Abstract
Purpose:
To investigate if study design factors such as randomization, multi-center versus single center evidence, institutional surgical volume, and patient selection affect the outcomes for endovascular repair (EVAR) versus open surgical repair (OSR). Finally, we investigate trends over time in EVAR versus OSR outcomes.
Methods:
Search strategies for comparative studies were performed individually for: OVID’s MEDLINE, EMBASE, CINAHL, HAPI, and Evidence Based Medicine (EBM) Reviews (including Cochrane DSR, ACP Journal Club, DARE and CCTR), limited to 1990 and November 2006.
Results:
Identified literature: 84 comparative studies pertaining to 57,645 patients. These include 4 randomized controlled trials (RCTs), plus 2 RCTs with long-term follow-up. The other 78 comparative studies were nonrandomized with 75 reporting perioperative outcomes, of which 16 were multi-center, and 59 single-center studies. Of the single-center studies 31 were low-volume and 28 were high-volume centers. In addition, 5 studies had all patients anatomically eligible for EVAR, and 8 studies included high-risk patients only. Finally, 25 long term observational studies reported outcomes up to 3 years.
Outcomes:
Lower perioperative mortality and rates of complications for EVAR versus OSR varied across study designs and patient populations. EVAR adverse outcomes have decreased in recent times.
Conclusion:
EVAR highlights the problem of performing meta-analysis when the experience evolves over time.
Introduction
Two recent reviews have described the relative outcomes of endovascular repair (EVAR) versus open surgical repair (OSR) (CitationDrury et al 2005; CitationHo et al 2006) and of EVAR alone (CitationFranks et al 2007) for abdominal aortic aneurysms (AAA) based on evidence from both randomized control trials (RCTs) and nonrandomized control trials (nRCTs). Despite these recent reviews, there are limitations in the accumulated evidence for an assessment comparing EVAR and OSR. As the authors suggest, the major differences between the RCTs and the nRCTs relate to differences in baseline characteristics of the patient populations and overall study design. While the recent reviews have cautioned the interpretation of the differential outcomes of EVAR versus OSR found in RCT compared to nRCTs, they do not assess the degree to which the imbalances or different study design impact relative outcomes.
For example, patients in RCTs tend to have lower ASA risk levels than patients in nRCTs, and the average ASA risk tends to be higher for EVAR than OSR patients in nRCTs (CitationSbarigia et al 2005). This baseline imbalance for AAA repair has continued ever since EVAR was first introduced to benefit patients with AAA who were physiologically at high risk for OSR or who were anatomically suitable for EVAR (CitationParodi et al 1991). Despite the focus on high risk patients, the benefits of EVAR in lower risk patients has also recently been established in two large RCTs, DREAM (CitationPrinssen et al 2004) and EVAR-1 (CitationGreenhalgh et al 2004). Despite these two landmark trials, only one small RCT has been completed to assess differences in quality of life between EVAR and OSR patients (CitationSoulez et al 2005). In order to compare and contrast the outcome evidence of EVAR and OSR in RCTs and nRCTs, differences in patient baseline risk levels and the degree to which these risk levels differ between EVAR and OSR in observational studies need to be investigated.
A major limitation of the recent reviews is the inclusion of studies that should have otherwise been excluded. For example, some studies are multi-center in nature and the meta-analysis includes the results of the multi-center studies and the redundant constituent single center evidence (CitationBrewster et al 1998; CitationSicard et al 2001; CitationMakaroun et al 2002; CitationMatsumura et al 2003). This is particularly evident with the large device trials, the Lifeline Registry (CitationLREAR 2001) and the Eurostar Registry (CitationButh and Parodi 1999). In addition, other evidence that should be excluded is the studies that include early evidence from the Ancure multi-center trial, even though the device was temporarily removed from the market for underreporting complications (CitationMoore et al 2001).
Interestingly, EVAR was originally intended for high risk patients, but there has been little cumulative evidence reported for high risk patients only (CitationSbarigia et al 2005). To address the benefits to high risk patients only, a review of studies where both EVAR and OSR patients are deemed at high medical risk should be the starting point. Similarly, EVAR is usually offered to anatomically suitable candidates. Yet, no review has isolated studies where both EVAR and OSR patients are anatomically suitable for EVAR. To include anatomically suitable patients for a comparative review would eliminate a significant bias in terms of the effects of vasculature on surgical outcomes (CitationWelborn et al 2005).
A further bias is added in reviews if the authors do not incorporate inclusion criterion based on treatment volumes or control for treatment volume in the analysis. This is important because most centers have low annual volume and treatment volumes have been shown to be related to treatment outcomes for endovascular repair (CitationBush et al 2006) as well as other cardiac procedures (CitationFranks et al 2007). Accordingly, it is important to compare relative outcomes for EVAR and OSR adjusting for different treatment volumes.
Finally, EVAR technology has been evolving over time due to new device generations and improvements in surgical techniques such as crossover grafts (CitationMoise et al 2006). The recent review of EVAR alone studies described a decreasing rate of mortality over time (CitationFranks et al 2007). Reviews that ignore the potential impact of time trends on the relative rate of mortality or systemic events of EVAR over time may potentially introduce bias.
The objectives of this systematic literature review were to address issues left unanswered by earlier reviews, such as: 1) to describe and compare the patient characteristics from the identified observational and randomized controlled trials of EVAR and OSR patients; 2) to examine differences in outcome measures between the treatment groups by study design and characteristics; and 3) to evaluate relative trends in mortality and endoleak rates over time. More specifically, this report investigated the effects of baseline characteristics imbalance, study design (ie, nRCT versus RCT), center status (ie, multi-center versus single center), patient risk status, EVAR suitability, treatment volume (ie, low versus high), and year of study publication on patient outcomes.
Methods
Literature search
Literature search strategies were developed to identify papers comparing EVAR to OSR. Strategies for each of the following databases were developed and performed individually in OVID’s: MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Health and Psychosocial Instruments (HAPI), and Evidence-Based Medicine (EBM) Reviews (including Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club, Database of Abstracts of Reviews of Effects (DARE) and Cochrane Central Register of Controlled Trials (CCTR)). Results were limited to human and English language studies published between 1990 and November 2006 inclusive. Identification of duplicate citations was completed using Reference Manager (v.10; Thomson ResearchSoft, Philadelphia, PA, USA). RCTs, controlled clinical trials, comparative observational studies, case series studies, and population-based registries assessing the efficacy and/or safety of EVAR versus OSR were all included in the review.
Titles and abstracts (when available) of all search results were screened using predefined criteria in order to identify publications that discussed the use of EVAR and OSR for the management of AAA. When it could not be determined from the information available whether an item met the inclusion criteria or not, the article was reviewed in full-text.
Inclusion criteria included nonruptured AAA repair, of at least a mean AAA diameter of greater than 5 cm, and a publication date from 1990 onwards. Citations were excluded if they reported a mixed patient population including patients with thoracic-abdominal aneurysms, iliac aneurysms, ruptured or infected aorta, emergency aortic repair. No restriction based on clinical study design was used and nonrandomized trials and patient registries were included. All relevant or potentially relevant studies were then retrieved in full-text.
Review of full-text articles was conducted to identify publications with unique patient data using preset criteria. Also, clinical studies regarding the FDA-approved clinical trials related to the Ancure device (Guidant) were identified and excluded from further analysis due to the potential under-reporting of device related complications (CitationBren 2003).
Data extraction
For unique comparative clinical studies, data were abstracted using a standard form to record details of the study design and methods, patient baseline characteristics, technical aspects of EVAR and OSR and outcome measures of interest according to SVS reporting standards (CitationChaikof et al 2002).
Clinical outcomes were obtained from studies which described 30 day and post 30 day events. When longer term cumulative evidence was available (>30 days), 30 day events were not included in the analysis of the long term outcome data. Studies were classified as multi-center or single center. Where studies examined outcomes from a single center over a period of time greater than one year, annual treatment volume was estimated by dividing the number of patients in the study by the enrollment duration in years. Further, after determining the median surgical volume of institutions performing EVAR, surgical volume was divided into two categories: low volume institutions (identified as having, on average, less than 30 patients per year) and high volume institutions (completed 30 or more procedures per year).
Studies including only high-risk surgical patients were also identified. High-risk patients were classified as high-risk if any one of the following characteristics was present: age >80, ASA III or IV, or an existing systemic complication (cardiac, pulmonary, renal) (CitationHollier et al 1992) If available, further information was extracted from the studies regarding the suitability of patients who received OSR as to whether they could have received EVAR.
Statistical analysis
Statistical analysis was carried out using Excel 2002 (Microsoft Corp., Redmond, WA, USA) and Stata (StataCorp., College Station, TX, USA) with comparison of dichotomous outcomes expressed as odds ratios (OR). An OR less than 1 indicated a lower rate in EVAR than OSR. Continuous variables were analyzed using weighted mean differences (WMD). A WMD less than zero indicated a lower rate in EVAR than OSR. A level of significance (α) of 0.05 was used to indicate statistical significance.
Meta-regression analyses (including a random effect) were performed using Stata metareg command to test if there were differences in outcomes between EVAR and OSR with respect to study enrolment time. Where there were multi-year enrolment periods, the middle of the time period was used as the independent variable. Trends in comparative outcomes which were analyzed and included were operative mortality, systemic and vascular complications, and adjuvant procedures. Trends in EVAR specific outcomes such as the rate of endoleaks (I–IV) and conversion were also investigated. A level of significance (α) of 0.05 was used to indicate statistical significance in the trend coefficient.
Results
Literature search
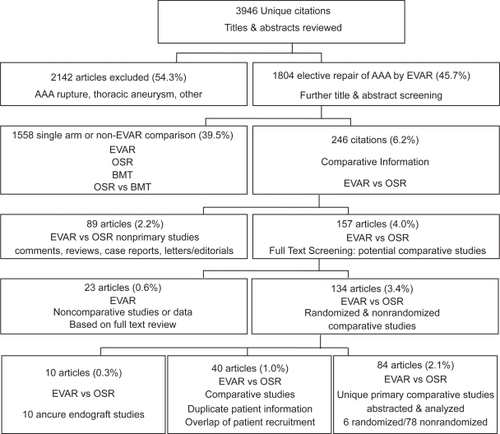
The literature search initially completed on 20 May 2005 and updated on 28 Nov 2006 identified a combined total of 3,946 unique citations (see ). The following evaluation is based on 84 comparative studies, containing unique patient data comparing EVAR to OSR.
Study characteristics
These 84 studies included 4 randomized controlled trials of which all 4 reported short-term outcomes (CitationCuypers et al 2001; CitationGreenhalgh et al 2004; CitationPrinssen et al 2004; CitationSoulez et al 2005) and 2 reported subsequent long-term outcomes (CitationBlankensteijn et al 2005; CitationGreenhalgh 2005). The other 78 comparative studies were observational evaluations (nRCT) (3 long term only) (CitationBirch et al 2000; CitationKibbe et al 2003; CitationLREAR 2005).
Overall, the identified studies contained data pertaining to 59,188 patients. In the 75 observational studies with perioperative outcomes reported (initial 30 days), there were 16,407 and 41,238 patients receiving EVAR and OSR, respectively. Of these observational studies, 16 were categorized as multi-center (CitationZarins et al 1999; CitationBeebe et al 2001; CitationCriado et al 2003; CitationMatsumura et al 2003; CitationAkkersdijk et al 2004; CitationAnderson et al 2004; CitationCarpenter and Endologix 2004; CitationGreenberg et al 2004; CitationForbes et al 2005; CitationHua et al 2005; CitationLeon et al 2005; CitationLREAR 2005; CitationBush et al 2006; CitationMendonca et al 2005; CitationSandridge et al 2006; CitationWald et al 2006). The other 59 papers were single center evaluations subdivided based on annual surgical volume of 30 cases per year, identified 31 low volume centers (Citationde Virgilio et al 1999; CitationKahn et al 1999; CitationSeiwert et al 1999; CitationTreharne et al 1999; CitationBecquemin et al 2000; CitationCohnert et al 2000; CitationMalina et al 2000; CitationSangiorgi et al 2001; CitationBerman et al 2002; CitationDavies et al 2002; CitationForbes et al 2002; CitationLigush et al 2002; CitationVan Sambeek et al 2002; CitationGawenda et al 2003; CitationHansman et al 2003; CitationPatel et al 2003; CitationTing et al 2003; CitationTurnipseed et al 2003; CitationBallard et al 2004; CitationGarcia-Madrid et al 2004; CitationZeebregts et al 2004; CitationAarts et al 2005; CitationBorchard et al 2005; CitationGoueffic et al 2005; CitationHayter et al 2005; CitationIannelli et al 2005; CitationRosenberg et al 2005; Citationde Donato et al 2006; CitationManis et al 2006; CitationVogel et al 2005; CitationParmer et al 2006), and 28 high volume centers (CitationBaxendale et al 1996; CitationDu Toit et al 1998; CitationCeelen et al 1999; CitationScharrer-Pamler et al 1999; CitationClair et al 2000; CitationGalle et al 2000; CitationOdegard et al 2000; CitationBertrand et al 2001; CitationMay et al 2001; CitationRowlands and Homer-Vanniasinkam 2001; CitationWijnen et al 2001; CitationCarpenter et al 2002; CitationTeufelsbauer et al 2002; CitationArko et al 2003; CitationDecker et al 2003; CitationDias et al 2003; CitationDryjski et al 2003; CitationJordan et al 2003; CitationJunnarkar et al 2003; CitationAho et al 2004; CitationAngle et al 2004; CitationCao et al 2004; CitationElkouri et al 2004; CitationPrault et al 2004; CitationWatson et al 2004; CitationMehta et al 2005; CitationEnglberger et al 2006; CitationPark et al 2006).
Five studies included information regarding patients that were surgically suitable for either procedure (CitationBecquemin et al 2000; CitationForbes et al 2002; CitationDias et al 2003; CitationGawenda et al 2003; CitationGarcia-Madrid et al 2004), 8 studies specifically examined the efficacy of high-risk patients only (CitationDu Toit et al 1998; CitationCarpenter et al 2002; CitationForbes et al 2002; CitationPatel et al 2003; CitationIannelli et al 2005; CitationMendonca et al 2005; Citationde Donato et al 2006; CitationParmer et al 2006) Long-term outcomes (>30 days) were reported in 25 observational studies with long term comparative outcomes of up to 3 years mean follow-up (CitationZarins et al 1999; CitationBecquemin et al 2000; CitationBirch et al 2000; CitationCohnert et al 2000; CitationBeebe et al 2001; CitationMay et al 2001; CitationSangiorgi et al 2001; CitationCarpenter et al 2002; CitationArko et al 2003; CitationCriado et al 2003; CitationDias et al 2003; CitationKibbe and Matsumura 2003; CitationTing et al 2003; CitationBallard et al 2004; CitationCao et al 2004; CitationElkouri et al 2004; CitationGarcia-Madrid et al 2004; CitationGreenberg et al 2004; CitationZeebregts et al 2004; CitationGoueffic et al 2005; CitationLREAR 2005; CitationMendonca et al 2005; CitationBush et al 2006; CitationCarpenter 2006; CitationParmer et al 2006).
Differences between the EVAR and OSR treatment groups for various baseline characteristics and estimates of short-term mortality, systemic complications, procedural outcomes, local/vascular complications, and EVAR specific outcomes are described below.
Differences between patient characteristics and relative outcomes are discussed in the following order: RCTs versus observational studies, multi-center versus single center observational studies, studies that have patients that are suitable for both procedures (EVAR and OSR), studies with only high risk patients, and finally high volume versus low volume single center evidence.
Patient characteristics
RCT vs observational studies
As shown in , patients enrolled in the EVAR and OSR arms of the 4 RCTs identified in this review had similar baseline characteristics except for pulmonary history (OR = 1.81, P < 0.05). In contrast, in nonrandomized studies, patients receiving EVAR were more likely to be male (OR 1.64), have higher ASA III and IV ratings (OR 1.33, 1.49, respectively), and less likely to be ASA I or II (OR 0.20, 0.72). In all observational studies, EVAR patients had a higher surgical risk with greater baseline comorbidities than in OSR patients.
Table 1 Difference in patient characteristics by study design and risk status
Multi-center vs single center observational studies
In single or multi-center institutions, patients receiving EVAR were more likely than patients receiving OSR to have comorbidities such as smoking history, diabetes, hyperlipidemia, cardiac disease, and peripheral vascular disease (PVD) and less likely to have hypertension (). No statistical difference existed for AAA diameter and age for multi-center or single center evidence. Within the single center evidence, more patients had higher level of ASA in EVAR than OSR (ASA I to IV: OR 0.20, 0.72, 1.33, 2.38). In patients that received EVAR, there were more males, a greater number of individuals with a smoking history, more patients with diabetes, cardiac disease, peripheral vascular disease, and less hypertension. Multi-center evidence included less patients receiving EVAR that have cardiovascular disease, pulmonary, or renal disease, while single center evidence reported the opposite. Fewer patients had stroke in EVAR in multi-center studies, and more had hyperlipidemia in EVAR in single center studies.
Studies with patients anatomically suitable for both EVAR and OSR
Only 5 studies stated that both EVAR and OSR patients were anatomically suitable for EVAR. While no statistical differences were found between AAA diameter and age, there was a much higher rate of ASA III and IV (OR 2.93, 12.34, respectively) in the EVAR patients than in the OSR patients as compared to the other study designs.
Studies with only high risk patients
When examining the baseline characteristics of the 8 studies that had high risk patients only, there were no statistical baseline difference in AAA diameter and age. As observed with EVAR studies reporting patient suitability, there were statistically significantly more ASA IV patients that received EVAR than in all nRCTs (OR 10.80).
High volume vs. low volume centers
The differences in baseline characteristics between the EVAR and OSR patients were still apparent when the single center studies are stratified based on their estimated procedural volume (). In institutions with high volume, patients receiving EVAR were also more likely than patients receiving OSR to have comorbidities such as smoking history, diabetes, hyperlipidemia, cardiac disease, and peripheral vascular disease (PVD) and less likely to have hypertension ().
Comparison of perioperative mortality and complications
RCT vs. observational studies
In the 75 nRCT studies, lower 30-day mortality existed in EVAR than OSR. The mortality difference reported in the nRCTs and in the RCTs was OR = 0.429 and 0.336, respectively (). Of all of the possible study designs and patient populations, RCTs reported the lowest OR for mortality (0.336). Within the published RCTs, there were no statistical differences between EVAR and OSR with respect to the occurrence of a MI, CHF, or arrhythmia, however, not all clinical outcomes were reported in the published papers (). In all designs lower rates of systemic complications were reported for all measures in EVAR treated patients compared to the OSR group, with the exception of limb ischemia which was higher in EVAR (OR 1.866).
Table 2 Mortality and systemic complications
Multi-center vs single center observational studies
Similar mortality results existed in multi-center versus single center evidence. In addition, the OR of all complications for both multi-center and single center studies were less than 1, indicating the benefit of EVAR vs OSR, in terms of all systemic complications (cardiac, pulmonary, renal, stroke, and ischemia). In general, the multi-center studies reported lower OR for EVAR vs OSR for cardiac and renal complications, similar OR for pulmonary complications. The evidence for stroke and ischemia was mixed.
Studies with patients anatomically suitable for both EVAR and OSR
No statistical difference between EVAR and OSR existed in mortality in anatomically suitable patients. This is important considering that the difference between EVAR and OSR baseline characteristics was the highest in this patient population. There was insufficient evidence to compare systemic complications.
Studies with only high risk patients
No statistical difference existed in mortality in high-risk patients, despite ASA IV patients being 10.80 times more likely to receive EVAR than OSR. Similarly, there were less complications in EVAR than OSR for cardiac (OR 0.076) and pulmonary complications (OR 0.075) ().
High volume vs. low volume centers
Similar differences in mortality existed in high volume versus low volume centers, when compared to all observational studies. In addition, there appeared to be little difference in systemic complications between high volume and low volume centers. This is despite high volume centers having a higher level of ASA IV patients, while low volume centers reported higher levels of other comomorbidities with the exception of pulmonary disease.
Surgical outcomes and local/vascular complications
Across all types of studies, and treatment volume risk status, surgical outcomes were better for EVAR in terms of OR time, blood loss, ICU length of stay (LOS), and total hospital, but these differences were not always statistically significant (,).
Table 3 Surgical outcomes
Table 4 Local/vascular complications
EVAR-specific outcomes
A meta-analysis of the studies reporting endoleak data is presented in .
Table 5 EVAR outcomes
Endoleaks
The rate of type 1 endoleaks was similar across study designs and patient populations, but was absent in the RCTs and highest in the suitable patient group. In contrast, the rate of type 2 endoleaks was lowest in the suitable patient group and in the RCTs.Type 3 and type 4 endoleaks, conversion, graft kinks, or folds are rare.
The rate of graft obstruction is high in the suitable patient group, and the rate of graft migration was highest in the high risk group.
Trends in endoleaks and ischemia over time
Examining the reported rate against the mid recruitment time of the study demonstrates that Type 1 endoleaks have been decreasing over time (). In comparison, the rate of type 2 endoleaks is relatively constant. Based on the study reports there has been a statistical increase in the rate of type 3 endoleaks over time (but this is the result of one outlier), and a decrease in the rate of type 4 endoleaks. Finally, the difference in the rate of limb ischemia has been decreasing over time (P < 0.01).
Table 6 Endoleaks and limb ischemia
In addition, when the trends in the difference in perioperative mortality were determined against the mid-enrolment time of the study, there was a small but statistically significant difference that persisted over time. The mortality difference has been changing at a rate of 0.066% per year (P < 0.001).
Mid-term mortality
No statistical difference in mid-term mortality was found between EVAR and OSR over time. This evidence was based on a total of 6,289 patients with EVAR and 3,547 who received OSR and were followed in comparative nRCT up to a maximum of 36 months. In RCTs, 914 patients received EVAR, and 784 patients received OSR with a mean follow-up of 28 months of follow-up (table not provided).
Discussion
In the question of EVAR versus OSR, the RCT evidence is limited. The enrolment period for DREAM was November 2000 to December 2003, and for EVAR 1 enrolment periods were September 1999 to December 2003. Based on this work and earlier work (CitationFranks et al 2007), this RCT evidence may be dated. Future evidence may come from an ongoing large multi-center trial, The Open Versus Endovascular Repair (OVER) Trial for Abdominal Aortic Aneurysms, which had an enrolment period, beginning in 2001 and ending in 2010. The recruitment period will be more up-to-date and thus, based on the results from this analysis, we should expect lower perioperative mortality rates in both EVAR and OSR, but similar mortality differences, as well as lower rates of endoleaks and ischemia. In the absence of more RCTs, observational data may provide the only current evidence.
There are some differences in evidence in nRCTs and RCTs. There are baseline imbalances that exist in nRCTs. In nRCTS, lower rates of outcomes are reported for all measures except limb ischemia which is higher in EVAR. A recent report has identified that there are only small differences between one large registry (Eurostar) and one large RCT (DREAM) (CitationLeurs et al 2007).
There are a number of large multi-center studies, including the Eurostar registry, Lifeline Registry for post device trial outcomes, and the large multi-center device trials. Also present are state, veteran affairs, and US national registries. In this study, care was taken to exclude the single center studies that make up the device trial evidence. There are some baseline differences between the multi-center and single center evidence. However, outcomes are mostly similar; the exceptions being that endoleaks (type 1 and type 2 endoleaks) are reportedly higher in single center studies.
The difference between low and high volume centers have been reported for other cardiac procedures (CitationNarayan et al 2004) and for AAA repair (CitationBush et al 2006). In this report, there were no differences in baseline differences in AAA diameter or age, and slightly more ASA IV patients in EVAR in low volume centers. Balancing this is that high volume centers had more patients receiving EVAR with smoking history, hyperlipidemia and other comorbidities. This translates into similar outcomes in low volume and high volume centers, with the exception of chronic renal failure being higher in EVAR for high volume centers.
Studies that have patients, who are suitable for both EVAR and OSR should have results similar to RCTs because of similar anatomical suitability. Collectively, the total number of these patients is the same as an RCT (larger than DREAM, smaller than EVAR 1). Mortality difference and cardiac, pulmonary and renal outcomes are similar to the RCTs. One key difference is the total length of hospital stay, which is much higher in EVAR in the nRCT suitable patients. This suggests that the differences in ASA III or IV lead to differences in OR times, ICU stay and hospital stay.
The RCTs and most nRCTs have a mixed population, with high risk and low risk patients. In this report, 8 studies that looked exclusively at high-risk patients were identified, These high risk patients incurred a lower rate of cardiac and pulmonary problems in EVAR than OSR. This suggests a significant benefit for high risk patients in terms of reduced systemic outcomes even when they are at a higher relative risk.
Long term data was analyzed to compare EVAR versus OSR for long term mortality. No statistical difference was found for up to 36 months. Almost all recent evidence has reported no significant difference in long term mortality (CitationDrury et al 2005; CitationHo et al 2006). Differences that existed earlier (CitationWalschot et al 2002) no longer exist.
Outcomes reported for EVAR have been improving over time and there are a number of reasons for this. First, there has been improvements in outcomes for EVAR surgical procedures through an evolving experience curve (CitationForbes et al 2004) preselecting suitable patients that would benefit from EVAR, or adjusting the device and perioperative adjuvant procedures to reduce later complications such as endoleaks and limb ischemia. Second, there have been improvements in the EVAR device almost eliminating type 3 or 4 endoleaks (device failures). Third, there has been an increased tendency to have lower risk patients receive EVAR (CitationAnderson et al 2004). In comparison, other studies have reported improving outcomes for patients that receive OSR (CitationBlack and Cambria 2006). This is the first study that demonstrates that the mortality risk difference is only slightly falling with time. This is supported by earlier comparative meta-analysis and recent comparative meta-analysis.
EVAR highlights the problem of performing a fixed point in time meta-analysis of a procedure that is continually evolving and technologically improving over time.
Disclosure
The authors report no conflicts of interest in this work.
References
- AartsFvan SterkenburgSBlankensteijnJD2005Endovascular aneurysm repair versus open aneurysm repair: comparison of treatment outcome and procedure-related reintervention rateAnn Vasc Surg1969970416075343
- AhoPSNiemiTLindgrenL2004Endovascular vs open AAA repair: Similar effects on renal proximal tubular functionScand J Surg9352615116821
- AkkersdijkGJMPrinssenMBlankensteijnJD2004The impact of endovascular treatment on in-hospital mortality following non-ruptured AAA repair over a decade: A population based study of 16,446 patientsEur J Vasc Endovasc Surg2841615177230
- AndersonPLAronsRRMoskowitzAJ2004A statewide experience with endovascular abdominal aortic aneurysm repair: Rapid diffusion with excellent early resultsJ Vasc Surg3910914718804
- AngleNDorafsharAHMooreWS2004Open versus endovascular repair of abdominal aortic aneurysms: What does each really cost?Ann Vasc Surg186121815534745
- ArkoFRHillBBReevesTR2003Early and late functional outcome assessments following endovascular and open aneurysm repairJ Endovasc Ther102912751922
- BallardJLAbou-ZamzamAMTeruyaTH2004Quality of life before and after endovascular and retroperitoneal abdominal aortic aneurysm repairJ Vasc Surg3979780315071445
- BaxendaleBRBakerDMHutchinsonA1996Haemodynamic and metabolic response to endovascular repair of infra-renal aortic aneurysmsBr J Anaesth7758158957971
- BecqueminJPBourriezAd’AudiffretA2000Mid-term results of endovascular versus open repair for abdominal aortic aneurysm in patients anatomically suitable for endovascular repairEur J Vasc Endovasc Surg196566110875781
- BeebeHGCronenwettJLKatzenBT2001Results of an aortic endograft trial: Impact of device failure beyond 12 monthsJ Vasc Surg33S55S6411174813
- BermanSSGenrileATBerensES2002Institutional economic losses associated with AAA repair are independent of techniqueJ Endovasc Ther9282812096941
- BertrandMGodetGKoskasF2001Endovascular treatment of abdominal aortic aneurysms: Is there a benefit regarding postoperative outcome?Eur J Anaesthesiol182455011350462
- BirchSEStaryDRScottAR2000Cost of endovascular versus open surgical repair of abdominal aortic aneurysmsAust N Z J Surg70660610976896
- BlackJHIIICambriaRP2006Current results of open surgical repair of descending thoracic aortic aneurysmsJ Vasc Surg43Suppl6A11A
- BlankensteijnJDde JongSEPrinssenMDutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group2005Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysmsN Engl J Med352239840515944424
- BorchardKLBirchSEHewittPM2005Endovascular abdominal aortic aneurysm repair: a 7 year experience at the Launceston General HospitalAust N Z J Surg753027
- BrenL2003Company caught in coverup of medical devicesFDA Consumer MagazineNov–Dec12
- BrewsterDCGellerSCKaufmanJA1998Initial experience with endovascular aneurysm repair: Comparison of early results with outcome of conventional open repairJ Vasc Surg2799210059652461
- BushRLJohnsonMLCollinsTC2006aOpen versus endovascular abdominal aortic aneurysm repair in VA hospitalsJ Am Coll Surg2025778716571424
- ButhJParodiJ1999EUROSTAR updateRound Table Series – Royal Society of Medicine138
- CaoPVerziniFParlaniG2004Clinical effect of abdominal aortic aneurysm endografting: 7-year concurrent comparison with open repairJ Vasc Surg40841815557895
- CarpenterJP2006The Powerlink bifurcated system for endovascular aortic aneurysm repair: four-year results of the US multicenter trialJ Cardiovasc Surg472394316760859
- CarpenterJPBaumRABarkerCF2002Durability of benefits of endovascular versus conventional abdominal aortic aneurysm repairJ Vasc Surg35222811854718
- CarpenterJPEndologixI2004Midterm results of the multicenter trial of the powerlink bifurcated system for endovascular aortic aneurysm repairJ Vasc Surg408495915557896
- CeelenWSonnevilleTRandonC1999Cost-benefit analysis of endovascular versus open abdominal aortic aneurysm treatmentActa Chirurgica Belgica9964710352734
- ChaikofELBlankensteijnJDHarrisPL2002Reporting standards for endovascular aortic aneurysm repairJ Vasc Surg3510486012021727
- ClairDGGrayBO’HaraPJ2000An evaluation of the costs to health care institutions of endovascular aortic aneurysm repairJ Vasc Surg321485210876217
- CohnertTUOelertFWahlersT2000Matched-pair analysis of conventional versus endoluminal AAA treatment outcomes during the initial phase of an aortic endograftingJ Endovasc Ther79410010821095
- CriadoFJFairmanRMBeckerGJ2003Talent LPS AAA stent graft: Results of a pivotal clinical trialJ Vasc Surg377091512663967
- CuypersPWGardienMButhJ2001Randomized study comparing cardiac response in endovascular and open abdominal aortic aneurysm repairBr J Surg8810596511488790
- DaviesMJArhanghelschiIGrauerR2002Anaesthesia for endoluminal repair of abdominal aortic aneurysmsAnaesth Intensive Care30667011939444
- de DonatoGChisciETrovatoR2006Open and endovascular treatment of abdominal aortic aneurysm in octogenarians: A single center approachItalian J Vasc Endovasc Surg137781
- de VirgilioCBuiHDonayreC1999Endovascular vs open abdominal aortic aneurysm repair: A comparison of cardiac morbidity and mortalityArch Surg1349475110487588
- DeckerDSpringerWDeckerP2003Changes in TH1/TH2 immunity after endovascular and conventional infrarenal aortic aneurysm repair: Its relevance for clinical practiceEur J Vasc Endovasc Surg252546112623338
- DiasNVIvancevKMalinaM2003Does the wide application of endovascular AAA repair affect the results of open surgery?Eur J Vasc Endovasc Surg261889412917837
- DruryDMichaelsJAJonesL2005Systematic review of recent evidence for the safety and efficacy of elective endovascular repair in the management of infrarenal abdominal aortic aneurysmBr J Surg89374616034817
- DryjskiMO’Brien-IrrMSHassettJ2003Hospital costs for endovascular and open repair of abdominal aortic aneurysmJ Am Coll Surg197647012831926
- Du ToitDFSaaimanADe BeerR1998Endovascular stent-graft repair of abdominal aortic aneurysms – Single- center experience and acute outcomeCardiovasc J S Afr88C273C81
- ElkouriSGloviczkiPMcKusickMA2004Perioperative complications and early outcome after endovascular and open surgical repair of abdominal aortic aneurysmsJ Vasc Surg3949750514981437
- EnglbergerLSavolainenHJandusP2006Activated coagulation during open and endovascular abdominal aortic aneurysm repairJ Vasc Surg431124916765226
- ForbesTLDeRoseGKribsS2002A cost-effectiveness analysis of standard versus endovascular abdominal aortic aneurysm repairCan J Surg45420412500916
- ForbesTLDeRoseGKribsSW2004Cumulative sum failure analysis of the learning curve with endovascular abdominal aortic aneurysm repairJ Vasc Surg39102814718826
- ForbesTLLawlorDKDeRoseG2005National audit of the recent utilization of endovascular abdominal aortic aneurysm repair in Canada: 2003 to 2004J Vasc Surg42410416171580
- FranksSCSuttonAJBownMJ2007Systematic review and meta-analysis of 12 years of endovascular abdominal aortic aneurysm repairEur J Vasc Endovasc Surg21547117166748
- GalleCDe MaertelaerVMotteS2000Early inflammatory response after elective abdominal aortic aneurysm repair: A comparison between endovascular procedure and conventional surgeryJ Vasc Surg322344610917982
- Garcia-MadridCJosaMRiambauV2004Endovascular versus open surgical repair of abdominal aortic aneurysm: A comparison of early and intermediate results in patients suitable for both techniquesEur J Vasc Endovasc Surg283657215350557
- GawendaMZaehringerMBrunkwallJ2003Open versus endovascular repair of para-anastomotic aneurysms in patients who were morphological candidates for endovascular treatmentJ Endovasc Ther107455114533968
- GouefficYBecqueminJ-PDesgrangesP2005Midterm survival after endovascular versus open repair of infrarenal aortic aneurysmsJ Endovasc Ther12475715683271
- GreenbergRKChuterTAMSternberghWCIII2004Zenith AAA endovascular graft: Intermediate-term results of the US multicenter trialJ Vasc Surg3912091815192559
- GreenhalghRM2005Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurym (EVAR trial 1): Randomised controlled trialLancet365217915978925
- GreenhalghRMBrownLCKwongGP2004Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: Randomised controlled trialLancet364843815351191
- HansmanMFNeuzilDQuigleyTM2003A comparison of 50 initial endoluminal endograft repairs for abdominal aortic aneurysm with 50 concurrent open repairsAm J Surg185441412727564
- HayterCLBradshawSRAllenRJ2005Follow-up costs increase the cost disparity between endovascular and open abdominal aortic aneurysm repairJ Vasc Surg429121816275447
- HoPYiuWKCheungGC2006Systematic review of clinical trials comparing open and endovascular treatment of abdominal aortic aneurysmSurg Pract12437
- HollierLHTaylorLMOchsnerJ1992Recommended indications for operative treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the Society for Vascular Surgery and the North American Chapter of the International Society for Cardiovascular SurgeryJ Vasc Surg151046561597887
- HuaHTCambriaRPChuangSK2005Early outcomes of endovascular versus open abdominal aortic aneurysm repair in the National Surgical Quality Improvement Program-Private Sector (NSQIP-PS)J Vasc Surg41382915838467
- IannelliGMonacoMDi TommasoL2005Endovascular vs. open surgery of abdominal aortic aneurysm in high-risk patients: a single center experienceThorac Cardiovasc Surg53291416208615
- JordanWDAlcocerFWirthlinDJ2003Abdominal aortic aneurysms in ‘high-risk’ surgical patients: Comparison of open and endovascular repairAnn Surg2376233012724628
- JunnarkarSLauLLEdreesWK2003Cytokine activation and intestinal mucosal and renal dysfunction are reduced in endovascular AAA repair compared to surgeryJ Endovasc Ther1019520212877599
- KahnRAMoskowitzDMManspeizerHE1999Endovascular aortic repair is associated with greater hemodynamic stability compared with open aortic reconstructionJ Cardiothorac Vasc Anesth1342610069283
- KibbeMRMatsumuraJS2003The Gore Excluder US Multi-Center Trial: Analysis of adverse events at 2 yearsSemin Vasc Surg161445012920685
- LeonLRJrLabropoulosNLaredoJ2005To what extent has endovascular aneurysm repair influenced abdominal aortic aneurysm management in the state of Illinois?J Vasc Surg415687415874918
- LeursLJButhJHarrisPL2007Impact of study design on outcome after endovascular abdominal aortic aneurysm repair. A comparison between the randomized controlled DREAM-trial and the observational EUROSTAR-registryEur J Vasc Endovasc Surg33172617097901
- [LREAR] Lifeline Registry of Endovascular Aneurysm Repair Steering Committee2001Lifeline Registry: collaborative evaluation of endovascular aneurysm repairJ Vasc Surg3411394611780606
- [LREAR] Lifeline Registry of EVAR Publications Committee2005Lifeline registry of endovascular aneurysm repair: long-term primary outcome measuresJ Vasc Surg4211016012445
- LigushJJPearceJDEdwardsMS2002Analysis of medical risk factors and outcomes in patients undergoing open versus endovascular abdominal aortic aneurysm repairJ Vasc Surg36492912218972
- MakarounMSChaikofENaslundT2002Efficacy of a bifurcated endograft versus open repair of abdominal aortic aneurysms: a reappraisalJ Vasc Surg352031011854716
- MalinaMNilssonMBrunkwallJ2000Quality of life before and after endovascular and open repair of asymptomatic AAAs: A prospective studyJ Endovasc Ther7372911032255
- ManisGFeuermanMHinesGL2006Open aneurysm repair in elderly patients not candidates for endovascular repair (EVAR): Comparison with patients undergoing EVAR or preferential open repairVasc Endovasc Surg4095101
- MatsumuraJSBrewsterDCMakarounMS2003A multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysmJ Vasc Surg372627112563194
- MayJWhiteGHWaughR2001Improved survival after endoluminal repair with second-generation prostheses compared with open repair in the treatment of abdominal aortic aneurysms: A 5-year concurrent comparison using life table methodJ Vasc Surg332 SupplS21S611174808
- MehtaMRoddySPDarlingRCIII2005Infrarenal abdominal aortic aneurysm repair via endovascular versus open retroperitoneal approachAnn Vasc Surg19374815735945
- MendoncaCTMoreiraRCRRibasTJr2005Comparison between open and endovascular treatment of abdominal aortic aneurysms in high surgical risk patientsJournal Vascular Brasileiro423242
- MoiseMAWooEYVelazquezOC2006Barriers to endovascular aortic aneurysm repair: past experience and implications for future device developmentVasc Endovasc Surg40197203
- MooreWSBrewsterDCBernhardVM2001Aorto-uni-iliac endograft for complex aortoiliac aneurysms compared with tube/bifurcation endografts: Results of the EVT/Guidant trialsJ Vasc Surg33Part 21120
- NarayanPCaputoMRogersCA2004Early and mid-term outcomes of surgery of the ascending aorta/arch: is there a relationship with caseload?Eur J Cardiothorac Surg256768215082266
- OdegardALundbomJMyhreHO2000The inflammatory response following treatment of abdominal aortic aneurysms: A comparison between open surgery and endovascular repairEur J Vasc Endovasc Surg195364410828237
- ParkBDanesSDreznerAD2006Endovascular abdominal aortic aneurysm repair at Hartford Hospital: A six year experienceConn Med703576216869465
- ParmerSSFairmanRMKarmacharyaJ2006A comparison of renal function between open and endovascular aneurysm repair in patients with baseline chronic renal insufficiencyJ Vasc Surg447061116930930
- ParodiJCPalmazJCBaroneHD1991Transfemoral intraluminal graft implantation for abdominal aortic aneurysmsAnn Vasc Surg549191837729
- PatelAPLanganEMIIITaylorSM2003An analysis of standard open and endovascular surgical repair of abdominal aortic aneurysms in octogenariansAm Surg69744714509320
- PraultTLStevensSLFreemanMB2004Open versus endo: Early experience with endovascular abdominal aortic aneurysm repair beyond the clinical trialsHeart Surg Forum7E4596115799925
- PrinssenMVerhoevenELButhJ2004A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysmsN Engl J Med35116071815483279
- RosenbergBLComstockMCButzDA2005Endovascular abdominal aortic aneurysm repair is more profitable than open repair based on contribution margin per daySurgery1372859215746778
- RowlandsTEHomer-VanniasinkamS2001Pro- and anti-inflammatory cytokine release in open versus endovascular repair of abdominal aortic aneurysmBr J Surg8813354011578287
- SandridgeLCBaglioniAJJrKongableGL2006Evaluation of the effect of endovascular options on infrarenal abdominal aortic aneurysm repairAm Surg72700416913313
- SangiorgiGD’AverioRMaurielloA2001Plasma levels of metalloproteinases-3 and -9 as markers of successful abdominal aortic aneurysm exclusion after endovascular graft treatmentCirculation10412 Suppli288i9511568071
- SbarigiaESpezialeFDucasseE2005What is the best management for abdominal aortic aneurysm in patients at high surgical risk? A single-center reviewInt Angiol2470415877002
- Scharrer-PamlerRKapferXOrendKH1999Endoluminal grafting of infrarenal aortic aneurysmsThorac Cardiovasc Surg471192110363612
- SeiwertAJWolfeJWhalenRC1999Cost comparison of aortic aneurysm endograft exclusion versus open surgical repairAm J Surg1781172010487261
- SicardGARubinBGSanchezLA2001Endoluminal graft repair for abdominal aortic aneurysms in high-risk patients and octogenarians: Is it better than open repair?Ann Surg2344273711573036
- SoulezGTherasseEMonfaredAA2005Pain and quality of life assessment after endovascular versus open repair of abdominal aortic aneurysms in patients at low riskJ Vasc Interv Radiol16109310016105921
- TeufelsbauerHPrusaAMWolffK2002Endovascular stent grafting versus open surgical operation in patients with infrarenal aortic aneurysms: A propensity score-adjusted analysisCirculation106782712176947
- TingACWChengSWKHoP2003Endovascular repair for abdominal aortic aneurysms: Initial experience of an endograft programmeAsian J Surg26172112527489
- TreharneGDThompsonMMWhiteleyMS1999Physiological comparison of open and endovascular aneurysm repairBr J Surg86760410383575
- TurnipseedWTeferaGCarrS2003Comparison of minimal incision aortic surgery with endovascular aortic repairAm J Surg1862879112946834
- Van SambeekMRHMvan DijkLCHendriksJM2002Endovascular versus conventional open repair of acute abdominal aortic aneurysm: Feasibility and preliminary resultsJ Endovasc Ther9443812223004
- VogelTRNackmanGBCrowleyJG2005Factors impacting functional health and resource utilization following abdominal aortic aneurysm repair by open and endovascular techniquesAnn Vasc Surg19641716075344
- WaldRWaikarSSLiangosO2006Acute renal failure after endovascular vs open repair of abdominal aortic aneurysmJ Vasc Surg43460616520155
- WalschotLHBLaheijRJFVerbeekALM2002Outcome after endovascular abdominal aortic aneurysm repair: A meta-analysisJ Endovasc Ther982911958330
- WatsonDRTanJWisemanL2004Challenges associated with the integration of endovascular repair of abdominal aortic aneurysms in a community hospitalHeart Surg Forum7E508E1315799935
- WelbornMBIIIYauFSModrallJG2005Endovascular repair of small abdominal aortic aneurysms: A paradigm shift?Vasc Endovasc Surg3938191
- WijnenMHCuypersPButhJ2001Differences in renal response between endovascular and open repair of abdominal aortic aneurysmsEur J Vasc Endovasc Surg21171411237792
- ZarinsCKWhiteRASchwartenD1999Aneurx stent graft versus open surgical repair of abdominal aortic aneurysms: Multicenter prospective clinical trialJ Vasc Surg292923089950987
- ZeebregtsCJGeelkerkenRHvan der PalenJ2004Outcome of abdominal aortic aneurysm repair in the era of endovascular treatmentBr J Surg91563815122606