Abstract
Approximately 25% of the adult population worldwide is hypertensive and thus at risk of cardiovascular morbidity and mortality. Despite the availability of many antihypertensive drugs, at least 50% of patients do not achieve blood pressure (BP) targets and thus remain at increased cardiovascular risk. Fixed-dose (FD) irbesartan/hydrochlorothiazide (HCTZ) is an antihypertensive combination therapy approved for the treatment of patients whose BP is not adequately controlled on monotherapy and for initial treatment of patients likely to need multiple drugs to achieve their BP goal. The efficacy and tolerability of FD irbesartan/HCTZ has been demonstrated in both patient populations in large multicenter studies. In patients failing antihypertensive monotherapy, FD irbesartan/HCTZ (150/12.5 mg) has been shown to be more effective than FD valsartan/HCTZ (80/12.5 mg) and at least comparable to FD losartan/HCTZ (50/12.5 mg). In patients with moderate or severe hypertension receiving FD irbesartan/HCTZ as initial therapy, this combination achieved more rapid BP reductions compared with irbesartan monotherapy and enabled a greater proportion of patients with severe hypertension to achieve their BP target. FD irbesartan/HCTZ is thus a valuable addition to the clinician’s armamentarium for the management of hypertension and should help more patients achieve their BP target.
Management issues in treating hypertension
Hypertension is a major cause of morbidity and mortality and an important public health challenge worldwide. It has been estimated that hypertension is responsible for approximately two-thirds of all strokes and 50% of heart attacks worldwide.Citation1 In addition, hypertension causes 7.1 million premature deaths per year worldwide and is responsible for 4.5% of the global burden of disease.Citation1 This startling impact of hypertension on health worldwide in part reflects the high prevalence of hypertension. According to a recent review of published literature, approximately a quarter of the adult population worldwide (26.4%) was hypertensive in 2000 and this is expected to increase to 29.2% by 2025.Citation2 Appropriate management of hypertension is therefore an important priority worldwide, especially given the impact that effective blood pressure (BP) control can have on morbidity and mortality.
Effective BP control has been shown to significantly reduce morbidity and mortality, as demonstrated in a meta-analysis of data from trials of active antihypertensive treatment compared with placebo.Citation3 According to this analysis, active antihypertensive treatment in hypertensive patients significantly reduced the risk of fatal and nonfatal stroke by approximately 40% and coronary heart disease (CHD) by approximately 15%, while all-cause mortality was reduced by approximately 15% and cardiovascular mortality by approximately 20%. In addition, a meta-analysis of data from 61 prospective observational studies has shown that the risk of vascular mortality is strongly and directly related to BP in middle-aged and elderly individuals. A 2-mmHg reduction in systolic blood pressure (SBP) has been reported to result in a 7% reduction in the risk of ischemic heart disease and a 10% reduction in the risk of stroke mortality, while larger reductions in SBP produce even greater reductions in vascular morbidity and mortality.Citation4 These data provide a strong rationale for the use of antihypertensive therapy to reduce the burden of morbidity and mortality associated with this highly prevalent condition.
However, despite the availability of a large number of antihypertensive agents, BP control remains inadequate in many patients. For example, a study which analyzed data collected in surveys conducted in the 1990s reported rates of BP control ranging from 5% to 10% in European countries, 17% in Canada and 29% in the US.Citation5 More recently, Wang et alCitation6 reported rates of BP control of 27% to 40% in five European countries (France, Germany, Italy, Spain, the UK) and 53% in the US, suggesting that rates of BP control are improving. However, over half of patients still fail to reach BP targets, and are therefore at increased risk of cardiovascular morbidity and mortality. It is mainly SBP targets that are not reachedCitation7 and systolic hypertension is most frequent in the elderly.Citation8 It has recently been suggested that control of SBP alone should be the guide to treatment.Citation9
Poor patient compliance with antihypertensive therapy and circumstances that do no allow physicians to adhere to treatment guidelines are frequent problems and are often the reason for patients not reaching their BP goal. For example, a recent survey of primary care physicians in 17 countries worldwide reported that 72% of physicians said that patients are not compliant with antihypertensive treatment when asked why over 50% of patients fail to achieve their BP targets.Citation10 A US study of patients receiving free medical care found that fewer than a third of patients on antihypertensive medication were still taking their prescribed medication a year later.Citation11 Another study investigating the rate of discontinuation or change in antihypertensive therapy in patients newly prescribed a course of antihypertensive medication has reported that 50% to 60% of patients discontinue or change therapy within 6 months.Citation12 Improving compliance is therefore an important task for all healthcare providers.Citation13 An important move to overcome this issue has been the development of fixed-dose (FD) combination therapies, since most patients require combination therapy to achieve BP targets.Citation14
Fixed-dose combinations
Most guidelines suggest that initial combination treatment should include a thiazide diuretic and either an angiotensin receptor blocker (ARB), an angiotensin-converting enzyme inhibitor (ACE-I), a calcium channel blocker (CCB), or a beta-blocker.Citation14,Citation15 In addition, for patients with chronic renal disease or type 2 diabetes, combinations including an ARB or ACE-I are recommended.Citation15–Citation20 FD combinations are now available consisting of combinations of different antihypertensive drugs which fit these recommendations, including: an ARB plus a thiazide diuretic; an ARB plus a calcium antagonist, an ACE inhibitor plus a thiazide diuretic; a beta-blocker with a diuretic; and an ACE inhibitor plus a calcium antagonist.Citation14
The usefulness of FD ARB/hydrochlorothiazide (HCTZ) combinations in effectively treating hypertension, including difficult-to-treat and severely hypertensive patients, has been demonstrated for several different ARBs.Citation14 Promising results have also been reported for FD combinations regarding improvements in clinical endpoints, as well as achieving BP targets.
There is evidence to suggest that combination therapy can be better tolerated than certain monotherapies. The use of low-dose combination therapy is associated with fewer adverse events than with the higher doses of single agents that would be required to achieve the same level of BP control.Citation21 In addition, combining HCTZ with an ARB attenuates the hypokalemic and fasting glucose-modifying effects of HCTZ.Citation22 As discussed in a later section, there is evidence to suggest that FD combinations are also associated with better compliance.Citation23–Citation25
In the recent ACCOMPLISH study (Avoiding Cardiovascular Events in Combination Therapy in Patients Living with Systolic Hypertension), HCTZ was compared with amlodipine on the basis of benazepril treatment in patients with compelling indications for the use of CCBs (eg, any atherosclerotic disease). Patients with a compelling indication for the use of HCTZ (eg, heart failure) were excluded. Cardiovascular morbidity and mortality were reduced by 20% with the ACE-I/CCB combination.Citation26 In addition, in this study, BP control rates were 75% in the ACE-I/CCB arm and 72% in the ACE-I/HCTZ arm. These BP control rates are better than those reported in other recent major trials in hypertensive patients.Citation26 The study design may have favored the ACE-I/CCB arm as the primary endpoint included measures of myocardial ischemia in which CCBs are more effective and excluded heart failure in which diuretics are more effective.Citation14,Citation18,Citation26
FD combinations of benazepril/HCTZ and benazepril/amlodipine have been reported to significantly reduce BP and albuminuria (p < 0.0001), in patients with type 2 diabetes and mild hypertension, although a significantly greater reduction in albuminuria was achieved with the ACE inhibitor/HCTZ combination.Citation27 In another study, the combination of trandolapril/verapamil was found to be superior to losartan/HCTZ in reducing new-onset diabetes in patients with hypertension and impaired glucose tolerance titrated to achieve a SBP of <130 mmHg; the incidence of new-onset diabetes was 11.0% for trandolapril/verapamil vs 26.6% for losartan/HCTZ (p = 0.002).Citation28
FD ARB/CCB combinations (amlodipine with either olmesartan or valsartan) are also effective in treating hypertension.Citation29,Citation30 BP control rates of 74% have been reported after 16 weeks of valsartan/amlodipine 160/10 mg treatment,Citation29 and of 53% after 8 weeks of olmesartan/amlodipine 20/10 mg treatment.Citation30 Combining a CCB with an ARB may reduce the incidence of peripheral edema as seen with olmesartan/amlodipine compared with amlodipine alone:Citation30 the incidence of edema was reduced from 13.0% to 36.8% for amlodipine monotherapy (dose range 5–10 mg) to 18.0% to 26.5% when olmesartan was combined with amlodipine (dose range 10/5 mg-40–10 mg), and was lowest for olmesartan monotherapy (dose range 10–40 mg, 9.9%–18.5%).
This review focuses on the benefits and tolerability of the FD ARB/HCTZ combination of irbesartan/HCTZ.
Overview of pharmacology, mode of action, and pharmacokinetics of irbesartan and HCTZ, alone and in combination
A valuable FD combination therapy that has received approval in Europe and the US is FD irbesartan/HCTZ. FD irbesartan/HCTZ is approved for the treatment of patients whose BP is not adequately controlled on irbesartan or HCTZ alone (US and European license),Citation31,Citation32 and in the US (but not in Europe) for initial treatment of patients likely to need multiple drugs to achieve their BP goals.Citation31 FD irbesartan/HCTZ is available as 150/12.5 mg, 300/12.5 mg and 300/25 mg and therapy should be started with the low dose and uptitrated. This paper reviews the efficacy and safety data for FD irbesartan/HCTZ, both for patients inadequately controlled on antihypertensive monotherapy and as initial therapy for patients with moderate or severe hypertension.
FD irbesartan/HCTZ has been developed in response to promising results reported for combination therapy with both agents given as individual tablets, as discussed in later sections of this paper,Citation22,Citation33,Citation34 and the fact that combining irbesartan and HCTZ is a logical option for patients requiring combination therapy since the 2 agents act via distinct mechanisms of action. Irbesartan exerts its antihypertensive effects by inhibiting the activation of angiotensin II type 1 (AT1) receptors. This elicits vasodilation and reduces the secretion of vasopressin and aldosterone, thereby reducing BP. HCTZ is a thiazide diuretic. It exerts its antihypertensive effects by inhibiting Na+/Cl− reabsorption from the distal convoluted tubules in the kidney. By reducing osmotic pressure in this way, HCTZ reduces the reabsorption of water in the distal convoluted tubules and thereby reduces plasma volume and cardiac output. The combined effect of these actions is to reduce BP.
Both irbesartan and HCTZ are active following oral administration and do not require biotransformation. They are efficiently absorbed following oral administration, having an oral bioavailability of 60% to 80% (irbesartan) and 50% to 80% (HCTZ), and peak concentrations are reached 1.5 to 2 hours (irbesartan) and 1 to 2.5 hours (HCTZ) after administration.Citation32 The intake of food does not affect the bioavailability of either agent.
Irbesartan exhibits linear dose-proportional pharmacokinetics over the dose range 10 to 600 mg. Repeated dosing results in limited accumulation (<20%) and steady state concentrations are achieved within 3 days with once-daily administration.Citation35 Irbesartan is largely excreted unchanged (80%–85%), but is also metabolized in the liver via glucuronide conjugation and oxidation. Irbesartan and its metabolites are eliminated largely in the feces (80%) but also in the urine (20%). Its terminal elimination half-life is 11 to 15 hours, which compares favorably with most other ARBs.Citation36
Pharmacokinetic data on the irbesartan/HCTZ combination are not available but co-administration of irbesartan and HCTZ has no effect on the pharmacokinetics of either drug,Citation37 but increases the BP lowering activity observed,Citation32 as discussed further in the following section of this review.
Efficacy of FD irbesartan/HCTZ combination
The benefits of combining irbesartan and HCTZ were demonstrated in an early study using a matrix design to investigate the effects of combinations of different doses of irbesartan (0, 37.5, 100 or 300 mg) plus HCTZ (0, 6.25, 12.5 or 25 mg) on BP.Citation22 The reduction in DBP by 8 weeks ranged from 3.5 mmHg for placebo, to 5.1 to 8.3 mmHg for HCTZ monotherapy, 7.1 to 10.2 mmHg for irbesartan monotherapy, and 8.1 to 15.0 mmHg for combination therapy, clearly indicating a synergistic effect for the addition of irbesartan to HCTZ and vica versa. Similarly, the proportion of responders (ie, patients achieving normalized DBP or trough DBP decreased by ≥10 mmHg) at 8 weeks increased from 24% for placebo, to 36% to 53% for HCTZ monotherapy, 35% to 58% for irbesartan monotherapy, and 44% to 80% for combination therapy.
FD irbesartan/HCTZ in patients failing on antihypertensive monotherapy
The benefits of FD irbesartan/HCTZ combination therapy have subsequently been demonstrated in a number of trials in patients with mild hypertension who failed to achieve BP control with monotherapy, and in patients with moderate or severe hypertension. The efficacy data for the main studies are summarized in .
Table 1 Summary of efficacy data for main studies of fixed-dose irbesartan/hydrochlorothiazide (HCTZ)
The efficacy of FD irbesartan/HCTZ 300/25 mg was first demonstrated in a study of hypertensive patients who had failed to gain BP control after at least 2 months of high-dose monotherapy or low-dose combination therapy.Citation38 In this study, 57 patients with SBP/DBP ≥140/90 mmHg received FD irbesartan/HCTZ 300/25 mg once daily for 12 weeks. A significant reduction in clinic and ambulatory mean BP was observed at the end of the study period compared with baseline. Mean 24-hour SBP was reduced from 146.0 to 123.3 mmHg and mean 24-hour DBP was reduced from 89.8 to 76.5 mmHg, both differences were statistically significant (p < 0.001). In addition, therapy achieved a mean lowering of ambulatory SBP/DBP at peak of 25.2/14.7 mmHg and 22.3/12.3 mmHg at trough. Thus FD irbesartan/HCTZ was found to produce clinically meaningful reductions in SBP/DBP in patients failing to achieve BP targets on monotherapy or low-dose combination therapy.
FD irbesartan/HCTZ at the lower dose of 150/12.5 mg has shown superior efficacy to other ARB/HCTZ combinations in comparative trials. The COmparative Study of Efficacy of Irbesartan/HCTZ with Valsartan/HCTZ Using Home Blood Pressure Monitoring in the TreAtment of Mild-to-Moderate Hypertension (COSIMA) study, compared the BP-lowering effects of irbesartan/HCTZ 150/12.5 mg and FD valsartan/HCTZ 80/12.5 mg.Citation39 Patients (n = 800) with untreated or uncontrolled mild-to-moderate essential hypertension initially received HCTZ 12.5 mg for 5 weeks. 464 patients failing to achieve BP control (SBP < 140 mmHg) at the end of this period were randomized to receive FD irbesartan/HCTZ 150/12.5 mg or FD valsartan/HCTZ 80/12.5 mg once daily for 8 weeks. Effects on BP were assessed by both office BP measurements and home BP monitoring (HBPM). Irbesartan/HCTZ produced greater reductions in both office and home BP compared with valsartan/HCTZ over the treatment period; according to HBPM a reduction in SBP of 13.0 mmHg was achieved with irbesartan/HCTZ compared with 10.6 mmHg with valsartan/HCTZ (p = 0.0094), while the reduction in DBP achieved was 9.5 mmHg for irbesartan/HCTZ compared with 7.4 mmHg for valsartan/HCTZ (p = 0.0007). In addition, the BP normalization rate (SBP/DBP < 135/85 mmHg) according to HBPM was significantly greater with irbesartan/HCTZ than with valsartan/HCTZ (50.2% vs 33.2%, p = 0.0003).
FD irbesartan/HCTZ 150/12.5 mg has also been shown to be more efficacious than FD losartan/HCTZ 50/12.5 mg.Citation40 In this study, patients with mild-to-moderate hypertension were randomized to receive FD irbesartan/HCTZ 150/12.5 mg (n = 16) or FD losartan/HCTZ 50/12.5 mg (n = 15) for 4 weeks. While both treatments significantly reduced BP from baseline, the reduction in ambulatory DBP was significantly greater with irbesartan/HCTZ than with losartan/HCTZ; the adjusted mean change from baseline in 24-hour ambulatory DBP was −10.5 mmHg with irbesartan/HCTZ compared with −6.1 mmHg with losartan/HCTZ, (p = 0.001). There was also a non-significant trend towards a greater reduction in mean 24-hour ambulatory SBP with irbesartan/HCTZ (−16.0 vs −11.1 mmHg). The response rate (ie, percentage of patients achieving a mean 24-hour ambulatory DBP of <90 mmHg or a reduction in mean 24-hour ambulatory DBP of ≥10 mmHg) was slightly higher for FD irbesartan/HCTZ but the difference was not statistically significant (86.7% vs 80.0%).
The Irbesartan/Hydrochlorothiazide Blood Pressure Reductions in Diverse Patient Populations (INCLUSIVE) trial, extended the findings of previous irbesartan/HCTZ trials by evaluating the efficacy of low and high FD irbesartan/HCTZ in a broad range of patients, including many in which BP goal attainment is particularly challenging, for example, the elderly, African Americans, and patients with type 2 diabetes mellitus and/or metabolic syndrome.Citation41 This was a large multicenter prospective open-label single-arm study. 844 patients with uncontrolled SBP (140–159 mmHg or 130–159 mmHg for patients with type 2 diabetes) on anti-hypertensive monotherapy were recruited from 119 centers in the USA. Patients initially discontinued previous anti-hypertensive therapy and received placebo for 4–5 weeks. They then received HCTZ 12.5 mg for 2 weeks followed by FD irbesartan/HCTZ 150/12.5 mg for 8 weeks and FD irbesartan/HCTZ 300/25 for 8 weeks (ie, active treatment was given for a total of 18 weeks). Changes in BP were assessed over the period of active treatment, ie, 18 weeks.
Over the course of the study, the mean reductions in SBP and DBP were 21.5 and 10.4 mmHg, respectively, and both were statistically significant (p < 0.001). At Week 18, mean SBP/DBP was 132.9 ± 13.8/81.1 ± 9.7 mmHg. In addition, 77% of patients achieved their SBP goal (<140 mmHg or <130 mmHg for patients with type 2 diabetes), 83% achieved their DBP goal (<90 mmHg or <80 mmHg for patients with type 2 diabetes), and 69% achieved SBP and DBP goals. Thus over three-quarters of patients previously uncontrolled on monotherapy achieved SBP and/or DBP control with FD irbesartan/HCTZ ().
Several subgroup analyses of the INCLUSIVE data have confirmed the efficacy of the treatment regimen in patients whose hypertension is often difficult to control. Of the patients included in this study, 30% (n = 254) had type 2 diabetes, 46% (n = 386) had metabolic syndrome (MS) and 21% (n = 177) had both MS and type 2 diabetes. A subgroup analysis of patients with type 2 diabetes (n = 227) found that the mean change from baseline in SBP/DBP for this subgroup was −18.2/−8.7 mmHg.Citation42 This reduction was only slightly less than for the total study population and was statistically significant compared with baseline (p < 0.001). In addition, 56% of diabetes patients achieved their SBP goal of 130 mmHg, 63% achieved their DBP goal of 80 mmHg, and 40% achieved both SBP and DBP goals. Analysis of data for the subgroup of patients with MS (n = 345) yielded a similar reduction in BP compared with the total study population (change from baseline in SBP/DBP, −21.0/−10.4 mmHg) and both were statistically significant (p < 0.001). In this subgroup, 73% achieved the SBP goal, 77% achieved the DBP goal and 61% achieved both SBP and DBP goals. For patients with both MS and type 2 diabetes (n = 157), 57% achieved the SBP goal, 59% the DBP goal and 39% reached both SBP and DBP goals. Thus FD irbesartan/HCTZ was found to achieve SBP goals in approximately three-quarters of patients with MS and over half of patients with type 2 diabetes, despite the more stringent BP goals in patients with type 2 diabetes.Citation42 BP control is particularly important for these patients who are at increased cardiovascular risk.
The possible impact of race/ethnicity and age on the response to FD irbesartan/HCTZ was also assessed.Citation43,Citation44 While almost two-thirds of patients in the INCLUSIVE trial were Caucasian (n = 515, 61%), almost a quarter were African American (n = 191, 23%) and 14% (n = 119) were Hispanic/Latino. Mean changes in SBP/DBP were similar for all subgroups and were statistically significant compared with baseline (p < 0.001). The percentages of patients achieving SBP/DBP goals were also similar for the different racial/ethnic subgroups: Caucasian, 70%; African-American, 66%; Hispanic/Latino, 65%.Citation43 A quarter of patients included in the study were elderly (aged 65 years or older, n = 212, 25%).Citation44 Analysis of data for this subgroup showed that the mean reductions in SBP and DBP achieved were similar to those in the total study population (23.0/10.9 mmHg; p < 0.001). At the end of the study, mean SBP/DBP was 134.0 ± 14.7/75.1 ± 8.4 mmHg, and dual SBP/DBP goals were achieved in 72% of patients. This response rate is similar to that achieved in the total study population (69%) and shows that FD irbesartan/HCTZ is highly efficacious in elderly as well as younger patients. A secondary analysis of data from the INCLUSIVE study found that higher baseline SBP, female sex, type 2 diabetes, and statin therapy were predictive of additional BP-lowering, suggesting that FD irbesartan/HCTZ may be a particularly appropriate choice of therapy in such patients.Citation45
FD irbesartan/HCTZ as initial therapy in moderate or severe hypertension
As patients with moderate or severe hypertension are likely to require 2 or more antihypertensive medications to attain BP goal, there is a clear rationale for treatment with combination therapy from the outset. The value of initial FD irbesartan/HCTZ in patients with moderate or severe hypertension has been investigated in 2 large multicenter studies, the RAPiHD moderate and severe trials.
The RAPiHD severe study, a randomized, double-blind, active-control, multicenter study, investigated the efficacy of FD irbesartan/HCTZ in untreated patients with severe hypertension (DBP ≥ 110 mmHg) or patients with a DBP of ≥100 mmHg while receiving antihypertensive monotherapy.Citation46 Patients initially received placebo for 1 week after which all patients with a DBP of ≥110 mmHg were randomized 2:1 to receive FD irbesartan/HCTZ or irbesartan monotherapy (as active control). FD irbesartan/HCTZ was initiated at a dose of 150/12.5 mg and force-titrated to 300/25 mg at the end of the first week of treatment (n = 468), while irbesartan was initiated at a dose of 150 mg and force-titrated to 300 mg (n = 227). Therapy at the higher dose was given for 6 weeks.
At Week 5, significantly more patients on combination therapy achieved a DBP of <90 mm Hg (the primary endpoint) compared with monotherapy recipients (47.2% vs 33.2%; p = 0.0005). Similarly, the percentage of patients achieving dual BP goals (SBP/DPB < 140/90 mmHg) was significantly greater for FD irbesartan/HCTZ: 34.6% vs 19.2%, p < 0.0001 (). Indeed, a significant difference in achievement of the DBP goal was evident after only 1 week of therapy (15.2% vs 9.2%, p = 0.03). In addition, the mean reduction in SBP/DBP was greater with FD combination therapy; at Week 5, the mean difference between combination and monotherapy in DBP and SBP was 4.7 mmHg and 9.7 mmHg, respectively (p < 0.0001). Thus initial therapy with FD irbesartan/HCTZ achieved more rapid BP reductions than irbesartan monotherapy in patients with severe hypertension, and in so doing significantly reduced their exposure to severe hypertension.
Of the patients included in the RAPiHD severe study, approximately a quarter (n = 199, 29%) had SBP ≥ 180 mmHg at baseline. Analysis of data for this subgroup of patients showed that a reduction in SBP/DBP of 41/23 mmHg was achieved with combination therapy at Week 5 compared with 29/19 mmHg with monotherapy (p < 0.0001 for SBP and p = 0.0071 for DBP).Citation47 In addition, as early as 1 week after the start of therapy, more than 70% of patients receiving combination therapy were no longer in the stage 3 hypertension category.
The RAPiHD moderate study enrolled patients with moderate hypertension, defined as SBP of 160 to 179 mmHg and DBP of <110 mmHg in untreated patients; or SBP ≥ 150 to <180 mmHg and DBP ≥ 95 to <110 mmHg in patients uncontrolled on monotherapy.Citation48 Following a 3-week placebo washout period, patients were randomized 3:1:1 to irbesartan/HCTZ 300/25 mg (n = 328), irbesartan 300 mg monotherapy (n = 106) or HCTZ monotherapy 25 mg (n = 104). Treatment was initiated at half dose, with forced titration to full dose after 2 weeks followed by 10 further weeks of treatment. The primary efficacy endpoint was the change in SBP after 8 weeks of active therapy.
FD irbesartan/HCTZ was found to induce a significantly greater reduction in SBP and DBP than either monotherapy. The mean reduction in SBP was 27.1 mmHg with irbesartan/HCTZ, compared with 22.1 mmHg with irbesartan monotherapy (p = 0.0016) and 15.7 mmHg with HCTZ (p < 0.0001), while the mean reductions in DBP from baseline to Week 8 were 14.6 mmHg in the irbesartan/HCTZ group compared with 11.6 mmHg in the irbesartan group (p = 0.0013) and 7.3 mm Hg in the HCTZ group (p < 0.0001). Both the rate of decline and the total degree of decline achieved were greatest with irbesartan/HCTZ and least with HCTZ. In addition, a significantly greater percentage of patients reached a treatment goal of SBP < 140 mmHg and DBP < 90 mmHg by Week 8 with irbesartan/HCTZ (53.4%), compared with irbesartan (40.6%; p = 0.0254) and HCTZ (20.2%; p < 0.0001) alone (). Thus FD irbesartan/HCTZ treatment achieved more rapid BP reduction than irbesartan monotherapy or HCTZ monotherapy in patients with moderate hypertension.
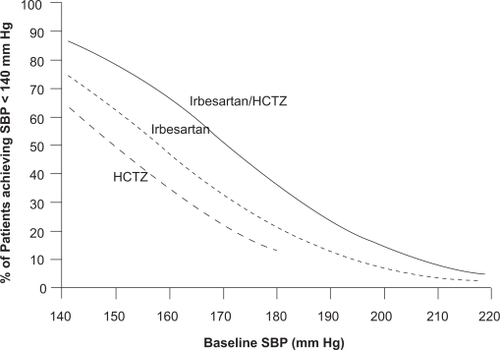
A post hoc pooled analysis of data from both this study and the randomized study in patients with severe hypertension found that the need for initial combination therapy increased with increasing baseline BP and lower BP goals across a range of BP levels spanning moderate and severe hypertension ().Citation49 For example, the probability of achieving a post-treatment value of <140 mmHg for a patient with a baseline SBP of 185 mm Hg was approximately 30% with irbesartan/HCTZ and 16% with irbesartan monotherapy, while for patients with a baseline SBP of 160 mmHg, the respective probabilities were 66% and 48%.
Figure 1 Probability of achieving a SBP <140 mmHg at Weeks 7/8 across a range of baseline SBP following treatment with irbesartan/hydrochlorothiazide (HCTZ), irbesartan, and HCTZ. Results from the RAPiHD study. Reproduced with permission from Franklin S, Lapuerta P, Cox D, Donovan M. Initial combination therapy with irbesartan/hydrochlorothiazide for hypertension: an analysis of the relationship between baseline blood pressure and the need for combination therapy. J Clin Hypertens (Greenwich). 2007; 9(12 Suppl):15–22.Citation59 Copyright © 2007 John Wiley and Sons, Inc.
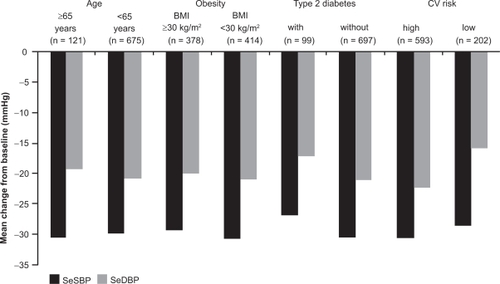
Another post hoc pooled analysis of data from both of these studies reported that SBP/DBP reductions (27–31/16–22 mmHg) were similar regardless of age, obesity, and type 2 diabetes status ().Citation50 SBP/DBP reductions with FD irbesartan/HCTZ were, however, greater in high- vs low-risk patients (30.4/22.1 mmHg vs 28.4/15.6 mmHg). When adjusted for factors including baseline age, BMI, type 2 diabetes, sex, race, cholesterol, target organ damage, and acute coronary syndrome, mean reductions in SBP were significantly higher in patients aged 65 years or older than in those younger than 65 years (24.6 vs 21.0 mmHg; difference, 3.6 mmHg; p = 0.015).
Figure 2 Mean reductions from baseline in SBP and DBP following initial fixed-dose irbesartan/hydrochlorothiazide (HCTZ) 300/25 mg treatment, according to age, body mass index (BMI), type 2 diabetes, and cardiovascular (CV) risk. Results from the RAPiHD study. Reproduced with permission from Weir MR, Neutel JM, Bhaumik A, Obaldia ME, Lapuerta P. The efficacy and safety of initial use of irbesartan/hydrochlorothiazide fixed-dose combination in hypertensive patients with and without high cardiovascular risk. J Clin Hypertens (Greenwich). 2007; 9(12 Suppl):23–30.Citation60 Copyright © 2007 John Wiley and Sons, Inc.
Taken together, the results from the 2 RAPiHD studies indicate the value of starting treatment with FD irbesartan/HCTZ in patients with moderate or severe hypertension.
Studies on the effectiveness of irbesartan/HCTZ in primary care
Further evidence for the efficacy of FD irbesartan/HCTZ for the treatment of hypertension in patients uncontrolled on monotherapy and as initial therapy in patients with moderate or severe hypertension comes from the results of a Chinese study, several observational studies and two post-marketing studies.
A multicenter, open-label, single-arm study in Chinese patients has reported on the efficacy of FD irbesartan/HCTZ in patients (n = 968) with mild-to-moderate hypertension.Citation51 Patients received FD irbesartan/HCTZ 150/12.5 mg for the first 2 weeks and the dose was increased to 300/12.5 mg (after 2 weeks) and 300/25 mg (after 4 weeks) if required to achieved a DBP of <85 mmHg. Treatment was given for 8 weeks. Of the patients who completed the study, 69% received irbesartan/HCTZ 150/12.5 mg, 23% received the intermediate dose level (300/12.5 mg) and 8% received the highest dose level (300/25 mg). After 8 weeks, a mean reduction in SBP/DBP of 22.0/16.1 mmHg was achieved (p < 0.01) and BP control was achieved in 84% of patients.
Two German studies have reported on the benefits of irbesartan/HCTZ in patients with hypertension and diabetes. In one observational study involving 9057 patients with hypertension and diabetes, the improvement in BP control was assessed over a 6-month period in patients receiving irbesartan or irbesartan/HCTZ.Citation52 At baseline, only 20% of patients had SBP levels within target (ie, <140 mmHg) and 57% had DBP levels within target (ie, <90 mmHg). However, at 6 months, BP control rates had improved to 63% for SBP and 93% for DBP. In the second observational study, patients with hypertension and type 2 diabetes (n = 31793) were switched from previous antihypertensive therapy to irbesartan (38%) or irbesartan/HCTZ (61%).Citation53 Effects on BP were assessed after 3 months on irbesartan therapy. Mean SBP and DBP were reduced by 22.5 mmHg and 10.7 mmHg, respectively, while SBP normalization (SBP < 140 mmHg) and DBP normalization (DBP < 90 mmHg) was achieved in 43% and 74% of patients, respectively. In addition, mean albuminuria decreased by 27.7 mg/dL. The results of these 2 observational studies thus confirm the benefit of irbesartan/HCTZ in patients with hypertension and diabetes reported in the INCLUSIVE study and suggest that irbesartan and irbesartan/HCTZ can help achieve good BP control rates in routine clinical practice.
Further evidence for the benefits of FD irbesartan/HCTZ in daily clinical practice comes from a 3-month, prospective, open-label, multicenter, phase IV study of irbesartan or irbesartan/HCTZ in 72,479 hypertensive patients (92% of whom were overweight or obese) in 6989 general practices across Germany.Citation54 Over the course of the 3-month study, a mean reduction in SBP/DBP of 23/12 mmHg was achieved and 48% of patients achieved BP normalization (SDP/DBP <140/90 mmHg). In addition, 79% of patients met their individual treatment goals, as defined by their physician.
Finally, another study has assessed the effect of irbesartan and irbesartan/HCTZ on BP in 14,200 patients with uncontrolled hypertension with or without the MS.Citation55,Citation56 Both irbesartan and irbesartan/HCTZ produced significant reductions in SBP/DBP over the 9-month study period (irbesartan monotherapy, 26.8/13.3 mmHg, p < 0.0001; irbesartan/HCTZ, 27.9/14.2 mmHg, p < 0.0001), and BP normalization was achieved by 66% of patients receiving monotherapy and 79% of those receiving irbesartan/HCTZ. Approximately two-thirds (n = 9281, 65%) of patients included in the study had MS. Reductions in SBP/DBP achieved with monotherapy and combination therapy in this subgroup of patients were similar to those for the total study population (irbesartan monotherapy, 26.3/13.0 mmHg, p < 0.0001; irbesartan/HCTZ: 27.5/14.1 mmHg, p < 0.0001). Reductions in cardiovascular risk factors were also observed in patients with MS.
The results from these large multicenter studies provide further evidence for the benefits of irbesartan/HCTZ in the management of BP, both in patients with hypertension alone and those with other cardiovascular risk factors including diabetes, MS and obesity.
Clinical significance of SBP/DBP reductions
The results of these studies show that irbesartan/HCTZ, used either to uptitrate from HCTZ monotherapy or as initial combination therapy, is highly effective for reducing SBP/DBP and achieving SBP/DBP goals. Achievement of such SBP/DBP reductions can be expected to translate into significant improvements in clinical endpoints.Citation57 Analysis of data from 29 randomized trials including 162,341 patients, showed that the risk of major cardiovascular events was reduced by interventions to reduce BP and that greater risk reductions were produced by antihypertensive regimens that targeted lower BP goals.Citation57 In addition to the benefits associated with BP reductions, there is evidence to suggest that early achievement of BP reductions can produce greater reductions in stroke risk compared with delayed reductions, as observed in the VALUE (Valsartan Antihypertensive Long-term Use Evaluation) study.Citation58 Analysis of data from this study showed that achieving BP control (SBP < 140 mmHg) within the first 6 months of the study was associated with significant benefits for subsequent major outcomes, including the risk of fatal and nonfatal cardiac events, fatal and nonfatal stroke, and all-cause death. In addition, achieving a BP response after just 1 month of treatment (defined as having no increase in BP in patients switched from previous therapy or achieving a decrease in SBP of ≥10 mmHg in previously untreated patients) predicted for a reduced risk of cardiac and stroke events, and improved survival. This suggests that the rapid achievement of BP goals with FD ARB/HCTZ combinations is likely to significantly improve clinical endpoints compared with initial therapy with monotherapy.
Safety and tolerability of irbesartan/HCTZ combination
It is well established that both HCTZ and irbesartan are well tolerated, and further studies have demonstrated that the addition of irbesartan to HCTZ and vice versa does not increase the incidence of adverse events. For example, in the 8-week study of Kochar et alCitation22 in which patients received fixed combinations of irbesartan (0, 37.5, 100 or 300 mg) plus HCTZ (0, 6.25, 12.5, or 25 mg), there was no significant difference in the incidence of commonly occurring adverse events between placebo, irbesartan monotherapy or irbesartan/HCTZ combination therapy, and no dose-related adverse events were observed. Indeed, for a number of common adverse events, such as headache, irbesartan monotherapy and irbesartan/HCTZ were associated with a lower incidence of events than placebo. Also, the addition of irbesartan to HCTZ reversed HCTZ-induced hypokalemic effects and the increases in serum uric acid levels seen with HCTZ.
The adverse event rates for the major trials with FD irbesartan/HCTZ are presented in . In the INCLUSIVE trial, FD irbesartan/HCTZ 150/12.5 mg and irbesartan/HCTZ 300/25 mg were found to be well tolerated over the 18 weeks of therapy ().Citation41 Only dizziness and hypotension occurred more frequently during treatment with irbesartan/HCTZ than with placebo (dizziness: placebo, 1%; irbesartan/HCTZ 150/12.5 mg, 2%; irbesartan/HCTZ 300/25 mg, 3%; hypotension: placebo, 0%; irbesartan/HCTZ 150/12.5 mg, 0.1%; irbesartan/HCTZ 300/25 mg, 1%). No patients were discontinued from the study because of adverse events.
Table 2 Safety data for main studies of fixed-dose irbesartan/hydrochlorothiazide (FD irbesartan/HCTZ): incidence of adverse events
Safety and tolerability is a critical consideration in aggressive hypertensive therapy which can be associated with hypotension, syncope, headache and hypokalemia. However, in patients with severe hypertensionCitation46 or with moderate hypertension,Citation48 starting treatment with FD irbesartan/HCTZ was as well tolerated as starting with irbesartan monotherapy. The adverse event and discontinuation rates are given in . In patients with moderate hypertension, 7 of the 22 patients receiving combination therapy withdrew due to dizziness or hypotension but these events occurred primarily following forced titration in patients in whom BP was already controlled. Citation48 In patients with severe hypertension, there was no syncope reported during the study, and the incidence of hypotension was <1% in patients taking FD irbesartan/HCTZ combination therapy.Citation46
In comparator trials, FD irbesartan/HCTZ had a similar adverse event profile to losartan/HCTZCitation40 and valsartan/HCTZ.Citation39 Long-term safety data for patients receiving irbesartan/HCTZ (although not as fixed-dose combination therapy) also support the favorable safety profile.Citation33,Citation34
In summary, accumulating safety data for irbesartan/HCTZ combination therapy indicate that this ARB/HCTZ antihypertensive combination is well tolerated by patients with hypertension; most adverse events are of mild or moderate intensity and transient in duration. In general, there is no evidence of an increase in the incidence of adverse effects as therapy is uptitrated, with the possible exception of dizziness and hypotension. However, the overall incidence of all individual adverse events is low. The incidence of adverse events with irbesartan/HCTZ appears to be similar to that for irbesartan monotherapy, while compared with HCTZ monotherapy, irbesartan/HCTZ appears to be better tolerated. This reflects the fact that HCTZ decreases serum potassium levels in a dose-related manner. However, this effect is less pronounced with the addition of increasing doses of irbesartan, and irbesartan 300 mg appears to provide greatest benefit in reversing the hypokalemic effects of HCTZ. In addition, HCTZ 25 mg results in a small increase in serum uric acid, but the addition of irbesartan reduces this effect. Thus no clinically significant occurrences of electrolyte imbalance have been observed in trials with irbesartan/HCTZ combination therapy or with irbesartan monotherapy. In conclusion, irbesartan/HCTZ is well tolerated and has a similar safety profile to that of irbesartan monotherapy.
Adherence and patient acceptance of FD irbesartan/HCTZ
Rapid and effective reduction of BP in hypertensive patients helps to prevent major cardiovascular events but patient non-adherence to antihypertensive medication often hinders the attainment of their optimal BP goal, as already discussed. FD irbesartan/HCTZ offers the important advantage of reducing the number of tablets to be taken by a patient and may therefore be expected to improve compliance and hence the efficacy of antihypertensive therapy over therapy with an ARB and HCTZ as separate tablets. Indeed the benefits of combination therapy in a single tablet over receiving medications as individual tablets in terms of improved compliance have clearly been demonstrated in three studies of persistence with anti-hypertensive therapy. One study retrospectively compared persistence for patients prescribed enalapril/HCTZ as a single pill or enalapril plus a diuretic as separate pills, and for patients prescribed lisinopril/HCTZ or lisinopril plus a diuretic as separate pills. At 12 months after the initial prescription, the percentage of patients persisting with therapy was 18.8% higher for lisinopril/HCTZ compared with lisinopril plus a diuretic and 21.7% higher for enalapril/HCTZ compared with enalapril plus a diuretic. These differences were statistically significant (p < 0.05). Two further studies have determined patient persistence with FD amlodipine/benazepril or an ACE-I plus a CCB.Citation20,Citation24 Gerbino et alCitation20 reported a persistence rate of 87.9% for FD amlodipine/benazepril compared with 69.2% for prescription of separate tablets, a difference that was statistically significant (p < 0.0001), while Dickson et alCitation24 also reported a statistically significant difference in favor of FD combination therapy in their study in elderly patients (63.4% vs 49.0%, p < 0.0001).
Conclusions
FD irbesartan/HCTZ has been shown to be effective for producing BP control in patients failing on antihypertensive monotherapy and as initial therapy in patients with moderate or severe hypertension who often require multiple agents to achieve BP control. It is effective in patients with type 2 diabetes, MS, and elderly patients as well as younger patients, and the response is similar regardless of race/ethnicity. In patients with severe or moderate hypertension, initial treatment with FD irbesartan/HCTZ achieves more rapid BP reductions than either agent as monotherapy and enables a greater proportion of subjects with severe hypertension to achieve BP targets. This is likely to be of significant therapeutic benefit in such patients since studies have shown that a more rapid achievement of BP reductions predicts for better cardiovascular outcomes. FD irbesartan/HCTZ is well tolerated, as for irbesartan monotherapy, and appears to reduce the incidence of hypokalemia and raised serum uric acid levels observed with HCTZ. The ARB/HCTZ combination therapies have an important role in the management of hypertension in clinical practice.
Acknowledgements
Editorial support for this article was provided by PPSI/Pam Milner and was funded by Bristol-Myers Squibb and sanofiaventis. The author retains sole responsibility for the content of this manuscript.
Disclosures
The author has received a number of research grants from pharamaceutical companies, including sanofiaventis.
References
- WHO, facts and figuresWorld Health Organization2008
- KearneyPMWheltonMReynoldsKMuntnerPWheltonPKHeJGlobal burden of hypertension: analysis of worldwide dataLancet2005365945521722315652604
- CifkovaRErdineSFagardRPractice guidelines for primary care physicians: 2003 ESH/ESC hypertension guidelinesJ Hypertens200321101779178614508180
- LewingtonSClarkeRQizilbashNPetoRCollinsRAge-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studiesLancet200236093491903191312493255
- Wolf-MaierKCooperRSKramerHHypertension treatment and control in five European countries, Canada, and the United StatesHypertension2004431101714638619
- WangYRAlexanderGCStaffordRSOutpatient hypertension treatment, treatment intensification, and control in Western Europe and the United StatesArch Intern Med2007167214114717242314
- ManciaGGrassiGSystolic and diastolic blood pressure control in antihypertensive drug trialsJ Hypertens20022081461146412172300
- FranklinSSJacobsMJWongNDL’ItalienGJLapuertaPPredominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) IIIHypertension200137386987411244010
- WilliamsBLindholmLHSeverPSystolic pressure is all that mattersLancet200837196312219222118561995
- BramlagePClinical practice and recent recommendations in hypertension management – reporting a gap in a global survey of 1259 primary care physicians in 17 countriesCurrent Medical Research and Opinions2007234783791
- McCombsJSNicholMBNewmanCMSclarDAThe costs of interrupting antihypertensive drug therapy in a Medicaid populationMed Care19943232142268145599
- JonesJKGorkinLLianJFStaffaJAFletcherAPDiscontinuation of and changes in treatment after start of new courses of anti-hypertensive drugs: a study of a United Kingdom populationBMJ199531170002932957633238
- WaeberBBurnierMBrunnerHRCompliance with antihypertensive therapyClin Exp Hypertens1999215–697398510423118
- ManciaGDe BackerGDominiczakAGuidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens20072561105118717563527
- ChobanianAVBakrisGLBlackHRThe Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 reportJAMA2003289192560257212748199
- American Diabetes AssociationStandards of medical care in diabetesDiabetes Care200427Suppl 1S15S3514693923
- HanssonLZanchettiACarruthersSGEffects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study GroupLancet19983519118175517629635947
- JamersonKABakrisGLWunCCRationale and design of the Avoiding Cardiovascular events through COMbination therapy in Patients Living with Systolic Hypertension (ACCOMPLISH trial): the first randomized controlled trial to compare the clinical outcome effects of first-line combination therapies in hypertensionAm J Hypertens200417979380115363822
- LewisEJHunsickerLGClarkeWRRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med20013451285186011565517
- K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney diseaseAm J Kidney Dis2004435 Suppl 1S1S29015114537
- LawMRWaldNJMorrisJKJordanREValue of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trialsBMJ20033267404142712829555
- KocharMGuthrieRTriscariJKassler-TaubKReevesRAMatrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertensionAm J Hypertens199912(8 Pt 1):79780510480473
- GerbinoPPShoheiberOAdherence patterns among patients treated with fixed-dose combination versus separate antihypertensive agentsAm J Health Syst Pharm200764121279128317563050
- DicksonMPlauschinatCACompliance with antihypertensive therapy in the elderly: a comparison of fixed-dose combination amlodipine/benazepril versus component-based free-combination therapyAm J Cardiovasc Drugs200881455018303937
- BangaloreSFixed-dose combination improves medication complianceJ Clin Hypertens (Greenwich)2006Suppl AA72
- JamersonKWeberMABakrisGLBenazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patientsN Engl J Med2008359232417242819052124
- BakrisGLTotoRDMcCulloughPAPurkayasthaDDavisPEffects of different ACE inhibitor combinations on albuminuria: results of the GUARD studyKidney Int20081303130918354383
- BakrisGMolitchMHewkinADifferences in glucose tolerance between fixed-dose antihypertensive drug combinations in people with metabolic syndromeDiabetes Care200629122592259717130190
- AllemannYFraileBLambertMBarbierMFerberPIzzoJLEfficacy of the combination of amlodipine and valsartan in patients with hypertension uncontrolled with previous monotherapy: the Exforge in Failure after Single Therapy (EX-FAST) studyJ Clin Hypertens (Greenwich)200810318519418326958
- ChrysantSGMelinoMKarkiSLeeJHeyrmanRThe combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety studyClin Ther200830458760418498909
- Avalide prescribing information. 2007. Available from: www.avaproavalide.com/pi_pop.aspx
- CoAprovel summary of product characteristics. 2007. Available from: emc.medicines.org.uk/emc/assets/c/html/DisplayDoc.asp?DocumentID=15112
- RaskinPGuthrieRFlackJReevesRSainiRThe long-term anti-hypertensive activity and tolerability of irbesartan with hydrochlorothiazideJ Hum Hypertens1999131068368710516738
- LittlejohnTIIISainiRKassler-TaubKChrysantSGMarburyTLong-term safety and antihypertensive efficacy of irbesartan: pooled results of five open-label studiesClin Exp Hypertens19992181273129510574413
- MarinoMRLangenbacherKFordNFUdermanHDPharmacokinetics and pharmacodynamics of irbesartan in healthy subjectsJ Clin Pharmacol19983832462559549663
- SchindlerCFerrarioCMOlmesartan for the treatment of arterial hypertensionFuture Cardiol200844357372
- MarinoMLagenbacherKEffect of hydrochlorothiazide on the pharmacokinetics and pharmadodynamics of angiotensin II blocker irbesartanDrug Invest1997383391
- CocaACalvoCSobrinoJOnce-daily fixed-combination irbesartan 300 mg/hydrochlorothiazide 25 mg and circadian blood pressure profile in patients with essential hypertensionClin Ther200325112849286414693309
- BobrieGDeloncaJMoulinCGiacominoAPostel-VinayNAsmarRA home blood pressure monitoring study comparing the antihypertensive efficacy of two angiotensin II receptor antagonist fixed combinationsAm J Hypertens200518111482148816280286
- NeutelJMSmithDAmbulatory blood pressure comparison of the anti-hypertensive efficacy of fixed combinations of irbesartan/hydrochlorothiazide and losartan/hydrochlorothiazide in patients with mild-to-moderate hypertensionJ Int Med Res200533662063116372579
- NeutelJMSaundersEBakrisGLThe efficacy and safety of low- and high-dose fixed combinations of irbesartan/hydrochlorothiazide in patients with uncontrolled systolic blood pressure on monotherapy: the INCLUSIVE trialJ Clin Hypertens (Greenwich)200571057858616227760
- SowersJRNeutelJMSaundersEAntihypertensive efficacy of Irbesartan/HCTZ in men and women with the metabolic syndrome and type 2 diabetesJ Clin Hypertens (Greenwich)20068747048016849900
- OfiliEOFerdinandKCSaundersEIrbesartan/HCTZ fixed combinations in patients of different racial/ethnic groups with uncontrolled systolic blood pressure on monotherapyJ Natl Med Assoc200698461862616623075
- CushmanWCNeutelJMSaundersEEfficacy and safety of fixed combinations of irbesartan/hydrochlorothiazide in older vs younger patients with hypertension uncontrolled with monotherapyAm J Geriatr Cardiol2008171273618174757
- SaundersECableGNeutelJPredictors of blood pressure response to angiotensin receptor blocker/diuretic combination therapy: a secondary analysis of the irbesartan/hydrochlorothiazide blood pressure reductions in diverse patient populations (INCLUSIVE) studyJ Clin Hypertens (Greenwich)2008101273318174768
- NeutelJMFranklinSSOparilSBhaumikAPtaszynskaALapuertaPEfficacy and safety of irbesartan/HCTZ combination therapy as initial treatment for rapid control of severe hypertensionJ Clin Hypertens (Greenwich)200681285085717170610
- FranklinSSNeutelJMDonovanMBouzamondoHIrbesartan/hydrochlorothiazide as initial therapy: subanalysis in patients with systolic BP > 180 mmHg and diastolic BP > 110 mmHg, efficacy and safetyJ Hypertens2008Suppl 1S159
- NeutelJMFranklinSSLapuertaPBhaumikAPtaszynskaAA comparison of the efficacy and safety of irbesartan/HCTZ combination therapy with irbesartan and HCTZ monotherapy in the treatment of moderate hypertensionJ Hum Hypertens20082226627417928878
- FranklinSLapuertaPCoxDDonovanMInitial combination therapy with irbesartan/hydrochlorothiazide for hypertension: an analysis of the relationship between baseline blood pressure and the need for combination therapyJ Clin Hypertens (Greenwich)2007912 Suppl 5152218046108
- WeirMRNeutelJMBhaumikAde ObaldiaMELapuertaPThe efficacy and safety of initial use of irbesartan/hydrochlorothiazide fixed-dose combination in hypertensive patients with and without high cardiovascular riskJ Clin Hypertens (Greenwich)2007912 Suppl 5233018046109
- SunNLJingSChenJ[The control rate of irbesartan/hydrochlorothiazide combination regimen in the treatment of Chinese patients with mild to moderate hypertension]Zhonghua Xin Xue Guan Bing Za Zhi200533761862116080809
- SchmiederREKreklerM[Observational study of blood pressure control and microalbuminuria in type 2 diabetics on irbesartan or irbesartan/HCTZ]MMW Fortschr Med2005147Suppl 39710116261944
- StrutzFBramlagePPaarWDEffect of three months’ treatment with irbesartan on blood and pulse pressure of hypertensive type 2 diabetic patients: open, observational study in 31,793 patientsCurr Med Res Opin20052191433144016197662
- SharmaAMBramlagePKirchWAntihypertensive effect of irbesartan and predictors of response in obesity-associated hypertension: a prospective, open-label studyClin Drug Investig20052512765776
- SchraderJBramlagePLudersSThoenesMSchirmerAPaarDWBP goal achievement in patients with uncontrolled hypertension: results of the treat-to-target post-marketing survey with irbesartanClin Drug Investig20072711783796
- KintscherUBramlagePPaarWDThoenesMUngerTIrbesartan for the treatment of hypertension in patients with the metabolic syndrome: a sub analysis of the Treat to Target post authorization survey. Prospective observational, two armed study in 14,200 patientsCardiovasc Diabetol200761217407587
- TurnbullFEffects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trialsLancet200336293951527153514615107
- WeberMAJuliusSKjeldsenSEBlood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE TrialLancet200436394262049205115207957
- FranklinSLapuertaPCoxDDonovanMInitial combination therapy with irbesartan/hydrochlorothiazide for hypertension: an analysis of the relationship between baseline blood pressure and the need for combination therapyJ Clin Hypertens (Greenwich)2007912 Suppl152218046108
- WeirMRNeutelJMBhaumikAObaldiaMELapuertaPThe efficacy and safety of initial use of irbesartan/hydrochlorothiazide fixed-dose combination in hypertensive patients with and without high cardiovascular riskJ Clin Hypertens (Greenwich)2007912 Suppl233018046109