Abstract
Coronary stent thrombosis is a serious problem in the drug-eluting stent era. Despite aggressive antiplatelet therapy during and after percutaneous coronary intervention (PCI), the incidence of sub-acute stent thrombosis remains approximately 0.5%–2%, which may represent a catastrophic clinical situation. Both procedural factors and discontinuation of antiplatelet therapy are normally associated with this event. We report on simultaneous stent thromboses of two drug-eluting stents implanted in two different vessels, which resulted in a life-threatening clinical condition. Possible contributing factors that led to synergistic thrombotic effects are discussed.
Simultaneous thromboses of two coronary artery stents: fostering bad synergies
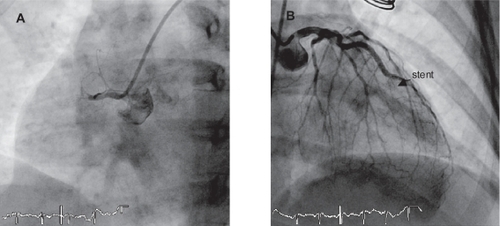
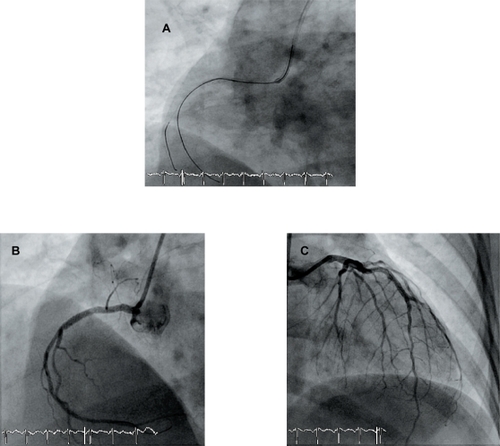
The patient is a 47-year-old woman from Morocco with a history of insulin-requiring type 2 diabetes mellitus (DM), dyslipidemia, and obesity (body mass index: 33), admitted to our institution during the acute phase of ST elevation myocardial infarction (STEMI). One week before the current admission, the patient had been admitted to another center with progressive angina pectoris (functional class III). Coronary angiography revealed severe coronary stenoses in the proximal segment of the right coronary artery (RCA) and in the distal segment of the left anterior descending (LAD) artery. At that time, PCI of both lesions was performed by implanting a 2.25 × 8 mm sirolimus-eluting stent (Cypher®, Cordis, Johnson and Johnson) in the distal LAD and a 2.5 × 13 mm stent in the proximal RCA. The patient was discharged on dual antiplatelet therapy (acetylsalycilic acid, ASA) 100 mg per day and clopidogrel 75 mg per day). Five days after the procedure, however, the patient decided to stop ASA although remained on clopidogrel. Two days later, the patient suffered from STEMI involving both anterior and inferior territories on the ECG. She also suffered repeated episodes of ventricular fibrillation, but was successfully defibrillated. The patient required inotropic support to maintain arterial pressure stability and was intubated because of repeated arrythmias. Emergency coronary angiography was performed and revealed thromboses of both stents (). RCA thrombosis was tackled first with abciximab bolus + infusion. The passage of the guidewire through the stent was very difficult requiring the use of different wires. A hydrophilic wire was initially passed through the stent, but neither a thrombus aspiration catheter nor a 1.5 × 10 mm angioplasty balloon could cross through the stent successfully. Not until a second wire was sited in parallel (), while the balloon remained inserted over the first wire at the point where it stopped, could we successfully dilate the stent with noncompliant balloons (2.5 and 3.0 mm in diameter). Finally a new drug-eluting stent (DES) (Xience™, Abbott Vascular 3.0 × 28 mm) was used to cover the entire segment including the more proximal segment of the RCA up to the distal part of the previously implanted stent. A DES was chosen because the patient was diabetic. A good angiographic result (TIMI 3 flow) was obtained (). The LAD artery was treated sequentially. In this vessel, thrombus aspiration was effective and the result was optimized using a noncompliant balloon dilatation (2.5 × 9 mm) gaining a final TIMI 3 flow (–C). The patient could be transferred to her referral hospital and the initial outcome was uneventful. Five days later, she was discharged on dual antiplatelet high-dose regimen (ASA 300 mg per day and clopidogrel 150 mg per day). Before discharge, she received intensive counseling on the need for compliance with medication, and on secondary prevention to avoid further thrombotic events.
Figure 2 Panel A: successful passage of the wire, while the balloon is inserted over another wire at the place where it stopped. Panel B and C: Final angiographic result on RCA (B) and LAD (C).
Discussion
Numerous reports describe the occurrence of acute (<24 hours), subacute (<30 days), late (>30 days), and very late (>12 months) stent thrombosis after DES implantation.Citation1,Citation2 However, the true incidence of stent thrombosis may be underestimated in clinical trials and could occur at substantially higher rates in the real world, where more complex lesions are treated.Citation3,Citation4 Several factors that contribute to stent thrombosis have been recognized, such as procedure-, patient-, and lesion-related factors, thrombogenity of the stent, impaired re-endothelization, and antiplatelet therapy (). In recent years, aggressive antiplatelet therapy during PCI, including use of ASA, thienopyridines, and glycoprotein IIb/IIIa inhibitors, has reduced the risk of post-procedural thrombotic complications.Citation5 Nonetheless, the incidence of acute stent thrombosis remains approximately 0.5%–2%.
Table 1 Factors implicated in pathophysiology of in-stent thrombosis
In our case, the simultaneous thrombosis of both stents suggested a systemic disturbance, to which many factors might have contributed. Among the procedure-related factors, smaller final lumen dimensions, especially with stent malapposition and/or underexpansion appear to be important for the development of in-stent thrombosis.Citation6 These procedural problems are more important with DES, in which the stent length, stent underexpansion, and residual stenosis have been associated with risk for stent thrombosis.Citation7 These problems, which may be involved in both bare metal and DES thromboses, can be prevented during the intervention by means of judicious stent deployment and implantation. Good selection of the stent size, proper coverage of the entire lesion length, and the achievement of good expansion of the stent are mandatory during PCI. Technical difficulties encountered during recanalization of the current thrombosed stent in the RCA suggested both underexpansion and incomplete stent apposition which, with the small stent size (2.5 mm), may have been involved in the pathogenesis of the thrombosis. Furthermore, stent size in the LAD was also small (2.25 mm). It is worthwhile considering other potential concomitant factors such as resistance to antiplatelet therapy (clopidogrel and/or ASA), compliance to therapy (which plays a major role in this case), and the grade of inflammation. Antiplatelet resistance and inflammation are known determinants of accelerated atherosclerosis in diabetics.Citation8
Certain stent designs and materials may predispose to thrombogenity. Thus the open-cell stent, compared with the closed-cell, appeared to generate greater platelet activation during the 30 days after implantation in one study.Citation9 Strut thickness, and polymer type and thickness, may also play an important role. Nonerodable polymers of the Cypher and Taxus provoke chronic eosinophilic infiltration of the arterial wall, suggestive of hypersensitivity reactions in a small number of cases.Citation10 Furthermore, drugs eluted from DES may exert a prothrombotic effect. Rapamycin (sirolimus), for example, may increase both thrombin- and tumor necrosis factor-α-induced endothelial tissue factor expression and activity.Citation11 At the same time, paclitaxel enhances tissue factor expression and activity in endothelial cells.Citation12 In addition, both drugs may easily penetrate into the artery wall owing to their lipophylic properties, with chronic retention of the drug in the surrounding artery tissue, which may also contribute to the prothrombotic environment after DES deployment.Citation13 Another contributing factor may be the delayed or impaired endothelialization of DES. In vitro rapamycin and paclitaxel can inhibit proliferation and migration of vascular smooth muscle cells, and may also suppress endothelial cells.Citation14
Other factors that may influence the healing are likely to be lesion-related, such as primary stenting in acute MI due to the presence of a pronounced inflammatory and thrombogenic environment of the exposed necrotic core to flowing blood, accompanied by enhanced platelet reactivity,Citation3,Citation15 and patient-related, such as antiplatelet therapy discontinuation, renal failure, DM, and a lower ejection fraction, which have all been reported in clinical studies.Citation3 In particular, discontinuation of antiplatelet therapy has been observed to be strongly associated with DES thrombosis.Citation1 The appropriate duration of the long-term antiplatelet regimen for prevention of DES thrombosis still needs to be assessed in randomized prospective trials. A further problem has emerged: hyporesponsiveness to antiplatelet therapy by some groups of patients, in particular diabetic patients.Citation16 Patients with DM are characterized by a prothrombotic status, related to endothelial dysfunction, impaired fibrinolysis, increased coagulation factors, and increased platelet reactivity and turnover.Citation17,Citation18 Despite the clinical benefit achieved with antiplatelet agents, these patients continue to have an increased risk of ischemic events compared with nondiabetics.Citation19 Diabetic patients also have reduced responsiveness to oral antiplatelet therapy, either ASA or clopidogrel,Citation20–Citation22 which is potentially related to the need for insulin therapy.Citation23 In particular, the hyporesponsiveness to clopidogrel may be partially averted with a high dose of clopidogrel (150 mg/day).Citation24,Citation25
In conclusion, our patient presented several factors that fostered bad synergies. Her diabetes could have heightened platelet reactivity. In this milieu, the early suspension of ASA might act as a precipitating factor in a setting of potential under-expansion and small DES. The involvement of both coronary arteries supports the systemic activation of the thrombotic state. Good stent selection and proper implantation may prevent many stents from being thrombosed in prothrombotic environment.
Limitations
Unfortunately, the patient was on infusion of IIb/IIIa antagonists, so that platelet function could not be measured with optical aggregometry. Moreover, the discussion on the probable underexpansion or malapposition of the thrombosed stent based on the technical difficulties encountered during the coronary angioplasty can only be considered a hypothesis. To demonstrate this issue intravascular ultrasound should have been performed.
Diabetes mellitus: a prothrombotic state. Implications for outcomes after coronary revascularization
Introduction
Diabetes mellitus (DM) affects 150 million people worldwide. In particular, type 2 DM is endemic and the incidence is increasing.Citation26 The leading cause of disability and premature mortality among diabetics is cardiovascular disease.Citation27 DM increases the risk for coronary heart disease, stroke, and peripheral arterial disease from 2-fold to 4-fold.Citation28,Citation29 The increased risk is independent of and in addition to other cardiovascular risk factors.Citation30 Importantly, the risk of myocardial infarction (MI) is 3- to 5-fold higher in type 2 DM. A diabetic subject with no history of MI has the same long-term risk as a non-DM subject with a past history of MI. Patients with DM usually show a diffuse and severe coronary artery disease. Coronary artery revascularization of diabetics continues to be a challenge: these patients suffer from a higher rate of repeated revascularization and worse outcomes after PCI, compared with non-DM patients. The increased atherothrombotic risk in DM patients is related to their pro-inflammatory and prothrombotic status. Platelets from diabetic subjects show increased adhesiveness and an exaggerated aggregation. Reduced responsiveness of diabetic patients to antiplatelet therapy has also been documented. The introduction of DES has improved outcomes in diabetic patients. Although DES are now widely used, only limited data are available for systematic evaluation of their safety in this specific population. Importantly, recent data have raised concerns about increased risks of stent thrombosis (ST) and mortality over longer periods of follow-up. This review focuses on the mechanisms leading to the prothrombotic status which characterizes DM patients, and its implications for coronary revascularization outcomes, including stent thrombosis, in particular antiplatelet therapy responsiveness and possible alternatives to improve clinical outcomes.
Diabetes: a prothrombotic state
DM is associated with an increased atherothrombotic risk. Patients with DM and coronary artery disease show a high rate of recurrence after MI.Citation16 Atherothrombotic disease is accelerated in subjects with type 1 and type 2 DM, accompanied by diverse underlying mechanisms, despite the common trace of hyperglycemia. The main feature of type 2 DM is insulin resistance, which precedes the development of hyperglycemia.Citation31 In contrast, in type 1 DM, hyperglycemia is the dominant abnormality with insulin resistance appearing in longer standing patients who develop renal disease.Citation32 Insulin resistance and hyperglycemia have several important effects. altering coagulation and platelet function, contributing to a prothrombotic status.
Insulin resistance determines increased levels of the fibrinolytic inhibitor, Plasminogen Activator Inhibitor-1 (PAI-1), the link between type 2 DM and fibrinolysis suppression.Citation33 Furthermore, insulin resistance is associated with the increased expression and production of different coagulation factors promoting platelet adhesion to the vascular sub-endothelium.Citation34
Insulin resistance affects the cellular phases of hemostasis, also impairing endothelial and platelet function, and endothelial-dependent vasodilatation.Citation35–Citation37. Platelet function is regulated by insulinCitation38,Citation39 which in normal conditions antagonizes the effect of a number of agonists;Citation40 the adhesion or aggregation of platelets is upregulated in insulin-resistant subjects.Citation17,Citation41 Up-regulation of the P2Y12 ADP receptor signaling pathway has been shown in type 2 DM platelets, thus contributing to increased platelet reactivity in these patients.Citation16
The effect of insulin resistance on the function of platelets is related to levels of intracytosolic calcium, a mediator of platelet activation.Citation42 Insulin decreases the intracellular concentration of calcium in platelets of insulin-sensitive subjects in vivo and in vitro, and appears to increase the intra-platelet calcium concentrations in insulin-resistant subjects, promoting platelet aggregation and activation.Citation43
Hyperglycemia in turn affects platelet and endothelial function, participating in the prothrombotic status of these patients. Protein glycation and the formation of advanced glycation end (AGE) products seem to be the underlying mechanisms.Citation44 Endothelial alterations lead to increased production of tissue factor,Citation44 a strong pro-coagulant, and alterations in soluble coagulation and fibrinolytic factors. Hyperglycemia provokes platelet hyperreactivity and enhanced thromboxane biosynthesis. Moreover, glycation of platelet membrane proteins may cause the enhanced expression of receptors such as P-selectin and glycoprotein IIb/IIIa, facilitating platelets interactions. Furthermore, hyperglycemia provokes nonenzymatic glycation of low density lipoprotein (LDL) and very low density lipoprotein (VLDL) which in turn may induce platelet dysfunctionCitation45 ().
Table 2 Insulin resistance and hyperglycemia effects on brinolysis, coagulation, and platelet function
The general influence of platelets abnormalities in DM results in hypersensitivity of diabetic platelets to agonists. Indeed platelets in diabetic subjects appear to be in an activated state even in the absence of vascular injury, and respond more frequently even to subthreshold stimuli, as evidenced by greater expression of the fibrinogen-binding glycoprotein IIb/IIIa receptor, which constitutes the final common pathway of platelet activation and allows for cross-linking of individual platelets by fibrinogen molecules, and formation of thrombus.Citation46
Current antiplatelet therapy options
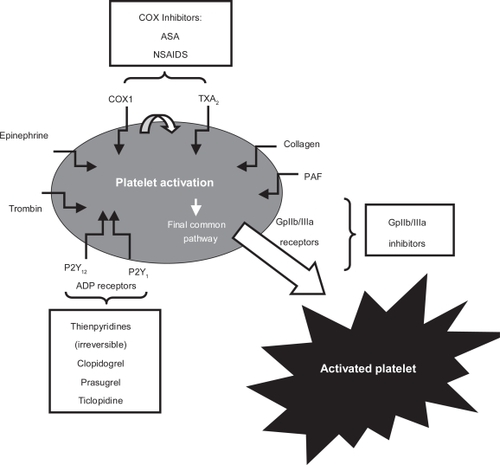
The complexity of platelet activation and subsequent aggregation provides many potential sites for inhibition. Three classes of platelet-inhibiting drugs, ASA, thienopyridines and platelet glycoprotein IIb/IIIa inhibitors, are most commonly used to prevent and to treat disorders of arterial vascular thrombosis, with different mechanisms of action. ASA inhibits thromboxane-A2 (TXA2) production; thienopyridines, clopidogrel and ticlopidine, antagonize ADP-induced activation; and GPIIb/IIIa receptor antagonists (abciximab, tirofiban, and eptifibatide) prevent platelet fibrinogen binding (). The mechanism of action of ASA and thienopyridines and the role of DM in antiplatelet therapy resistance will now be discussed.
Aspirin
ASA is more than 100 years old and provides marked benefits in the primary and secondary prevention of coronary, cerebral, and peripheral vascular disease.Citation47 ASA inhibits TXA2 production by acetylating a serine residue at position 529 within the active site of the enzyme cyclo-oxygenase.Citation48 Inhibition is irreversible and lasts for the lifespan of the platelet (7–10 days).Citation49 The inhibition of TXA2 production prevents TXA2-mediated granule release and aggregation, while aggregation via thromboxane- independent mechanisms, such as that induced by thrombin or elevated shear stress, can still occur. It is the first antiplatelet agent of choice for secondary prevention of ischemic events in patients with atherothrombotic disease, including patients with DM. The American Diabetes Association (ADA) recommends the use of ASA as a secondary prevention measure in diabetic patients with atherosclerotic disease.Citation50 This recommendation is supported by data from two large meta-analyses of major secondary prevention trials by the Antithrombotic Trialists’ Collaboration.Citation47,Citation51
The use of ASA in primary prevention in the general population is still somewhat controversial, although experts agree on its usefulness for primary prevention in patients with DM. The ADA recommends 81–325 mg/day of enteric-coated ASA as a preventive strategy in high-risk diabetic individuals, defined by these risk factors:Citation50
family history of coronary artery disease
cigarette smoking
hypertension
weight >120% of ideal body weight
micro- or macroalbuminuria
total cholesterol > 200 mg/dL (LDL cholesterol >100, HDL cholesterol < 55 in women and < 45 in men, and triglycerides > 200)
The American Heart Association (AHA) has issued similar guidelines and recommends 75–160 mg/day of ASA as primary prevention in high-risk individuals, defined as those with a 10-years risk of coronary artery disease over 10%.Citation52
The Primary Prevention Project evaluated low dose ASA (100 mg/day) for the prevention of cardiovascular events in almost 4500 individuals with one or more risk factors: it showed that after a mean follow-up of 3.6 years, ASA reduced the frequency of cardiovascular death and total cardiovascular events.Citation53 In a larger population (n = 22,701) of healthy men including 533 diabetics, the US Physicians’ Health Study found a significantly lower incidence of MI among DM subjects on ASA therapy than among those on placebo therapy.Citation54 These results are also supported by the Early Treatment Diabetic Retinopathy Study (ETDRS), which enrolled type 1 and type 2 diabetic men and women, about 48% of whom had a history of cardiovascular disease. This study, a primary and secondary prevention trial, showed that the relative risk of MI in the first 5 years in those randomized to ASA therapy was significantly lower than those randomized to placebo.Citation55 Finally, the Hypertension Optimal Treatment (HOT) study, which examined antihypertensive treatment in 18,790 individuals, 1501 of whom had DM, showed that ASA therapy resulted in an additional 15% reduction in the risk of cardiovascular events over that seen with antihypertensive therapy.Citation56 These studies support the aforementioned AHA guidelines.
ASA “resistance”
Despite the use of ASA, a high percentage of patients still suffer from atherothrombotic complications, giving rise to the concept of “ASA resistance”. Various studies have validated the relationship between ASA resistance and the risk of ischemic events. However, the definition of the ASA resistance phenomenon remains controversial. Strictly speaking, “resistance” is defined as the failure of a specific antiplatelet agent to inhibit its target. Thus ASA resistance should be defined as the failure of ASA to block arachidonic acid-induced platelet aggregation, inhibiting production of platelet thromboxane A2.Citation57 In the scientific literature, the term resistance has been applied to failure to prevent occurrence of atherothrombotic vascular events in patients taking ASA (or other antiplatelet agents). However this phenomenon should more appropriately be defined as “therapeutic failure”.Citation58 Indeed numerous pathways are involved in thrombotic events, which therefore cannot be explained by inadequate inhibition of that target of an individual antiplatelet agent. Several laboratory assays have been used to explore for ASA resistance. Moreover, many of the currently available assays are also sensitive to other variables; inter-test correlations have seldom been reported, resulting in uncertainty about the capacity of these tests to detect ASA failure to the extent comparable with that of optical aggregation, considered by many experts to be the gold standard (). This uncertainty may explain why the reported range of ASA resistance varies broadly, from 5% to 40%, depending on the assay used for identification and the population studied.Citation59–Citation61 Various reports have shown that when responsiveness to aASA is assessed using COX-1 specific assays, resistance to ASA is virtually absent, which is primarily the result of patient noncompliance with treatment.Citation62 The redundancy of platelet activation pathways and receptors, not inhibited by ASA, contribute to the presence of variability of ASA-induced antiplatelet effects when using non-COX-1 specific assays. More specifically, pathways involving non-TXA2-dependent activators such as thrombin, ADP, epinephrine, and collagen can bypass the ASA-mediated inhibitory effect leading to platelet activation and thrombosis.Citation63 Catecholamine-induced platelet aggregation is one such pathway that might not be adequately inhibited by ASA. Among patients with a previous MI, ASA has reportedly achieved adequate antiplatelet effects at rest, but failed to inhibit exercise-induced increases in platelet aggregation.Citation64 Similarly, stimulation of P2Y1/P2Y12 receptors by ADP, of α2β1 integrin and GPVI by collagen, and of PAR1/PAR4 receptors by thrombin on platelet membrane, leads to platelet activation in spite of adequate COX-1 inhibition.Citation65 All these pathways are increased in DM patients, because of a greater prevalence of ASA “resistance” when assessed with non-COX-1 specific assays.Citation66–Citation68 The concomitant administration of commonly used analgesics may modulate the effect of low-dose ASA. A clinical dosing regimen of ibuprofen may competitively inhibit the sustained inhibitory effect of COX-1 on platelets.Citation69 An imbalance between oxidants and antioxidants has also been suggested as an influence on aASA reactivity: Cipollone and colleagues demonstrated that increased nonenzymatic formation of isoprostanes, particularly F2-isoprostanes like 8-iso-PGF2α may provide an important biochemical link between an altered oxidant/antioxidant balance and ASA-insensitive TX biosynthesis in patients with unstable angina.Citation70
Table 3 Tests used to evaluate antiplatelet effect of ASA
The role of genetics in a patient’s response to aASA is controversial, because polymorphisms of platelet membrane glycoproteinsCitation71 of von Willebrand factor or of the collagen receptor gene have been associated with ASA resistance.Citation72 How the concomitant presence of diabetes and these genetic polymorphisms affects the prevalence of ASA resistance remains unknown.
With all these limitations, subgroups of DM patients have been considered clinically unresponsive to the cardio-protective effects of ASA. The Heart Outcomes Prevention Evaluation trial, for example, demonstrated a 50% higher rate of cardiovascular events in those with, compared with those without, DM despite ASA therapy.Citation73 In the Primary Prevention Project, ASA use was not associated with cardiovascular protection in those with DM, but a 40% decrease in cardiovascular death in those without.Citation74
Thienopyridines
Thienopyridines are orally-active antagonists of the platelet ADP (P2Y12) receptor.Citation75 Clopidogrel and ticlopidine are the two currently available thienopyridines, although clopidogrel is the thienopyridine of choice because it has a more favorable safety profile than ticlopidine.Citation76 The antiplatelet effects of thienopyridines are irreversible due to the formation of a disulfide bond with the receptor and last for the lifespan of the platelets. They are inactive prodrugs that are converted by the hepatic cytochrome P450 system into an active thiol metabolite, which interacts with the P2Y12 receptor, in an inactive carboxy metabolite. These agents are of benefit in coronary, peripheral, or cerebrovascular atherosclerosis, and their combination with ASA is routine in patients undergoing PCI and in patients with acute coronary syndromes.Citation77–Citation79 Current guidelines for the management of unstable angina and non-ST elevation MI (NSTEMI) recommend promptly adding clopidogrel to ASA in patients presenting with these clinical syndromes.Citation30 Furthermore, clopidogrel should be used in patients being treated with medical therapy or coronary revascularization for up to 9–12 months. Current guidelines also recommend administering clopidogrel to patients who are hypersensitive or intolerant to ASA.Citation77 Clopidogrel has also been approved recently by the US Food and Drug Administration for patients with STEMI, based on the results of two large-scale clinical trials.Citation77,Citation80,Citation81
The CAPRIE (Clopidogrel versus ASA in Patients at Risk of Ischemic Events) trial was a randomized, blinded trial, involving more than 19,000 patients, designed to assess the relative efficacy of clopidogrel and ASA in reducing the risk of a composite outcome cluster of ischemic stroke, MI, or vascular death.Citation82 A retrospective analysis of the CAPRIE study showed, for the first time, the superiority of clopidogrel compared with ASA in the diabetic subgroup. This superiority was attributed to the more potent antiplatelet effect of clopidogrel, and its more efficient inhibition of hyperreactive diabetic platelets: only 15.6% of diabetic patients on clopidogrel therapy developed the composite vascular primary endpoint vs 17.7% of those on ASAtherapy alone (p = 0.042); the insulin subgroup showed greater absolute reduction.Citation81,Citation83,Citation84
The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial evaluated the efficacy and safety of clopidogrel when given with ASA to patients suffering from acute coronary syndromes, without ST-segment elevation for 3 to 12 months (n = 12562).Citation52 In this trial, the rate of primary outcome (composite vascular death, MI or stroke) was much higher in the diabetic cohort of patients. The use of clopidogrel in this subgroup reduced the rate of this endpoint (14.2% rate of primary endpoint in diabetic cohort on clopidogrel vs 16.7% in diabetic cohort on placebo) without it reaching statistical significance.Citation79 The high event rates may in part be attributed to the persistence of increased platelet reactivity in DM patients even when on dual antiplatelet therapy compared with non-DM patients.
Clopidogrel response variability
The methodology most commonly reported in the literature to measure clopidogrel response is conventional light transmittance aggregometry, in which platelet-rich plasma is prepared from blood usually anticoagulated with citrate and then stimulated with ADP.Citation85 Notably, the degree of platelet responsiveness in patients treated with clopidogrel has been found to follow a normal bell curve.Citation85,Citation86 The concept of variability in response to clopidogrel has long been recognized during investigations of platelet reactivity, especially after elective coronary stent implantation.Citation87 Several studies investigated the use of a 300 mg loading dose of clopidogrel immediately after stenting and found highly variable responses among individuals.Citation88–Citation90 Although higher loading dose regimens are associated with better and faster response profiles, a broad variability in the antiplatelet effects continues to be observed.Citation91,Citation92 The durability of platelet inhibition by clopidogrel has also been studied showing the sustained antiplatelet effect of clopidogrel after 5 days, but also a significant heterogeneous response to the medication.Citation93 Thus the antiplatelet effects after both the acute and chronic phases of clopidogrel therapy vary.Citation86 Importantly, increased rates of coronary stent thrombosis and recurrent ischemic events after PCI, have been noted in poor clopidogrel responders. Variability in clopidogrel response is a multifactorial process, in which clinical, cellular, and genetic factors are involved.Citation86 Among the clinical factors, DM has been associated with a greater prevalence of poor responsiveness.Citation16 In particular, diabetic patients have been shown to have a poor response to clopidogrel in both the acute and chronic phases of therapy.Citation67 Of note, insulin-requiring diabetics are those who persist with the strongest platelet reactivity despite dual antiplatelet therapy.Citation23 DM patients usually have a poor response to dual antiplatelet therapy, but a variety of antiplatelet effects has also been observed in these patients.Citation67 Recent findings have shown that enhanced platelet reactivity selectively determined in DM patients, enables identification of those with a greater long-term risk of atherothrombotic events.Citation19
Overall, the persistence of elevated platelet reactivity, and reduced response to ASA and clopidogrel therapy, enhance the atherothrombotic risk of DM patients. Multiple causes have been suggested. Poor glycemic control is an important cause of increased platelet reactivityCitation18,Citation23,Citation94 In this way, platelet reactivity can be reduced with tight control of glucose levels.Citation95 Other mechanisms intrinsic to the diabetic platelet, which involve intracellular signaling pathways, play a critical role in platelet reactivity. These may include increased oxidative stress leading to enhanced peroxidation of arachidonic acid to form biologically active isoprostanes,Citation18 increased platelet turnover,Citation67 increased cytosolic levels of calcium,Citation96,Citation97 insulin resistance, and upregulation of the P2Y12pathway.Citation67,Citation98
Treating ASA and clopidogrel resistance
The treatment for failed antiplatelet therapy, especially in diabetic patients, is as yet undefined. Initially, physicians should ensure patient compliance, and minimize drug–drug interactions. In diabetic patients, physicians must also establish optimal control of glucose levels, cholesterol levels, and blood pressure, thus minimizing platelet reactivity.
The optimal dose is controversial. There is no good evidence to date that increasing the ASA dose would be useful, especially because of an increased risk of bleeding.Citation99 Of note, increasing the dose of ASA is not associated with further inhibition of COX-1.Citation99 Increasing the loading or maintenance doses of clopidogrel may be an option.Citation25,Citation99 Increasing the loading dose increases drug responsiveness and has been associated with improved clinical outcomes.Citation100,Citation101 This approach is valid only for the acute phase of treatment, however, because patients must rely on daily maintenance therapy for long-term prevention of ischemic events. The Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study evaluated the functional impact of a 150-mg maintenance dose of clopidogrel compared with a standard 75-mg dose selectively in type 2 DM patients with a suboptimal response to standard dose therapy. High maintenance therapy was associated with enhanced antiplatelet effects compared with the 75-mg dose.Citation25 Although a high dose resulted in marked platelet inhibition, many patients remained above the therapeutic threshold of post-treatment platelet reactivity used in this study, suggesting the need for alternative antithrombotic regimens in these high-risk patients. This issue has prompted recent interest in triple antiplatelet therapy in DM patients, using cilostazol in addition to ASA and clopidogrel. Triple therapy has been shown to be associated with a reduced risk of stent thrombosis.Citation102,Citation103 In the bare metal stent (BMS) era, triple therapy was shown to be beneficial for high risk patients, including diabetics, in reducing restenosis rates.Citation102 In the DES era, recent findings from the DECLARE-DIABETES study showed triple therapy to be associated with reduced target lesion revascularization (TLR) and major adverse cardiac events (MACE) at 9 months.Citation104 The mechanisms underlying this benefit may arise from the greater antiplatelet effects achieved, as well as the effects of cilostazol on endothelial cells and smooth vascular muscle cells. The OPTIMUS-2 study evaluated the functional impact of adding cilostazol to ASA and clopidogrel therapy in type 2 DM patients. The study showed that cilostazol compared with placebo was associated with marked inhibition of P2Y12 signaling.Citation105 Current guidelines specify a class IIb indication with a level of evidence C that the dose of clopidogrel can be increased to 150 mg per day if <50% inhibition of platelet aggregation is demonstrated only in patients in whom stent thrombosis may be catastrophic or lethal (such as unprotected left main, bifurcating left main and last patent coronary vessel).Citation106 However, although the use of a 150-mg maintenance dose of clopidogrel in patients with type 2 DM and with <50% platelet inhibition has been shown to be associated with enhanced antiplatelet effects, these effects are nonuniform and many patients persist with inadequate platelet inhibition.Citation107 Probably the use of more potent P2Y12 inhibitors, with their uniform and potent effect, could help us resolve this problem. Prasugrel is a third-generation P2Y12 inhibitor, with more potent and less variable antiplatelet effects compared with clopidogrel.Citation108,Citation109 Recently, the TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction) trial showed significantly reduced rates of ischemic events, including stent thrombosis, in patients presenting with acute coronary syndromes undergoing PCI treated with prasugrel compared with clopidogrel.Citation110 The net clinical benefit achieved with prasugrel in the general study population still has statistical significance for better clinical outcomes although diminished by an increased risk of bleeding. Importantly, in this trial the greatest risk reduction (rate of primary end-point, defined as death from cardiovascular causes, nonfatal MI or nonfatal stroke, in diabetic patients on prasugrel 12.2% vs diabetic patients on clopidogrel 17.0% with 30% relative risk reduction) was observed in the diabetic population (n = 3146). Importantly, in these patients prasugrel was not associated with an increased risk of major bleedings compared with clopidogrel. The functional impact of prasugrel vs clopidogrel among selected diabetic patients is currently being evaluated in the OPTIMUS-3 study.
Percutaneous coronary revascularization in diabetics
Diabetic patients have more progressive, diffuse, and multivessel coronary disease compared with nondiabetics,Citation111,Citation112 and have been shown to have worse outcomes after both PCI, especially with BMS, and surgery, compared with nondiabetic patients.Citation113 This prognosis includes a 35% to 45% higher incidence of TLR and a 33% to 86% higher incidence of angiographic restenosis compared with nondiabetic patients with BMS.Citation113,Citation114 The advent of DES has completely changed the scenario of percutaneous revascularization in diabetic patients. Most clinical experience with DES comes from the Cypher® (sirolimus) and Taxus® (paclitaxel) stents, introduced in 2002 and 2003, respectively. Compared with BMS, the first generation DES significantly reduced restenosis rates and MACE, and show significantly lower rates of TLR, target vessel revascularization (TVR) and target vessel failure (TVF).Citation8,Citation115
Despite the routine use of DES in diabetic patients, only limited data are available for systematic evaluation of their long term safety in this population. Most of the data come from published subgroup analysis of randomized trials between DES and BMS, and registry data from single or multiple centers.Citation115 Five studies, that is, DIABETES,Citation116 ISAR-DIABETES,Citation117 a recently published head-to-head comparison of sirolimus-eluting stent (SES) and paclitaxel (PES)-eluting stent in 120 diabetics patients,Citation118 the SCORPIUS Trial,Citation119 and the DESSERT Study,Citation120 focused solely on diabetics ().
Table 4 Studies performed in diabetic patients treated with DES
The DIABETESCitation116 (Diabetes and Sirolimus-Eluting Stent) trial is a multicenter, randomized, placebo-controlled trial involving 160 diabetic patients, 80 of whom were treated with BMS and 80 with SES. Late lumen loss (LLL) assessed by QCA at 9 month follow-up was the primary end-point. The SES treated group showed a significant reduction of LLL (relative reduction 87%). The study considered a sub-randomization according to the type of diabetes; the SES benefit was independent from diabetes status. The study also demonstrated similar repeat revascularization rates among both insulin- and noninsulin-treated diabetics; and confirmed the relationship between glycemic control and progression of atherosclerosis. These results with SES have recently been confirmed by two trials especially designed for diabetic patients: the SCORPIUS TrialCitation119 and the Italian Multicenter Randomized DESSERT Study.Citation120
The ISAR (In-Stent Angiographic Restenosis)-DIABETESCitation117 a prospective trial, was designed to show the noninferiority of the PES compared with SES, defined as a difference in the extent of in-segment LLL of no more than 0.16 mm, at 6–8 months follow-up. The study included 250 diabetic patients randomized to receive SES (n = 150) or PES (n = 150). It found that the use of SES in diabetics was associated with a decrease in the extent of late loss, in insulin-treated (p = 0.02) and noninsulin-treated (p = 0.03) patients, suggesting a reduced risk of restenosis, but the study was not sufficiently powered to assess the incidence of clinical restenosis.
Tomai and colleagues compared the efficacy of SES and PES in diabetic patients with multiple de novo coronary artery lesions, to prevent restenosis.Citation118 They randomized 60 patients for a total of 120 lesions (SES, n = 60; PES, n = 60) and concluded that SES, when directly compared with PES, is associated with a decrease in the extent of in-stent LLL at 8 months, suggesting a reduced risk of restenosis.
Although DES reduces angiographic and clinical restenosis compared with BMS, in late 2006 concerns over DES safety emerged. Several studies and meta-analysis suggested increases in adverse clinical end-points with DES, especially after the first year of stent implantation.Citation121,Citation122 The overall rate of acute and subacute ST (occurring within 24 hours or within 30 days after stent implantation respectively) appears to be no different for DES and BMS, but analysis incorporating long-term follow-up has shown a small but finite risk of late ST associated with DES. Of note, despite the small increase in late ST, overall rates of death and MI are similar between DES and BMS.Citation121–Citation124 The magnitude of very late ST is not well defined, but is in the range of 0.2%–0.4% excess events per year after year 1 through year 4.Citation125,Citation126 Importantly, these numbers are from clinical trials and not from real world practice, where patients show more complex scenarios.
Surgery remains the gold standard treatment for multivessel disease in diabetics. The ongoing CARDIA (Coronary Artery Revascularization in DIAbetes) trialCitation127 and the FREEDOM (Future REvascularization Evaluation in Patients with Diabetes mellitus: Optimal management of Multivessel disease) trialCitation128 will shed light on the outcomes of DES implantation in diabetics compared with current surgical techniques.
Stent thrombosis in diabetics: incidence and predictors
ST with BMS and DES often presents in a catastrophic way, by triggering death or acute MI.Citation3,Citation129 The incidence of ST could easily be underestimated, because a patient’s cause of death is not always determined and angiography is not always performed after MI. To control for this possibility, cases in which patients have experienced sudden death or acute MI not definitively proved to be secondary to ST have been adjudicated judged as thrombotic events in studies examining incidence and predictors of ST.Citation3,Citation129 The Academic Research ConsortiumCitation130 developed consensus definitions delineating 3 levels of certainty of ST: definite, probable, and possible. Definite ST involves the pathologic or angiographic confirmation of thrombus within a stent in a patient who presents with an acute coronary syndrome. Probable ST pertains to any unexplained death within 30 days after PCI or any target-vessel acute MI occurring without angiographic confirmation of a culprit lesion. Possible ST includes any unexplained death occurring later than 30 days after the index procedure (). ST is also classified according to time of presentation. Acute ST appears within 24 hours after PCI; subacute ST presents between 24 hours and 30 days; late ST between 1 and 12 months; very late ST occurs later than 1 year.
Table 5 Academic research consortium de nitions of stent thrombosis
A number of factors are associated with DES thrombosis. In a series of 2,229 patients, of whom 29 experienced ST, risk factors included bifurcation lesions, renal failure, PCI in the setting of acute MI and DM.Citation129 The strongest association was between ST and the premature discontinuation of clopidogrel. The etiology of ST is multifactorial.Citation131 Patient, lesion, procedural, and stent characteristics can all play a role. Other factors include mechanisms unique to the DES, namely, hypersensitivity, inflammatory responses, and delayed endothelialization.Citation132 Given that these reactions occur predominantly with late ST, the presumed trigger is hypersensitivity to the stent polymer, because active drug should no longer be present. This phenomenon has not been documented with BMS.
The increased risk in diabetic patients might be related to the pro-inflammatory and prothrombotic status typical of this population along with the more diffuse and aggressive nature of atherosclerosis (longer lesion lengths, smaller vessel size, and greater plaque burden) which might lead to less optimal procedural results.Citation8,Citation133–Citation135
The nonresponsiveness to antiplatelet therapy also has a role: diabetics have been considered clinically unresponsive to the cardioprotective effects of ASA and clopidogrel.Citation16 Patients with ST have high in vitro post-treatment platelet reactivity despite the dual antiplatelet treatment, suggesting that platelet aggregation nonresponsiveness to clopidogrel may be an important cause.Citation136 Buonamici and colleaguesCitation137 assessed whether nonresponsiveness to clopidogrel as revealed by high in-vitro post-treatment platelet reactivity was predictive of DES thrombosis. In their interesting work, a total of 804 patients who had successful SES or PES implantation were assessed for post-treatment platelet reactivity after a loading dose of 600 mg of clopidogrel. Patients with platelet aggregation by 10 μmol adenosine 5-diphosphate ≥70% were defined as nonresponders. All patients received chronic dual antiplatelet treatment (ASA 325 mg and clopidogrel 75 mg daily) for 6 months. The primary end-point was the incidence of definite/probable early, subacute, and late ST at 6-month follow-up. The incidence of 6-month definite/probable ST was 3.1% (). All ST were subacute or late. Of 804 patients, 105 (13%) were not responsive to clopidogrel. The incidence of ST was 8.6% in nonresponders and 2.3% in responders (p < 0.001). The authors concluded that nonresponsiveness to clopidogrel is a strong independent predictor of ST in patients receiving SES or PES. Moreover in this study, DM was associated with low response (overall 169 DM patients: 19% were responders and 36% nonresponders, p > 0.001).
Table 6 ST classification according to time of presentation
To date, few large-scale studies have focused on very late ST, later than 1 year after DES implantation. Specific predictors for late ST have not yet been identified. Moreover, DES thrombosis in randomized trials could not be comparable to those observed in clinical practice, frequently including off-label indications. Interestingly the Multicenter Spanish Registry ESTROFACitation138 was designed to assess the incidence, predictors, and outcome of DES thrombosis (angiography-documented) in real-world clinical practice, with 3 years follow-up. In a total of 23,500 patients treated with DES the cumulative incidence of ST was 2% at 3 years. Antiplatelet treatment had been discontinued in 95 cases (31.6%). No differences in incidences were found among stent types. Independent predictors for subacute ST analyzed in a subgroup of 14,120 cases were diabetes, renal failure, acute coronary syndrome, STEMI, stent length, and left anterior descending artery stenting; while predictors for late ST were STEMI, stenting in left anterior descending artery, and stent length. The authors concluded that patient profiles differed between early and late ST.
Daemen and colleaguesCitation121 performed a large multicenter cohort study assessing all angiographically documented ST, after unrestricted use of SES and PES (8146 patients; SES n = 3823; PES n = 4323) between 2002 and 2005. Their purposes were to estimate the incidence and time course of ST with DES in routine clinical practice; identify predictors and differences between early (<30 days) and late (>30 days) ST; and assess differences between SES and PES. They observed angiographically documented ST in 152 patients (cumulative incidence at 3 years 2.9%). Early ST was noted in 91 (60%) patients, and late ST in 61 (40%) patients. Late ST occurred steadily at a constant rate of 0.6% per year up to 3 years after stent implantation. Incidence of early ST was similar for SES (1.1%) and PES (1.3%), but late ST was more frequent with PES (1.8%) than with SES (1.4%; p = 0·031). At the time of ST, dual antiplatelet therapy was being taken by 87% (early) and 23% (late) of patients (p < 0·0001). Independent predictors of overall ST were acute coronary syndrome at presentation (HR 2.28, 95% CI 1.29–4.03) and diabetes (2.03, 1.07–3.83). The results of the present study suggest that late ST with DES occurs more frequently than expectedCitation116 and that rates increase steadily during long-term follow-up. The authors suggest that sustained occurrence over a long-term period might be explained in part by the delayed healing response after implantation of DES. This maybe due to the delayed re-endothelization and hypersensitivity reactions to the antiproliferative drugs, or more probably, to the synthetic polymers.Citation131,Citation132 This study confirms the predictive value of diabetes and acute coronary syndrome at presentation.
A specific assessment of DES safety in DM has yet to be demonstrated. Whether DES are similarly safe and effective in the higher risk cohort of diabetic patients remains controversial. Several meta-analysis and registries have been performed showing contrasting data.
Kumbhan and colleaguesCitation139 conducted a meta-analysis including randomized trials comparing either PES or SES with a BMS or PES with SES, in diabetic patients during a follow-up of a maximum of 12 months. A total of 16 studies were identified, which included 2951 diabetic patients. A reduction in target lesion revascularization (TLR) was found with DES compared with BMS (RR 0.35, 95% CI 0.27–0.46, P < 0.001). Similar reductions were noted in the incidence of MACE (RR 0.42, 95% CI 0.31−0.56, P <0.001), in-segment restenosis (RR 0.31, 95% CI 0.25–0.40, P < 0.001), and non-Q-wave MI (RR 0.57, 95% CI 0.32–0.99, P = 0.046). Event rates were similar for Q-wave MI (RR 0.72, 95% CI 0.25–2.07, P = 0.54), death (RR 0.64, 95% CI 0.32–1.28, P = 0.20), and ST (RR 0.41, 95% CI 0.13–1.27, P = 0.12).
The specific safety of SES compared with BMS has been addressed in a recent meta-analysis by Spaulding and colleagues.Citation140 The authors reported greater long-term mortality in patients with DM treated with SES compared with those treated with BMS, an effect that was absent in patients without DM. These data must be interpreted with caution: in the studies analyzed, survival among BMS treated patients was far better than expected, which may have accounted for the observed differences. Besides, spurious results due to the modest-sized diabetic cohort (n = 428) in this series may be another cause of error. On the other hand, the analysis of 1-year data collected by the e-Cypher registry Citation141 suggests a high degree of safety of SES, with a rate of ST similar to that observed in randomized trials. Insulin treated DM among other factors was recognized as a clinical predictor of stent thrombosis at 12 months.
The EVASTENT Matched-Cohort RegistryCitation142 assessed the frequency and causes of ST specifically in diabetic and nondiabetic patients after SES implantation. In this matched multicenter cohort registry of 1731 during a 1-year follow-up, MACE occurred in 78 patients (4.5%), cardiac death in 35 (2.1%), and stent thrombosis in 45 (2.6%): 30 definite, 23 subacute, and 22 late, including 9 at 6 months. In univariate analysis, the 1-year ST rate was 1.8 times higher in diabetic than in nondiabetic patients (3.2% vs 1.7%; log rank p < 0.03); diabetic patients with multivessel disease experienced the highest rate and nondiabetic single-vessel disease patients the lowest (4.3% vs 0.8%; p < 0.001). In multivariate analysis, in addition to the interruption of antithrombotic treatment, independent ST predictors were previous stroke, renal failure, lower ejection fraction, calcified lesion, length stented, and insulin-requiring diabetes.
The safety of PES vs BMS has also been assessed. Analysis of the five pivotal PES trials focused specifically on the diabetic patientCitation143 demonstrated similar rates of death, MI, and ST with both stents at 4-year follow-up. Patients treated with PES and BMS had similar baseline characteristics among both the diabetic and nondiabetic cohorts in these trials. At 4-year follow-up, there were no significant differences between PES and BMS among diabetic patients in the rates of death (8.4% vs 10.3%, respectively, p = 0.61), MI (6.9% vs 8.9%, p = 0.17), or ST (1.4% vs 1.2%, p = 0.92). ST was adjudicated by the Academic Research Consortium (ARC) as not being restricted to the angiographically determined events. Treatment of diabetic patients with PES compared with BMS was associated with a significant and durable reduction in TLR over the 4-year follow-up period (12.4% vs 24.7%, p < 0.0001). The relative safety and efficacy of PES compared with BMS in diabetic patients extended to both those requiring and not requiring insulin. The rates of both efficacy and safety end-points may vary in a less selected patient population in which stents are implanted in more complex and higher-risk situations. Frequent scenarios in DM patients are: true bifurcation lesions, multivessel disease, and acute MI. Interestingly, Kuchulakanti and colleaguesCitation144 assessed the correlates and outcomes of angiographically proven ST with DES (PES and SES) in a population of 2974 consecutive patients. Compared with patients without ST, patients with ST had a higher frequency of diabetes, acute post-procedural renal failure, and chronic renal failure. There were more bifurcation lesions, type C lesions, and a trend for smaller-diameter stents. Discontinuation of clopidogrel was more frequent in these patients (36.8% vs 10.7%; p = 0.0001). The author stressed that careful management is warranted in patients with renal failure and in those undergoing treatment for in-stent restenosis and bifurcations. These data reflect outcomes from a real world population. Specific and sufficiently powered, well-defined, randomized studies are needed to understand the real safety of DES in DM.
Clinical and therapeutic implications
The concern of late ST has emerged with the widespread use of DES, during the last 5 years. To date long-term safety has not been addressed in a powered, randomized trial, specifically designed for diabetic patients. These aforementioned studies, and meta-analysis results, seem to minimize the problem, showing similar rates of late ST, but these data must be interpreted with caution for many reasons. The heterogeneity and end-point definitions differed among the pooled trials; diabetic patients were often a subgroup in the study population because these trials were not designed to study diabetics exclusively. Because original data were not accessed, no information on patient glycemic control was available, and discrepancies arising from variability in the definition of diabetes, and ST, could not be resolved.
Available data so far show that diabetes is an independent predictor of ST; despite the use of dual antiplatelet therapy, patients with DM have a higher risk of developing adverse clinical outcomes. Recent observations suggest that this risk may relate to platelet dysfunction typical of DM, leading to inadequate platelet inhibition.
Research results in general imply a complex and multi-disciplinary treatment for this type of patient. When PCI is indicated for a diabetic patient, DES is the device of choice. At the same time, tight glycemic control and compliance with guidelines for antiplatelet drug management are mandatory. A specific antiplatelet regimen in DM may be useful. Novel antiplatelet agents with a strong action against the hyper-activated “diabetic platelet” may be needed.
Conclusions
Coronary revascularization in diabetic patients remains a challenge. The introduction of DES has improved PCI outcomes, but the problem of atherothrombotic complications, including ST, persist for which DM is recognized as an independent predictor. Inadequate responsiveness to currently available antiplatelet agents, including ASA and clopidogrel, may contribute to these poor outcomes. Novel antiplatelet agents under advanced clinical investigation may provide future treatment alternatives to tackle the “diabetic platelet”. Indeed, dedicated studies on selected diabetic patients are warranted, to understand the real magnitude and significance of the problem, and find an appropriate solution.
Disclosure
The authors report no conflicts of interest in this work.
References
- Mc FaddenEPStabileERegarELate thrombosis in drug-eluting stent after discontinuation of antiplatelet therapyLancet20043641519152115500897
- MorenoRFernandezCHernandezRDrug-eluting stent thrombosis: results from a pooled análisis including 10 randomized studiesJ Am Coll Cardiol20054595495915766835
- IakovouISchmidtTBonizzoniEIncidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stentsJAMA20052932126213015870416
- OngATMcFaddenEPRegarEde JaegerePPvan DomburgRTSerruysPWLate angiographic stent thrombosis (LAST) events with drug-eluting stentsJ Am Coll Cardiol2005452088209215963413
- SteinhublSRBergerPBMannJTIFryETDeLagoAEarly and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trialJAMA20022882411242012435254
- CheneauEleborgneLMintzGSKotaniJPichardADSatlerLFCanosDCastagnaMWeissmanNJWaksmanRPredictors of subacute stent thrombosis: results of a systematic intravascular ultrasound studyCirculation2003108434712821553
- FujiiKCarlierSGMintzGSStent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound studyJ Am Coll Cardiol20054599599815808753
- ColaCSabatéMRevascularization in high risk patients: diabetes mellitusMinerva Cardioangiol20075555757717912163
- GurbelPACallahanKPMalininAISerebruanyVLGillisJCould stent design affect platelet activation? Results of the Platelet Activation in STenting (PAST) studyJ Invasive Cardiol20021458458912368510
- NebekerJRVirmaniRBennettCLHypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and reports (RADAR) projectJ Am Coll Cardiol20064717518116386683
- SteffelJLatiniRAAkhmedovARapamycin, but not FK-506, increases endothelial tissue factor expression: implications for drug-eluting stent designCirculation20051122002201116172265
- StahliBECamiciGGSteffelJPaclitaxel enhances thrombin-induced endothelial tissue factor expression via c-Jun terminal NH2 kinase activationCirc Res20069914915516794185
- FinnAVKolodgieFDHarnekJDifferential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stentsCirculation200511227027815998681
- MatterCMRozenbergIJaschkoAEffect of tacrolimus or sirolimus on proliferation of vascular smooth muscle and endothelial cellsJ Cardiovasc Pharmacol20064828629217204907
- ParkDWParkSWParkKHFrequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-upAm J Cardiol20069835235616860022
- AngiolilloDJAntiplatelet therapy in type 2 diabetes mellitusCurr Opin Endocrinol Diabetes Obes20071412413117940430
- VinikAErbasTSun ParkTNolanRPittengerGPlatelet dysfunction and type II diabetesDiabetes Care2001241476148511473089
- FerroniPBasiliSFalcoADavìGPlatelet activation in type 2 diabetes mellitusJ Thromb Haemost200421282129115304032
- AngiolilloDJBernardoESabateMImpact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery diseaseJ Am Coll Cardiol2007501541154717936152
- WatalaCGolanskiJPlutaJReduced sensitivity of platelet from type 2 diabetic patients to acetylsalicylic (aspirin)-its relation to metabolic controlJ Thromb Res2004113101113
- MoriTAVandongenRDouglasAJMcCullochRKBurkeVDifferential effect of aspirin on platelet aggregation in IDDMDiabetes1992412612661551486
- AngiolilloDJFernandez-OrtizABernardoEInfluence of aspirin resistance on platelet function profiles in patients on long-term aspirin and clopidogrel after percutaneous coronary internventionAm J Cardiol200697384316377281
- AngiolilloDJBernardoERamirezCInsulin therapy is associated with platelet dysfunction in patients with type 2 diabetes mellitus on dual oral antiplatelet treatmentJ Am Coll Cardiol20064829830416843179
- AngiolilloDJFernandez-OrtizABernardoEClopidogrel responders and interindividual variability in platelet inhibition following a high clopidogrel loading dose regimen during coronary interventionEur Heart J2004251903191015522469
- AngiolilloDJShoemakerSBDesaiBRandomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery diseaseCirculation200711570871617261652
- KingHAubertREHermanWHGlobal burden of diabetes, 1995–2025: prevalence, numerical estimates, and projectionsDiabetes Care199821141414319727886
- NathanDMLong-term complications of diabetes mellitusN Engl J Med1993328167616858487827
- BrandFNAbbottRDKannelWBDiabetes, intermittent claudication, and risk of cardiovascular eventsDiabetes1989385045092925008
- StamlermJVaccaroONeatonJDWentworthDDiabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention TrialDiabetes Care1993164344448432214
- PahorMPsatyBMFurbergCDNew evidence on the prevention of cardiovascular events in hypertensive patients with type 2 diabetesJ Cardiovasc Pharmacol199832Suppl 2S18S239736437
- ReavenGRole of insulin resistance in human diseaseDiabetes198837159516073056758
- GrantPJDiabetes mellitus as a prothrombotic conditionJ Inter Med2007262157172
- Juhan-VagueIRoulCAlessiMArdissoneJHeimMVaguePIncreased plasminogen activator inhibitor activity in non insulin dependent diabetic patients. Relationship with plasma insulinThromb Haemost1989613703732678583
- WagnerDCell biology of von Willebrand factorAnnu Rev Cell Biol199062172462275814
- CorrettiMAndersonTBenjaminEGuidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task ForceJ Am Coll Cardiol20023925726511788217
- PerticoneFCaravoloRCandigliotaMObesity and body fat distribution induce endothelial dysfunction by oxidativestress. Protective effect of vitamin CDiabetes20015015916511147782
- TaylorAPathophysiology of hypertension and endothelial dysfunction in patients with diabetes mellitusEndocrinol Metab Clin North Am20013098399711727407
- GryglewskiRBottingRVaneJMediators produced by the endothelial cellHypertension1988125305483060428
- HajekAJoistJBakerRJarrettLDemonstration and partial characterization of insulin receptors in human plateletsJ Clin Invest19796310601065447828
- TrovatiMAnfossiGInfluence of insulin and insulin resistance on platelet and vascular smooth muscle cell functionJ Diabetes Complications200216354011872364
- WesterbackaJYki-JarvinenHRissanenAVehkavaaraSSyrjälä MRLInhibition of platelet-collagen interaction. An in vivo action of insulin is abolished by insulin resistance in obesityArterioscler Thromb Vasc Biol20022216717211788478
- KrollMSchaferABiochemical mechanisms of platelet activationBlood198974118111952669994
- BaldiSNataliABuzzigoliGGavlanASironiAFerranniniEIn vitro effect of insulin on intracellular calcium concentrations: relation to insulin resistanceMetabolism199645140214078931646
- KhechaiFOllivierVBrideyFEffect of advanced glycation end product-modified albumin on tissue factor expression by monocytesArterioscler Vasc Biol19971728852890
- Lieuw-A-FaMvan HinsberghVTeerlinkTIncreased levels of N(epsilon)-(carboxyethyl) lysine in type 1 diabetic patients with impaired renal function:correlation with markers of endothelial dysfunctionNephrol Dial Transplant20041963163614767019
- WatalaCMay the alterations in lipid fluidity-mediated platelet hypersensitivity contribute to accelerated aging of platelets in diabetes mellitus?Med Hypotheses1991361421451779916
- Antithrombotic Trialists CollaborationCollaborative meta-analyses of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patientsBMJ2002324718611786451
- SmithJBWillisALAspirin selectively inhibits prostaglandin production in human plateletsNat New Biol19712312352375284361
- PatrignaniPFilabozziPPatronoCJSelective cumulative inhibition of platelet thromboxane production by low-dose aspirin in hearlthy subjectsClin Invest19826913661372
- ColwellJAmerican Diabetes Association. Aspirin therapy in diabetes (Position Statement)Diabetes Care200326S87S8812502626
- Antiplatelet Trialists CollaborationCollaborative overview of randomized trials of antiplatelets therapy. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patientsBMJ1994308811068298418
- PearsonTABlairSNDanielsSRAHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update. Consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseasesCirculation200210638839112119259
- Collaborative Group of the Primary Prevention ProjectLow-dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practiceLancet2001357899511197445
- Physicians’ Health Study Research GroupFinal report on the aspirin component of the ongoing Physicians’Health StudyN Engl J Med19893211291352664509
- ETDRS InvestigatorsAspirin effects on mortality and morbidity in patients with diabetes mellitus: Early Treatment Diabetic Retinopathy Study ReportJAMA1992268129213001507375
- HanssonLZanchettiACarruthersSGEffects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the hypertensive optimal treatment randomized trialLancet1998351175517629635947
- PamukcuBA review of aspirin resistance: definition, possible mechanisms, detection with platelet function tests, and its clinical outcomesJ Thromb Thrombolysis20072321322217186390
- BarnesGDLiJKline-RogersEDual antiplatelet agent failure: a new syndrome or clinical nonentity?Am Heart J200715473273517893001
- MacchiLChristiaensLBrabantSResistance to aspirin in vitro is associated with increased platelet sensitivity to adenosine diphosphateThromb Res200210811511912590946
- ZimmermannNWenkAKimUFunctional and biochemical evaluation of platelet aspirin resistance after coronary artery bypass surgeryCirculation200310854254712874188
- MichelsonADPlatelet function testing in cardiovascular diseasesCirculation2004110e489e49315533872
- TantryUSBlindenKPGurbelPAOverstimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulationJ Am Coll Cardiol2005461705170916256872
- MasonPJJacobsAKFreedmanJEAspirin resistance and atherothrombotic diseaseJ Am Coll Cardiol20054698699316168280
- HurlenMSeljeflotIArnesenHIncreased platelet aggregability during exercise in patients with previous myocardial infarction. Lack of inhibition by aspirinThromb Res20009948749410973679
- KawasakiTOzekiYIgawaTKambayashiJIncreased platelet sensitivity to collagen in individuals resistant to low dose aspirinStroke20003159159510700490
- AngiolilloDJFernandez-OrtizABernardoEInfluence of aspirin resistance on platelet function-profiles in patients on long-term aspirin and clopidogrel after percutaneous coronary interventionAm J Cardiol200697384316377281
- AngiolilloDJFernandez-OrtizABernardoEPlatelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatmentDiabetes2005542430243516046311
- DichiaraJBlindenKPTantryUSThe effect of aspirin dosing on platelet function in diabetic and nondiabetic patients: an analysis from the aspirin-induced platelet effect (ASPECT) studyDiabetes2007563014301917848625
- Catella-LawsonFReillyMKapoorSCyclooxygenase inhibitors and the platelet effects of aspirinN Engl J Med20013451809181711752357
- CipolloneFCiabattoniGPatrignaniPOxidant stress and aspirin insensitivity thromboxane biosynthesis in severe unstable anginaCirculation20001021007101310961965
- MacchiLChristiaensLBrabantSResistance in vitro to low-dose aspirin is associated with platelet PlA1 (GPIIIa) polymorphism but not with C807T (GPIa/IIa) and C-5T kozak (GPIbα) polymorphismsJ Am Coll Cardiol2003421115111913678940
- QuinnMTopolEJCommon variations in platelet glycoproteins: pharmacogenomic implicationsPharmacogenomics2001234135211722284
- EikelboomJWHirshJWeitzJIJohnstonMYiQYusufSAspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular eventsCirculation20021051650165511940542
- SaccoMPellegriniFRoncaglioniMCAvanziniFTognoniGNicolucciAPrimary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trialDiabetes Care2003263264327214633812
- QuinnMJFitzgeraldDJTiclopidine and clopidogrelCirculation19991001667167210517740
- BertrandMERupprechtHJUrbanPGershlickAHCLASSICS InvestigatorsDouble-blond study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS)Circulation200010262462910931801
- SabatineMSCannonCPGibsonCMClopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Addition of Clopidogrel to aspirin and fibrinolytis therapy for myocardial infarction with ST-segment elevation. CLARITY-TIMI 28 InvestigatorsN Engl J Med20053521179118915758000
- SteinhublSRBergerPBMannJTCREDO InvestigatorsClopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trialJAMA20022882411242012435254
- YusufSZhaoFMethaSRClopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevationN Engl J Med200134549450211519503
- BraunwaldEAntmanEMBeasleyJMAmerican College of Cardiology, American Heart AssociationCommittee on the management of Patients with Unstable Angina. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction-summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients with Unstable Angina)J Am Coll Cardiol2002401366137412383588
- ChenZMJiangLXChenYPCOMMIT (ClOpidogrel and metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trialLancet20053661607162116271642
- CAPRIE Steering CommitteeA randomized blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE)Lancet1996348132913398918275
- BhattDMarsoSHirschAAmplified benefit of clopidogrel versus aspirin in patients with diabetes mellitusAm J Cardiol20029062562812231089
- CAPRIE Steering CommitteeA randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events. (CAPRIE)Lancet1996348132913398918275
- HochholzerWTrenkDFrundiDNeumannFJWhole blood aggregometry for evaluation of the antiplatelet effects of clopidogrelThromb Res200711928529116603231
- AngiolilloDJFernandez-OrtizABernardoEAlfonsoFMacayaCBassTACostaMAVariability in individual responsiveness to clopidogrel: clinical implications, management and future perspectivesJ Am Coll Cardiol2007491505151617418288
- WiviottSDAntmanEMClopidogrel resistance: a new chapter in a fast-moving storyCirculation20041093064306715226220
- GurbelPABlindenKPHiattBLO’ConnorCMClopidogrel for coronary stenting. Response, variability, drug resistance and the effect of pre-treatment platelet reactivityCirculation20031072908291312796140
- JaremoPLindahlTLFranssonSGRichterAIndividual variations of platelet inhibition after loading doses of clopidogrelJ Intern Med200225223323812270003
- AngiolilloDJFernandez-OrtizABernardoEIdentification of low responders to a 300 mg clopidogrel loading dose in patients undergoing coronary stentingThromb Res200511510110815567460
- AngiolilloDJFernandez-OrtizABernardoEHigh clopidogrel loading dose during coronary stenting: effects on drug response and interindividual variabiltyEur Heart J2004251903191015522469
- GurbelPABlindenKPHayesKMYohoJAHerzogWRTantryUSThe relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stentingJ Am Coll Cardiol2005451392139615862408
- GurbelPABlindenKPDurability of platelet inhibition by clopidogrelAm J Cardiol2003911123112512714161
- GreselePGuglieminiGDe AngelisMAcute, short-term hyperglycemia enhances shear stress-induced platelet activation in patients with type 2 diabetes mellitusJ Am Coll Cardiol2003411013102012651051
- DavìGAvernaMCatalanoIBarbagalloCMGiovencoECarroccioANotarbartoloAStranoAPlatelet function in patients with type 2 diabetes mellitus: the effect of glycemic controlDiabetes Res Clin Pract198910712
- FerreiraIAEybrechtsKLMockingAIKronerCAkkermanJWIRS-1 mediates inhibition of Ca2+ mobilization by insulin via the inhibitory G-protein GiJ Biol Chem20042793254326414602724
- LiuDMaierAScholzeAHigh glucose enhances transient receptor potential channel canonical type 6-dependent calcium influx in human platelets via phosphatidylinositol 3-kinase-dependent pathwayArterioscler Thromb Vasc Biol20082874675118258814
- FerreiraIAMockingAIFeijgeMAPlatelet inhibition by insulin is absent in type 2 diabetes mellitusArterioscler Thromb Vasc Biol20062641742216339499
- PatronoCGarcia RodriguezLALandolfiRBaigentCLow-dose aspirin for the prevention of atherothrombosisN Engl J Med20053532373238316319386
- SibbingDvon BeckerathOSchomigAkastratiAvon BeckerathNDiabetes mellitus and platelet function after administration of aspirin and a single dose of clopidogrelJ Thromb Haemost200642566256816938125
- LotrionteMBiondi-ZoccaiGGAgostoniPMeta-analysis appraising high clopidogrel loading in patientes undergoing percuntaneous coronary interventionAm J Cardiol20071001199120617920357
- LeeSWParkSWHongMKTriple versus dual antiplatelet therapy after coronary stenting: impact on stent thrombosisJ Am Coll Cardiol2005461833183716286167
- Biondi-ZoccaiGGLotrionteMAnselminoMSystematic review and meta-analysis of randomized clinical trials appraising the impact of cilostazol after percutaneous coronary interventionAm Heart J20081551081108918513523
- LeeSWParkSWKimYHDrug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus the DECLARE-DIABETES trial (A Randomized Comparison of Triple Antiplatelet Therapy with Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation in Diabetic Patients)J Am Coll Cardiol2008511181118718355656
- AngiolilloDJCapranzanoPGotoSA randomized study assessing the impact of cilostazol on platelet function profiles in patients with diabetes mellitus and coronary artery disease on dual antiplatelet therapy: results of the OPTIMUS-2 studyEur Heart J2008292202221118567918
- AngiolilloDJCostaMAShoemakerSBFunctional effects of high clopidogrel maintenance dosing in patients with inadequate platelet inhibition on standard dose treatmentAm J Cardiol200810144044518312754
- VorchheimerDABadimonJJFusterVPlatelet glycoprotein IIb/IIIa receptors antagonists in cardiovascular diseaseJAMA19992811407141410217057
- WiviottSDTrenkDFrelingerALPRINCIPLE-TIMI 44 InvestigatorsPrasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trialCirculation20071162923293218056526
- WallentinLVarenhorstCJamesSPrasugrel achieves greater and faster P2Y12 receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery diseaseEur Heart J200829213018055486
- WiviottSDBrunwaldEMcCabeCHTRITON-TIMI 38 InvestigatorsPrasugrel versus clopidogrel in patients with acute coronary syndromesNew Engl J Med20073572001201517982182
- LempGFVander ZwaagRHughesJPAssociation between the severity of diabetes mellitus and coronary arterial atherosclerosisAm J Cardiol198760101510193314456
- GorayaTYLeibsonCLPalumboPJCoronary atherosclerosis in diabetes mellitus: a population-based autopsy studyJ Am Coll Cardiol20024094695312225721
- FlahertyJDDavidsonCJDiabetes and coronary revascularizationJAMA20052931501150815784875
- KarhaJBhattDLPercutaneous coronary intervention in diabeticsRev Endocr Metab Disord2004527728515211100
- DharamJKumbhaniSMBavryAAKamdarAHeltonTJBhattDLThe effect of drug-eluting stents on intermediate angiographic and clinical outcomes in diabetic patients: Insights from randomized clinical trialsshowedAm Heart J200815564064718371470
- SabatéMJimenez-QuevedoPAngiolilloDJRandomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus-eluting stent (DIABETES) trialCirculation20051122175218316203930
- DibraAKastratiAMehilliJISAR-DIABETES Study InvestigatorsPaclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patientsN Engl J Med200535366367016105990
- TomaiFReimersBDe LucaLHead-to-head comparison of sirolimus- and paclitaxel-eluting stent in the same diabetic patient with multiple coronary artery lesions: a prospective, randomized, multicenter studyDiabetes Care200831151917909090
- BaumgartDKlaussVBaerFSCORPIUS Study InvestigatorsOne-year results of the SCORPIUS study: a German multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patientsJ Am Coll Cardiol2007501627163417950142
- MarestaAVaraniEBalducelliMDESSERT InvestigatorsComparison of effectiveness and safety of sirolimus-eluting stents versus bare-metal stents in patients with diabetes mellitus (from the Italian Multicenter Randomized DESSERT Study)Am J Cardiol20081011560156618489933
- DaemenJWenaweserPTsuchidaKEarly and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort studyLancet200736966767817321312
- StoneGWMosesJWEllisSGSafety and efficacy of sirolimus and paclitaxel-eluting coronary stentsN Engl J Med2007356998100817296824
- KastratiAMehilliJPacheJAnalysis of 14 trials comparing sirolimus-eluting stents with bare-metal stentsN Engl J Med20073561030103917296823
- MauriLHsiehW-HMassaroJMHoKKLD’AgostinoRCutlipDEStent thrombosis in randomized clinical trials of drug-eluting stentsN Engl J Med20073561020102917296821
- StettlerCWandelSAllemannSOutcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysisLancet200737093794817869634
- HodgsonJMStoneGWLincoffAMSociety for Cardiovascular Angiography and InterventionsLate stent thrombosis: considerations and practical advice for the use of drug-eluting stents: a report from the Society for Cardiovascular Angiography and Interventions Drug-eluting Stent Task ForceCatheter Cardiovasc Interv200769332733317219373
- KapurAMalikISBaggerJPThe Coronary Artery Revascularisation in Diabetes (CARDia) trial: background, aims, and designAm Heart J2005149131915660030
- FREEDOM IFuture revascularization evaluation in patients with Diabetes Mellitus: optimal management of multivessel disease. FREEDOM Trial, 2006.
- OngATHoyeAAokiJThirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantationJ Am Coll Cardiol20054594795315766834
- CutlipDEWindeckerSMehranRClinical end points in coronary stent trials: a case for standardized definitionsCirculation20071152344235117470709
- LuscherTFSteffelJEberliFRDrug-eluting stent and coronary thrombosis: biological mechanisms and clinical implicationsCirculation20071151051105817325255
- VirmaniRGuagliumiGFarbALocalized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious?Circulation200410970170514744976
- WestNERuygrokPNDiscoCMClinical and angiographic predictors of restenosis after stent deployment in diabetic patientsCirculation200410986787314757691
- NilesNWMcGrathPDMalenkaDGroup. NNECDSSurvival of patients with diabetes and multivessel coronary artery disease after surgical or percutaneous coronary revascularization: results of a large regional prospective studyJ Am Coll Cardiol2001371008101511263600
- LincoffAMImportant triad incardiovascular medicine: diabetes, coronary intervention and platelet glycoprotein IIb/IIIa receptor blokadeCirculation20031071556155912654616
- GurbelPABlindenKPSamaraWYohoJAHayesKFisshaMZTantryUSClopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST studyJ Am Coll Cardiol2005461827183216286166
- BuonamiciPMarcucciRMiglioriniAImpact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosisJ Am Coll Cardiol2007492312231717572245
- de la Torre-HernándezJMAlfonsoFHernándezFDrug-eluting stent thrombosis: results from the multicenter Spanish registry ESTROFA (Estudio ESpanol sobre TROmbosis de stents FArmacoactivos)J Am Coll Cardiol20085198699018325436
- KumbhanDJBavryAAKamdarARHeltonTJBhattDLThe effect of drug-eluting stents on intermediate angiographic and clinical outcomes in diabetic patients: Insights from randomized clinical trialsAm Heart J200815564064718371470
- SpauldingCDaemenJBoersmaECutlipDESerruysPWA pooled analysis of data comparing sirolimus-eluting stents with baremetal stentsN Engl J Med200735698999717296825
- UrbanPGershlickAHGuagliumiGSafety of coronary sirolimus-eluting stents in daily clinical practice: one-year follow-up of the e-Cypher registryCirculation20061131434144116534015
- MachecourtJDanchinNLablancheJRisk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and non-diabetic patients. The EVASTENT matched-cohort registryJ Am Coll Cardiol20075050150817678732
- KirtaneAJEllisSGDawkinsKDPaclitaxel-eluting coronary stents in patients with diabetes mellitus pooled analysis from 5 randomized trialsJ Am Coll Cardiol20085170871518279734
- KuchulakantiPKChuWWTorgusonRCorrelates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stentsCirculation20061131108111316490815