Abstract
Hypertension and dyslipidemia are two of the most commonly co-occurring cardiovascular risk factors which together cause an increase in coronary heart disease-related events that is more than simply additive for anticipated event rates with each condition. Data have shown that even relatively small reductions in both blood pressure and cholesterol levels can lead to large reductions in the risk for cardiovascular events. However, though there are robust data on the beneficial effect of concomitant reduction in these risk factors, the reality is that this is achieved in <10% of patients. There is nonadherence with prescribed therapies with up to 50% of patients stopping their medications of their own volition for a variety of reasons. There is a reasonable evidence base to suggest that simplifying drug regimens and reducing pill burden will enhance patient adherence. The fixed-dose combination containing the antihypertensive agent amlodipine besylate and the statin atorvastatin is the first combination of its kind, which is both efficacious and safe and could potentially improve medication compliance, thereby improving the outcomes in these patients.
Introduction
Coronary artery disease (CAD) is the leading cause of morbidity and mortality worldwide accounting for in excess of 930,000 deaths with an estimated direct and indirect cost of US$448.5 billion in 2008.Citation1 It is a multifactorial disease, emphasizing the need to treat an individuals’ overall cardiovascular risk, rather than single risk factors in isolation.Citation2
Hypertension and dyslipidemia are two of the most commonly co-occurring cardiovascular risk factors. In a recent study utilizing data from the third National Health and Nutrition Examination Survey (NHANES) it was estimated that almost 15% of US adults (representing approximately 30 million persons) have both hypertension and dyslipidemia.Citation3 It was also shown that more than 64% of patients with hypertension also have dyslipidemia; conversely, approximately 47% of patients with dyslipidemia have hypertension.Citation3 These two risk factors together cause an increase in coronary heart disease-related events that is more than simply additive for anticipated event rates with each disease.
Antihypertensive and lipid-lowering therapy and current practice
Antihypertensive and lipid-lowering medications substantially reduce the risk of CAD, stroke, and death in patients with cardiovascular risk factors.Citation4–Citation6 Data have highlighted the importance of prompt and ‘aggressive’ control of blood pressure (BP) and cholesterol for patients with hypertension alone and for patients with additional cardiovascular risk factors including dyslipidemia and diabetes.Citation5,Citation7,Citation8 Recent trials indicate that patients with hypertension and concomitant multiple cardiovascular risk factors can benefit from lipid-lowering therapy regardless of their baseline lipid levels.Citation5
Although the importance of treating hypertension and dyslipidemia is well established in treatment guidelines, the current rate of control is unsatisfactory. In a managed care population of 154,235 patients, >90% of patients in whom both hypertension and dyslipidemia had been diagnosed had not met treatment goals for both conditions.Citation9 Suboptimal treatment patterns exist despite national and international guidelines.Citation10,Citation11 Moreover there is nonadherence with prescribed therapies; up to 50% of patients choose to stop their medications of their own volition for a variety of reasons.Citation12 Factors reported to influence adherence include patient education and attitudes towards treatment, cost, complexities of treatment regimen, numbers of concomitant medications, and side effects.Citation13,Citation14
There is, therefore, a reasonable evidence to suggest that simplifying drug regimens and reducing pill burden may enhance patient adherence.Citation13,Citation14 A retrospective study of patient adherence to antihypertensive and lipid-lowering therapy demonstrated improvements in adherence if both therapies were initiated simultaneously, and if fewer other medications were taken concomitantly.Citation13 The logic of combining multiple risk interventions for this multifactor disease is self evident and might be expected to enhance patient adherence and, therefore, improve achievement of treatment targets and reduce overall cardiovascular risk.
Combination therapy in cardiovascular disease
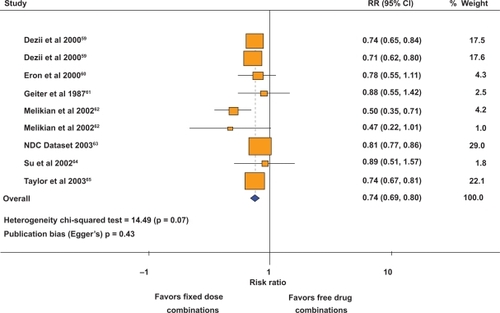
Polypharmacy and complex treatment regimens have been identified as important, modifiable risk factors for medication noncompliance. Poor compliance to medication regimen contributes to the practice-outcome gap, in which clinical guidelines are implemented but expected benefits are not realized. Fixed-dose combinations have the potential to improve compliance by reducing the pill burden (polypharmacy). A meta-analysis of nine studies which compared fixed-dose combinations versus free-drug components of the regimen, showed that fixed-dose combinations decreased the rate of nonadherence by 26% compared with free-drug component regimens ().Citation15 A subgroup analysis of the four studies on hypertension showed that fixed-dose combination decreased the risk of medication noncompliance by 24% compared with free-drug combination regimens.Citation15
Figure 1 Effect of fixed-dose combinations versus free-drug combination on the risk of medication nonadherence. Copyright © 2007, Elsevier. Adapted from Bangalore S, Kamalakkannan G, Parkar S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120:713–719.
The fixed-dose combination containing the antihypertensive agent amlodipine and the statin, atorvastatin, is the first combination of its kind designed to treat two risk factors for cardiovascular disease (CVD). This article provides an overview of this combination.
Overview of pharmacology of atorvastatin and amlodipine
Amlodipine component
Amlodipine besylate, a 3rd generation dihydropyridine calcium channel blocker (CCB), is approved for the treatment of hypertension and both vasospastic and chronic stable angina, alone or in combination with other agents. The primary action of amlodipine is to inhibit calcium entry through voltage-gated transmembrane l-type channels, thus decreasing intracellular calcium concentration and inducing smooth muscle relaxation.Citation16 Several important processes in atherosclerosis are influenced by calcium channel blockers, as they require calcium-dependent energy. Amlodipine also mediates nitric oxide release via a kinin-dependent mechanismCitation17 and modulates the metabolism of collagens within the extracellular matrix, and thus potentially has anti-atherosclerotic-plaque-stabilizing properties as well.Citation18,Citation19 It has further been proposed that amlodipine’s apparent anti-atherosclerotic properties are related to its strong lipophilicity and membrane location, allowing it to modulate the atherosclerotic process via both calcium-dependent and calcium-independent pathways.Citation19
Atorvastatin component
Atorvastatin calcium is a synthetic lipid-lowering agent and is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which catalyzes the conversion of HMG-CoA to mevalonate. Inhibition of HMG-CoA reductase leads to upregulation of low-density lipoprotein cholesterol (LDL-C) receptors in the liver, mediated by activation of sterol regulatory element-binding proteins resulting in enhanced clearance of LDL from the circulation and thus has an important role in preventing atherosclerosis. Atorvastatin, a second-generation statin, was introduced in 1996 and reduces LDL-C by 41%–61% in hypercholesterolemic patients.
Pharmacokinetic properties
Amlodipine/atorvastatin
The rate and extent of absorption of both amlodipine and atorvastatin after administration of a fixed-dose combination tablet has been shown to be similar to those after co-administration of matching doses of each single agent in healthy volunteers in a randomized, two-way crossover study.Citation20 In elderly patients, the clearance of amlodipine is reduced compared with in younger recipients, causing an increase of approximately 40%–60% in the area under the plasma concentration-time curve (AUC). As a result, a lower initial dose of amlodipine may be required in this patient group. The pharmacokinetics of amlodipine is not significantly affected in patients with renal impairment. However, in patients with hepatic impairment, clearance is reduced to a similar extent as that demonstrated in elderly patients, and a lower initial dosage may be required. In patients with moderate to severe heart failure, the increase in amlodipine AUC was similar to that observed in elderly and patients with hepatic dysfunction. Atorvastatin is associated with higher plasma concentrations in the elderly (aged 65 years) than in younger patients, with a corresponding increase in lipid-lowering efficacy. Plasma concentrations of atorvastatin are markedly increased in patients with hepatic failure and the dosage may need to be reduced.
Rationale for single-pill amlodipine/atorvastatin therapy
US epidemiological data have suggested that, on average, less than 10% of patients with concomitant hypertension and dyslipidemia are at target levels for both conditions.Citation9,Citation21 The large benefits that can result from simultaneous treatment of hypertension and dyslipidemia and the current suboptimal management of these conditions demonstrate that novel solutions are needed to treat the growing number of patients who have both of these important cardiovascular risk factors. Single-pill amlodipine/atorvastatin therapy represents such a solution.
The pharmacokinetic and pharmacodynamic properties of amlodipine and atorvastatin make them well suited for combination in a single pill to manage cardiovascular risk.Citation22 The half-lives of both agents facilitate once-daily dosing, and both can be administered at any time of day with or without food.Citation23 Neither drug has any adverse effects on the other’s efficacy or tolerability.Citation24–Citation26
In addition a potential synergistic and dose-dependent increase in nitric oxide release was observed with combination treatment compared with individual components in a study on human vein endothelial cells taken from healthy volunteers.Citation27 Moreover, combination therapy of amlodipine plus atorvastatin improved vascular compliance, an indicator of structural and functional vascular changes, and the beneficial effect on small arteries appeared to be more than additive.Citation28,Citation29 In normocholesterolemic obese hypertensive patients amlodipine plus atorvastatin reduced inflammatory markers and insulin resistance more than amlodipine therapy alone.Citation30 Amlodipine has demonstrated some anti-atherosclerotic properties, whereas the beneficial effects of atorvastatin on atherosclerosis, via a reduction in cholesterol levels, are more marked.Citation18
Hypertension is often associated with impaired fibrinolysis, usually expressed by increased levels of plasminogen activator inhibitor type 1 (PAI-1) and decreased activity of tissue plasminogen activator (t-PA).Citation31 Combination therapy with amlodipine and atorvastatin improved the fibrinolytic balance more than either single agent in hypertensive hypercholesterolemic patients with insulin resistance.Citation31
Study in transgenic ApoE * 3–Leiden mice demonstrates that amlodipine treatment alone did not significantly reduce atherosclerotic lesion development, whereas treatment with atorvastatin decreased lesion area substantially. The combination of amlodipine and atorvastatin tended to reduce atherosclerosis even more, possibly especially in modest statin responders, which may have implications for clinical practice.Citation32 Mason and colleagues have observed that the combination of atorvastatin and amlodipine produces a synergistic reduction in oxidative damage to human LDL, an effect not observed with other combinations of amlodipine and statins.Citation33
The Regression Growth Evaluation Statin Study (REGRESS) trial was designed to determine the effect of lipid-lowering therapy with pravastatin in symptomatic patients with normal to moderately raised cholesterol levels. Although the REGRESS trial was not designed to evaluate combination therapy, the results suggest strongly that addition of CCBs to statin acts synergistically in retarding the progression of established coronary atherosclerosis.Citation34
Key outcome trials
Amlodipine
Amlodipine effectively lowers blood pressure and reduces the risk of cardiovascular morbidity and mortality. The major outcome trials of amlodipine are listed in .
Table 1 Key outcome studies of amlodipine
Hypertension trials
The Irbesartan Diabetic Nephropathy Trial (IDNT)Citation35 evaluated the effects of amlodipine or irbesartan or placebo in hypertensive patients with diabetic nephropathy. Although irbesartan was superior to amlodipine and placebo for the primary composite end point (doubling of the baseline serum creatinine concentration, the development of end-stage renal disease, or death from any cause), amlodipine reduced the time to a secondary, cardiovascular composite end point as effectively as irbesartan.Citation35
In the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT),Citation36 more than 40,000 high risk hypertensive patients were randomly assigned to receive chlorthalidone, amlodipine, lisinopril, or doxazosin. Amlodipine was as effective as chlorthalidone in reducing the primary combined endpoint of fatal coronary heart disease or nonfatal myocardial infarction. Moreover, it was more effective than lisinopril in reducing the risk of stroke. However, incidence of heart failure was 38% higher in patients assigned to amlodipine than patients assigned to chlorthalidone.
Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial was designed to test the hypothesis that, for the same BP control, valsartan would reduce cardiac morbidity and mortality more than amlodipine in hypertensive patients at high cardiovascular risk.Citation8 Amlodipine treatment was associated with a more prompt and robust reduction in BP than valsartan treatment, particularly early in the trial. The primary composite end point of cardiac mortality and morbidity was reduced equally by both groups.Citation8 However, amlodipine was associated with a statistically significant 16% reduction in the incidence of myocardial infarction and a near-significant reduction in stroke (). Subanalysis of VALUE results reported that valsartan monotherapy reduced the risk of heart failure and new onset diabetes to a greater extent compared to amlodipine monotherapy.Citation37 These data are consistent with the findings in ALLHAT that amlodipine does not prevent heart failure as effectively as some other antihypertensive drugs.
Anglo-Scandinavian Cardiac Outcomes Trial – Blood Pressure Lowering Arm (ASCOT-BPLA)Citation38 compared a “standard” antihypertensive regimen (β-blocker [atenolol] plus/minus diuretic [thiazide]) with a more “contemporary” regimen (CCB [amlodipine] plus/minus ACE inhibitor [perindopril]) on the combined primary outcome (nonfatal myocardial infarction and fatal coronary heart disease).Citation39 ASCOT-BPLA was terminated early due to benefits in cardiovascular mortality and all-cause mortality in patients treated with amlodipine versus atenolol-based treatment. Results from the ASCOT Conduit Artery Function Evaluation (CAFE) Citation40 study assessed the effects of atenolol- versus amlodipine-based therapy on central arterial blood pressure and showed that central arterial blood pressure was more favorably influenced by the amlodipine- than the atenolol-based regimen when compared with peripheral BP. Central, arterial pulse pressure was observed to be a more important and an independent predictor of cardiovascular and renal outcomes.
Heart failure trials
The Prospective Randomized Amlodipine Survival Evaluation Study (PRAISE) trialCitation41 evaluated the safety of amlodipine in patients with severe heart failure with an ejection fraction <30%. Among patients with ischemic heart disease, there was no difference between the amlodipine and placebo groups in the occurrence of either death from any cause and hospitalization for major cardiovascular events. However, among patients with nonischemic cardiomyopathy, amlodipine reduced the combined risk of fatal and nonfatal events by 31% and decreased the risk of death by 46% and showed that it did not increase cardiovascular morbidity or mortality in patients with severe heart failure.Citation41
Coronary artery disease trials
The other area of major randomized controlled trials of amlodipine have been in the cohort of patients with atherosclerosis. The Prospective Randomized Evaluation of the Vascular Effects of Norvasc Trial (PREVENT) was a multicenter, randomized, placebo-controlled, clinical trial designed to test whether amlodipine would slow the progression of early coronary atherosclerosis in patients with angiographically documented coronary artery disease.Citation42 Although there was no difference in the coronary angiographic endpoint, there was a significant reduction in the progression of carotid atherosclerosis as well as a significant reduction in the risk of stroke.
Comparison of Amlodipine vs Enalapril to Limit Occurrences of Thrombosis (CAMELOT)Citation43 study compared the incidence of cardiovascular events among patients with angiographically documented coronary artery disease and normal BP randomized to amlodipine, enalapril, or placebo.Citation43 After 24 months, there was a significant reduction in the incidence of cardiovascular events in the amlodipine arm compared with placebo. Compared with baseline, intravascular ultrasound (IVUS) showed progression in the placebo group, a trend toward progression in the enalapril group (p = 0.08), but no progression in the amlodipine group (p = 0.31).
Atorvastatin
Atorvastatin, like other statins, has been shown to reduce LDL cholesterol and reduce the risk of cardiovascular morbidity and mortality (). In addition, statins have pleiotropic effects.
Table 2 Key outcome studies of atorvastatin
Coronary artery disease trials
The Pravastatin or Atorvastatin Evaluation and Infection Therapy – Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) trial compared standard treatment (pravastatin 40mg daily) with more intensive treatment (atorvastatin 80 mg daily) in patients who had been hospitalized for an acute coronary syndrome within the preceding 10 days.Citation44 The primary outcome was a composite of death from any cause, myocardial infarction, severe unstable angina, revascularization or stroke. A 16% reduction in the hazard ratio favored atorvastatin. The findings from PROVE IT-TIMI 22 have been confirmed by the results from the Treating to New Targets (TNT) Study.Citation45
The TNT study examined the effectiveness of low-dose versus high-dose atorvastatin therapy on major cardiovascular events. TNT demonstrated that the use of atorvastatin 80 mg to reduce LDL-C to 77 mg/dL provided additional clinical benefits in stable coronary heart disease patients, compared with reduction of LDL-C to 100mg/dL with atorvastatin 10 mg. The composite primary outcome – first occurrence of a major cardiovascular event – showed a relative risk reduction of 22%.Citation46 TNT’s findings confirm the growing body of evidence that reducing LDL-C below current guideline-recommended levels confers significant clinical benefits.
The Incremental Decrease in Endpoints through Aggressive Lipid Lowering (IDEAL) study compared patients with a history of acute myocardial infarction treated with a high dose of atorvastatin (80mg/day) with those receiving the standard dose of simvastatin (20mg/day). Citation47 Mean LDL-C levels were 104mg/dL in the simvastatin group and 81 mg/dL in the atorvastatin group during treatment. The risk reduction between the treatment groups (11%) in the primary endpoint of major coronary events failed to reach significance (p = 0.07). However, significant reductions favoring the atorvastatin treatment group were observed for the occurrence of secondary cardiovascular endpoints such as coronary events and nonfatal acute myocardial infarction.
The Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) study used the same doses of atorvastatin and pravastatin as the PROVE IT study in patients with angiographically demonstrated CAD. The results of the REVERSAL study showed that atorvastatin 80 mg halted plaque progression (as monitored by IVUS), while pravastatin did not.Citation48
The Aggressive Lipid-Lowering Initiation Abates New Cardiac Events (ALLIANCE) trial compared a focused treatment strategy using atorvastatin with usual medical care. The study showed that aggressive treatment with atorvastatin was associated with significantly lower LDL cholesterol levels over usual care accompanied by improved outcomes in the composite primary end point of cardiovascular events and particularly nonfatal myocardial infarction.Citation49
The Greek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study assessed the effect of atorvastatin on mortality and morbidity in patients with coronary heart disease. The treatment regimen was atorvastatin, 10–80 mg/day, titrated to LDL-C <100 mg/dL, or usual care. Total mortality was lower with atorvastatin than with usual care. Similar reductions with atorvastatin compared to usual care were seen in coronary mortality and coronary morbidity.Citation50
Hypertension and diabetes trials
In the recent Collaborative Atorvastatin Diabetes Study (CARDS), atorvastatin 10mg reduced the death rate among patients with type 2 diabetes mellitus and relatively low-cholesterol levels by 27% compared with placebo.Citation7 The CARDS study was terminated after approximately two years early due to the highly significant reduction in cardiovascular events, including heart attack and stroke, in those patients receiving atorvastatin treatment.
The lipid-lowering arm of the ASCOT trial investigated, in a factorial design, the effects of simultaneous treatment with antihypertensive and lipid-lowering therapy (atorvastatin 10 mg) among hypertensive patients with normal to mildly elevated lipid levels and at least three other cardiovascular risk factors.Citation5 The lipid-lowering study arm was terminated nearly two years early due to the highly significant (36%) decrease in the cumulative incidence of nonfatal myocardial infarction and CHD mortality among patients receiving treatment to lower both BP and lipids compared with patients receiving treatment for hypertension alone.
Key studies evaluating amlodipine and atorvastatin combination therapy
Clinical trials have been conducted (comparative and non comparative) to assess the efficacy and safety of the combination therapy. Single-pill therapy in the Treatment of Concomitant Hypertension and Dyslipidemia (GEMINI),Citation22 GEMINI- Asia Pacific, Middle East, Africa, Latin America (GEMINI-ALAA),Citation51 An international, multicenter, open label study to assess the effectiveness of amlodipine–atorvastatin combination in subjects with hypertension and dyslipidemia in the UK and Canada (JEWEL I), JEWEL Europe (JEWEL II),Citation52 Clinical Utility of Caduet in Simultaneously Achieving Blood Pressure and Lipid End Points (CAPABLE)Citation53 are noncomparative, titration-to-goal, multicenter studies, which showed the efficacy and effectiveness of the combination medication at achieving both LDL and BP goals. AVALON and RESPOND are two randomized double-blind, multicenter trials that compared the efficacy of the coadministration of amlodipine and atorvastatin with that of single-agent therapy or placebo over eight weeks.Citation25,Citation54 The Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA) is a randomized, double-blind, multicenter trial that compared the efficacy of amlodipine plus atorvastatin with that of placebo over 3.3 years.Citation55
ASCOT-LLA study evaluated data from patients who had received atorvastatin 10mg once daily or placebo in addition to their antihypertensive regimen as described previously.Citation55 In ASCOT-LLA, the relative risk of nonfatal myocardial infarction and fatal coronary heart disease was reduced by 36% in the group receiving atorvastatin plus either antihypertensive regimen compared with the group receiving placebo plus either antihypertensive regimen.Citation5
In the multicenter Atorvastatin and Amlodipine in Patients with Elevated Lipids and Hypertension (AVALON)Citation25 trial more patients receiving combination therapy achieved their BP goal than patients receiving atorvastatin, and more patients receiving combination therapy achieved their LDL-C goal than patients receiving amlodipine.Citation25 Similarly, significantly more patients receiving combination therapy achieved both their BP and LDL-C goals compared with those receiving single-agent therapy.Citation25 The mean Framingham estimated 10-year CHD risk was significantly with combination therapy than with single-agent therapy.
In the Efficacy and Safety of Fixed-Dose Combinations of Amlodipine and Atorvastatin in the Treatment of Patients with Concomitant Hypertension and Dyslipidemia (RESPOND)Citation54 study, hypertensive patients with dyslipidemia the concomitant use of amlodipine plus atorvastatin did not modify the efficacy achieved with either agent alone.Citation54 In an analysis of risk, the mean Framingham estimated 10-year CHD risk was reduced from mean baseline values of 15.8%–18.0% to endpoint values of 7.3%–10.7% in patients receiving combination therapy.Citation54
Tolerability/safety data
In the double-blind phase of the AVALON trial, the rate of treatment discontinuation for any reason was similar in groups receiving amlodipine 5 mg plus atorvastatin 10 mg (7.7%), amlodipine 5mg alone (7.0%), or atorvastatin 10 mg alone (7.5%), but slightly higher in the placebo group (9.6%). Adverse events reported most frequently in the combination therapy group compared with the placebo group during this phase were peripheral edema (5.3% vs 2.1%), myalgia (4.8% vs 2.1%), and sinusitis (2.9% vs 0.8%).Citation25 In RESPOND trial combination-treated patients did not experience any increase in treatment-related side effects compared with amlodipine or atorvastatin monotherapy. The most common treatment-related side effects were peripheral edema (9.4% vs 2.7%), headache and dizziness compared to placebo. These events were mild to moderate in severity. The incidence of treatment-related myalgia in combination-treated patients was low (1.0%) and similar to that in patients treated with amlodipine alone (1.4%), atorvastatin alone (1.1%), or placebo (1.8%).Citation54 GEMINI study showed that amlodipine/atorvastatin combination pill has a safety profile consistent with its components. These data demonstrated that co-administered amlodipine plus atorvastatin is well tolerated in patients with hypertension and additional risk factors, and that the adverse events observed are similar in nature, severity and frequency to those seen with amlodipine or atorvastatin administered alone.
Table 3 Noncomparative studies
Pharmacoeconomic considerations/quality of life
Treatment with a single tablet of amlodipine/atorvastatin has been shown to be more cost effective than two-tablet therapy and may be slightly more effective when real world adherence levels are considered.Citation56,Citation57 It has been shown that the clinical and economic consequences of adding atorvastatin to an existing amlodipine-based antihypertensive regimen using a single-pill formulation versus a two-pill regimen among patients similar to the ASCOT–LLA population showed the single-pill formulation to be less costly and could be slightly more effective when real world adherence levels are considered.Citation57
Conclusion
Concomitant hypertension and dyslipidemia are very common and are associated with a high risk of cardiovascular disease. Despite the widespread availability of safe and efficacious medications for the treatment of hypertension and dyslipidemia, the management of these conditions is far from optimal. Indeed, epidemiological studies have indicated that 90% of patients with concomitant hypertension and dyslipidemia fail to achieve their therapeutic targets for both conditions. Moreover, the optimal LDL goal in patients with risk factors has been steadily declining, necessitating the treatment of bigger population subsets.
Amlodipine and atorvastatin both have excellent efficacy and safety profiles for the treatment of hypertension and dyslipidemia, respectively. Clinical trials have shown that co-administration of these two agents, across the dose range, does not modify the efficacy of either medication. Moreover, the efficacy and safety of single-pill amlodipine/atorvastatin therapy has also been demonstrated in patients at different levels of risk for CVD. The association of amlodipine and atorvastatin in a single pill formulation with flexible dosing combinations offers the possibility of simplifying the process for treating hypertension and/or angina and dyslipidemia and thereby improving medication adherence.Citation66 The concept of dual-therapy pill also highlights the importance of managing both the risk factors simultaneously, both for the practitioner and the patient and open the floodgates to development and release of other cross-risk-factor, single-pill combinations, and a future polypill.
Disclosure
The authors report no conflicts of interest in this work.
References
- RosamondWFlegalKFurieKHeart disease and stroke statistics – 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics SubcommitteeCirculation20081174e25e14618086926
- VolpeMAldermanMHFurbergCDBeyond hypertension toward guidelines for cardiovascular risk reductionAm J Hypertens20041117(11 Pt 1)1068107415533736
- National Center for Health Statistics2242008Accessed on Jan 10, 2009. Available from: http://www.cdc.gov/nchs/
- Heart Protection Study Collaborative GroupMRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trialLancet2002360932672212114036
- SeverPSDahlofBPoulterNRPrevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trialLancet200336193641149115812686036
- TurnbullFEffects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trialsLancet200336293951527153514615107
- ColhounHMBetteridgeDJDurringtonPNPrimary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trialLancet2004364943568569615325833
- JuliusSKjeldsenSEWeberMOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet200436394262022203115207952
- PettittDKarterAJPengTYThe impact of concurrent dyslipidemia and diabetes on hypertension management and goal attainmentVancouver, CanadaPoster presentation at the 26th Annual Meeting of the Society of General Internal Medicine2003
- EUROASPIRE I and II GroupEuropean Action on Secondary Prevention by Intervention to Reduce Events. Clinical reality of coronary prevention guidelines: a comparison of EUROASPIRE I and II in nine countries. EUROASPIRE I and II Group. European Action on Secondary Prevention by Intervention to Reduce EventsLancet20013579261995100111293642
- PearsonTALauroraIChuHKafonekSThe lipid treatment assessment project (L-TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goalsArch Intern Med2000160445946710695686
- InsullWThe problem of compliance to cholesterol altering therapyJ Intern Med199724143173259159603
- ChapmanRHBennerJSPetrillaAAPredictors of adherence with antihypertensive and lipid-lowering therapyArch Intern Med2005165101147115215911728
- SchroederKFaheyTEbrahimSHow can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trialsArch Intern Med2004164772273215078641
- BangaloreSKamalakkannanGParkarSMesserliFHFixed-dose combinations improve medication compliance: a meta-analysisAm J Med2007120871371917679131
- KrumHCritical assessment of calcium antagonistsAust Fam Physician19972678418459232924
- ZhangXHintzeTHAmlodipine releases nitric oxide from canine coronary microvessels: an unexpected mechanism of action of a calcium channel-blocking agentCirculation19989765765809494028
- JukemaJWvan der HoornJWAmlodipine and atorvastatin in atherosclerosis: a review of the potential of combination therapyExpert Opin Pharmacother20045245946814996641
- MasonRPMarchePHintzeTHNovel vascular biology of third-generation L-type calcium channel antagonists: ancillary actions of amlodipineArterioscler Thromb Vasc Biol200323122155216314512371
- ChungMCalcagniAGluePBramsonCBioavailability of amlodipine besylate/atorvastatin calcium combination tabletJ Clin Pharmacol20064691030103716920898
- BattlemanDPetersonEEstimated prevalence of comorbid hypertension and dyslipidemia and therapeutic goal attainment among US adults in 2000, utilizing data from the National Health and Nutrition Examination Survey (NHANES III)J Manag Care Pharm200410186
- BlankRLaSalleJReevesRMaroniJTarasenkoLSunFSingle-pill therapy in the treatment of concomitant hypertension and dyslipidemia (the amlodipine/atorvastatin gemini study)J Clin Hypertens (Greenwich)20057526427315886529
- ChungMCalcagniAGluePBramsonCEffect of food on the bioavailability of amlodipine besylate/atorvastatin calcium combination tabletJ Clin Pharmacol200646101212121616988211
- PrestonRAHarveyPHerfertOReduction in Framingham cardiovascular risk with concomitant treatment of hypertension/dyslipidemia with amlodipine/atorvastatinAm J Hypertens200518A226
- MesserliFHBakrisGLFerreraDEfficacy and safety of coad-ministered amlodipine and atorvastatin in patients with hypertension and dyslipidemia: results of the AVALON trialJ Clin Hypertens (Greenwich)200688571581; quiz 582–583.16896273
- PrestonRASunFTarasenkoLSafety and tolerability of coad-ministered amlodipine and atorvastatin in patients with concomitant hypertension and dyslipidemia in the Respond studyAm J Hypertens200518A92A93
- MasonRPA rationale for combined therapy with a calcium channel blocker and a statin: evaluation of basic and clinical evidenceCurr Drug Targets Cardiovasc Haematol Disord20055648950116503869
- CohnJNeutelJHoustonMEarly improvements in vascular compliance following coadministration of amlodipine and atorvastatin in patients with concomitant hypertension and dyslipidemia. The Avalon Arterial Wall Compliance (AWC) trialSan Francisco, CAProgram and abstracts from the 20th Annual Scientific Meeting of the American Society of HypertensionMay 14–182005
- LeibovitzEBeniashviliMZimlichmanRFreimanAShargorodskyMGavishDTreatment with amlodipine and atorvastatin have additive effect in improvement of arterial compliance in hypertensive hyperlipidemic patientsAm J Hypertens200316(9 Pt 1)71571812944027
- FogariRPretiPZoppiAEffects of amlodipine-atorvastatin combination on inflammation markers and insulin sensitivity in normocholesterolemic obese hypertensive patientsEur J Clin Pharmacol2006621081782216896785
- FogariRDerosaGLazzariPEffect of amlodipine-atorvastatin combination on fibrinolysis in hypertensive hypercholesterolemic patients with insulin resistanceAm J Hypertens200417982382715363826
- DelsingDJJukemaJWvan de WielMADifferential effects of amlodipine and atorvastatin treatment and their combination on atherosclerosis in ApoE * 3-Leiden transgenic miceJ Cardiovasc Pharmacol2003421637012827028
- MasonRPWalterMFDayCAJacobRFIntermolecular differences of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors contribute to distinct pharmacologic and pleiotropic actionsAm J Cardiol2005965A11F23F
- JukemaJWZwindermanAHvan BovenAJEvidence for a synergistic effect of calcium channel blockers with lipid-lowering therapy in retarding progression of coronary atherosclerosis in symptomatic patients with normal to moderately raised cholesterol levels. The REGRESS Study GroupArterioscler Thromb Vasc Biol19961634254308630669
- LewisEJHunsickerLGClarkeWRRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med20013451285186011565517
- Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)JAMA2002288232981299712479763
- JuliusSWeberMAKjeldsenSEThe Valsartan Antihypertensive Long-Term Use Evaluation (VALUE) trial: outcomes in patients receiving monotherapyHypertension200648338539116864741
- DahlofBSeverPSPoulterNRPrevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trialLancet2005366948989590616154016
- PoulterNRWedelHDahlofBRole of blood pressure and other variables in the differential cardiovascular event rates noted in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA)Lancet2005366948990791316154017
- WilliamsBO’RourkeMThe Conduit Artery Functional Endpoint (CAFE) study in ASCOTJ Hum Hypertens200115Suppl 1S69S7311685915
- PackerMO’ConnorCMGhaliJKEffect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study GroupN Engl J Med199633515110711148813041
- PittBByingtonRPFurbergCDEffect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT InvestigatorsCirculation2000102131503151011004140
- NissenSETuzcuEMLibbyPEffect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trialJAMA2004292182217222515536108
- CannonCPBraunwaldEMcCabeCHIntensive versus moderate lipid lowering with statins after acute coronary syndromesN Engl J Med2004350151495150415007110
- LaRosaJCGrundySMWatersDDIntensive lipid lowering with atorvastatin in patients with stable coronary diseaseN Engl J Med2005352141425143515755765
- ShepherdJBarterPCarmenaREffect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) studyDiabetes Care20062961220122616731999
- PedersenTRFaergemanOKasteleinJJHigh-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trialJAMA2005294192437244516287954
- NissenSETuzcuEMSchoenhagenPEffect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trialJAMA200429191071108014996776
- KorenMJHunninghakeDBClinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance studyJ Am Coll Cardiol20044491772177915519006
- AthyrosVGMikhailidisDPPapageorgiouAARelationship between LDL-C and non-HDL-C levels and clinical outcome in the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) StudyCurr Med Res Opin20042091385139215383187
- ErdineSRoYMTseHFGemini-AALA InvestigatorsSingle-pill amlodipine/atorvastatin helps patients of diverse ethnicity attain recommended goals for blood pressure and lipids (the Gemini-AALA study)J Hum Hypertens200923319621018800143
- HobbsFDGensiniGManciniGBCan combining different risk interventions into a single formulation contribute to improved cardiovascular disease risk reduction? Rationale and design for an international, open-label program to assess the effectiveness of a single pill (amlodipine/atorvastatin) to attain recommended target levels for blood pressure and lipids (The JEWEL Program)Int J Cardiol2006110224225016338012
- FlackJMVictorRWatsonKImproved attainment of blood pressure and cholesterol goals using single-pill amlodipine/atorvastatin in African Americans: the CAPABLE trialMayo Clin Proc2008831354518174006
- PrestonRAHarveyPHerfertOA randomized, placebo-controlled trial to evaluate the efficacy, safety, and pharmacodynamic interaction of coadministered amlodipine and atorvastatin in 1660 patients with concomitant hypertension and dyslipidemia: the respond trialJ Clin Pharmacol200747121555156918048574
- SeverPDahlofBPoulterNPotential synergy between lipid-lowering and blood-pressure-lowering in the Anglo-Scandinavian Cardiac Outcomes TrialEur Heart J200627242982298817145722
- LindgrenPBuxtonMKahanTAmlodipine +atorvastatin is cost effective compared to atenolol +atorvastatin, amlodipine or atorvastatin alone: the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT)Eur Heart J200728Abstr Suppl85717303589
- SmithTW CSTangSSKClinical and economic consequences of single pill combination of amlodipine/atorvastatin compared with a two-pill regimen in hypertension patients with additional cardiovascular risk factorsEur Heart J200728Abstr Suppl85717303589
- TattiPPahorMByingtonRPOutcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDMDiabetes Care19982145976039571349
- DeziiCMA retrospective study of persistence with single-pill combination therapy vs. concurrent two-pill therapy in patients with hypertensionManag Care200099Suppl2611729417
- EronJJYetzerESRuanePJEfficacy, safety, and adherence with a twice-daily combination lamivudine/zidovudine tablet formulation, plus a protease inhibitor, in HIV infectionAIDS20001467168110807190
- GeiterLJO’BrienRJCombsDLSniderDEJrUnited States Public Health Service Tuberculosis Therapy Trial 21: preliminary results of an evaluation of a combination tablet of isoniazid, rifampin and pyrazinamideTubercle1987682 Suppl41463318048
- MelikianCWhiteTJVanderplasAAdherence to oral antidiabetic therapy in a managed care organization: a comparison of monotherapy, combination therapy, and fixed-dose combination therapyClin Ther20022446046711952029
- NDC dataset (personal communication from Novartis – November 2005).
- SuWJPerngRPFixed-dose combination chemotherapy (Rifater/Rifinah) for active pulmonary tuberculosis in Taiwan: a two-year follow-upInt J Tuberc Lung Dis200261029103212475151
- TaylorAAShoheiberOAdherence to antihypertensive therapy with fixed-dose amlodipine besylate/benazepril HCl versus comparable component-based therapyCongest Heart Fail2003932433214688505
- PatelBVLeslieRSFoodyJMAdherence with single pill amlodipine/atorvastatin vs a two pill regimenVasc Health Risk Manage200843673681