Abstract
Endoplasmic reticulum (ER) stress is involved in the pathogenesis of several diseases including Alzheimer disease and Parkinson disease. Many recent studies have shown that ER stress is related to the pathogenesis of diabetes mellitus, and with the death of pancreatic β-cells, insulin resistance, and the death of the vascular cells in the retina. Diabetic retinopathy is a major complication of diabetes and results in death of both neural and vascular cells. Because the death of the neurons directly affects visual function, the precise mechanism causing the death of neurons in early diabetic retinopathy must be determined. The ideal therapy for preventing the onset and the progression of diabetic retinopathy would be to treat the factors involved with both the vascular and neuronal abnormalities in diabetic retinopathy. In this review, we present evidence that ER stress is involved in the death of both retinal neurons and vascular cells in diabetic eyes, and thus reducing or blocking ER stress may be a potential therapy for preventing the onset and the progression of diabetic retinopathy.
Introduction
Diabetic retinopathy is a major complication in patients with diabetes and it can lead to severe visual decrease in a high percentage of diabetic patients (CitationOshitari 2006; CitationOshitari and Roy 2007). Although the precise mechanism(s) for the onset and progression of diabetic retinopathy has still not been determined, recent studies have indicated that neuronal and vascular abnormalities are associated with the pathogenesis of early diabetic retinopathy (CitationBarber et al 1998; CitationTakano et al 1999; CitationAsnaghi et al 2003; CitationMartin et al 2004; CitationOshitari et al 2005). The neuronal abnormalities in the early stage of diabetic retinopathy are difficult to observe and evaluate by routine clinical tests, but ophthalmologists should consider neuronal abnormalities, including the death of retinal ganglion cell (RGCs), when evaluating eyes with diabetic retinopathy. This is important because the death of retinal neurons is irreversible and directly affects the visual function (CitationOshitari 2006; CitationOshitari and Roy 2007).
The endoplasmic reticulum (ER) is a critical intracellular organelle, which has several vital functions such as protein synthesis (CitationChevet et al 2001), protein transport (CitationPalade 1975), and acts as a reservoir of Ca2+ (CitationNielsen and Podolsky 1972). The accumulation of unfolded proteins or an upset in the Ca2+ homeostasis in the ER will activate the ER stress response, eg, the unfolded protein response (UPR) and the ER overload response (CitationRao et al 2004; CitationLindholm et al 2006). Most importantly, ER stress activates several cell death pathways including the caspase-12-dependent pathway (CitationNakagawa et al 2000), apoptosis signal-regulating kinase 1 (ASK1) – c-Jun N-terminal kinase (JNK) pathway (CitationNishitoh et al 2002) and the PKR-like endoplasmic reticulum kinase (PERK) – C/ERB homologous protein (CHOP) pathway (CitationMa et al 2002) ().
Figure 1 Hypothesized scheme of the ER stress-mediated cell death pathways. At least, three major cell death pathways are associated with ER stress-induced cell death.
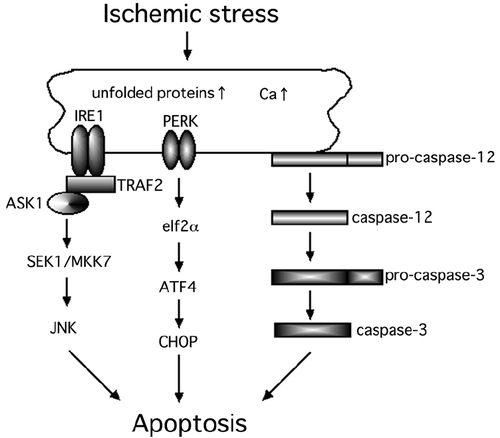
The results of recent studies have shown that ER stress-mediated cell death is associated with the death of pancreatic β-cells in patients with diabetes (CitationOyadomari et al 2001, Citation2002; CitationLaybutt et al 2007). In addition, a recent study reported that the ER stress-induced apoptosis is related to changes in the glucose concentration and results in the death of pericytes (CitationIkesugi et al 2006). CitationRoybal and colleagues (2004) suggested that the ER stress-mediated activating transcriptional factor 4 (ATF4) activation was associated with an over-expression of vascular endothelial growth factor (VEGF). In addition, recent studies indicate that ER stress-induced apoptosis is involved in the death of neurons in the brain and retina under different physiological conditions (CitationGuo et al 1997; CitationTobisawa et al 2003; CitationImai and Takahashi 2004; CitationLarner et al 2004; CitationTajiri et al 2004; CitationWootz et al 2004; CitationHayashi et al 2005; CitationAwai et al 2006; CitationShimazawa et al 2007).
One therapeutic strategy that might be used to prevent the development and progression of type 2 diabetes is the inhibition of ER stress. This would then block the ER stress-induced pancreatic β-cell death, and may also prevent the onset and progression of diabetic retinopathy. We shall discuss the possible role of ER stress in the pathogenesis of diabetic retinopathy and describe potential therapeutic strategies to block the development and the progression of diabetic retinopathy.
ER stress-mediated pancreatic β-cell death in diabetes
The ER is involved in the maintenance of cellular homeostasis by inducing the UPR. The UPR of mammals is mediated by at least three types of ER transmembrane proteins; IRE1 (protein kinase and site-specific endoribonuclease) (CitationTirasophon et al 1998; CitationWang et al 1998), PERK (CitationHarding et al 2000), and ATF6 (CitationYe et al 2000). Under diabetic conditions, the pancreatic β-cells are continuously exposed to oxidative stress, eg, exposure to reactive oxygen species (ROS; CitationKaneto et al 2005) and to nitrous oxide (NO; CitationOyadomari et al 2001). The ER is highly developed in pancreatic β-cells because of the continuous insulin secretion. Thus, it seems that even under physiological conditions, a potential ER stress is present because there are many unfolded proteins and premature proteins in the ER of pancreatic β-cells (CitationHarding et al 2001; CitationWeir et al 2001). These unfolded and premature proteins can easily become targets of ROS and NO (CitationOyadomari et al 2001; CitationKaneto et al 2005). Once the ER stress is increased in pancreatic β-cells, the JNK- and CHOP-mediated cell death pathways are activated (CitationOyadomari et al 2001, Citation2002; CitationKaneto et al 2005). The activation of the JNK pathway under diabetic stress is known to induce the serine phosphorylation of insulin receptor substance 1, which in turn leads to insulin resistance (CitationÖzcan et al 2004). When the number of pancreatic β-cell is decreased, ER stress is increased in the remaining pancreatic β-cells to compensate for the reduced insulin secretion leading to pancreatic β-cell dysfunction (CitationWeir et al 2001; CitationPoitout et al 2002). Thus, ER stress-mediated pancreatic β-cell death is critical and a key alteration for the pathogenesis of type 2 diabetes.
ER stress involved in vascular abnormalities in eyes with diabetic retinopathy
The loss of pericytes from the microvessels in diabetic retinas is one of the characteristic pathological changes in the early stage of diabetic retinopathy. The results of a recent study indicated that the UPR, activated by ER stress, is induced in retinal pericytes by the changes in the glucose concentration (CitationIkesugi et al 2006). Thus, ER stress-mediated cell death is the common pathology in the death of pancreatic β-cells and pericytes in diabetes.
VEGF plays important roles in the pathogenesis of diabetic retinopathy (CitationShweiki et al 1992; CitationAmin et al 1997; CitationLu et al 1998; CitationIshida et al 2000; CitationQaum et al 2001; CitationEl-Remessy et al 2003). The expression of VEGF is increased in diabetic retinas by the high-glucose, ischemia, and hypoxia, and this leads to the development of neovascularization and/or increased vascular permeability (CitationShweiki et al 1992; CitationAmin et al 1997; CitationLu et al 1998; CitationIshida et al 2000; CitationQaum et al 2001; CitationEl-Remessy et al 2003). Abcouwer et al showed that the glutamine deprivation-induced ER stress is associated with an up-regulation of VEGF expression in human retinal pigment epithelial cells (CitationAbcouwer et al 2002). CitationRoybal and colleagues (2004) suggested that the homocysteine-induced ER stress is related to the over-expression of VEGF under the control of ATF4. Hyperglycemia has been suggested to increase the intracellular homocysteine levels in the retinal pigment epithelium cells. Thus, there is good evidence that the diabetic stress-induced ER stress is involved in vascular abnormalities such as pericyte loss and neovascularization.
ER-stress-mediated neuronal cell death
Recent studies have shown that RGCs die at the early stage of diabetes (CitationBarber et al 1998; CitationAsnaghi et al 2003; CitationOshitari and Roy 2005). The neuronal abnormalities, such as RGC death, are irreversible and may precede the vascular abnormalities including the increased vascular permeability in diabetic retinas. This is observed even in retinas with a clinical diagnosis of non-diabetic retinopathy, however neuronal abnormalities, such as the reduction of retinal nerve fiber thickness, can be detected by optical coherence tomography even at this stage (CitationSugimoto et al 2005).
CitationGuo and colleagues (1997) suggest that an upset of the Ca2+ homeostasis in the ER caused by mutations in presenilin-1 is associated with the neuronal cell death in Alzheimer’s disease. Recently, many studies have reported that the ER stress-mediated neuronal cell death is related to the pathogenesis of various neuronal diseases in the brain and retina, eg, polyglutamine diseases (CitationNishito et al 2002), Parkinson’s disease (CitationImai and Takahashi 2004), amyotrophic lateral sclerosis (CitationTobisawa et al 2003; CitationWootz et al 2004), acute brain disorders (CitationLarner et al 2004; CitationTajiri et al 2004; CitationHayashi et al 2005), and retinal ischemia (CitationAwai et al 2006; CitationShimazawa et al 2007).
Other studies have shown that the PERK-CHOP pathway, one of the ER stress-mediated pathways that is induced in ischemic retinas, is related to neuronal cell death (CitationAwai et al 2006; CitationShimazawa et al 2007). It is well-known that under ischemic stress, an increase of intracellular Ca2+ level disturbs the ER Ca2+ homeostasis which in turn leads to ER stress-induced neuronal degeneration (CitationVerkhratsky and Toescu 2003). Unfolded proteins that accumulate in the ER in ischemic retinas become targets of ROS and NO. Thus, it seems reasonable that in ischemic retinas, the ER stress-mediated cell death pathways are related to the neuronal degeneration.
We have examined the IRE1-JNK pathway to determine if it is associated with the neuronal death in ischemic retinas using the ischemia-reperfusion injury model (unpublished data). Our results suggested that the expressions of IRE1α and tumor necrosis factor receptor-associated factor 2 (TRAF2) were significantly increased in the ischemic retinas compared to that in control retinas. In addition, we found that the expression of ASK1, SAPK/ERK kinase 1 (SEK1), and JNK were expressed in the same degenerating neurons of ischemic retinas (unpublished data). Thus, not only the PERK-CHOP pathway but also the IRE1-JNK pathway is associated with neuronal death in ischemic retinas.
The exact mechanism leading to the death of RGCs has not been conclusively determined especially at the early stage of diabetic retinopathy. One possible link between vascular abnormalities and neuronal abnormalities may be the changes in the glial cells at the early stage of diabetic retinopathy (). Glial processes make contact with both the blood vessels and neurons, and form the blood-retinal barrier (CitationKim et al 2006) (). Glial cells can maintain the blood-retinal barrier by expressing VEGF, and there are many studies that have shown interactions between glial cells and neuronal cells in the retina (CitationFruttiger et al 1996; CitationRauen et al 1999; CitationHarada et al 2000). During the onset and the progression of diabetic retinopathy, Müller cells are changed, eg, up-regulation of glial fibrillary acidic protein (GFAP) and accumulation of gamma-aminobutyric acid (CitationIshikawa et al 1996; CitationLieth et al 1998; CitationBarber et al 2000; CitationRungger-Brändle et al 2000). CitationPannicke and colleagues (2006) suggested that glial abnormalities, eg, swelling of the cell body, may lead to the retinal edema detected in diabetic retinas. Thus, neuro-glial interactions may be involved at the onset and the progression of diabetic retinopathy, and glial changes may be related to both neuronal and vascular abnormalities at the early stage of diabetic retinopathy ().
Figure 2 Hypothesized scheme of the pathogenesis of early diabetic retinopathy. The characteristic changes of early diabetic retinas may be glial changes, which in turn leads to vascular and neuronal abnormalities such as increased vascular permeability or neuronal cell death. The ideal therapies for diabetic retinopathy may be the improvement of both vascular and neuronal abnormalities.
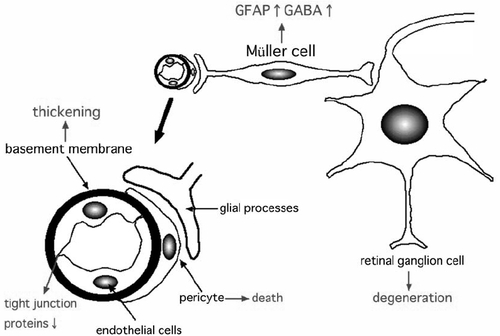
The glutamate levels are known to be elevated in the vitreous of diabetic patients (CitationAmbati et al 1997), which could lead to neuronal cell death. Because an over-stimulation of neurons by glutamate upsets the Ca2+ homeostasis in the ER, ER stress may be present in degenerating neurons under diabetic stress.
Because there is very little evidence of a direct association of ER stress and the pathogenesis of diabetic retinopathy, ER stress may be an epiphenomenon and/or only one of the players, perhaps with a minor role, in the development of diabetic retinopathy. Thus, although ER-stress-related factors may be promising targets for the prevention and the progression of diabetic retinopathy, additional studies are needed to determine the relationship between ER stress and neuronal cell death in diabetic retinas.
Potential therapeutic strategies for diabetic retinopathy
An epidemiological study performed by the Japanese Ministry of Welfare in 2005 showed that diabetic retinopathy was the second most common eye disease to cause blindness in the Japanese. Over 3,000 patients with diabetic retinopathy lose their vision each year in Japan. This indicates that the current management and therapies for diabetic retinopathy are not sufficient to prevent the progression to blindness in patients with diabetic retinopathy. To reduce the number of patients who lose their vision from diabetic retinopathy, new therapeutic strategies must be established to prevent the onset and the progression of the diabetic retinopathy.
At present, the standard treatments for diabetic retinopathy include: control of the blood glucose levels and the blood pressure (CitationKlein et al 1995; CitationDiabetes Control and Complications Trial Research Group 1995a, Citation1995b; CitationUK Prospective Diabetes Study (UKPDS) Group 1998; CitationDiabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group 2000; CitationWriting Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group 2002; CitationMatthews et al 2004), focal laser photocoagulation (CitationEarly Treatment Diabetic Retinopathy Study Research Group 1985, Citation1987, Citation1991a), pan-retinal laser photocoagulation (CitationDiabetic Retinopathy Study Research Group 1978, Citation1981), and early vitrectomy (CitationDiabetic Retinopathy Vitrectomy Study Research Group 1985a, Citation1985b, Citation1988a, Citation1988b, Citation1990). Although the diabetic retinopathy continues to progress in some patients in spite of good control of blood glucose and blood pressure, a tight control of blood glucose levels and blood pressure is the first choice for the treatment of diabetic retinopathy.
Focal laser treatment significantly decreased the risk of visual disturbances in diabetic patients with macular edema. However, side effects, eg, foveal burns, central visual field defects and retinal fibrosis, are not uncommon. Pan-retinal photocoagulation also has many side effects such as visual field constriction, night blindness, and exacerbation of macular edema. However, pan-retinal laser photocoagulation significantly decreased the risk of visual disturbances in diabetic patients with severe non-proliferative and proliferative retinopathy. Early vitrectomy reduced the risk of visual disturbances in diabetic patients with proliferative retinopathy and vitreous hemorrhage. Again, there are many side effects of vitrectomy including vitreous hemorrhage, retinal detachment, neovascular glaucoma, and infection.
Intravitreal or sub-Tenon injections of triamcinolone acetonide (TA) has been recently used to treat the macular edema common in diabetic patients, and during the early post-TA period, there were improvements in both the macular edema and visual acuity (CitationMartidis et al 2002; CitationAvitabile et al 2005; CitationSorbin and D’Amico 2005; CitationJonas et al 2006a). Steroids are known to up-regulate the expression of tight junctions proteins, eg, occludin and ZO-1, in endothelial cells, and this increase reduce the increased vascular permeability in the retina (CitationAntonetti et al 2002). Unfortunately, repeated injections of TA are frequently required, and there are many side effects such as increased intraocular pressure, cataracts, and infections (CitationGillies et al 2004; CitationJonas et al 2005, Citation2006b; CitationWestfall et al 2005). Although long-term follow-up studies of TA must be made, the treatments by TA may be considered together with laser treatments and vitrectomy in diabetic patients with severe macular edema.
There are many medical therapies that are being tried to prevent the development and progression of diabetic retinopathy, eg, aspirin (CitationEarly Treatment Diabetic Retinopathy Study Research Group 1991b; CitationChew et al 1995), anti-VEGF agents (CitationCunningham et al 2005; CitationAvery et al 2006; CitationChun et al 2006; CitationSpaide et al 2006), protein kinase C inhibitors (CitationPKC-DRS Study Group 2005, Citation2006; CitationPKC-DMES Study Group 2007), growth hormone inhibitors (CitationKirkegaard et al 1990), and aldose reductase inhibitors (CitationSorbinil Retinopathy Trial Research Group 1990). However, CitationMohamed and colleagues (2007) stated that these treatments cannot be recommended for routine use because evidence to support their use has not been published.
The most important factor to consider in the management and the treatment of diabetic retinopathy is the protection of visual function. Because the onset and progression of neuronal and vascular abnomralities lead to visual dysfunction, the targets of the therapeutic methods should be the factors that are common to both vascular and neuronal abnormalities of diabetic retinopathy. Thus, we have stated in an earlier review article that two of the candidates common to both vascular and neuronal abnormalities in diabetic retinopathy are tumor necrosis factor-alpha and Bax (CitationOshitari 2006). ER stress-related factors should also be considered as targets of new therapeutic strategies for diabetic retinopathy as well as the targets of new therapies for type 2 diabetes because ER stress is also related to pancreatic β-cell death and insulin resistance in patients with type 2 diabetes.
The results of a recent study showed that oral administration of chemical chaperones, 4-phenyl butyric acid or taurine-conjugated ursodeoxycholic acid, alleviated ER stress and improved the action of systemic insulin in diabetic animals (CitationÖzcan et al 2006). Although the precise mechanism involved in the improvement of type 2 diabetes is unclear, these chemical chaperones may stabilize protein conformation and improve the folding capacity of the ER, which in turn would reduce the ER stress in these diabetic animals. These chemical chaperones may become a standard treatment for type 2 diabetes because of their safety profiles in vivo (CitationMaestri et al 1996; CitationKaplan and Gershwin 2005).
Recently, a Bax inhibitor-1 (BI-1) was identified to be an anti-apoptotic protein in mammals (CitationChae et al 2003), and this is relevant because BI-1 can regulate a cell death pathway involved in ER stress (CitationChae et al 2004). In addition, an over-expression of BI-1 can protect against the neuronal cell death induced by ER stress (CitationDohm et al 2006). Although the mechanism for this protective effect was not determined, a recent study showed that BI-1 can inhibit ER stress proteins such as CHOP, IRE1α or phospho-JNK (CitationLee et al 2007). The activity of BI-1 may provide clues on developing new ways to regulate the ER stress-induced apoptosis. For example, brain-derived neurotrophic factor (BDNF), which is known to reduce the neuronal degeneration of diabetic retinas in vivo (CitationSeki et al 2004), prevents ER stress-mediated neuronal cell death by suppressing caspase-12 activation in vitro (CitationShimoke et al 2004).
Although these neuroprotective therapies may be promising, the prevention of neuronal cell death under chronic diabetic stress may have limitations. Even if one major cell death pathway is blocked, another cell death pathway may become activated. Even when apoptosis can be blocked, other types of cell death such as necrosis or autophagy may be induced (CitationKoh et al 1995; CitationHartmann et al 2001; CitationVandenabeele et al 2006). Thus, even if we can establish neuroprotective therapies for diabetic retinopathy, the first choice of the treatment for diabetic retinopathy must still be the standard treatment of controlling blood glucose levels and blood pressure to reduce the causes of diabetic stress.
In conclusion, ER stress is involved in the pathogenesis of type 2 diabetes and diabetic retinopathy. The reduction of ER stress by chemical chaperones such as 4-phenyl butyric acid or taurine-conjugated ursodeoxycholic acid may become one of the standard treatments for type 2 diabetes. Such treatments may be helpful in preventing the development of diabetic retinopathy. Additional studies are required to determine the optimal methods to reduce ER stress in patients with diabetic retinopathy.
Acknowledgements
This study was supported by the grant from The Eye Research Foundation for the Aged. We thank Prof. Duco Hamasaki for editing this manuscript.
References
- AbcouwerSFMarjonPLLoperRKResponse of VEGF expression to amino acid deprivation and inducers of endoplasmic reticulum stressInvest Ophthalmol Vis Sci2002432791812147617
- AmbatiJChalamKVChawlaDKElevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathyArch Ophthalmol1997115116169298058
- AminRHFrankRNKennedyAVascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathyInvest Ophthalmol Vis Sci19973836479008628
- AntonettiDAWolpertEBDeMaioLHydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludinJ Neurochem2002806677711841574
- AsnaghiVGerhardingerCHoehnTA role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the ratDiabetes2003525061112540628
- AveryRLPearlmanJPieramiciDJIntravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathyOphthalmology2006113169517011951
- AvitabileTLongoAReibaldiAIntravitreal triamcinolone compared with macular laser grid photocoagulation for the treatment of cystoid macular edemaAm J Ophthalmol200514069570216226521
- AwaiMKogaTInomataYNMDA-induced retinal injury is mediated by an endoplasmic reticulum stress-related protein, CHOP/GADD153J Neurochem200696435216269013
- BarberAJAntonettiDAGardnerTWAltered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research GroupInvest Ophthalmol Vis Sci2000413561811006253
- BarberAJLiethEKhinSANeural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulinJ Clin Invest1998102783919710447
- ChaeHJKeNKimHREvolutionarily conserved cytoprotection provided by Bax Inhibitor-1 homologs from animals, plants, and yeastGene20033231011314659883
- ChaeHJKimHRXuCBI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stressMol Cell2004153556615304216
- ChevetECameronPHPelletierMFThe endoplasmic reticulum: integration of protein folding, quality control, signaling and degradationCurr Opin Struct Biol200111120411179901
- ChewEYKleinMLMurphyRPEffects of aspirin on vitreous/preretinal hemorrhage in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report no. 20Arch Ophthalmol19951135257826294
- ChunDWHeierJSToppingTMA pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edemaOphthalmology200611317061217011952
- CunninghamETJrAdamisAPAltaweelMA phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edemaOphthalmology200511217475716154196
- Diabetes Control and Complications Trial Research GroupProgression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications TrialOphthalmology1995a10264761
- Diabetes Control and Complications Trial Research GroupThe relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trialDiabetes1995b4496883
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research GroupRetinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapyN Engl J Med2000342381910666428
- Diabetic Retinopathy Study Research GroupPhotocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findingsOphthalmology19788582106345173
- Diabetic Retinopathy Study Research GroupPhotocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8Ophthalmology1981885836007196564
- Diabetic Retinopathy Vitrectomy Study Research GroupTwo-year course of visual acuity in severe proliferative diabetic retinopathy with conventional management. Diabetic Retinopathy Vitrectomy Study (DRVS) report #1Ophthalmology1985a92492502
- Diabetic Retinopathy Vitrectomy Study Research GroupEarly vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. The Diabetic Retinopathy Vitrectomy Study Research GroupArch Ophthalmol1985b103164452
- Diabetic Retinopathy Vitrectomy Study Research GroupEarly vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Results of a randomized trial – Diabetic Retinopathy Vitrectomy Study Report 3. The Diabetic Retinopathy Vitrectomy Study Research GroupOphthalmology1988a95130720
- Diabetic Retinopathy Vitrectomy Study Research GroupEarly vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Clinical application of results of a randomized trial – Diabetic Retinopathy Vitrectomy Study Report 4. The Diabetic Retinopathy Vitrectomy Study Research GroupOphthalmology1988b95132134
- Diabetic Retinopathy Vitrectomy Study Research GroupEarly vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Four-year results of a randomized trial: Diabetic Retinopathy Vitrectomy Study Report 5Arch Ophthalmol1990108958642196036
- DohmCPSiedenbergSLimanJBax inhibitor-1 protects neurons from oxygen-glucose deprivationJ Mol Neurosci2006291816757804
- Early Treatment Diabetic Retinopathy Study Research GroupPhotocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1Arch Ophthalmol198510317968062866759
- Early Treatment Diabetic Retinopathy Study Research GroupTreatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2Ophthalmology198794761743658348
- Early Treatment Diabetic Retinopathy Study Research GroupEarly Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7Ophthalmology1991a9874156
- Early Treatment Diabetic Retinopathy Study Research GroupEffects of aspirin treatment on diabetic retinopathy. ETDRS report number 8Ophthalmology1991b9875765
- El-RemessyABBehzadianMAAbou-MohamedGExperimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptorAm J Pathol20031621995200412759255
- FruttigerMCalverARKrugerWHPDGF mediates a neuron-astrocyte interaction in the developing retinaNeuron1996171117318982160
- GilliesMCSimpsonJMBillsonFASafety of an intravitreal injection of triamcinolone: results from a randomized clinical trialArch Ophthalmol20041223364015006845
- GuoQSopherBLFurukawaKAlzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicalsJ Neurosci1997174212229151738
- HaradaTHaradaCNakayamaNModification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degenerationNeuron2000265334110839371
- HardingHPZhangYBertolottiAPerk is essential for translational regulation and cell survival during the unfolded protein responseMol Cell2000589790410882126
- HardingHPZengHZhangYDiabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survivalMol Cell2001711536311430819
- HartmannATroadecJDHunotSCaspase-8 is an effector in apoptotic death of dopaminergic neurons in Parkinson’s disease, but pathway inhibition results in neuronal necrosisJ Neurosci20012122475511264300
- HayashiTSaitoAOkunoSDamage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neuronsJ Cereb Blood Flow Metab200525415315678111
- IkesugiKMulhernMLMadsonCJInduction of endoplasmic reticulum stress in retinal pericytes by glucose deprivationCurr Eye Res2006319475317114120
- ImaiYTakahashiRHow do Parkin mutations result in neurodegeneration?Curr Opin Neurobiol200414384915194120
- IshidaSShinodaKKawashimaSCoexpression of VEGF receptors VEGF-R2 and neuropilin-1 in proliferative diabetic retinopathyInvest Ophthalmol Vis Sci20004116495610845581
- IshikawaAIshiguroSTamaiMAccumulation of gamma-amino-butyric acid in diabetic rat retinal Muller cells evidenced by electron microscopic immunocytochemistryCurr Eye Res199615958648921217
- JonasJBDegenringRFKreissigIIntraocular pressure elevation after intravitreal triamcinolone acetonide injectionOphthalmology2005112593815808249
- JonasJBKamppeterBAHarderBIntravitreal triamcinolone acetonide for diabetic macular edema: a prospective, randomized studyJ Ocul Pharmacol Ther2006a22200716808682
- JonasJBKreissigISpandauUHInfectious and noninfectious endophthalmitis after intravitreal high-dosage triamcinolone acetonideAm J Ophthalmol2006b1415798016490517
- KanetoHKawamoriDMatsuokaTAOxidative stress and pancreatic beta-cell dysfunctionAm J Ther2005125293316280646
- KaplanMMGershwinMEPrimary biliary cirrhosisN Engl J Med200535312617316177252
- KimJHKimJHParkJABlood-neural barrier: intercellular communication at glio-vascular interfaceJ Biochem Mol Biol2006393394516889675
- KirkegaardCNφrgaardKSnorgaardOEffect of one year continuous subcutaneous infusion of a somatostatin analogue, octreotide, on early retinopathy, metabolic control and thyroid function in Type I (insulin-dependent) diabetes mellitusActa Endocrinol (Copenh)1990122766722197845
- KleinBEKleinRMossSEA cohort study of the relationship of diabetic retinopathy to blood pressureArch Ophthalmol199511360167748130
- KohJYGwagBJLobnerDPotentiated necrosis of cultured cortical neurons by neurotrophinsScience199526857357725105
- LarnerSFHayesRLMcKinseyDMIncreased expression and processing of caspase-12 after traumatic brain injury in ratsJ Neurochem200488789014675152
- LaybuttDRPrestonAMAkerfeldtMCEndoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetesDiabetologia2007507526317268797
- LeeGHKimHKChaeSWBax inhibitor-1 regulates endoplasmic reticulum stress-associated reactive oxygen species and heme oxygenase-1 expressionJ Biol Chem2007282216182817526500
- LiethEBarberAJXuBGlial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research GroupDiabetes199847815209588455
- LindholmDWootzHKorhonenLER stress and neurodegenerative diseasesCell Death Differ2006133859216397584
- LuMKurokiMAmanoSAdvanced glycation end products increase retinal vascular endothelial growth factor expressionJ Clin Invest19981011219249502762
- MaYBrewerJWDiehlJATwo distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein responseMol Biol2002318135165
- MaestriNEBrusilowSWClissoldDBLong-term treatment of girls with ornithine transcarbamylase deficiencyN Engl J Med199633585598778603
- MartidisADukerJSGreenbergPBIntravitreal triamcinolone for refractory diabetic macular edemaOphthalmology2002109920711986098
- MartinPMRoonPVan EllsTKDeath of retinal neurons in streptozotocin-induced diabetic miceInvest Ophthalmol Vis Sci2004453330615326158
- MatthewsDRStrattonIMAldingtonSJRisks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69Arch Ophthalmol200412216314015534123
- MohamedQGilliesMCWongTYManagement of diabetic retinopathy: a systematic reviewJAMA20072989021617712074
- NakagawaTZhuHMorishimaNCaspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-betaNature20004039810310638761
- NielsenSPPetersenOHTransport of calcium in the perfused submandibular gland of the catJ Physiol1972223685975045737
- NishitohHMatsuzawaATobiumeKASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeatsGenes Dev20021613455512050113
- OshitariTNon-viral gene therapy for diabetic retinopathyDrug Dev Res20066783541
- OshitariTRoySDiabetes: a potential enhancer of retinal injury in rat retinasNeurosci Lett2005390253016154273
- OshitariTRoySCommon therapeutic strategies for diabetic retinopathy and glaucomaCurr Drug Ther2007222432
- OyadomariSKoizumiATakedaKTargeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetesJ Clin Invest20021095253211854325
- OyadomariSTakedaKTakiguchiMNitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathwayProc Natl Acad Sci USA200198108455011526215
- ÖzcanUCaoQYilmazEEndoplasmic reticulum stress links obesity, insulin action, and type 2 diabetesScience20043064576115486293
- ÖzcanUYilmazEÖzcanLChemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetesScience200631311374016931765
- PaladeGIntracellular aspects of the process of protein synthesisScience197518986717812524
- PannickeTIandievIWurmADiabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retinaDiabetes200655633916505225
- PKC-DMES Study GroupEffect of ruboxistaurin in patients with diabetic macular edema: thirty-month results of the randomized PKC-DMES clinical trialArch Ophthalmol20071253182417353401
- PKC-DRS Study GroupThe effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trialDiabetes20055421889715983221
- PKC-DRS2 GroupAielloLPDavisMDEffect of ruboxistaurin on visual loss in patients with diabetic retinopathyOphthalmology200611322213016989901
- PoitoutVRobertsonRPMinireview: Secondary beta-cell failure in type 2 diabetes – a convergence of glucotoxicity and lipotoxicityEndocrinology20021433394211796484
- QaumTXuQJoussenAMVEGF-initiated blood-retinal barrier breakdown in early diabetesInvest Ophthalmol Vis Sci20014224081311527957
- RaoRVEllerbyHMBredesenDECoupling endoplasmic reticulum stress to the cell death programCell Death Differ2004113728014765132
- RauenTFischerFWiessnerMGlia-neuron interaction by high-affinity glutamate transporters in neurotransmissionAdv Exp Med Biol1999468819510635021
- RoybalCNYangSSunCWHomocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4J Biol Chem2004279148445214747470
- Rungger-BrändleEDossoAALeuenbergerPMGlial reactivity, an early feature of diabetic retinopathyInvest Ophthalmol Vis Sci20004119718010845624
- SekiMTanakaTNawaHInvolvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neurotrophic factor for dopaminergic amacrine cellsDiabetes2004532412915331553
- ShimazawaMInokuchiYItoYInvolvement of ER stress in retinal cell deathMol Vis2007135788717438523
- ShimokeKUtsumiTKishiSPrevention of endoplasmic reticulum stress-induced cell death by brain-derived neurotrophic factor in cultured cerebral cortical neuronsBrain Res200410281051115518647
- ShweikiDItinASofferDVascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesisNature199235984351279431
- SobrinLD’AmicoDJControversies in intravitreal triamcinolone acetonide useInt Ophthalmol Clin2005451334116199972
- Sorbinil Retinopathy Trial Research GroupA randomized trial of sorbinil, an aldose reductase inhibitor, in diabetic retinopathyArch Ophthalmol19901081234442119168
- SpaideRFFisherYLIntravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhageRetina200626275816508426
- SugimotoMSasohMIdoMDetection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathyOphthalmologica20052193798516286799
- TakanoMSangoKHorieHDiabetes alters neurite regeneration from mouse retinal explants in cultureNeurosci Lett1999275175810580703
- TajiriSOyadomariSYanoSIschemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOPCell Death Differ2004114031514752508
- TirasophonWWelihindaAAKaufmanRJA stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cellsGenes Dev1998121812249637683
- TobisawaSHozumiYArawakaSMutant SOD1 linked to familial amyotrophic lateral sclerosis, but not wild-type SOD1, induces ER stress in COS7 cells and transgenic miceBiochem Biophys Res Commun200330349650312659845
- UK Prospective Diabetes Study (UKPDS) GroupIntensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33)Lancet1998352837539742976
- VandenabeelePVanden BergheTFestjensNCaspase inhibitors promote alternative cell death pathwaysSci STKE200635844
- VerkhratskyAToescuECEndoplasmic reticulum Ca2+ homeostasis and neuronal deathJ Cell Mol Med200373516114754504
- WangXZHardingHPZhangYCloning of mammalian Ire1 reveals diversity in the ER stress responsesEMBO J1998175708179755171
- WeirGCLaybuttDRKanetoHBeta-cell adaptation and decompensation during the progression of diabetesDiabetes200150S154911272180
- WestfallACOsbornAKuhlDAcute endophthalmitis incidence: intravitreal triamcinoloneArch Ophthalmol20051231075716087840
- WootzHHanssonIKorhonenLCaspase-12 cleavage and increased oxidative stress during motoneuron degeneration in transgenic mouse model of ALSBiochem Biophys Res Commun2004322281615313203
- Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research GroupEffect of intensive therapy on the microvascular complications of type 1 diabetes mellitusJAMA20022872563912020338
- YeJRawsonRBKomuroRER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPsMol Cell2000613556411163209