Abstract
Dyslipidemia is a major risk factor in the initiation and progression of cardiovascular diseases such as atherosclerosis. Several pharmacological agents have been developed over the past 50 years which target various lipid components such as low density lipoprotein (LDL) cholesterol, triglyceride, and high density lipoprotein (HDL) cholesterol. Similar to other risk factors such as hypertension and diabetes mellitus, the management of dyslipidemia can be complicated and may require combination therapy for effective treatment. This review discusses the biochemical mechanisms of action and clinical uses for simvastatin (the most widely available and commercially prescribed statin) and niacin, and the combination of these agents in the management and treatment of dyslipidemia.
Introduction
‘Multiple dyslipidemia’ is a collective term for the low HDL-cholesterol, elevated triglycerides (TG), and small, dense LDL-cholesterol that is often found in insulin-resistant patients with abdominal obesity, metabolic syndrome, or type 2 diabetes (CitationKrauss et al 2004). Current guidelines for the management of cardiovascular risk focus firmly on the control of individual cardiovascular risk factors, such as dyslipidemia, hypertension, and obesity (CitationNCEP 2002; CitationGrundy et al 2004). For dyslipidemia, these guidelines focus firmly on the control of LDL-cholesterol as the primary objective, with the control of other lipid components viewed as a secondary objective.
The clinical benefits and safety of statin-based therapy, particularly simvastatin, are proven by many well-designed intervention trials (CitationKastelein et al 2005). Despite the impressive benefits with statins, there remains a significant residual cardiovascular risk for events. The National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III guidelines recognize low HDL (1.03 mmol/L or 40 mg/dL) as an independent major risk factor for coronary heart disease and as a potential target for therapeutic intervention.
The presentation of dyslipidemic phenotypes is heterogeneous, and combination therapy with agents (statins) to reduce LDL-cholesterol with agents (eg, niacin and fibrates) that correct low HDL-cholesterol and hypertriglyceridemia provides a rational strategy for addressing cardiovascular risk arising from these multiple sources. Niacin is an effective pharmacological agent available to increase levels of HDL-cholesterol (CitationNCEP 2002), and this compound also induces substantial reductions in TG. Furthermore, niacin therapy shifts the LDL subclass profile towards larger, less atherogenic particles (CitationMcGovern et al 2005). This article will discuss the choice of simvastatin and extended release niacin (niacin ER) combination therapy in the management of multiple dyslipidemia.
Pharmacology and pharmacokinetics of niacin ER and simvastatin
Simvastatin
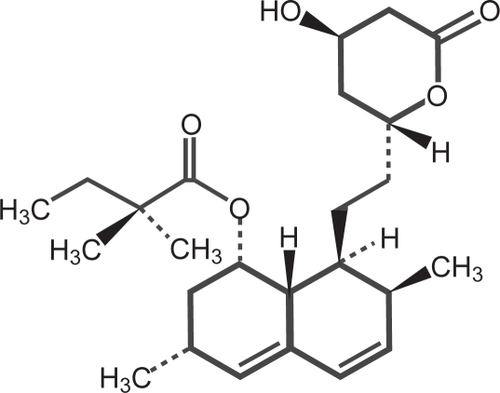
Simvastatin is butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3-7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)-ethyl]-1naphthalenylester [1S[1α,3α,7β,8β(2S*4S*),-8aβ]] (). Simvastatin is a white to off-white, non-hygroscopic, crystalline powder that is practically insoluble in water and freely soluble in chloroform, methanol, and ethanol. The empirical formula of simvastatin is C25H38O5 and its molecular weight is 418.57 (CitationKos Pharma 2008).
Mechanism of action of simvastatin
Simvastatin is a prodrug and is hydrolyzed to its active β-hydroxyacid form, simvastatin acid, after administration. Simvastatin is a specific inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate, an early and rate-limiting step in the biosynthetic pathway for cholesterol. In addition, simvastatin reduces very low density lipoprotein (VLDL) and TG and increases HDL-cholesterol. The pleitropic effects of statins including simvastatin have been well studied (CitationRobinson et al 2005).
Niacin
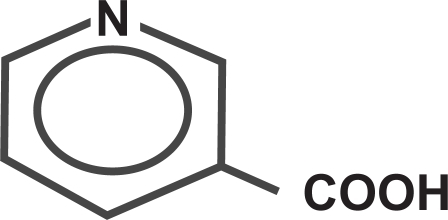
Niacin is nicotinic acid, or 3-pyridinecarboxylic acid. Niacin is a white, non-hygroscopic crystalline powder that is soluble in water, boiling ethanol, or propylene glycol. It is insoluble in ethyl ether. The empirical formula of niacin is C6H5NO2 and its molecular weight is 123.11 () (CitationKos Pharma 2008).
Mechanism of action of niacin
Niacin was found to inhibit lipolysis in adipocytes and reported as early as 1960 by CitationButcher et al (1968), who investigated its effect on cyclic AMP. The investigators also found that niacin inhibits cyclic AMP production in epinephrine (adrenaline)-exposed adipocytes. Later work suggested the presence of a G-protein-coupled cell surface receptor of the Gig/Go type (CitationAktories et al 1983; CitationLorenzen et al 2001). In 2003, the niacin (nicotinic acid) receptor was identified as the human orphan receptor HM74 or the highly homologous, higher affinity HM74A, as well as the mouse homolog PUMA-G (protein upregulated in macrophages by interferon-[gamma]) (CitationTunaru et al 2003; CitationWise et al 2003; CitationSoga et al 2003). HM74A (GPR109A) and PUMA-G are also identified as the pharmacologically active niacin receptors (CitationSoudijn et al 2007). The receptors mediate inhibition of adenyl cyclase in adipocytes and thereby block lipolysis, accounting for the marked reduction of free fatty acids caused by niacin. Furthermore, there was an approximately 30% reduction in TG in fat-fed normal mice. In receptor-deficient mice, no reductions in plasma free fatty acid and triglyceride levels were seen (CitationTunaru et al 2003). Substantial mRNA expression of HM74A/PUMA-G was found in brown and white adipose tissue, spleen, adrenal, and lung, but expression was essentially absent in liver (CitationWise et al 2003).
Inhibition of lipolysis in adipocytes is a most important effect of niacin, but it is not yet proven that antilipolysis accounts for all or even most of the effects on plasma lipo-proteins. Generally, plasma free fatty acid levels appear to increase production of VLDL, especially in states of elevated free fatty acids and VLDL overproduction (eg, obesity or diabetes). After an initial steep fall in plasma free fatty acids after ingestion of plain crystalline niacin, however, their levels rebound 2 h later, whereas hepatic production of VLDL remains suppressed for several additional hours (CitationWang et al 2001). An alternative mechanism, inhibition of hepatic diacylglycerol acyltransferase 2 in niacin, has also been proposed. In millimolar concentrations, niacin was shown to inhibit TG formation in HepG2 cells by diacylglycerol acyltransferase 2 inhibition and concomitantly to increase apolipoprotein B degradation (Jin et al 1991).
Niacin in millimolar concentrations also inhibited the uptake of HDL–apolipoprotein A-I by HepG2 cells (a model for liver) without affecting the uptake of cholesteryl ester from HDL (CitationJin et al 1997). This is a potential mechanism by which niacin raises HDL levels. Moreover, niacin is more effective in raising HDL than any other currently available drug (CitationGuyton et al 2000). The increase in HDL–apolipoprotein A-I may augment reverse cholesterol transport, allowing removal of greater amounts of cholesterol from the vascular wall. Recently, niacin (1 mM) was shown to activate the peroxisome proliferator-activated receptor-[gamma] in a monocytoid cell line, presumably via a metabolite of prostaglandin D2. Both cholesterol efflux and the transcription of efflux-related receptors, namely CD36 and ATP-binding cassette protein A1, were increased by niacin. The effect on CD36 was cyclo-oxygenase dependent, whereas the effect on ATP-binding cassette protein A1 was blocked by an inhibitor of protein kinase A (downstream from adenylyl cyclase) (CitationRubic et al 2004).
Formulations of niacin and its metabolism
Three types of niacin formulations are available: immediate release, sustained release and extended release. They differ in terms of their pharmacokinetic characteristics (McKenney et al 2003). Immediate release niacin (niacin IR) is absorbed quickly and produces peak serum concentrations within 30–60 min. It is metabolized predominantly via conjugation to nicotinuric acid, which is thought to be responsible for the flushing associated with niacin therapy. Niacin IR is generally given 3 times per day because of its rapid pharmacokinetics. Sustained release niacin (niacin SR) formulations are absorbed much more slowly, often taking 12–24 h (McKenney et al 2003). As a result, this preparation is metabolized to a greater extent via oxidation to nicotinamide and several pyrimidine breakdown products thought to be responsible for the hepatotoxicity associated with some SR formulations. Sustained release niacin is generally taken twice daily, but the absorption characteristics vary considerably across products. Extended release niacin (niacin ER) has a dissolution rate of 8–12 h and is absorbed at a rate intermediate to the other two types of niacin. As a result, niacin ER undergoes a more balanced metabolism via both pathways, which is believed to contribute to its improved safety profile. Niacin ER is administered once daily. Niacin ER is the only FDA approved form of niacin available within the United States for the treatment of dyslipidemia.
Absorption and bioavailability of the combination of niacin ER and simvastatin
The relative bioavailability of niacin (nicotinuric acid; Cmax, and total urinary excretion as the surrogate), simvastatin, and simvastatin acid was evaluated in healthy volunteers (n = 42) after administration of two 1000/20 mg niacin/simvastatin combination tablets. Niacin exposure(Cmax and AUC) after Simcor® (combination pill of simvastatin and niacin ER) was similar to that of a niacin ER formulation. However, simv-astatin and simvastatin acid AUC after Simcor® increased by 23% percent and 41%, respectively, compared with those of a simvastatin IR. The mean time to Cmax (Tmax) for niacin ranged from 4.6 to 4.9 h and simvastatin from 1.9 to 2.0 h. Afgter administration of 2 tablets of 1000/20 mg Simcor®, the mean Cmax, Tmax, and AUC(0-t) for simvastatin acid, were 3.29 ng/mL, 6.56 h, and 30.81 ng h/mL respectively (CitationKos Pharma 2008).
Clinical trials on niacin ER and simvastatin
Numerous clinical trials have shown the efficacy and safety of simvastatin in the treatment of hyperlipidemia for secondary and primary prevention in dyslipidemic patients (CitationScandinavian Simvastatin Survival Study Group 1994). The efficacy and safety of combination of niacin ER and simvastatin have been studied recently (CitationGoldberg et al 2000, CitationTaylor et al 2004).
The HATS trial studied the progression of atherosclerosis at nine predefined proximal sites in the coronary circulation of 160 patients with coronary disease and low HDL-cholesterol (>35 mg/dL in men and <40 mg/dL in women) (CitationBrown et al 2001). Three years of treatment with a combination of simvastatin and immediate-release nicotinic acid increased HDL-cholesterol by 26% and decreased LDL-cholesterol by 42%, relative to baseline. These improvements were accompanied by regression of atherosclerosis, measured as percentage luminal diameter of coronary arteries, compared with progression of atherosclerosis on placebo. In comparison to placebo, the 3-year primary event rate was reduced by 90 percent in patients receiving simvastatin/nicotinic acid therapy (p < 0.03).
The Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER 2) (CitationTaylor et al 2004) study confirmed that niacin ER shares these anti-atherogenic benefits. ARBITER 2 was a randomized, double-blind comparison of niacin ER (1000 mg once daily) and placebo when added to existing statin therapy in 167 men or women with HDL-cholesterol <45 mg/dL and LDL-cholesterol <130 mg/dL. Treatment duration was 1 year. The primary endpoint was the change in carotid intima-media thickness (cIMT), assessed using ultrasound. This endpoint is highly predictive of coronary events, as shown by a 9-year follow-up of 146 men who underwent periodic carotid ultrasound and coronary angiography (CitationHodis et al 1998). The cIMT was significantly related to the risk of coronary events (p < 0.02), and an annual increase in this parameter of 0.03 mm was associated with a two-fold increase in the risk of myocardial infarction or coronary heart disease death, and a three-fold increase in the risk of any coronary event (p < 0.001). The authors concluded that measurement of cIMT using ultrasound provided prognostic information beyond that available from coronary angiography and that cIMT provides a meaningful surrogate marker for coronary atherosclerosis.
The ARBITER 2 population was at high risk of coronary events. All patients had known coronary heart disease at baseline as a recruitment criterion, 28 percent had diabetes, 75% were hypertensive, and 51% had NCEP/ATPIII metabolic syndrome. Mean HDL-cholesterol at baseline was 39 mg/dL and mean LDL-cholesterol at baseline was 89 mg/dL. Mean HDL-cholesterol did not change in the placebo group but was 20% higher after treatment with niacin ER. Niacin ER also reduced TG compared with placebo. The lack of effect of study treatment on LDL-cholesterol was expected, as this parameter was already well controlled by pre-existing statin treatment before niacin was administered. Overall, the effects on the lipid profile of adding niacin to the regimen were consistent with the known therapeutic profile of this agent (CitationGoldberg et al 2000; CitationNCEP 2002).
During treatment with placebo, there was an increase in cIMT, whereas no significant change occurred during treatment with niacin ER, confirming the anti-atherogenic properties of this agent. Indeed, ARBITER 2 was the first clinical trial to demonstrate the superiority of lipid-modifying combination therapy (niacin ER with statin) over monotherapy (statin alone) by using cIMT as a clinically validated surrogate for coronary atherosclerosis. At the end of the ARBITER 2 study, 104 patients completed a further 12 months of open-label treatment with niacin ER. The patients who were on placebo were switched to niacin and those on niacin continued therapy. The results of this study were termed as ARBITER 3. Initial results of ARBITER 3 show that the expected increase in HDL-cholesterol occurred in patients switching to niacin ER and that atherosclerosis regressed, with a mean reduction in cIMT of 0.04 mm from baseline (p = 0.008) after 12–24 months of treatment with niacin.
The SEACOAST trial (CitationBallantyne et al 2007) which was recently reported is a 24-week double-blind, randomized, controlled trial in more than 640 patients with elevated non-HDL (type II hyperlipidemia or mixed dyslipidemia) compared simvastatin alone to a combination of niacin ER and simvastatin. This was a pharmaceutical industry sponsored trial. The study was designed to evaluate the safety and efficacy of Simcor® (combination pill of simvastatin and niacin ER) following simvastatin monotherapy. Patients enrolled in the trial were assigned to either a low-dose (20 mg) or high-dose (40 mg) simvastatin group. Patients in the low-dose group were randomized to receive niacin ER 2000 mg/simvastatin 20 mg, niacin ER 1000 mg/simvastatin 20 mg, or simvastatin 20 mg. Patients in the high-dose group were randomized to receive niacin ER 2000 mg/simvastatin 40 mg, niacin ER 1000 mg/simvastatin 40 mg or simvastatin 80 mg. Those in the simvastatin control groups received a 50 mg dose of niacin IR to maintain blinding.
Patients in the low-dose group receiving combination treatment achieved 14% (1000 mg/20 mg) and 23% (2000 mg/20 mg) reductions in non-HDL compared with a 7% reduction with 20 mg simvastatin therapy alone. Additionally, combination treatment significantly improved HDL by 18% (1000 mg/20 mg) and 25% (2000 mg/20 mg) compared with 7% with 20 mg simvastatin alone. Similarly, significant reductions in TG of 27% (1000 mg/20 mg) and 38% (2000 mg/20 mg) were seen in those treated with combination therapy compared with a 15% reduction with simvastatin monotherapy.
In the high-dose group, patients treated with combination therapy showed similar (non-inferior) improvements in non-HDL of 11% (1000 mg/40 mg) and 17% (2000 mg/40 mg) compared with a 10% improvement with 80 mg simvastatin therapy alone. Additionally, the high-dose combination group demonstrated significant improvements in HDL of 15% (1000 mg/40 mg) and 22% (2000 mg/40 mg) compared with a 1% decrease among those receiving 80 mg simvas-tatin monotherapy. Triglyceride levels among the high-dose combinations groups dropped 23% and 32%, respectively, in contrast to a 0.3% increase in those randomized to 80 mg simvastatin monotherapy. The study was not designed to measure any mortality or outcome data.
Safety and tolerability of niacin ER and simvastatin combination
The most common and important side effect of niacin is cutaneous flushing, with variable severity among patients. Other side effects include hepatic toxicity, hyperglyce-mia, gout, and rare cases of retinal macular edema. All of these effects are dose dependent and fully reversible given timely discontinuation. Gastrointestinal side effects, dizziness, pruritis, headache, and back pain are not uncommon. Much of the existing perception of niacin adverse effects is from studies of the IR and the very-slow release preparations (CitationKnopp et al 1985). Hepatic toxicity and flushing have been favorably affected by recent preparations that attempt to strike a beneficial balance between the duration and the peak levels of plasma drug exposure. The recent publication of efficacy and safety data for the combination of a statin (including simvastatin) and niacin ER in the treatment of dyslipidemia (CitationBallantyne et al 2007; CitationKaras et al 2008; CitationBays 2008) strongly suggests that the combination therapy would be more tolerable than reported in the recent past.
Flushing
Flushing occurs in almost all subjects who take lipid-modifying doses of niacin IR. It is the major reason for discontinuation of the drug, which has been described at rates as high as 25%–40% (CitationKnopp et al 1985; CitationGibbons et al 1995; CitationMcKenney et al 1994). In the Coronary Drug Project (CitationCDP Group 1975), the prescribed dose of niacin was 1000 mg 3 times daily, but mean adherence was only 66%. Flushing appears to be decreased and tolerance improved with the use of niacin ER, because rates of discontinuation of this formulation due to flushing have not exceeded 6% in short-term trials (CitationKnopp et al 1998; CitationGoldberg et al 2000; CitationGuyton et al 2000).
Flushing is caused by the release of prostaglandin D2 and possibly other eicosanoids from cells in the skin (CitationMorrow et al 1992). Tachyphylaxis of flushing occurs usually with continued use of niacin. Regular, consistent dosing over days, weeks, and months reduces frequency and severity of flushing, accompanied by lesser increases of prostaglandin D2 after niacin ingestion (CitationStern et al 1991). Administering aspirin or other inhibitors of cyclooxygen-ase 30 min to 1 h before niacin intake can substantially reduce flushing (CitationOberwittler et al 2006). An aspirin dose of 325 mg is commonly advised; an 81-mg dose may not be enough. Recently, an inhibitor of the prostaglandin D2 receptor 1 was found to inhibit niacin-induced flushing in mice and in early human trials; this compound is under clinical development (CitationCheng et al 2006).
The terms “no-flush niacin” and “flush-free niacin” are conventionally used for inositol hexanicotinate. In this compound, 6 molecules of nicotinic acid (niacin) are covalently attached to inositol by ester bonds. No published study has demonstrated that inositol hexanicotinate releases free niacin, demonstrably increases circulating niacin, or alters plasma lipid levels. Thus, there is no evidence that niacin in this formulation is bioavailable (Meyers et al 2006).
Laropiprant, a prostaglandin D2 receptor-1 antagonist, has shown considerable promise in reducing the associated flushing with niacin use and shown to improve compliance and dosing of niacin ER (CitationCheng et al 2006; CitationPaolini et al 2008). This drug in combination with niacin ER is currently awaiting FDA approval.
Hyperglycemia
The potential for exacerbation of hyperglycemia in patients with diabetes represents another barrier to the successful therapeutic use of niacin to correct low HDL-cholesterol. Initial studies that suggested the substantial deterioration of glycemic control in patients with diabetes occurred in patients taking niacin IR. However, these studies used niacin doses as high as 4.5 g/day, which are rarely used in the statin era to treat hypercholesterolemia (CitationGarg et al 1990). Clinical studies discussed below, including the CDP Study, noted only mild hyperglycemic effects as a result of niacin administration (CitationCDP 1975; CitationCanner et al 1986). Niacin ER at a dose of 2000 mg/day was associated with a 5% increase in fasting plasma glucose (CitationGuyton et al 1998). This hyperglycemia is caused by insulin resistance. Only a few studies have quantified insulin resistance in niacin-treated subjects. Some studies have suggested that the decrease in insulin sensitivity may average 20%–28% after 2 weeks of administration of niacin IR at 1000 mg twice daily (CitationAlvarsson et al 1996; CitationKelly et al 2000; CitationRasouli et al 2005; CitationChang et al 2006). In subjects aged older than 60 years, insulin sensitivity decreased by 55%–60% after niacin administration. Insulin secretory responsiveness does not decrease. In fact, insulin secretion may be increased as part of the homeostatic response to the decrease in the effectiveness of insulin action (CitationKahn et al 1989). The mechanism of peripheral insulin resistance may be related to a rebound increase in circulating fatty acids but not to increased muscle triglyceride content (CitationPoynten et al 2003).
The discussion of niacin-induced insulin resistance is important for two clinical reasons. First, hyperglycemia itself appears to be associated with worsened cardiovascular outcomes (CitationAdler et al 1999). However, it must be recalled that the overall impact of niacin is to reduce the residual cardiovascular risk associated with statin alone treatment of dyslipidemia. The second consequence of insulin resistance might be to increase the frequency of new onset of diabetes in patients at risk, such as those with the metabolic syndrome. Although this is a theoretical possibility, the 5-year CDP did not show significant differences between niacin treated and placebo-treated subjects in new prescriptions for insulin, new prescriptions for oral hypoglycemic drugs, or instances of dipstick-positive glycosuria (CitationCDP Research Group 1975).
In the ADMIT study, 64 patients with diabetes and peripheral arterial disease, receiving an average niacin dose of 2.55 g/day, were compared with 61 similar patients receiving placebo over 48 weeks. The mean change in hemoglobin A1c (HbA1c) was 0.3% greater in the group receiving niacin (p < 0.04), and insulin use was 13% more frequent in niacin users over the course of the trial, compared with 4% with placebo (p < 0.09) (CitationElam et al 2000). Likewise, HbA1c increased by 0.3% in 52 patients receiving niacin ER at 1500 mg/day, compared with no change in 49 patients receiving placebo. In this study, niacin-treated patients tended to require additional hypoglycemic therapy more often than placebo-treated patients (24%–29% of those taking niacin vs 16% with placebo, p < 0.32) (CitationGrundy et al 2002). CitationThoenes et al (2007) reported that in 50 patients with the metabolic syndrome treated with niacin ER for 52 weeks, there was a significant improvement in endothelial function and a favorable effect on the lipid profile. Furthermore, there was no sig-nificant change in glucose intolerance in the study group.
Clinical trials with doses of niacin used clinically today appear to result in only minor deterioration of glycemic control in most patients with diabetes. The favorable effects of niacin on HDL-cholesterol, TG, lipoprotein (a), and LDL particle size, along with its lesser effect in lowering LDL-cholesterol, probably outweigh the small detriment in glycemic control in diabetes.
Hepatotoxicity
CitationMcKenney et al (1994) directly compared IR and SR niacin in a randomized clinical trial with dosage escalation from 500 to 3000 mg/day over 30 weeks. None of the 23 patients taking niacin IR developed hepatotoxic effects, whereas 12 of 23 patients (52%) taking niacin SR developed similar effects. The increase in liver toxicity with niacin SR mainly occurred with doses >1500 mg/day.
Niacin ER and simvastatin can cause abnormal liver tests. In a simvastatin-controlled, 24-week study with Simcor® in 641 patients, there were no persistent increases (to more than 3x the upper limit of normal) in serum transaminases (CitationBallantyne et al 2007). Persistent increases (to more than 3 times the upper limit of normal) in serum transaminases have occurred in approximately 1% of patients who received simvastatin in clinical studies (CitationScandinavian Simvastatin Survival Study Group 1994). When drug treatment was interrupted or discontinued in these patients, the trans-aminases levels usually fell slowly to pretreatment levels. Liver function tests should be performed on all patients during therapy with combination therapy with simvastatin and niacin ER. It is recommended that liver function tests be performed before treatment begins, every 12 weeks for the first 6 months, and periodically thereafter, at approximately 6-month intervals (CitationKos Pharma 2008).
Myopathy and other adverse events
Niacin ER monotherapy has specifically amassed considerable clinical trial data that do not support an increased risk for muscle adverse experiences with niacin monotherapy. Similarly, the safety of niacin ER in combination with statins has likewise been assessed, and studies have demonstrated no sign of increased muscle toxicity over that of statins alone (HATS trial and SEACOAST trial). Many drugs that are potent inhibitors of CYP3A4 can increase the risk of myopa-thy by reducing the elimination of simvastatin. Hence when simvastatin is used with a potent inhibitor of CYP3A4, elevated plasma levels of HMGCoA reductase inhibitory activity can increase the risk of myopathy and rhabdomyolysis, particularly with higher doses of simvastatin. In patients with renal failure and elderly, caution should be exercised in using high doses of statins with niacin ER and may need frequent monitoring of muscle and liver enzymes.
Patient perspectives
It is important to counsel patients about the role of niacin in improving their lipid profile and reducing their cardiovascular risk. Physicians should review the expected side effects with their patients before starting therapy and reinforce the importance of staying on therapy. Although most patients can expect to experience some flushing even with niacin ER formulations, tolerance to the flushing generally develops with continued use. In addition, several measures can reduce the frequency and severity of flushing. First, niacin therapy needs to be initiated at a low dose and then slowly titrated upward to achieve the treatment goal. Second, patients may take aspirin 325 mg or another non-steroidal anti-inflammatory drug 30 min before the niacin dose to reduce prostaglandin-mediated flushing (CitationOberwittler et al 2006). Third, physicians should advise patients to avoid spicy foods, hot beverages, and hot showers shortly after taking niacin. Patient should avoid any interruptions in niacin therapy to continue any tolerance to flushing that has developed. If niacin is interrupted for more than 1 week, treatment needs to be restarted at the lowest dose and again titrated upward. The niacin ER formulation is given at bedtime, which may further reduce the awareness of flushing. The simplified regimen may also improve patient adherence. Practitioners should discuss these steps with their patients and should provide instructions in a written form (see ).
Table 1 Recommended dosing regimen of niacin ER
Conclusion
The combination of niacin ER with a statin addresses two key sources of cardiovascular risk: low HDL-cholesterol and elevated LDL-cholesterol. The availability of Niaspan®, a prolonged-release formulation of nicotinic acid with improved tolerability compared with the immediate-release version facilitates the delivery of this therapy. The recently published SEACOAST trial (CitationBallantyne et al 2007) demonstrated the safety and efficacy of combination simvastatin and niacin ER in small group of patient population for period of 6 months. The randomized, double-blind ARBITER 2 study (CitationTaylor et al 2004) demonstrated the benefits of Niaspan® plus a statin in terms of inhibition of atherosclerosis. Although ARBITER 2 was not powered to detect significant differences in event rates, there was a trend towards a reduction in cardiovascular events in the Niaspan group, compared with placebo.
The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL-C/High Triglyceride and Impact on Global Health Outcomes (AIM-HIGH) study will explore quantitatively the potential of aggressive intervention with Niaspan® and a statin to improve cardiovascular event rates. This trial, jointly sponsored by the US National Heart, Lung, and Blood Institute and Kos Pharmaceuticals Inc (now Abbott Pharmaceuticals), is currently recruiting a population of 3300 patients with established coronary heart disease, atherogenic dyslipidemia (low HDL-cholesterol and high TG), and LDL-cholesterol controlled to current US guideline goals (NCEP criteria) at 60 sites in the US and Canada. Patients will be randomized to receive double-blind treatment with Niaspan® plus a statin or to statin monotherapy for 6 years. The primary outcome measure will be a composite of cardiovascular death, non-fatal myocardial infarction, non-hemorrhagic stroke, or hospitalization for acute coronary syndrome with objective evidence of ischemia. AIM-HIGH will define the potential of aggressive management of both low HDL-cholesterol and hyperlipidemia to reduce cardiovascular event rates.
The Heart Protection Study 2: Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) is an ongoing trial combining simvastatin and extended-release niacin plus a D prostanoid 1 receptor antagonist laropiprant, to inhibit the prostaglandin D2–mediated flushing side effect in comparison with simvastatin monotherapy in 20,000 patients with coronary heart disease.
In our opinion, the AIM HIGH and HPS2-THRIVE studies will give us answers to the question whether the combination of simvastatin with niacin ER will reduce residual cardiovascular risk in comparison to statin therapy alone. At this point of time, the combination of simvastatin with niacin ER is an effective treatment of the specific components of dyslipidemia, namely improving the profiles of elevated TG and LDL-cholesterol and low levels of HDL-cholesterol.
Disclosures
None of the authors has any conflicts of interest to disclose.
References
- AdlerAINeilHAManleySE1999Hyperglycemia and hyperinsu-linemia at diagnosis of diabetes and their association with subsequent cardiovascular disease in the United Kingdom Prospective Diabetes Study (UKPDS 47)Am Heart J138S353S910539797
- Aim High: Niacin plus statin to prevent vascular eventsClinical trials gov-NCT 00120289
- AktoriesKSchultzGJakobsKH1983Islet-activating protein prevents nicotinic acid-induced GTPase stimulation and GTP but not GTP gamma S-induced adenylate cyclase inhibition in rat adipocytesFEBS Lett15688926133780
- AlvarssonMGrillV1996Impact of nicotinic acid treatment on insulin secretion and insulin sensitivity in low and high insulin respondersScand J Clin Lab Invest56563708903118
- BallantyneCMDavidsonMMcKenneyJ2007Safety and efficacy of a combination of extended-release niacin and simvastatin in patients with dyslipedemia (SEACOAST) – a dose ranging study [abstract]AHA Scientific ConferenceChicago
- BaysH2008Safety of niacin and simvastatin combination therapyAm J Cardiol1013818243856
- ButcherRWBairdCESutherlandEW1968Effects of lipolytic and antilipolytic substances on adenosine 3’, 5’-monophosphate levels in isolated fat cellsJ Biol Chem2431705124384670
- BrownBGZhaoXQChaitA2001Simvastatin and niacin, anti-oxidant vitamins, or the combination for the prevention of coronary diseaseN Engl J Med34515839211757504
- CannerPLBergeKGWengerNK1986Fifteen-year mortality in Coronary Drug Project patients: long-term benefit with niacinJ Am Coll Cardiol81245553782631
- ChangAMSmithMJGaleckiAT2006Impaired beta-cell function in human aging: response to nicotinic acid-induced insulin resistanceJ Clin Endocrinol Metab913303916757523
- ChengKWuTJWuKK2006Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humansProc Natl Acad Sci U S A1036682716617107
- Coronary Drug Project Research GroupClofibrate and niacin in coronary heart diseaseJAMA1975231360381
- ElamMBHunninghakeDBDavisKB2000Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT [Arterial Disease Multiple Intervention Trial] study. A randomized trialJAMA28412637010979113
- GanjiSHKamannaVSKashyapML2003Niacin and cholesterol: role in cardiovascular diseaseJ Nutr Biochem1429830512873710
- GargAGrundySM1990Nicotinic acid as therapy for dyslipidemia in non-insulin-dependent diabetes mellitusJAMA26472362374275
- GibbonsLWGonzalezVGordonN1995The prevalence of side effects with regular and sustained-release nicotinic acidAm J Med99378857573093
- GinsbergHN2000Insulin resistance and cardiovascular diseaseJ Clin Invest106453810953019
- GoldbergAAlagonaPJrCapuzziDM2000Multiple-dose efficacy and safety of an extended-release form of niacin in the management of hyperlipidemiaAm J Cardiol851100510781759
- Grundy2004
- GrundySMVegaGLMcGovernME2002Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of Niaspan trialArch Intern Med16215687612123399
- GuytonJRBlazingMAHagarJ2000Extended-release niacin vs gemfibrozil for the treatment of low levels of high-density lipoprotein cholesterolArch Intern Med16011778410789612
- GuytonJRBlazingMAHagarJ2000Extended-release niacin versus gemfibrozil for treatment of low levels of high density lipoprotein cholesterolArch Intern Med16011778410789612
- GuytonJRGoldbergACKreisbergRA1998Effectiveness of once-nightly dosing of extended release niacin alone and in combination for hypercholesterolemiaAm J Cardiol82737439761083
- HodisHNMackWJLaBreeL1998The role of carotid arterial intima-media thickness in predicting clinical coronary eventsAnn Intern Med12826299471928
- Health Protection Study-2-(HPS2-THRIVE). clinicaltrials.gov-00461360.
- JinFYKamannaVSKashyapML1999Niacin accelerates intracel-lular ApoB degradation by inhibiting triacylglycerol synthesis in human hepatoblastoma (HepG2) cellsArterioscler Thromb Vasc Biol191051910195935
- JinFYKamannaVSKashyapML1997Niacin decreases removal of high-density lipoprotein apolipoprotein A-I but not cholesterol ester by Hep G2 cells. Implication for reverse cholesterol transportArterioscler Thromb Vasc Biol17202089351367
- KahnSEBeardJCSchwartzMW1989Increased beta-cell secretory capacity as mechanism for islet adaptation to nicotinic acid-induced insulin resistanceDiabetes3856282653928
- KasteleinJJP2005The realities of dyslipidaemia: what do the studies tell us?Eur Heart J7Suppl FF27F33
- KarasRHAlSheikh-AliAA2008The safety of niacin in the US Food and Drug Administration Adverse Event Reporting DatabaseAm J Cardiol101103
- KellyJJLawsonJACampbellLV2000Effects of nicotinic acid on insulin sensitivity and blood pressure in healthy subjectsJ Hum Hypertens145677210980588
- KnoppRHGinsbergJAlbersJJ1985Contrasting effects of unmodified andtime-release forms of niacin on lipoproteins in hyper-lipidemic subjects: clues to mechanism of action of niacinMetabolism34642503925290
- KnoppRHAlagonaPDavidsonM1998Equivalent efficacy of a time-release form of niacin (Niaspan) given once a-night versus plain niacin in the management of hyperlipidemiaMetabolism4710971049751239
- Kos Pharmaceuticals (currently) Abbott Pharmaceuticals DN1628V4-simcor-021408 Prescribing Information 2008.
- KraussRM2004Lipids and lipoproteins in patients with type 2 diabetesDiabetes Care27149650415161808
- LorenzenAStannekCLangHCharacterization of a G protein-coupled receptor for nicotinic acidMol Pharmacol2001593495711160872
- McGovernME2005Niaspan®: creating a new concept for raising HDL–cholesterolEur Heart J7Suppl FF41F7
- McKenneyJMProctorJDHarrisS1994A comparison of the efficacy and toxic effects of sustained- vs. immediate-release niacin in hypercholesterolemic patientsJAMA27167278309029
- MeyersCDCarrMCParkS2003Varying cost and free nicotinic acid content in over-the-counter niacin preparations for dyslipidemiaAnn Intern Med139996100214678919
- MorganJMCapuzziDMGuytonJR1996Treatment effect of Niaspan, a controlled-release niacin, in patients with hypercholes-terolemia: a placebo-controlled trialJ Cardiovasc Pharmacol Ther119520210684417
- MorrowJDAwadJAOatesJA1992Identification of skinas a major site of prostaglandin D2 release following oral administration of niacin in humansJ Invest Dermatol98812151373750
- National Cholesterol Education Program (NCEP)2002Expert panel on detection (third report), evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final reportCirculation106314342112485966
- OberwittlerHBaccara-DinetMClinical evidence for use of acetyl salicylic acid in control of flushing related to nicotinic acid treatmentInt J Clin Pract20066070715
- PaoliniJFMitchellYBReyesR2008Effects of laropiprant on nicotinic acid-induced flushing in patients with dyslipedemiaAm J Cardiol101625818308010
- PoyntenAMGanSKKriketosAD2003Nicotinic acid-induced insulin resistance is related to increased circulating fatty acids and fat oxidation but not muscle lipid contentMetabolism5269970412800094
- RasouliNHaleTKahnSE2005Effects of short-term experimental insulin resistance and family history of diabeteson pancreatic beta-cell function in nondiabetic individualsJ Clin Endocrinol Metab9058253316091496
- RobinsonJGSmithBMaheshwariN2005Pleiotrophic effects of statins – benefits beyond LDL reduction?J Am Coll Cardiol4618556216286171
- RubicTTrottmannMLorenzRL2004Stimulation of CD36 and the key effector of reverse cholesterol transport ATP-binding cassette A1 in monocytoid cells by niacinBiochem Pharmacol67411915037193
- Scandinavian Simvastatin Survival Study Group1994Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease : Scandinavian Simvastatin Survival Study (4S)Lancet344138397968073
- SogaTKamoharaMTakasakiJ2003Molecular identification of nicotinic acid receptorBiochem Biophys Res Commun303364912646212
- SoudijnWvan WijngaardenIIjzermanAP2007Nicotinic acid receptor subtypes and their ligandsMed Res Rev274173317238156
- SternRHSpenceJDFreemanDJ1991Tolerance to nicotinic acid flushingClin Pharmacol Ther5066701855354
- TaylorAJSullenbergerLELeeHJ2004Arterial Biology for the Treatment Effects of Reducing Cholesterol 2 (ARBITER 2 Study)Circulation1103512715537681
- ThoenesMOguchiANagamiaS2007The effects of extended-release niacin on carotid intimal medial thickness, endothelial function, and inflammatory markers in patients with the metabolic syndromeInt J Clin Pract611942817935553
- TunaruSKeroJSchaubA2003PUMA-G and HM74 are receptors for nicotinic acid and mediate its antilipolytic effectNat Med9352512563315
- WangWBasingerANeeseRA2001Effect of nicotinic acid administration on hepatic very low density lipoprotein-triglyceride productionAm J Physiol Endocrinol Metab280E540E711171611
- WiseAFoordSMFraserNJ2003Molecular identification of high and low affinity receptors for nicotinic acidJ Biol Chem27898697412522134