Abstract
The aim of this multi-center, open-label, randomized, parallel-group trial was to compare the efficacy of rosuvastatin with that of simvastatin in achieving the 1998 European Atherosclerosis Society (EAS) lipid treatment goals. 504 patients (≥18 years) with primary hypercholesterolemia and a 10-year cardiovascular (CV) risk >20% or history of coronary heart disease (CHD) or other established atherosclerotic disease were randomized in a 2:1 ratio to receive rosuvastatin 10 mg or simvastatin 20 mg once daily for 12 weeks. A significantly higher proportion of patients achieved 1998 EAS low-density lipoprotein cholesterol (LDL-C) goal after 12 weeks of treatment with rosuvastatin 10 mg compared to simvastatin 20 mg (64 vs 51.5%, p < 0.01). Similarly, significantly more patients achieved the 1998 EAS total cholesterol (TC) goal and the 2003 EAS LDL-C and TC goals (p < 0.001) with rosuvastatin 10 mg compared with simvastatin 20 mg. The incidence of adverse events and the proportion of patients who discontinued study treatment were similar between treatment groups. In conclusion, in the DISCOVERY-Beta Study in patients with primary hypercholesterolemia greater proportion of patients in the rosuvastatin 10 mg group achieved the EAS LDL-C treatment goal compared with the simvastatin 20 mg group. Drug tolerability was similar across both treatment groups.
Introduction
Despite a substantial decrease in cardiovascular disease (CVD) mortality over the past 30 years in several industrialized countries, coronary heart disease (CHD) remains the leading cause of morbidity and mortality (CitationThom 1989; CitationTunstall-Pedoe et al 2003). The decline in CHD mortality has been accompanied by a simultaneous decrease in the main risk factors. This has led to speculation that during the 1970s the decline in CHD mortality rate was mainly due to primary prevention, but during the 1980s improved treatment and secondary prevention have increasingly contributed to the decrease (CitationVartiainen et al 1994). At the same time, in most developing countries, the incidence of CVD is increasing towards epidemic levels (CitationReddy and Yusuf 1998). Also, in Eastern Europe CHD morbidity and mortality rates, particularly among men, are high compared with other areas of Europe (CitationSans et al 1997). Estonia, an eastern European country and formerly part of the former Soviet Union, has been facing high CVD and CHD rates. In Estonia, CVD and CHD account, respectively, for 55% and 32% of all deaths (CitationEstonian Medical Statistical Buerau 1997; CitationLaks et al 1999). The age-standardized mortality rates by the World Health Organization multinational MONItoring of trends and determinants in CArdiovascular disease (WHO MONICA) Project protocol criteria for 35- to 64-year-old people per 100,000 population in Estonia ranged from 208 to 317 for men and from 31 to 61 for women, substantially higher than rates in southern and central European centers of the WHO MONICA Project (CitationLaks et al 1999; CitationTunstall-Pedoe et al 1999).
Findings that an excess of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) particles are the main causes of atherosclerosis and major risk factors for CHD have been confirmed by molecular biology, animal experiments, and clinical and epidemiological trials (CitationLaRosa et al 1990; CitationVartiainen et al 1994; CitationLevine et al 1995; CitationCoresh et al 1996). The importance of elevated TC and LDL-C levels are described in many guidelines (CitationWood et al 1998; CitationAdult Treatment Panel III 2001; CitationDe Backer et al 2003). Substantial lowering of LDL-C level has been associated with risk reduction and a reduction in CHD events (Citationde Lorgeril et al 1999; CitationIto et al 2001). Despite the introduced guidelines and the availability of lipid-lowering therapies (LLT), many patients fail to meet TC and LDL-C goals (CitationPearson et al 2000). Results from large-scale trials have confirmed the effectiveness of the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) in the primary and secondary prevention on CHD. However, the residual morbidity and mortality remains high (CitationGotto and Grundy 1999). Therefore, the development of more effective lipid-lowering drugs has potential to further improve CV risk reduction.
The DIrect Statin COmparison of LDL-C Values: an Evaluation of Rosuvastatin therapY (DISCOVERY) Study was series of 9 independently powered studies performed in different countries and regions worldwide, including: The Netherlands (CitationBots and Kastelein 2005); Triple Country – Iceland, Ireland, Finland (CitationStrandberg et al 2004); Asia (CitationZhu et al 2007); Alpha – Eastern Europe, Central and South Africa, the Middle East (CitationBinbrek et al 2006); Belux – Belgium, Luxembourg (CitationHerregods et al 2006); Canada (CitationGupta and Constance 2005); Penta – Latin America, Portugal (CitationFonseca et al 2005); Beta (Estonia); and the United Kingdom (CitationMiddleton and Fuat 2006). These studies aimed to compare the efficacy of rosuvastatin 10 mg with that of other statins (given at their respective recommended starting doses) in achieving recommended lipid levels in the clinical setting. Simvastatin 20 mg was chosen as the comparator, because it was the standard of care in Estonia at the time of the start of the study. The DISCOVERY study program started in March 2002. The primary objective of this trial was to provide the results for the DISCOVERY-Beta Study, which compared the efficacy of rosuvastatin 10 mg with that of simvastatin 20 mg to achieve the 1998 European Atherosclerosis Society (EAS) LDL-C treatment goal (CitationWood et al 1998). The 1998 EAS LDL-C goal served as primary endpoint in the Discovery series studies, making it possible to use the data for the meta-analysis of the series.
Methods
Trial design and patients
The DISCOVERY-Beta was a 12-week multi-center, open-label, randomized, parallel-group study conducted between March and September 2004 at 18 centers in Estonia. Men and women (aged ≥18 years) were enrolled with primary hypercholesterolemia (LDL-C > 3.5 mmol/L if statin-naïve or >3.1 mmol/L if switching) and a 10-year CV risk >20% or a history of CHD or other established atherosclerotic disease (cerebrovascular disease as transient ischemic attack, ischemic stroke, or carotid artery disease; CHD as acute myocardial infarction [AMI], unstable angina, or coronary revascularization; peripheral arterial disease [PAD] as aortic aneurysm, intermittent claudication, lower limb arterial revascularization, or amputation due to the complications of atherosclerosis) and fasting triglycerides (TG) ≤4.52 mmol/L at visit 2 (week 0). Subjects were selected by cardiologists, internists, and general practitioners (when needed if previously consulted by cardiologist) from a primary or secondary prevention subject population. Statin-naïve subjects (those who had not received a statin within the previous 6 months) or subjects on a start dose (commonly used or accepted starting doses were atorvastatin 10 mg, fluvastatin 20 mg, or pravastatin 20 mg) or other LLT, which was ineffective (ie, subjects had not reached their LDL-C goal at that dose), were entered into this study. Dietary counseling for 6 weeks was given for statin-naïve patients before enrolling into the study. Main exclusion criteria were: known familial hypercholesterolemia; secondary dyslipidemia of any cause; history of serious adverse effect (SAE) or hypersensitivity to other HMG-CoA reductase inhibitors; pregnancy, breast-feeding, and women of childbearing potential not using chemical or mechanical contraception or with a positive pregnancy test; malignancy; use of disallowed concomitant medications (antibiotics clarithromycin or erythromycin, antifungal treatment with fluconazole, ketoconazole, or itraconazole, lipid-lowering agents, cyclosporin); history of alcohol or drug dependence; active liver disease or hepatic dysfunction with elevations of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >1.5 times upper limit of normal (ULN); renal impairment as defined by serum creatinine >220 μmol/L (2.5 mg/dL); uncontrolled diabetes; unstable angina; uncontrolled hypertension as either diastolic >95 mmHg or systolic blood pressure >200 mmHg; unexplained serum creatine kinase (CK) >3 times ULN; subjects in serious or unstable medical or psychological condition that compromises the subject safety or participation in the trial. Investigators of each center ensured that each patient gave written informed consent for participation in the study.
Patients were assessed for eligibility and, within 2 weeks, were randomized in a 2:1 ratio to receive rosuvastatin 10 mg or simvastatin 20 mg administered once daily for 12 weeks. Patients were allocated numbers as they entered the study, and a computer-generated randomization scheme was used to determine treatment allocation as each patient became eligible. Treatment codes for each patient were provided to each center in a sealed envelope, and investigators were unaware of the randomization scheme and treatment codes until the envelope was opened at the randomization visit.
Efficacy and tolerability assessments
At each visit (screening at week −2, randomization at week 0, final at week 12) blood samples were drawn to determine serum levels of TC, LDL-C, high-density lipoprotein cholesterol (HDL-C), and TG. Local laboratories were used in the study. Serum LDL-C level was determined using the Friedewald formula (CitationFriedewald et al 1972). Patients with TG ≥ 4.52 mmol/L were excluded from the study, so that the confounding effect of elevated TG level on the Friedewald formula was minimized.
The primary endpoint was the percentage of patients achieving the 1998 EAS LDL-C goal (<3.0 mmol/L) at week 12. Secondary efficacy endpoints included: 1. percentage of patients achieving the 1998 EAS TC goal (<5.0 mmol/L); 2. percentage change from pre-dose and final visit in serum LDL-C, TC, HDL-C, and TG levels in statin-naïve and switched patients; 3. comparing the efficacy of rosuvastatin 10 mg with simvastatin 20 mg by assessment of percentage of subjects who reach the 2003 EAS LDL-C (<2.5 mmol/L) or TC (<4.5 mmol/L) target goals for patients with clinically established CVD and/or diabetes mellitus (DM) after 12 weeks of therapy; 4. comparison of rosuvastatin 10 mg with simvastatin 20 mg after 12 weeks of treatment for the incidence and severity of adverse events (AEs) and abnormal laboratory values. Lipid levels were determined from fasting (12 hours) blood samples analyzed in local laboratories. The incidence and severity of AEs, including abnormal laboratory values (liver enzymes, creatinine, CK) were recorded to assess tolerability. At every visit, AEs were reported to investigators in response to a standard questionnaire. Any decline in a patient’s condition subsequent to study entry was considered an AE. Also, the relationship of SAEs to the treatment was assessed.
Statistical analysis
The trial has been sized to detect a difference of 15% between rosuvastatin 10 mg and simvastatin 20 mg, in terms of the percentage of subjects reaching the 1998 EAS LDL-C target goal at week 12. Using these data, and the fact that patients were randomized in a 2:1 ratio (rosuvastatin : simvastatin), it was calculated that 480 evaluable subjects were required to achieve 90% power for a two-sided significance level of 5%. Subjects were evaluated based on an intention to treat (ITT) population which consisted of subjects who had a baseline reading, one week 12 reading, and at least one dose of medication. If a subject was withdrawn prior to week 12, the last value after baseline was taken as the week 12 assessment. The numbers of subjects reaching the 1998 EAS lipid target goals on rosuvastatin 10 mg and simvastatin 20 mg at week 12 were compared using a logistic regression analysis with factors being fitted for treatment, subject type (naïve or switched) and the pre-dose lipid parameter value fitted as a covariate. For lipid parameters, analyses of percentage change for pre-dose (week 0) and at week 12 were performed separately for the naïve and switched patients because the week 0 value for statin-naïve patients followed 6 weeks of dietary advice, whereas this was not case for switched patients. The analyses were performed using the ANCOVA model, with factors fitted for treatment and the pre-dose (week 0) lipid parameter value as a covariate. The results are presented as least squares means (LSM) and the difference between the LSM, with p values and associated 95% confidence intervals. In addition, the percentage change in lipid parameters has been summarized.
Results
Patient characteristics
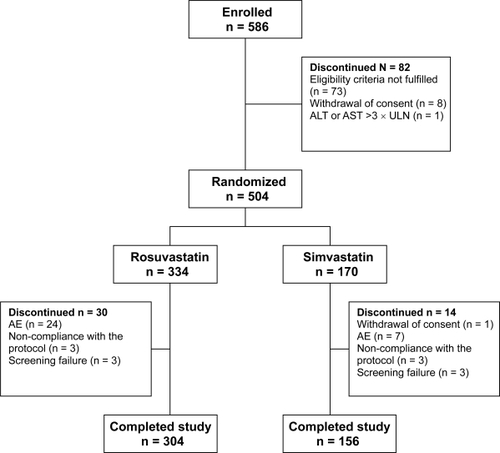
Of 586 patients screened, 504 (59.3 % women and 40.7% men) were randomized at visit 2 (week 0) into the treatment groups (rosuvastatin 10 mg 334 and simvastatin 20 mg 170 patients) and received at least 1 dose of the study drug, and had baseline and week 12 lipid measurements available (ITT population). Eighty-two patients were discontinued before randomization: in 73 subjects eligibility criteria were not fulfilled, 1 subject ALT was elevated >3 times ULN, and 8 subjects withdrew their consent () .
Figure 1 Patient disposition in study groups.
Baseline demographic, clinical characteristics, risk factors, and previous LLT for 504 randomized patients are presented in . Sex distribution, clinical characteristics of study subjects, and frequency of risk factors (body mass index [BMI], systolic and diastolic blood pressure [BP], documented atherosclerotic disease, type 2 DM, family history of premature CHD and PAD, smoking, low HDL or elevated TG) were similar between the two treatment groups. In total, 187 (37.1%) patients had documented atherosclerotic disease, 115 (22.8%) type 2 DM, 98 (19.4%) family history of premature CHD or PAD, 25 (5.0%) low HDL, 208 (41.3%) elevated TG, and 167 (33.1%) were smokers. 119 (23.6%) of the total study population had previously received LLT, 82 patients (24.6%) in the rosuvastatin group compared with 37 (21.8%) patients in the simvastatin group. The most frequently prescribed LLTs were pravastatin (60 patients, 11.9%) and atorvastatin (29 patients, 5.8%).
Table 1 Baseline demographic and clinical characteristics of the study population was statistically not different in the two treatment groups
Efficacy
The proportions of patients reaching the 1998 EAS LDL-C goal (primary endpoint), the 1998 and 2003 EAS TC, and the 2003 EAS LDL-C goals (all secondary endpoints) after 12 weeks of treatment with either rosuvastatin 10 mg or simvastatin 20 mg are presented in . Significantly more patients in the rosuvastatin group achieved the 1998 EAS LDL-C goal compared with those in the simvastatin group, 210 (64%) and 86 (51.5%) patients (p < 0.01). In addition, significantly more statin-naïve subjects achieved the goal in the rosuvastatin arm compared with simvastatin, 165 (67.1%) versus 71 (54.6%) patients (p < 0.001). The same trend occurred within switched patients 45 (54.9%) patients in the rosuvastatin group compared to 15 (40.5%) patients in the simvastatin group. A significantly higher number of patients in the rosuvastatin group achieved the 1998 EAS TC goal compared with patients in simvastatin arm, 195 (59.5%) versus 72 (43.1%) patients (p < 0.001). This analysis was made within the total ITT population, as there was no significant difference between statin-naïve and switched subjects. Similarly, a significantly greater number of patients receiving rosuvastatin 10 mg reached the 2003 EAS LDL-C and TC goals compared with patients in the simvastatin group, 146 (44.5%) and 37 (22.2%) patients (p < 0.001) and 141 (43%) and 43 (25.7%) patients (p < 0.001).
Table 2 Subjects (%) reaching the 1998 and the 2003 EAS lipid (mmol/L) target goals at the end of the study (week 12)
Percentage change from baseline levels of LDL-C, TC, HDL-C, and TG in both statin-naïve and switched patients are shown in .
Table 3 Changes in lipid profiles before and after 12 weeks treatment with rosuvastatin 10 mg and simvastatin 20 mg
In both treatment groups, in statin-naïve and switched subjects, TG levels declined from baseline, but these decreases were too small to be significant. At the same time, during the 12-week treatment period there were no changes in HDL-C levels in statin-naïve patients in the rosuvastatin and simvastatin groups, and changes in switched patients were small.
Safety
The occurrence of AEs was similar between groups. Both study statin treatments (rosuvastatin and simvastatin) were generally well tolerated, and the incidence of AEs and SAEs was low. The proportion of patients who discontinued study treatment because of AEs did not differ statistically – in the rosuvastatin group 7.2% and in the simvastatin group 4.1% (). The most common AEs leading to withdrawal from the study were gastrointestinal disorders such as abdominal pain and nausea, and the musculoskeletal disorder myalgia. SAEs were reported by 1.8% of study subjects. The only death (due to AMI) during the trial was in the rosuvastatin group, and was considered by the investigator to have been unrelated to the investigational product. All SAEs were judged by the investigators as having had no causal relationship to the study treatment, with the one exception of general discomfort (not related to myalgia) reported by a patient receiving simvastatin. The most common AE was nausea, in 13 patients (3.9%) in the rosuvastatin group and in 6 patients (3.5%) in simvastatin group. Myalgia, a known side effect of statin therapy, had a statistically not significant tendency to be reported more frequently with rosuvastatin – by 10 patients (3%) compared with 1 patient (0.6%) in simvastatin group. None of these reports were associated with a clinically significant increase in CK (>10 times ULN). Rhabdomyolysis occurred in none of the patients. Mean changes from baseline in circulating levels of ALT, CK, and creatinine at week 12 were small and similar between the rosuvastatin and simvastatin groups, and generally unremarkable. Only 1 patient from each group had a CK level at week 12 of special note (>3 times ULN).
Table 4 Adverse events in rosuvastatin and simvastatin treatment groups (%)
Subjects with multiple AEs in the same category are counted only once in that category; subjects with AEs > 1 category are counted once in each category.
Discussion
CVD is major health problem and a leading cause of global mortality (CitationThom 1989; CitationSans et al 1997; CitationReddy and Yusuf 1998; CitationTunstall-Pedoe et al 2003). Despite the decline in CHD mortality during recent decades in most Western countries, it remains high because of the aging population (CitationSleight 2003). CVD includes CHD, and is most frequently caused by atherosclerosis, a progressive condition that may remain asymptomatic for many years, with sudden death often being the first clinical manifestation (CitationThaulow et al 1993). Elevated LDL-C is an important contributory factor to development of atherosclerosis, and as such is recognized as a major risk factor for CHD. Consequently, LDL-C is a key therapeutic target for the prevention of CHD, with statins recommended as first-line drug treatment (CitationAdult Treatment Panel III 2001; CitationDe Backer et al 2003).
This randomized, multi-center study comparing lipid-modifying efficacy of two statins showed that on the basis of both the 1998 EAS and the stricter 2003 EAS LDL-C goals, rosuvastatin showed greater efficacy in reducing LDL-C compared with simvastatin with commonly used starting doses (rosuvastatin 10 mg and simvastatin 20 mg once a day) over 12 weeks of treatment in patients with primary hypercholesterolemia. Also, up to the end of the study, more hypercholesterolemic patients in the rosuvastatin group achieved a reduction in TC levels to the EAS target goals (CitationWood et al 1998; CitationDe Backer 2003) compared with simvastatin-treated patients. Our study confirms the results of previous trials comparing the efficacy of different statins, that rosuvastatin, at a commonly used starting dose, is highly effective in reducing LDL-C levels. Thus, in the DISCOVERY-Beta Study as in the earlier published MERCURY I (CitationSchuster et al 2004), STELLAR (CitationJones et al 2003), and Rosuvastatin Study Group trials (CitationBrown et al 2002), rosuvastatin was more effective than other statins used (atorvastatin, simvastatin, pravastatin) in decreasing LDL-C levels and allowing more patients to achieve the recommended treatment goals. This effect was evident from week 6 and continued to at least week 52 during the LLT.
The DISCOVERY-Beta Study randomized a large proportion of patients (24%) who had not reached the EAS lipid goals with LLTs. At the same time the reduction of LDL-C in statin-naïve patients with rosuvastatin 10 mg was significantly higher than with simvastatin 20 mg (43% and 34%, respectively).
HDL-C has taken on increased importance as a clinical prognostic factor for CVD, which is reflected in coronary preventive guidelines (CitationWood et al 1998; CitationAdult Treatment Panel III 2001; CitationDe Backer et al 2003). In our study analysis of the data from the fixed-dose treatment period, comparing rosuvastatin 10 mg with simvastatin 20 mg did not reveal any changes in HDL-C levels in both groups during the 12 weeks. The reasons could be the relatively high baseline values for HDL-C in both groups and/or some technical issues caused by the fact that local laboratories instead of one central laboratory were used for sample analysis. No definitive or primary reference methods exist for the separation of HDL, and because differences in the precipitation procedures can alter the population of particles precipitated, not all methods give the same result for HDL cholesterol, and therefore standardization of HDL cholesterol measurement is difficult (CitationMarques-Vidal et al 1999). However, questions remain as to the specificity of these assays under contrasting conditions of high and low LDL levels as typically observed in statin trials.
In our study rosuvastatin 10 mg was as effective as simvastatin 20 mg at reducing TG in both statin-naïve and switched patients, confirming results obtained with rosuvastatin 10 mg in earlier published reports (CitationBrown et al 2002; CitationDavidson et al 2002; CitationJones et al 2003; CitationBinbrek et al 2006). In the DISCOVERY-Beta Study, both rosuvastatin 10 mg and simvastatin 20 mg were well tolerated, with similar profiles of AEs reported with both agents. There appeared to be no differences between treatment groups. The types and numbers of AEs and overall safety profile were consistent with those reported for statin therapy (CitationKnopp 1999; CitationSchuster 2003). However, in our study we found no reports of myopathy or rhabdomyolysis, and rate of myalgia was low in both groups. Treatment discontinuation was similar in both arms of the study; however, in the rosuvastatin group there were 4 cases of AMI which were deemed by the investigators to be unrelated to the investigated product. Significant elevation in liver enzymes was similarly rare in both groups in both treatment populations.
Limitations
Because of the open-label design of the study, patient reporting of AEs could have been affected by their awareness of drug treatment or expectations from previous experiences with statins. However, the number and nature of AEs were generally consistent with those observed in previous trials. At the same time, the 12-week study period was long enough to demonstrate the LDL-C reducing effect and possible AEs of statin therapy (CitationBrown et al 2002; CitationOlsson et al 2002).
In conclusion, in the DISCOVERY-Beta Study in patients with primary hypercholesterolemia and high risk of CVD in clinical practice, greater reduction in LDL-C and TC levels was achieved with rosuvastatin 10 mg compared with simvastatin 20 mg, with more patients achieving the 1998 EAS and the 2003 EAS LDL-C and TC goals. Both statin treatments were well tolerated.
Acknowledgements
We gratefully acknowledge the investigators, co-investigators (see Appendix), study coordinators, and the patients who participated in the trial.
Disclosures
TS and KO are employees of AstraZeneca, Estonia. None of the other authors any conflicts of interest to disclose.
References
- BinbrekASElisAAl-ZaibagMfor the DISCOVERY Alpha Study Group2006Rosuvastatin versus atorvastatin in achieving lipid goals in patients at high risk for cardiovascular disease in clinical practice: a randomized, open-label, parallel-group, multicenter study (DISCOVERY Alpha Study)Curr Ther Res672143
- BotsAFEKasteleinJJP2005Achieving lipid goals in real life: the Dutch DISCOVERY StudyInt J Clin Pract5913879416351669
- BrownWVBaysHEHassmannDR2002Efficacy and safety of rosuvastatin compared with pravastatin and simvastatin in patients with hypercholesterolemia: a randomized, double-blind, 52-week trialAm Heart J14410364312486428
- CoreshJKwiterovichPOJr1996Small, dense low-density lipoprotein particles and coronary heart disease risk: a clear association with uncertain implicationsJAMA27691458782642
- DavidsonMMaPSteinEA2002Comparison of effects on low-density lipoprotein cholesterol and high-density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemiaAm J Cardiol892687511809427
- De BackerGAmbrosioniEBorch-JohansenKOther Societies on Cardiovascular Disease Prevention in Clinical Practice2003Europen guidelines on cardiovascular disease prevention in clinical practiceEur Heart J2416011012964575
- de LorgerilMSalenPMartinJ-L1999Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart StudyCirculation99779859989963
- Estonian Medical Statistics Bureau1997Some facts about health care in Estonia 1992–1996Tallinn64
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)2001JAMA28524869711368702
- FonsecaFAHRuizASilvaJM2005The DISCOVERY PENTA study: a DIrect Statin COmparison of LDL-C Value – an Evaluation of Rosuvastatin therapY compared with atorvastatinCurr Med Res Opin2113071516083541
- FriedewaldWTLevyRIFredricksonDS1972Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifugeClin Chem184995024337382
- GottoAMJrGrundySM1999Lowering LDL cholesterol: questions from recent meta-analyses and subset analyses of clinical trial data: issues from the Interdisciplinary Council on Reducing the Risk for Coronary Heart Disease, ninth Council meetingCirculation99E1E710051310
- GuptaMConstanceC2005Direct statin comparison of LDL-C values: an evaluation of rosuvastatin therapy (DISCOVERY – Canada). [abstract]Atheroscler Suppl6108
- HerregodsMCDaubresseJCMichelG2006DISCOVERY BELUX: An open-label, randomised, multi-centre, phase IIIb, parallel group study to compare the efficacy and safety of rosuvastatin and atorvastatin in subjects with type IIa and IIb hypercholesterolaemiaActa Cardiol612356
- ItoMKDeluccaGMAldridgeMA2001The relationship between low-density lipoprotein cholesterol goal attainment and prevention of coronary heart disease related eventsJ Cardiovasc Pharmacol Ther61293511509919
- JonesPHDavidsonMHSteinEAfor the STELLAR Study Group2003Comparison of efficacy and safety of rosuvastatin versus ator-vastatin, simvastatin, and pravastatin across doses (STELLAR Trial)Am J Cardiol921526012860216
- KnoppRH1999Drug treatment of lipid disordersN Engl J Med34149851110441607
- LaksTTuomilehtoJJõesteE1999Alarmingly high occurrence and case fatality of acute coronary heart disease events in Estonia: results from the Tallinn AMI register 1991–94J Int Med2465360
- LaRosaJCHunninghakeDBushDfor the Task Force on Cholesterol Issues, American Heart Association1990The cholesterol facts: a summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease: a joint statement by the American Heart Association and the National Heart, Lung, and Blood InstituteCirculation811721332184951
- LevineGNKeaneyJFJrVitaJA1995Cholesterol reduction in cardiovascular disease: clinical benefits and possible mechanismsN Engl J Med332512217830734
- Marques-VidalPFerrarioPKuulasmaaKfor the WHO MONICA Project1999Quality Assessment of Data on HDL Cholesterol in the WHO MONICA Project URL:http://www.ktl.fi/publications/monica/hdl/hdlqa.htm
- MiddletonAFuatA2006Achieving lipid goals in real life: the DISCOVERY-UK studyBr J Cardiol13726
- OlssonAGIstadHLuurilaOon behalf of the Rosuvastatin Investigators Group2002Effects of rosuvastatin and atorvastatin compared over 52 weeks of treatment in patients with hypercholesterolemiaAm Heart J14410445112486429
- PearsonTALauroraIChuH2000The lipid treatment assessment project (L-TAP): a multi-centre survey to evaluate the percentages of dyslipidemic subjects receiving lipid-lowering therapy and achieving low-density lipoprotein goalsArch Intern Med1604596710695686
- ReddyKSYusufS1998Emerging epidemic of cardiovascular disease in developing countriesCirculation975966019494031
- SansSKestelootHKromhoutDon behalf of the Task Force. The burden of cardiovascular diseases mortality in Europe1997Task Force of the European Society of Cardiology on cardiovascular mortality and morbidity statistics in EuropeEur Heart J18123148
- SchusterHBarterPJStenderS2004Effects of switching statins on achievement of lipid goals: Measuring Effective Reductions in Cholesterol Using Rosuvastatin Therapy (MERCURY I) studyAm Heart J1477051215077101
- SchusterH2003Rosuvastatin – a high effective new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor: review of clinical trial data at 10–40 mg doses in dyslipidemic patientsCardiology991263912824720
- SleightP2003Current opinions in the management of coronary heart diseaseAm J Cardiol92Suppl4N8N
- StrandbergTEFeelyJSigurdssonEL2004Twelve-week, multicenter, randomized, open-label comparison of the effects of rosuvastatin 10 mg/d and atorvastatin 10 mg/d in high-risk adults: A DISCOVERY studyClin Ther2618213315639694
- ThaulowEErikssenJSandvikL1993Initial clinical presentation of cardiac disease in asymptomatic men with silent myocardial ischemia and angiographically documented coronary artery disease (the Oslo Ischemia Study)Am J Cardiol72629338249835
- ThomTJ1989International mortality from heart disease: Rates and trendsInt J Epidemiol18Suppl I208
- Tunstall-PedoeHKuulasmaaKMähönenMfor the WHO MONICA (monitoring trends and determinants in cardiovascular disease) Project1999Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA Project populationsLancet35315475710334252
- Tunstall-PedoeHKuulasmaaKTolonenH with 64 other contributors for the WHO MONICA Project2003MONICA Monograph and Multimedia SourcebookWorld′s largest study of heart disease, stroke, risk factors, and population trends 1979–2002GenevaWHO
- VartianenEPuskaPPekkanenJ1994Changes in risk factors explain changes in mortality from ischaemic heart disease in FinlandBr Med J3092378044063
- WoodDDe BackerGFaegermanO1998Task Force Report: Prevention of coronary heart disease in clinical practice. Recommendations of the Second Joint Task Force of European and Other Societies on coronary preventionEur Heart J1914345039820987
- ZhuJRTomlinsonBYoungMR2007A randomised study comparing the efficacy and safety of rosuvastatin with atorvastatin for achieving lipid goals in clinical practice in Asian patients at high risk of cardiovascular disease (DISCOVERY-Asia study)Curr Med Res Opin2330556818196620
- ZijlstraFPatelAJonesMfor the PCAT collaboration2002Clinical characteristics and outcome of patients with early (<2 h), intermediate (2–4 h) and late (>4 h) presentation treated by primary coronary angioplasty or thrombolytic therapy for acute myocardial infarctionEur Heart J235505711922645
Appendix
The following centers and investigators (all in Estonia) participated in the trial.
North-Estonia Regional Hospital, Tallinn – T. Laks (National Co-ordinator), R Vettus, M. Zemtsovski, L. Anton, Ü. Planken, L. Kalinina, A. Leht, H. Tupits, P. Rudenko, A. Ambos; Mustamäe Outpatient Clinic, Tallinn – A. Kreis (Principal Investigator, PI), M. Leiner, K. Valkenklau, A. Kund, T. Kivimäe, M. Mäemets, E. Gustavson, E. Nukk, T. Simm; Sütiste Centre, Tallinn – Ü. Kaasik (PI), K. Kender, A. Rosenthal, T. Jürgenson; West-Tallinn Central Hospital, Tallinn – I. Lapidus (PI), H. Kaljusaar, L. Aug, A. Bljumovitš, O. Kolbassova, S. Masik; East-Tallinn Central Hospital – A. Reinold (PI), A. Kork, M. Kadarik, M. Reimand, O. Taaler; Pelgulinna Outpatient Clinic, Tallinn – P. Laan (PI), T. Nurmoja, S. Reinmets; Lasnamäe Health Centre, Tallinn – I. Loogna (PI), L. Meledina (PI), N. Pantelejeva, S. Ehiloo, J. Ivanov, R. Aivazjan, I. Dmitrieva; Nõmme Family Doctors’ Centre, Tallinn – P. Pärnakivi (PI), E. Ehatamm, M. Alba, K. Kuningas, J. Miller, P. Tammist, M. Doroš, T. Oruaas, E. Merilind, K. Merilind, R. Raamat; Keila Family Physicians Centre – M. Võsa (PI); Saue Family Doctors’ Centre - A. Mäeorg (PI), A. Soomets; Saku Health Centre – M. Petersen (PI), M. Stern; Kuressaare Health Centre – V. Nemvalts (PI), S. Väli, E. Nurmekivi, T. Tarkin; Narva Outpatient Clinic – O. Averina (PI); Rakvere Centre – L. Slobodtshikova (PI), K. Veidrik, A. Kullerkupp; Tartu Puusepa Centre - Ü. Roostalu (PI), M. Treial, L. Koppel; Gildi Outpatient Clinic, Tartu – H. Loogus (PI), M. Plaks, L. Pilv, S. Saarniit, M. Nirk, M. Peda, L. Pullerits; Elva Family Physician – T. Laasik (PI); Viljandi Outpatient Clinic – E. Keba (PI).