic blood pressure below 90 mmHg, symptomatic infection, and any conditions requiring help of others with drug administration (eg, impaired dexterity, serious visual defect).
The sample size of 48 patients in each group was obtained by assuming overall compliance (primary outcome measure) difference between the group of 10%, and common standard deviation of 15%, with a power of 90% and α = 0.05 (CitationRosner 2000). Therefore, the study was designed to cover 100 patients.
At the first visit, the eligibility criteria were assessed and written informed consent was obtained. All patients were provided with self-control diaries, in which they were asked to record the number of chest pain episodes per week and the number of short-acting nitrate doses taken in case of need (eg, nitroglycerine in tablets or aerosol, izosorbide dinitrate in aerosol) as well as any relevant information concerning health and medication.
After the next 2 weeks of observation, patients were randomized to the two groups: the once-daily (od) group was informed to take 20 mg betaxolol (Lokren®, Sanofi-Synthelabo, France) at 07:00; and the twice-daily (bd) group was informed to take 100 mg metoprolol tartrate (metoprolol, ICN Polfa Rzeszow, Poland) in two 50 mg doses, to be taken at 07:00 and 19:00.
The active treatment phase was designed for 8 weeks, and the patients received the amount of drug sufficient for 10 weeks therapy, in MEMS® vials.
Measurement of compliance by MEMS
Patient compliance was measured by an electronic monitoring system (MEMS, Medication Event Monitoring System, Aardex, Zug, Switzerland). The MEMS container consists of a standard tablet bottle and a cap containing a microprocessor that registers the date and time of every opening. The patients were fully familiarized with the aim and method of study and instructed how to use MEMS container correctly, that is, to open it for no other reason but to take out tablets directly before use.
Compliance with beta-blockers in angina pectoris
For the purpose of calculations, multiple MEMS openings within a short period of time (≤15 min) were filtered and not counted. All other recorded openings were considered to represent a single dose intake.
The following parameters were employed for patient compliance assessment:
Overall compliance, which was defined by the number of container openings divided by the number of prescribed doses for the treatment period, and expressed as a percentage. Overall compliance was chosen to be the primary outcome measure.
Days with correct number of doses taken were defined as a percentage of the treatment days with one or two doses taken for od and bd regimens, respectively.
Doses taken in correct time window were defined as a percentage of the doses taken between 05:00 and 09:00 for once-daily dosage and between 06:00 and 08:00 and 18:00 and 20:00 for twice-daily dosage.
Correct interdose intervals were defined as the number of correct interdose intervals divided by the number of inter-dose intervals between prescribed doses, and expressed as a percentage. Interdose interval was judged correct when the dose was taken within the period of 20–28 h after the previous dose intake in case of od regimen and within the period of 10–14 h after the previous dose in case of bd regimen.
Missing doses were calculated according to the absence of registered dose intake during the periods “midnight to midnight” (24 h) in case of od regimen and “midnight to midday” or “midday to midnight” (two 12 h periods) in case of bd regimen, respectively, and expressed as percentage of prescribed doses.
Therapeutic coverage was expressed as a percentage of the studied period covered with drug activity, arbitrary assuming a drug action up to 24 h after single-dose intake for od regimen and up to 12 h for bd regimen.
Drug effectiveness and tolerance; health-related quality of life
Drug effectiveness was assessed with the weekly number of chest pain episodes and weekly number of short-acting nitrates doses, both established on the basis of patients’ self-report diaries. The effect of treatment on quality of life was assessed with the means of especially designed 6-item questionnaire. Moreover, general health-related quality of life was assessed with the means of EuroQol-5D (EQ-5D) generic questionnaire, which covers 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression (CitationRabin and de Charro 2001).
Data processing and statistical analysis
Data from MEMS containers were transferred into a computer and processed using the PowerViev v. 1.3.2 program (Aardex, Zug, Switzerland). Qualitative variables were compared using a chi2 test. Quantitative data were expressed as means ± SD (standard deviation) and were compared using Mann-Whitney test. The statistical significance threshold was chosen at p < 0.05.
Results
Baseline characteristics
One hundred and twelve patients entered the study and were randomized: 56 patients to each od and bd group. Thirteen patients were excluded from the analysis for different reasons (1 patient due to premature withdrawal, 6 due to premature termination due to adverse effects, and 6 patients for protocol violation). The compliance analysis was finally performed for 96 patients (47 in betaxolol and 49 in metoprolol group) due to a MEMS container lost in 2 cases and failure to download compliance data from the MEMS cap in 1 case.
The patients’ demographic characteristics are given in . The two groups did not differ in terms of age, sex, and the average number of monitored days.
Table 1 Demographic characteristics of studied groups
Overall compliance
Overall compliance assessed electronically by MEMS was better with once-daily than with twice-daily treatment: patients on betaxolol took 86.5% of recommended doses on average, while patients on metoprolol took 76.1% (p < 0.005) ().
Table 2 Parameters of patient compliance in studied groups (parameters are expressed as mean ± SD)
Assessment of daily compliance by MEMS
The difference in the percentage of days with the correct number of doses taken between the groups was highly significant in favor of the betaxolol group (84.4% vs 64.0%, p < 0.0001). There was no significant difference in days with no dose and days with extra doses, although these two values were slightly lower in the od group.
The number of missed doses in the metoprolol group was nearly twice that of the betaxolol group (24.8% vs 14.5%, p < 0.05).
Timing compliance
In the betaxolol group, 58.6% of prescribed doses were taken in the correct time window compared with only 42.0% in the metoprolol group (p = 0.01) (). A statistically significant difference was also observed between the groups concerning correct interdose intervals: 77.4% of doses in the od group were taken with correct interdose intervals versus only 53.1% in the bd group (p < 0.0001). Similarly, therapeutic coverage was significantly better in the betaxolol group (85.6% vs 73.7%, p = 0.0005).
Treatment effectiveness
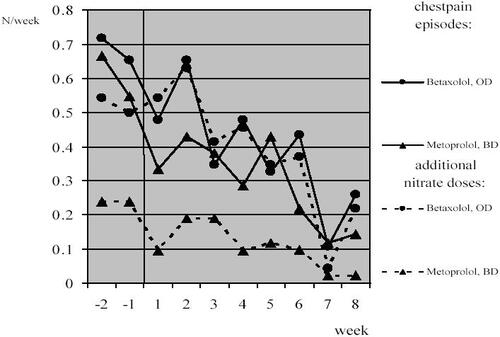
Treatment effectiveness assessment revealed similar decrease in the number of chest pain episodes per week compared with baseline (ie, averaged number for initial 2-weeks drug-free period) in both od and bd groups (0.42/week and 0.46/week, respectively, p > 0.05) as well as reduction in the weekly number of short-acting nitrate doses taken in case of need (0.30/week and 0.21/week, respectively, p > 0.05) ().
Health-related quality of life
The health-related quality of life parameters results are listed in and . None of the general health-related quality of life parameters assessed by EQ-5D (ie, all 5 dimensions scores as well as mean EQ-5D utility score and EQ-5D visual analog scale [VAS] results) differed between od and bd groups neither at randomization nor final visit. However, a positive change was observed in both groups in case of usual activities, pain/discomfort (especially in the od group), and anxiety/depression dimensions of EQ-5D, and in the betaxolol group in case of mobility, which altogether resulted in positive change to mean EQ-5D utility scores in both od and bd groups. Similarly, EQ-5D VAS scores have improved during the follow-up. These results were consistent with those of the specific questionnaire, which showed a marked improvement in general well-being (over 70% of patients in both groups), and an important increase in physical function in the od group only.
Table 3 EQ-5D dimension scores and visual analog health scale (VAS) scores at randomization (V0) and the final visit (V8)
Table 4 Changes to the quality of life dimensions after 8 weeks of active treatment
When filling the specific questionnaire at final visit, patients expressed a marked improvement in their general well-being (72% of patients) and modest one in terms of sleep (33% of patients), and mood (40% of patients). There was marked improvement in physical function in betaxolol group (43%) versus only slight in metoprolol group (15%). Both treatments had no influence on physical function and, notably, on sexual function ().
Tolerance and adverse effects
Adverse effects occurred in 13.4% of patients (in 10.7% within od and 16.1% within bd groups). The most common adverse effect was bradycardia (3.5% in both groups). Adverse effects were the reason for patient withdrawal in 6 cases: 2 in the betaxolol group, and 4 in the metoprolol group. In most cases, adverse effects were transient and slightly expressed, no severe adverse event was observed in the trial.
Discussion
Although the negative relation between non-compliance and clinical outcome seems to be obvious, the power of this relation differs greatly among the treatments (CitationHughes et al 2001). Indeed, even in the cardiovascular area, some drugs are more forgiving, and the condition for which they are prescribed is less life-threatening. In the case of lipid-lowering drugs, for example, there is no direct danger connected with skipping a dose, or even drug holidays (ie, discontinuation of treatment for 3 days or longer). On the other hand, there are drugs for which doctor’s advice needs to be followed very carefully. Relatively small non-compliance with warfarin may result in life-threatening hemorrhagic or thrombotic complications.
Among the antianginal drugs, beta-blockers are particularly sensitive to non-compliance. Rapid cessation of their use is connected with increased risk of exacerbation of angina and acute coronary event, including heart attack, and death. Subjects who stop using beta-blocker medication have a transient four-fold increase in the relative risk of cardiovascular event (CitationPsaty et al 1990). Those who do not adhere well to treatment regimen (ie, who took no more than 75% of prescribed medication) are 2.6 times more likely than good adherers to die within a year of follow-up (CitationHorwitz et al 1990).
Results of the present study prove that once-daily dosing of beta-blockers in stable angina pectoris leads to significantly better compliance than twice-daily as confirmed by overall compliance, days with the correct number of doses taken, and missing doses. Similar results were obtained for beta-blockers in hypertension, where those taking at least 90% of prescribed doses constituted 92.8% of the od group and 81.5% in the bd group (p = 0.009) (CitationBaird et al 1984). Other studies found comparable trends for other antianginal treatments (CitationBrun 1994; CitationDetry et al 1994; CitationKardas 2004). However, in some studies comparing once- and twice-daily dosing, better compliance for once-daily regimen was accompanied by higher percentage of no-dosing days. In a cross-over study comparing amlodipine once-daily and nifedypine SR twice-daily in the treatment of hypertension and angina pectoris, taking compliance improved in 30% of patients when switching from twice-daily to once-daily regimen but, at the same time, there was a 15% increase in the number of patients with one or more no-dosing days (CitationErne et al 1994). For this reason, twice-daily regimen was regarded as superior to once-daily regimen by some authors (CitationHaynes et al 2002). Moreover, in some studies the number of days with extra doses was higher for once-daily drugs than for twice-daily ones, which could lead to overdosing (CitationWinkler et al 2002). Contrary to these findings, in the present study, no difference with respect to days with no doses and days with extra doses between once-daily and twice-daily groups was found.
A satisfactory timing compliance does not always follow a good dosing one. In extreme cases, only 17%–33% of drug doses were taken within an acceptable time interval (ie, 12 ± 3 h after previous dose in case of twice-daily dosing), although overall compliance was nearly perfect at 97%–99.6% (CitationRudd et al 1992; CitationFavre et al 1997). However, betaxolol, used in the present study as an od drug due to its long elimination half-life, was proven to maintain the effect on both blood pressure and heart rate longer than some other beta-blockers, even when some doses are missed (CitationJohnson and Whelton 1994). Moreover, the present study revealed better timing compliance (in terms of doses taken in correct time window, correct interdose intervals, and therapeutic coverage) with this drug, than with its bd comparator.
Results of studies performed in this field point at the strict association between compliance and treatment effectiveness (CitationHughes et al 2001). This rule was proved in a number of cardiovascular conditions and treatments (CitationCramer 2002). Among over 4000 diabetic patients in a longitudinal study, od regimen was correlated with significantly better compliance and glycemic control after 6 months’ follow-up, with a significant decrease of HbA1c level (CitationGuillausseau 2004). Some recent studies, which employed electronic monitoring to assess compliance, clearly proved that once-daily regimen leads not only to better compliance, but also to better effectiveness (CitationKardas 2004, Citation2005).
Nevertheless, good adherence to beta-blockers is a necessary precondition for patients to benefit from this type of treatment (CitationWei et al 1994). It was observed that omission of short-acting beta-blocker resulted in significantly increased blood pressure and heart rate during the following two days (CitationJohnson and Whelton 1994). Betaxolol, the once-daily administered beta-blocker used in the present study, proved to be effective in the treatment of stable angina pectoris, reducing angina pectoris frequency and improving exercise capacity (CitationAlpert et al 1990; CitationNarahara 1990; CitationChrysant and Bittar 1994). Due to its long plasma t1/2 betaxolol was found to provide a long-lasting reduction in exercise-induced ischemia, longer than that found with atenolol (CitationMcLenachan et al 1992). Similarly, in this study patients on betaxolol revealed more pronounced improvement in their “pain/discomfort” dimension of quality of life assessment as well as physical function, compared with the metoprolol group.
Beta-blockers were found to be effective in increasing quality of life in case of heart failure as well as hypertension (CitationFowler 1998; CitationHaneda et al 1998; CitationReddy and Dunn 2000). Due to their antianginal effect, they also enhance quality of life in case of ischemic heart disease. Betaxolol has been recently found to enhance quality of life in patients with heart failure (CitationFigulla et al 2005). In a placebo-controlled study in hypertension, the effect of betaxolol treatment on well-being, physical state, sexual functioning, and sleep was similar to placebo, whereas cognitive acuity was slightly attenuated in this treatment group (CitationAmeling et al 1991). Contrary to that finding, in the present study both betaxolol and metoprolol had a beneficial influence on general well-being, which was particularly marked in pain, discomfort, and mood dimensions (Tables 3 and 4). Noteworthy, this beneficial effect, at least in some aspects of quality-of-life assessment, seems to be more pronounced for od betaxolol than bd metoprolol, and one may speculate to what extent it is associated with simpler regimen, better compliance, and greater effectiveness connected with this treatment.
In assessing results of this study, it is necessary to remember its limitations. At first, due to ethical reasons, all patients were informed about the aim of the study, which could positively affect compliance. However, it is believed that this effect decreases with the time of treatment, and an 8-week active treatment study period is long enough to minimize it. Another source of limitation is using self-re-porting diaries for recording symptoms, not the objective method, which could result in potential underestimation of anginal episodes. Indeed, limited usefulness of diaries has been proved recently by comparison with electronic measurement (CitationStone et al 2002). Nevertheless, neither the difference in compliance nor in effectiveness between the groups may be attributed to these factors, as both study arms were given the same conditions. Finally, beta-blockers used in this study not only differ in their pharmacokinetic properties (betaxolol, t1/2 14–20 h, metoprolol, t1/2 3–4 h), but they also have different pharmacodynamic properties. Therefore, in their typical dosage, 20 mg of betaxolol might induce slightly more beta-blockade than 100 mg of metoprolol tartrate (CitationBorchard 1998).
Conclusions
The study compared compliance in patients with stable angina pectoris, taking od versus bd beta-blockers. Using electronic measurement (MEMS), it has been found that patient compliance with od betaxolol is significantly higher than with bd metoprolol tartrate in terms of both dosing and timing. Moreover, patients on od betaxolol achieved better quality of life. Therefore, once-daily betaxolol seems to be an especially attractive beta-blocker which combines good compliance with low sensitiveness for missed doses and ensures not only high efficacy in clinical trials, but also high effectiveness in real life conditions. The results point also at general therapeutic advantage of once-daily administered beta-blockers over twice-daily administered ones in the treatment of stable angina pectoris, which is of high clinical relevance due to the life-threatening consequences of noncompliance in case of this treatment.
Acknowledgements
The author would like to express his thanks to family physicians participating in this trial: Maciej Baranski MD, Magdalena Basinska MD, Hanna Boguszewska MD, Tomasz Gula MD, Lidia Klichowicz MD, Pawel Klink MD PhD, Rafal Mazur MD, Magdalena Muras MD, Magdalena Myszkiewicz MD, Jacek Plucinski MD, Hubert Stefan MD.
The study was supported by grant from the Medical University of Lodz (No 502-16-261) and from Sanofi-Synthelabo Warsaw, Poland.
References
- AlpertMAMukerjiVVillarrealDEfficacy of betaxolol in the treatment of stable exertional angina pectoris: a dose-ranging studyAngiology199041365762162638
- AmelingEHde KorteDFMan in 't VeldAImpact of diagnosis and treatment of hypertension on quality of life: a double-blind, randomized, placebo-controlled, cross-over study of betaxololJ Cardiovasc Pharmacol199118752601723773
- BairdMGBentley-TaylorMMCarruthersSGA study of efficacy, tolerance and compliance of once-daily versus twice-daily metoprolol (Betaloc) in hypertension. Betaloc Compliance Canadian Cooperative Study GroupClin Invest Med19847951026380858
- BorchardUPharmacological properties of beta-adrenoreceptor blocking drugsJ Clin Bas Cardiol1998159
- BrunJPatient compliance with once-daily and twice-daily oral formulations of 5-izosorbide mononitrate: a comparative studyJ Int Med Res199422266727867871
- ChrysantSGBittarNBetaxolol in the treatment of stable angina pectorisCardiology199484316218187119
- ClaxtonAJCramerJPierceCA systematic review of the association between dose regimens and medication complianceClin Ther200123129631011558866
- CramerJAEffect of partial compliance on cardiovascular medication effectivenessHeart200288203612117861
- DetryJMBlockPDe BackerGPatient compliance and therapeutic coverage: amlodipine versus nifedipine (slow-release) in the treatment of angina pectoris. Belgian Collaborative GroupJ Int Med Res199422278867867873
- ErnePSaxenhoferHWaeberBTime of drug intake in hypertension and angina pectoris. A controlled monitoring studySchweiz Rundsch Med Prax1994831079837939074
- FavreODelacretazEBadanMRelationship between the pre-scriber‘s instructions and compliance with antibiotherapy in outpatients treated for an acute infectious diseaseJ Clin Pharmacol199737758
- FigullaHRKrzeminska-PakulaMWrabecKBetaxolol is equivalent to carvedilol in patients with heart failure NYHA II or III Results of a randomized multicenter trial (BETACAR Trial)Int J Cardiol2005doi10.1016/j.ijcard.2005.06.067
- FowlerMBBeta-blockers in heart failure. Do they improve the quality as well as the quantity of life?Eur Heart J199819Suppl PP17259886708
- GuillausseauPJObservance et optimisation du traitement antidiabétique oral: étude longitudinalePress Med20043315660
- HanedaTIdoAFujikaneTEffect of bisoprolol, a beta 1-selective beta-blocker, on lipid and glucose metabolism and quality of life in elderly patients with essential hypertensionNippon Ronen Igakkai Zasshi1998353389564739
- HaynesRBMcDonaldHPGargAXHelping patients follow prescribed treatment. Clinical applicationsJAMA20022882880312472330
- HopeCJWuJTuWAssociation of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failureAm J Health Sys Pharm20046120439
- HorwitzRIViscoliCMBerkmanLTreatment adherence and risk of death after a myocardial infarctionLancet199033654251975045
- HughesADBagustAHaycoxAThe impact of non-compliance on the cost-effectiveness of pharmaceuticals: a review of the literatureHealth Econ2001106011511747044
- JohnsonBFWheltonAA study design for comparing the effects of missing daily doses of antihypertensive rugsAm J Therapeutics199412607
- KardasPComparison of once daily versus twice daily oral nitrates in stable angina pectorisAm J Cardiol200494213615246905
- KardasPThe DIACOM study (effect of DosIng frequency of oral Antidiabetic agents on the COMpliance and biochemical control of type 2 diabetes)Diabetes Obes Metab20057722816219016
- MalhotraSKaranRSPandhiPDrug related medical emergencies in the elderly: role of adverse drug reactions and non-compliancePostgrad Med J200177703711677279
- McLenachanJMFindlayINWilsonJTTwenty-four-hour beta-blockade in stable angina pectoris: a study of atenolol and betaxololJ Cardiovasc Pharmacol19922031151381024
- NaraharaKADouble-blind comparison of once daily betaxolol versus propranolol four times daily in stable angina pectoris. Betaxolol Investigators GroupAm J Cardiol199065577822178381
- NewbyLKLaPointeNMChenAYLong-term adherence to evidence-based secondary prevention theraopies in coronary artery diseaseCirculation20061132031216401776
- PsatyBMKoepsellTDWagnerEHThe relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockersJAMA1990263165371968518
- RabinRde CharroFEQ-5D: a measure of health status from the EuroQol GroupAnn Med2001333374311491192
- ReddyPDunnABThe effect of beta-blockers on health-related qualityof life in patients with heart failurePharmacotherapy2000206798910853624
- RosnerBFundamentals of biostatistics20005th EditionPacifi c Grove, CADuxbury Press
- RoterDLHallJAMeriscaREffectiveness of interventions to improve patient compliance. A meta-analysisMed Care1998361138619708588
- RuddPAhmedSZacharyVCompliance with medication timing: implication from a medication trial for drug development and clinical practiceJ Clin Res Pharmacoepidemiol199261527
- StoneAAShiffmanSSchwartzJEPatient noncompliance with paper diariesBMJ20023241193412016186
- SullivanSDKrelingDHNoncompliance with medication regimens and subsequent hospitalizations: a literature analysis and cost of hospitalization estimateJ Res Pharm Econ199021933
- VrijensBUrquhartJPatient adherence to prescribed antimicrobial drug dosing regimensJ Antimicrob Chemother2005556162715772145
- WeiLFlynnRMurrayGDUse and adherence to beta-blockers for secondary prevetion of myocardial infarction: who is not getting the treatment?Pharmacoepidemiol Drug Saf199413761615386713
- WinklerATeuscherAUMuellerBMonitoring adherence to prescribed medication in type 2 diabetic patients treated with sulfonylureasSwiss Med Wkly20021323798512428192