Abstract
A score system integrating the evolution of efficacy and tolerability over time was applied to a subpopulation of the STRATHE trial, a trial performed according to a parallel group design, with a double-blind, random allocation to either a fixed-dose combination strategy (perindopril/indapamide 2 mg/0.625 mg, with the possibility to increase the dose to 3 mg/0.935 mg, and 4 mg/1.250 mg if needed, n = 118), a sequential monotherapy approach (atenolol 50 mg, followed by losartan 50 mg and amlodipine 5 mg if needed, n = 108), or a stepped-care strategy (valsartan 40 mg, followed by valsartan 80 mg and valsartan 80 mg+ hydrochlorothiazide 12.5 mg if needed, n = 103). The aim was to lower blood pressure below 140/90 mmHg within a 9-month period. The treatment could be adjusted after 3 and 6 months. Only patients in whom the study protocol was strictly applied were included in this analysis. At completion of the trial the total score averaged 13.1 ± 70.5 (mean ± SD) using the fixed-dose combination strategy, compared with −7.2 ± 81.0 using the sequential monotherapy approach and −17.5 ± 76.4 using the stepped-care strategy. In conclusion, the use of a score system allows the comparison of antihypertensive therapeutic strategies, taking into account at the same time efficacy and tolerability. In the STRATHE trial the best results were observed with the fixed-dose combination containing low doses of an angiotensin enzyme converting inhibitor (perindopril) and a diuretic (indapamide).
Introduction
During the last few decades numerous clinical trials have been performed in the field of hypertension. These trials were required for the development of new antihypertensive agents and to demonstrate their efficacy and tolerability in comparison with placebo or established blood-pressure-lowering agents. Pharmacological treatment of hypertension has been shown over the years to decrease significantly morbidity and mortality due to cardiovascular and renal diseases (CitationStaessen et al 2003; CitationTurnbull et al 2005; CitationWilliams 2005). Today it appears critical to achieve strict blood pressure control in hypertensive patients in order to provide maximum benefit from blood pressure lowering, and current guidelines recommend bringing blood pressure below 140/90 mmHg in every patient, and even below 130/80 mmHg if high blood pressure co-exists with diabetes mellitus and/or renal disease (CitationChobanian et al 2003; CitationMancia et al 2007). Different therapeutic strategies are available to reach these goal blood pressures, but increasing evidence indicates that the combination of two or more drugs is needed in most patients (CitationBrunner et al 1990; CitationDusing 2006). In terms of pharmacological treatment of hypertension, the ideal drug regimen should be effective, well tolerated, and easy to take every day, thereby facilitating long-term adherence. To what extent a given treatment fulfills these criteria remains, however, difficult to assess in clinical trials.
The present study was aimed to evaluate whether a score system integrating the evolution of efficacy and tolerability over time would enable a better characterization of advantages and disadvantages of antihypertensive drug regimens. To this end we created a score system and applied it to the STRATHE trial, a parallel group design trial which compared in double-blind fashion different therapeutic strategies (CitationMourad et al 2004).
Patients and methods
The STRATHE study was performed in France by 193 community physicians in 533 patients with a mean sitting systolic blood pressure ≥160 mmHg and/or a mean diastolic blood pressure ≥95 mmHg after a 4-week single-blind placebo run-in. After randomization to a fixed low-dose combination (perindopril/indapamide, 2 mg/0.625 mg, increased if required first to 3 mg/0.937 mg and later to 4 mg/1.25 mg) (n = 180 ), sequential monotherapy (atenolol 50 mg, followed as needed first by losartan 50 mg, and then by amlodipine 5 mg) (n = 176 ), or stepped care (valsartan 40 mg, increased if necessary first to valsartan 80 mg, with the possibility to combine then valsartan 80 mg and hydrochlorothiazide, 12.5 mg (n = 177) (CitationMourad et al 2004). In each study arm the aim was to lower blood pressure below 140/90 mmHg. To this end, the treatment could be adjusted as described above after 3 and 6 months of therapy. The final visit was planned after 9 months of treatment, or at 6 months if target blood pressure was achieved. Upgrading to a superior level was recommended when systolic blood pressure was ≥140 and ≤160 mmHg and/or diastolic blood pressure ≥90 mmHg, and ≤95 mmHg, but was mandatory when systolic pressure was >160 mmHg and/or diastolic blood pressure <95 mmHg. There was, however, still the possibility, regardless of the blood pressure achieved, to maintain the previous level if the investigators were concerned about treatment upgrading, for safety reasons. Blood pressure was measured in the sitting position using a mercury sphygmomanometer. Three readings were obtained at each visit, with at least a 1-min interval between each reading, and were then averaged. All study tablets were encapsulated to conceal their identity and had to be taken once a day in the morning.
The present analysis included patients having still their blood pressure (BP) in whom the study protocol was strictly applied, which means that the treatment was modified according to the randomized therapeutic strategies each time blood pressure was still ≥140/90 mmHg (fixed low-dose combination, n = 118; sequential monotherapy, n = 108 ; stepped-care approach, n = 103). A score system was created to assess the advantages and the disadvantages of the treatment approaches. The number of points was attributed in order to give equal importance to efficacy and tolerability in comparing the different therapeutic strategies as follows:
| Month 3: | BP <140/90 mmHg =+25 |
| ∅ | BP ≥140/90 mmHg = −25 |
| ∅ | side-effect(s) during months 1–3 = −25 |
| Month 6: | BP <140/90 mmHg = +25 |
| ∅ | BP ≥140/90 mmHg = −25 |
| ∅ | side-effect(s) during months 3–6 = −25 |
| Month 9: | BP <140/90 mmHg = +50 |
| ∅ | BP ≥140/90 mmHg = −50 |
| ∅ | side-effect(s) during months 6–9 = −25 |
At any time: withdrawal because of side-effect(s) = −50 Data are reported as means ± SD. The scores calculated as described above were not subjected to statistical analysis since they were not predefined endpoints (CitationMourad et al 2004). Also, this study includes only the subset of patients who underwent changes in therapy during the trial each time their blood pressure was still ≥140 mmHg for systolic and/or ≥90 mmHg for diastolic.
Results
The fraction of patients who normalized their blood pressure (<140/90 mmHg) during the 9 month follow-up was 72.9% in the fixed low-dose combination group, compared with 59.3% and 52.4% in the sequential monotherapy and stepped-care groups, respectively. shows the percentage of patients in the three treatment groups who normalized their blood pressure during the course of the trial (Months 3, 6 and 9).
Table 1 Percentage of patients having their blood pressure normalized (3140/90 mmHg) at months 3, 6, and 9
shows the percentage of patients exhibiting a cumulative positive, neutral (sum of positive and negative points = 0), or negative score at completion of the trial. A positive score was achieved more often using the fixed low-dose strategy (64.4%) than using the sequential monotherapy (51.9%) or the stepped-care (43.7%) approach.
Figure 1 Fraction of patients exhibiting a cumulative positive, neutral or negative score at the end of the trial.
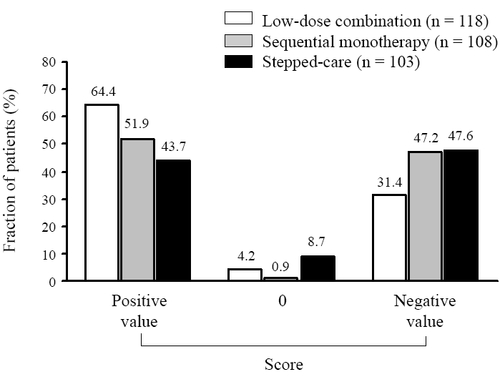
During the course of the trial the score increased by an average of 13.1 ± 70.5 points in the fixed low-dose combination group, but decreased by 7.2 ± 81.0 and 17.5 ± 76.4 points in the sequential monotherapy and stepped-care groups, respectively.
Discussion
Controlled clinical trials are critical for characterizing the efficacy and the tolerability of new antihypertensive agents. This has been done, for instance, during the developmental phase of the fixed low-dose combination containing the angiotensin converting enzyme inhibitor perindopril and the diuretic indapamide (CitationLaurent 2001). In addition morbidity and mortality trials should be performed whenever possible to define the most appropriated indications of each class of antihypertensive drugs (CitationWilliams 2005; CitationZanchetti 2005). During recent years the importance of bringing blood pressure below 140 mmHg for systolic and 90 mmHg for diastolic has been emphasized (CitationChobanian et al 2003; CitationMancia et al 2007). To achieve these target blood pressures the co-administration of two drugs is often required, accounting for the growing interest for fixed-dose combinations (CitationBrunner et al 1990; CitationDusing 2006). The advantage of combining drugs acting by different mechanisms in a single pill is to enhance the antihypertensive efficacy, but not at the expense of reduced tolerability (CitationLaw et al 2003), and to facilitate long-term persistence on treatment (CitationVan Wijk et al 2005). There is therefore a strong rationale for the use of fixed-dose combinations not only as second-line, but also as first-line therapy (CitationElliott 2002; CitationWelsh and Ferro 2004), and this view has been supported by international experts in hypertension guidelines (CitationChobanian et al 2003: CitationMancia et al 2007).
Beyond combination therapy as first step, the traditional strategies for treating hypertension with drugs comprise the stepped-care approach (monotherapy as initial treatment, followed if needed by a dose titration or the addition of a second drug) and the sequential monotherapy approach (rotation through several monotherapies from different classes until blood pressure control is reached) (CitationBrunner et al 1990). The design of the STRATHE trial is original as it enabled, under controlled conditions, a direct comparison of different therapeutic strategies in clinical practice (CitationMourad et al 2004). A first-line management of essential hypertensive patients based on a low-dose combination of perindopril and indapamide normalized blood pressure (<140/90 mmHg) in significantly more patients (62%) than a sequential mono-therapy strategy involving atenolol, losartan, and amlodipine (49%), and a stepped-care strategy involving valsartan and hydrochlorothiazide (47%), without difference, however, between the three groups with regard to the tolerability profile. The blood pressure normalization rate observed in the subset of patients of the STRATHE trial included in the present analysis is even better (72.9% in the fixed low-dose combination group, 59.3% in the sequential monotherapy group, and 52.4% in the stepped-care group). These patients were selected as their treatment was modified according to the study protocol at each visit if their blood pressure was still ≥140/90 mmHg, indicating that it is important to adjust antihypertensive treatment if blood pressure remains uncontrolled.
The choice of the most appropriate strategy to treat hypertensive patients is still difficult. To be considered are efficacy or tolerability criteria, as well as the presence of target organ damage or associated cardiovascular/renal disorders. Some drug regimens may require fewer adjustments than others at initiation of treatment, an advantage derived from a better antihypertensive efficacy combined with a preserved tolerability. The turbulence resulting from treatment adjustments might have important implications. In addition to a negative economic impact, frequent drug switches might lead patients to become non-compliant, or even to stop the treatment (CitationHughes and McGuire 1998; CitationDusing 2001; CitationUrquhart 2001). Notably, prompt blood pressure control now appears desirable in order to benefit maximally from the blood pressure lowering (CitationJulius et al 2004). Admittedly, the current way to analyze results of clinical trials in the field of hypertension does not enable assessment of how difficult or easy it is to normalize blood pressure in the individual patient. This prompted us to test whether a score system integrating the evolution of efficacy and tolerability over time would facilitate the weighing of advantages and disadvantages of different therapeutic strategies, and therefore render comparison between them more meaningful. For this purpose we used the observations obtained in the STRATHE trial. A better score was seen in the patients allocated to the fixed low-dose combination compared with the two other options. Notably, the number of points given to efficacy and tolerability criteria could be attributed differently in other clinical trials, depending, on the relative importance one wants to give to the various criteria to be taken into account.
In summary, it is possible to analyze the results of trials aimed to compare different treatment strategies in the field of hypertension using a score system. This approach gives an integrative view on the evolution of efficacy and tolerability, and reflects how difficult or easy it is to normalize blood pressure. Further studies are, however, needed to test prospectively whether this type of analysis is more informative than an analysis based on efficacy and tolerability criteria considered separately.
References
- BrunnerHRMenardJWaeberBTreating the individual hypertensive patient: considerations on dose, sequential monotherapy and drug combinationsJ Hypertens199083112157754
- ChobanianAVBakrisGLBlackHRSeventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureHypertension20034212065214656957
- DusingRAdverse events, compliance, and changes in therapyCurrent Hypertens Rep2001348892
- DusingROvercoming barriers to effective blood pressure control in patients with hypertensionCurr Med Res Opin20062215455316870079
- ElliottWJIs fixed combination therapy appropriate for initial hypertension treatment?Current Hypertens Rep2002427885
- HughesDMcGuireAThe direct costs to the NHS of discontinuing and switching prescriptions for hypertensionJ Hum Hypertens19981253379759987
- JuliusSKjeldsenSEWeberMOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet200436320223115207952
- LaurentSClinical benefit of very-low-dose perindopril-indapamide combination in hypertensionJ Hypertens200119suppl 4S9S14
- LawMRWaldNJMorrisJKValue of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trialsBMJ200332614273112829555
- ManciaGDe BackerGDominiczakAGuidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens20072511058717563527
- MouradJJWaeberBZannadFComparison of different therapeutic strategies in hypertension: a low-dose combination of perin-dopril/indapamide versus a sequential monotherapy or a stepped-care approachJ Hypertens20042223798615614033
- StaessenJAWangJGThijsLCardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003J Hypertens20032110557612777939
- TurnbullFNealBAlgertCEffects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trialsArch Intern Med200516514101915983291
- UrquhartJSome economic consequences of noncomplianceCurrent Hypertens Rep2001347380
- Van WijkBLKlungelOHHeerdinkERRate and determinants of 10-year persistence with antihypertensive drugsJ Hypertens2005232101716208154
- WelshLFerroADrug treatment of essential hypertension: the case for initial combination therapyInt J Clin Pract2004589566315587775
- WilliamsBRecent hypertension trials: implications and controversiesJ Am Coll Cardiol2005458132715766813
- ZanchettiAEvidence-based medicine in hypertension: what type of evidence?J Hypertens20052311132015894883