Abstract
Oxidative stress plays an important role in the progression of vascular endothelial dysfunction. The two major systems generating vascular oxidative stress are the NADPH oxidase and the xanthine oxidase pathways. Allopurinol, a xanthine oxidase inhibitor, has been in clinical use for over 40 years in the treatment of chronic gout. Allopurinol has also been shown to improve endothelial dysfunction, reduce oxidative stress burden and improve myocardial efficiency by reducing oxygen consumption in smaller mechanistic studies involving various cohorts at risk of cardiovascular events. This article aims to explain the role of xanthine oxidase in vascular oxidative stress and to explore the mechanisms by which allopurinol is thought to improve vascular and myocardial indices.
Introduction
The role of oxidative stress in disease has always been a contentious issue because attempts to reduce oxidative stress using antioxidant vitamins such as in the Heart Outcomes Prevention Evaluation (HOPE) study have consistently failed to demonstrate a mortality benefit.Citation1 There are many possible reasons for this, not least because of cohort selection and inappropriate dosing of antioxidants. Even if adequate doses were used, at many magnitudes higher than currently taken as part of multivitamin supplementation, there would be further issues with tolerability and safety. Therefore, recent research has concentrated on mechanisms to reduce the formation of reactive oxygen species (ROS) rather than a scavenging approach to already-formed ROS. The two major ROS generating systems are the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the xanthine oxidase (XO) systems.
Background
ROS consists of molecular oxygen and all of its aerobic cellular metabolites including superoxide (O2–) and hydroxyl radical (OH–). Other substances such as hydrogen peroxide (H2O2), peroxynitrite (ONOO–) and hypochlorous acid (HOCL) have oxidative properties although they are not free radicals.Citation2 McCord and Fridovich were one of the first to describe the deleterious effects of ROS in inflammation in an article describing the enzyme superoxide dismutase.Citation3 Since then, there has been an abundance of research demonstrating the role oxidative stress plays in the pathophysiology of abnormal vasorelaxation. There is also increasing evidence that oxidative stress plays a direct role in myocardial remodeling.Citation4,Citation5 above and beyond its effects on vasomotion.
Enhanced production of reactive oxygen species (ROS) is a major cause of endothelial dysfunction. This has been demonstrated in animal studies using p66SHC knockout mice which demonstrate reduced aortic endothelial cell superoxide production and a 30% prolonged lifespan.Citation6 The precise role of p66SHC remains unclear but in studies looking at its effects on the p53 signaling pathway demonstrate that it is involved in stress-activated p53-induced elevation of intracellular oxidants, apoptosis and regulation of the intracellular redox state.Citation7 This is consistent with the finding that ROS promotes apoptosis and microvascular rarefaction in spontaneously hypertensive rats.Citation8 Furthermore, in chronic heart failure (CHF) patients with the Glu298Asp variant (Glu to Asp amino acid substitution for codon 298 of endothelial nitric oxide synthase (eNOS)) have a significantly shorter eNOS half-life. This translates to a significantly lower event-free survival.Citation9 There is also evidence that the same polymorphism results in blunting of the endothelial-dependent vasodilation in healthy individuals.Citation10
NADPH oxidase catalyzes the reduction of oxygen through electron donation from either NADH (predominantly) or NADPH to generate superoxide (O2–). This system is thought to be the predominant driver of O2– formation. The most potent inducer of the NADPH oxidase system is angiotensin II,Citation11,Citation12 and there are angiotensin II antagonists in the form of ACE inhibitors, angiotensin II receptor antagonists and direct renin inhibitors (DRA) in clinical use. The other enzyme system, XO, has been largely ignored with regards to managing oxidative stress despite the availability of XO inhibitors allopurinol, oxypurinol and febuxostat. This is primarily because most of the data for the beneficial effect of these agents have come from smaller mechanistic studies and the lack of large clinical trial evidence to support widespread use. The two larger studies using the XO inhibitor oxypurinol either showed neutral.Citation13 (in LA-PLATA) or negative results (OPT-CHF)Citation14 which may have been due to an insufficient dose-effect or patient selection. A subsequent follow-up studyCitation14 found a significant benefit in patients with high baseline urate, which could imply either high XO activity or that urate itself is detrimental. A recent study in patients with type II diabetes suggests that urate lowering per se does not improve endothelial function.Citation15
Xanthine oxidase (XO)
Xanthine oxidoreductase (XOR) is part of a group of enzymes known as the molybdenum iron-sulfur flavin hydroxylases. It was first discovered in milk by Schardinger in 1902Citation16 and is thought to be involved in reactions that produce ROS such as nitrite which enable newborn infants to overcome gut-associated bacterial gastroenteritis.Citation17,Citation18 XOR is widely distributed throughout various organs including the liver, gut, lung, kidney, heart, brain and plasmaCitation19 with the highest levels being found in the gut and the liver.Citation20 In the myocardium, it is localized to the capillary endothelial cells.Citation21 The gene encoding for XOR is located at the short arm of chromosome 2.Citation22 It exists in two inter-convertible forms known as XO (EC 1.1.3.22) and xanthine dehydrogenase (XDH) (EC 1.17.1.4).Citation23 Both enzymes consist of two identical subunits of 145 kDa.
Mammalian XOR is present in vivo as the dehydrogenase form but is easily converted to XO by oxidation of the sulfhydryl residues or by proteolysis.Citation19 Although XDH has a much greater affinity for NAD+ compared to oxygen (and therefore is practically incapable of directly producing ROS), both XO and XDH can oxidize NADH which results in ROS formation.Citation24 Physiologically, XOR is involved in the hydroxylation of purines, pterins, and aldehydes but its primary role is as the rate-limiting enzyme in the conversion of hypoxanthine to xanthine and xanthine to urate (). XOR is the only enzyme capable of catalyzing the formation of urate in man.Citation25 In lower mammals, an enzyme, urate oxidase further metabolizes uric acid to allantoin but this enzyme is inactivated in primates.Citation26 There is also a suggestion from teleological studies that urate may have even evolved as a compensatory mechanism in higher primates that have lost the capacity to generate other antioxidants like ascorbate in vivo.Citation27
Figure 1 The purine degradation pathway. Reproduced with permission from Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004; 555(Pt 3):589–606.Citation16 Copyright © Blackwell Publishing.
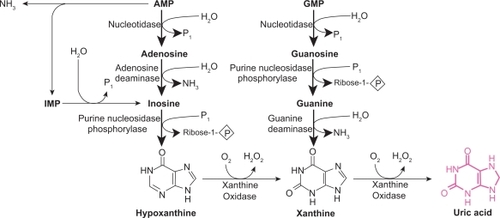
Figure 2 Mechanism of xanthine oxidoreductase XOR reaction with xanthine; A) reductive half reaction; B) oxidative half reaction. Reproduced with permission from Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004; 555(Pt 3):589–606.Citation16 Copyright © Blackwell Publishing.
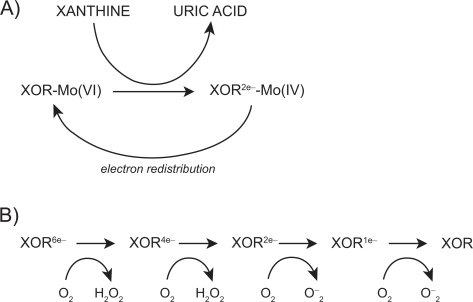
The mechanism by which XOR catalyzes hypoxanthine and xanthine conversion is extremely complex and has been previously described in detail.Citation16,Citation28 A fully reduced XO contains six electrons and its re-oxidation involves electron transfer to oxygen molecules which generates two H2O2 and two O2– speciesCitation28 for every fully reduced XO molecule ().
There is a large variability in human XOR expression which can be up to three-fold and on average 20% higher in men than in women.Citation29 Although basal expression of XOR is low in humans, hypoxia, IL-1, IL-6, TNF-α, lipo-polysaccharides as well as steroid treatment have been shown to upregulate transcription.Citation16 XO is significantly elevated in a variety of conditions including limb ischemia,Citation30 major surgeryCitation31 coronary artery disease (CAD)Citation32 and heart failure.Citation33 Circulating XO binds to glycosaminoglycans on the surface of endothelial cells where it is thought it acquires modified kinetics (higher Km and Ki, oxidant producing capacity, and increased stability).Citation34 There are suggestions that this form of induced, circulating and depositing XO appears to be more important in the pathogenesis of endothelial injury compared with XO constitutively produced from endothelial cells.Citation35
Despite this, the activity of endothelial bound XO is increased by more than 200% in patients with CHF.Citation36 Furthermore, studies using electron spin resonance have demonstrated that endothelial oxygen tension is thought to regulate XO activity at a post-translational level as demonstrated by a doubling in XOR activity post exposure to hypoxia without any increase in mRNA expression for 24 hours in bovine aortic endothelial cells.Citation37 Cells produce a marked elevation in XO levels when exposed to ischemiaCitation38 and XDH conversion to XO is also accelerated in hypoxia.Citation39 When infused acutely, XO produces a marked decrease in cardiac contractility, cardiac index and left ventricular systolic pressure.Citation40 In atherosclerotic plaques, urate levels are found to be elevated six-fold, reflecting accelerated purine oxidation within these plaques. Therefore XO production may not necessarily be reflected by systemic levels of XO metabolites.Citation41
The XO inhibitor allopurinol
Recent evidence indicates that allopurinol improves endothelial dysfunction in high risk primary prevention patients such as those with metabolic syndrome.Citation42
Allopurinol has also been shown to normalize endothelial dysfunction in type 2 diabetics with mild hypertension and reduced plasma malondialdehyde (MDA) levels.Citation43 MDA results from acid hydrolysis of lipid peroxides which are formed by free radical attack on plasma lipoproteins. It is therefore used as an indirect measure of oxidized low-density lipoprotein (LDL).
In the experimental murine myocardial infarction model, allopurinol significantly attenuated LV dilatation, hypertrophy, fibrosis and dysfunction. Once again, XO expression (as determined by electron spin resonance spectroscopy) and myocardial ROS generation were markedly increased in the post-mycardial infarction ischemic model.Citation44 This suggests a role for allopurinol in LV remodeling, a possibility that we are investigating at present in our unit. Allopurinol has also been shown to be beneficial in conditions such as post coronary artery bypass surgery where it reduced ischemic events and produced less ST segment depressionCitation45 as well as in hypercholesterolemic patients.Citation46 There are mixed data from ischemic-reperfusion studies. Pacher et al,Citation19 in an excellent in-depth review on this topic, have summarized the data in the .
Table 1 Effects of xanthine oxidase (XO) inhibitors in myocardial ischemia-reperfusion injury
Allopurinol in CHF was assessed by Doehner et alCitation47 and by Farquharson et al.Citation48 Doehner et al showed that the degree of improvement in forearm blood flow correlated with the degree of urate lowering. Interestingly, they also measured allantoin, a marker of oxygen free radical generation, which was reduced by 20% following 300 mg/day allopurinol. Farquharson et alCitation48 from our unit, found a 181% change in forearm blood flow with 300 mg allopurinol. They also found a 33% reduction in plasma MDA levels in patients treated with 300 mg allopurinol suggesting that the improvement in endothelial function and NO bioavailability seen was due (at least in part) to a reduction of ROS generation or oxidative stress burden. Allopurinol also reduced B-type natriuretic peptide (BNP) in stable CHF patients, although the reduction did not correlate with the fall in urate.Citation49
We have shown previously that high dose allopurinol is a very effective antioxidant in the vasculature because it abolishes the vitamin C-sensitive component of oxidative stress on vascular endothelial function, ie, in patients on high dose allopurinol, there is insufficient vascular oxidative stress being formed for vitamin C to neutralize the oxidative stress and further improve endothelial function.Citation50 This is further strengthened by evidence that the beneficial effect of vitamin C co-infusion in patients with CHF was greatest in patients with the highest levels of oxidative stress as measured by extracellular superoxide dismutase (ecSOD)Citation36 and XO activity.
Interestingly, it is the ability of allopurinol to inhibit XO, and therefore ROS, that results in its inclusion as a constituent in the University of Wisconsin solution for organ transport prior to transplantation.Citation51
The biomarker of lipid peroxidation, F2-isoprostane, is an indirect but validated marker of oxidative stress. Data from our group (unpublished) also demonstrate the ability of allopurinol to significantly reduce F2-isoprostane levels in patients with high baseline oxidative stress, further confirming the potent antioxidant effect of allopurinol. This also reflects XO activity within the vascular milieu in these patients because for the same degree of urate lowering as those with low baseline oxidative stress, only patients with high pre-existing oxidative stress were found to have a significant reduction in F2-isoprostanes. This is consistent with the known cascade effect of multiple superoxide and hydrogen peroxide generation for every urate molecule formation that is catalyzed by XO.Citation16,Citation62 In fact, NO and O2– react at a three-fold greater rate than the rate at which antioxidant defense mechanisms such as SOD can eliminate O2–.Citation63
In chronic diseases such as CHF, sustained high levels of ROS may exceed the capacity of cellular enzymatic and non-enzymatic antioxidantsCitation64 to counter its effects. Using electron spin resonance, Spiekermann et al demonstrated that both NADPH oxidase and xanthine oxidase are up-regulated in patients with coronary artery disease.Citation32 Others have demonstrated increased levels in CHF.Citation36,Citation65
Direct antioxidant action of allopurinol
Allopurinol directly scavenges free radicals as demonstrated by Das et al and othersCitation66–Citation68 in in vitro hearts where evidence of free radical scavenging occurred in the absence of XO activity. Animal studies in experimentally induced uveitis show that at very high doses (up to 50 mg/kg), allopurinol behaves as a free radical scavenger with intrinsic antioxidant properties. Crucially, this was only achieved far beyond the XO inhibition dose of 10 mg/kg and not at that dose itself. Further evidence for a possible direct antioxidant effect of allopurinol comes from models of experimental colitis where tungsten (a potent XO inhibitor) failed to improve symptoms whereas allopurinol did.Citation69 Augustin et al suggested that this direct effect was only seen at higher doses.Citation70 This was also seen in mice paracetamol toxicity models where lower doses (sufficient to block XO activity) of allopurinol failed to show antioxidant protection but higher doses did.Citation71 There have been other non-XO effects of allopurinol suggested such as copper chelation, preventing LDL oxidation as described above,Citation72 inhibition of heat shock protein (hsp) expressionCitation73 and calcium sensitization (below). Allopurinol treatment reduces early changes in inflammation such as leukocyte activation by reducing adherence, rolling and extravasation.Citation74
Mechanoenergetic uncoupling
This phenomenon refers to an imbalance between left ventricular performance and myocardial energy consumption.Citation75 The role of XO inhibition may either be to maintain cardiac output while reducing myocardial oxygen consumption or even increase cardiac output without increasing myocardial oxygen consumption. In dogs with pacing-induced heart failure, allopurinol improved myocardial contractility and eff iciency in oxygen utilization, prevented increases in systemic vasoconstriction and ameliorated reductions in myocardial contractility.Citation65,Citation76,Citation77 In murine post-ischemic cardiomyopathy models, allopurinol attenuated the increase in end-systolic and end-diastolic volumes,Citation78 increased survival, augmented ventricular function as well as reduced products of lipid peroxidation.Citation79 Khan et al found a direct protein-protein interaction between XO and neuronal NOS in the sarcoplasmic reticulum of cardiac myocytes.Citation80 Allopurinol improved myofilament calcium sensitivity as contraction force increased without a concomitant rise in systolic Ca2+ influx. The effects were not seen in endothelial NOS deficient mice, suggesting a role for neuronal NOS preventing XO inhibition of cardiac excitation-contraction coupling.Citation80 The finding that allopurinol is a potent myofilament Ca2+ sensitizer, particularly in the setting of ischemia, is thought to be due to the inhibition of basal XO production. As with the previous study by Khan et al, Perez et al found an almost exclusive increase in force generation without a lowering of inward transient Ca2+.Citation81
Despite the small sample size (n = 9), Cappola et al showed using cardiac catheterization that direct intracoronary infusions of allopurinol in these patients resulted in a marked decrease in myocardial oxygen consumption (MVO2) with no decrease in the rate of left ventricular pressure rise (dP/dT), stroke work or ventricular load.Citation82 Patients post coronary artery bypass grafting given allopurinol have also been shown to require less inotropic support.Citation45
Conclusion
The evidence for the role of oxidative stress in disease cannot be disputed. However, there are many questions that remain such as (1) exactly what is the contribution of oxidative stress to overall endothelial dysfunction; (2) at what stage should intervention take place; (3) what agents can we employ to effectively deal with this ubiquitous problem, which will be both safe and tolerable to our patients? The emerging evidence from therapies such as allopurinol are encouraging and should be put to the test in larger randomized studies to determine if the interesting data garnered from smaller mechanistic studies actually translate to a survival advantage with these agents.
Disclosures
The authors disclose no conflicts of interest.
References
- YusufSSleightPPogueJBoschJDaviesRDagenaisGEffects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study InvestigatorsN Engl J Med2000342314515310639539
- FensterBETsaoPSRocksonSGEndothelial dysfunction: clinical strategies for treating oxidant stressAm Heart J2003146221822612891188
- McCordJMFridovichISuperoxide dismutase. An enzymic function for erythrocuprein (hemocuprein)J Biol Chem196924422604960555389100
- ZhangMShahAMRole of reactive oxygen species in myocardial remodelingCurr Heart Fail Rep200741263017386182
- TakimotoEKassDARole of oxidative stress in cardiac hypertrophy and remodelingHypertension200749224124817190878
- FranciaPdelli GattiCBachschmidMMartin-PaduraISavoiaCMigliaccioEDeletion of p66shc gene protects against age-related endothelial dysfunctionCirculation2004110182889289515505103
- TrineiMGiorgioMCicaleseABarozziSVenturaAMigliaccioEA p53–p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosisOncogene200221243872387812032825
- KobayashiNDeLanoFASchmid-SchonbeinGWOxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive ratsArterioscler Thromb Vasc Biol200525102114212116037565
- McNamaraDMHolubkovRPostavaLRamaniRJanoskoKMathierMEffect of the Asp298 variant of endothelial nitric oxide synthase on survival for patients with congestive heart failureCirculation2003107121598160212668492
- GodfreyVChanSLCassidyAButlerRChoyAFardonTThe functional consequence of the Glu298Asp polymorphism of the endothelial nitric oxide synthase gene in young healthy volunteersCardiovasc Drug Rev200725328028817919260
- GriendlingKKMinieriCAOllerenshawJDAlexanderRWAngiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cellsCirc Res1994746114111488187280
- HarrisonDGriendlingKKLandmesserUHornigBDrexlerHRole of oxidative stress in atherosclerosisAm J Cardiol2003913A7A11A
- CingolaniHEPlastinoJAEscuderoEMMangalBBrownJPerezNGThe effect of xanthine oxidase inhibition upon ejection fraction in heart failure patients: La Plata StudyJ Card Fail200612749149816952781
- ClelandJGFColettaAPClarkALClinical trials update from the Heart Failure Society of America meeting: FIX-CHF-4, selective cardiac myosin activator and OPT-CHFEur J Heart Fail20068776476617049305
- WaringWSMcKnightJAWebbDJMaxwellSRLowering serum urate does not improve endothelial function in patients with type 2 diabetesDiabetologia200750122572257917928991
- BerryCEHareJMXanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implicationsJ Physiol2004 16;555(Pt 3): 58960614694147
- HancockJTSalisburyVOvejero-BoglioneMCCherryRHoareCEisenthalRAntimicrobial properties of milk: dependence on presence of xanthine oxidase and nitriteAntimicrob Agents Chemother200246103308331012234868
- StevensCRMillarTMClinchJGKanczlerJMBodamyaliTBlakeDRAntibacterial properties of xanthine oxidase in human milkLancet2000356923282983011022933
- PacherPNivorozhkinASzaboCTherapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinolPharmacol Rev20065818711416507884
- ParksDAGrangerDNXanthine oxidase: biochemistry, distribution and physiologyActa Physiol Scand Suppl198654887993529824
- CicoiraMZanollaLRossiAGoliaGFranceschiniLBrighettiGElevated serum uric acid levels are associated with diastolic dysfunction in patients with dilated cardiomyopathyAm Heart J200214361107111112075270
- IchidaKAmayaYNodaKMinoshimaSHosoyaTSakaiOCloning of the cDNA encoding human xanthine dehydrogenase (oxidase): structural analysis of the protein and chromosomal location of the geneGene199313322792848224915
- Della CorteEGozzettiGNovelloFStirpeFProperties of the xanthine oxidase from human liverBiochim Biophys Acta196919111641664309908
- ZhangZBlakeDRStevensCRKanczlerJMWinyardPGSymonsMCA reappraisal of xanthine dehydrogenase and oxidase in hypoxic reperfusion injury: the role of NADH as an electron donorFree Radic Res19982821511649645392
- PfefferKDHuecksteadtTPHoidalJRXanthine dehydrogenase and xanthine oxidase activity and gene expression in renal epithelial cells. Cytokine and steroid regulationJ Immunol19941534178917978046245
- UsudaNReddyMKHashimotoTRaoMSReddyJKTissue specificity and species differences in the distribution of urate oxidase in peroxisomesLab Invest19885811001113336202
- WilkinsonSRPrathalingamSRTaylorMCHornDKellyJMVitamin C biosynthesis in trypanosomes: A role for the glycosomeProc Natl Acad Sci U S A200510233116451165016087875
- HilleRMasseyVStudies on the oxidative half-reaction of xanthine oxidaseJ Biol Chem198125617909090956894924
- GuercioliniRSzumlanskiCWeinshilboumRMHuman liver xanthine oxidase: nature and extent of individual variationClin Pharmacol Ther1991125066636721752110
- TanSGelmanSWheatJKParksDACirculating xanthine oxidase in human ischemia reperfusionSouth Med J19958844794827716606
- PesonenEJLinderNRaivioKOSarnestoALapattoRHockerstedtKCirculating xanthine oxidase and neutrophil activation during human liver transplantationGastroenterology19981145100910159558291
- SpiekermannSLandmesserUDikalovSBredtMGamezGTatgeHElectron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilationCirculation2003107101383138912642358
- de JongJWSchoemakerRGde JongeRBernocchiPKeijzerEHarrisonREnhanced expression and activity of xanthine oxidoreductase in the failing heartJ Mol Cell Cardiol200032112083208911040111
- RadiRRubboHBushKFreemanBAXanthine Oxidase Binding to Glycosaminoglycans: Kinetics and Superoxide Dismutase Interactions of Immobilized Xanthine Oxidase-Heparin ComplexesArch Biochem Biophys199733911251359056242
- PanusPCWrightSAChumleyPHRadiRFreemanBAThe contribution of vascular endothelial xanthine dehydrogenase/oxidase to oxygen-mediated cell injuryArch Biochem Biophys199229426957021567225
- LandmesserUSpiekermannSDikalovSTatgeHWilkeRKohlerCVascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutaseCirculation2002106243073307812473554
- PossWBHuecksteadtTPPanusPCFreemanBAHoidalJRRegulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxiaAm J Physiol.1996270(6 Pt 1):L941L9468764218
- ZweierJLKuppusamyPLuttyGAMeasurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissuesProc Natl Acad Sci U S A19888511404640502836868
- DoehnerWAnkerSDUric acid in chronic heart failureSemin Nephrol2005251616615660337
- PrasadKKalraJBharadwajLCardiac depressant effects of oxygen free radicalsAngiology19934442572708457076
- BaldusSKosterRChumleyPHeitzerTRudolphVOstadMAOxypurinol improves coronary and peripheral endothelial function in patients with coronary artery diseaseFree Radic Biol Med20053991184119016214034
- YiginerOOzcelikFInancTAparciMOzmenNCingozbayBYAllopurinol improves endothelial function and reduces oxidant-inflammatory enzyme of myeloperoxidase in metabolic syndromeClin Res Cardiol200897533434018330493
- ButlerRMorrisADBelchJJHillAStruthersADAllopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertensionHypertension200035374675110720589
- EngberdingNSpiekermannSSchaeferAHeinekeAWienckeAMullerMAllopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug?Circulation2004110152175217915466649
- SistoTPaajanenHMetsa-KetelaTHarmoinenANordbackITarkkaMPretreatment with antioxidants and allopurinol diminishes cardiac onset events in coronary artery bypass graftingAnn Thorac Surg1995596151915237771834
- CardilloCKilcoyneCMCannonRO3rdQuyyumiAAPanzaJAXanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patientsHypertension199730(1 Pt 1):57639231821
- DoehnerWSchoeneNRauchhausMLeyva-LeonFPavittDVReaveleyDAEffects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studiesCirculation2002105222619262412045167
- FarquharsonCAJButlerRHillABelchJJFStruthersADAllopurinol improves endothelial dysfunction in chronic heart failureCirculation2002106222122612105162
- GavinADStruthersADAllopurinol reduces B-type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failureHeart200591674975315894768
- GeorgeJCarrEDaviesJBelchJJFStruthersAHigh-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acidCirculation2006114232508251617130343
- JamiesonNVLindellSSundbergRSouthardJHBelzerFOAn analysis of the components in UW solution using the isolated perfused rabbit liverTransplantation19884645125163176157
- JohnsonWDKayserKLBrenowitzJBSaediSFA randomized controlled trial of allopurinol in coronary bypass surgeryAm Heart J1991121(1 Pt 1):20241985364
- RashidMAWilliam-OlssonGInfluence of allopurinol on cardiac complications in open heart operationsAnn Thorac Surg19915211271302069440
- ParmleyLFMuftiAGDowneyJMAllopurinol therapy of ischemic heart disease with infarct extensionCan J Cardiol1992832802861576562
- KaliakinIEMit’kinAF[Effects of allopurinol on uric acid metabolism and lipid peroxidation in ischemic heart disease patients with stable angina]Kardiologiia199333215178084119
- CoghlanJGFlitterWDCluttonSMPandaRDalyRWrightGAllopurinol pretreatment improves postoperative recovery and reduces lipid peroxidation in patients undergoing coronary artery bypass graftingJ Thorac Cardiovasc Surg199410712482568283893
- TaggartDPYoungVHooperJKempMWalesbyRMageePLack of cardioprotective efficacy of allopurinol in coronary artery surgeryBr Heart J19947121771818130028
- YamazakiISomaTIchikawaYIwaiYKondoJMatsumotoA[Usefulness of allopurinol for prevention of myocardial reperfusion injury in open heart surgery]Nippon Kyobu Geka Gakkai Zasshi199543126317884257
- CastelliPCondemiAMBrambillascaCFundaroPBottaMLemmaMImprovement of cardiac function by allopurinol in patients undergoing cardiac surgeryJ Cardiovasc Pharmacol19952511191257723340
- CoetzeeARoussouwGMacgregorLFailure of allopurinol to improve left ventricular stroke work after cardiopulmonary bypass surgeryJ Cardiothorac Vasc Anesth19961056276338841871
- GuanWOsanaiTKamadaTHanadaHIshizakaHOnoderaHEffect of allopurinol pretreatment on free radical generation after primary coronary angioplasty for acute myocardial infarctionJ Cardiovasc Pharmacol200341569970512717099
- MunkresKDAgeing of Neurospora crassa IV. Induction of senescence in wild type by dietary amino acid analogs and reversal by antioxidants and membrane stabilizersMech Ageing Dev1976531711917715
- CaiHHarrisonDGEndothelial dysfunction in cardiovascular diseases: the role of oxidant stressCirc Res2000871084084411073878
- DeanfieldJEHalcoxJPRabelinkTJEndothelial function and dysfunction: testing and clinical relevanceCirculation2007115101285129517353456
- AmadoLCSaliarisAPRajuSVLehrkeSSt JohnMXieJXanthine oxidase inhibition ameliorates cardiovascular dysfunction in dogs with pacing-induced heart failureJ Mol Cell Cardiol200539353153615963530
- DasDKEngelmanRMClementROtaniHPrasadMRRaoPSRole of xanthine oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvageBiochem Biophys Res Commun198714813143192823807
- HoeyBMButlerJHalliwellBOn the specificity of allopurinol and oxypurinol as inhibitors of xanthine oxidase. A pulse radiolysis determination of rate constants for reaction of allopurinol and oxypurinol with hydroxyl radicalsFree Radic Res Commun1988442592632852627
- RicardoSDBertramJFRyanGBPodocyte architecture in puromycin aminonucleoside-treated rats administered tungsten or allopurinolExp Nephrol1995352702797583048
- KeshavarzianAMorganGSedghiSGordonJHDoriaMRole of reactive oxygen metabolites in experimental colitisGut19903177867902164491
- AugustinAJBokerTBlumenroderSHLutzJSpitznasMFree radical scavenging and antioxidant activity of allopurinol and oxypurinol in experimental lens-induced uveitisInvest Ophthalmol Vis Sci19943511389739047928187
- KnightTRKurtzABajtMLHinsonJAJaeschkeHVascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stressToxicol Sci200162221222011452133
- MalkielSHar-elRSchwalbHUretzkyGBormanJBChevionMInteraction between allopurinol and copper: possible role in myocardial protectionFree Radic Res Commun19931817158349148
- NishizawaJNakaiAMatsudaKKomedaMBanTNagataKReactive oxygen species play an important role in the activation of heat shock factor 1 in ischemic-reperfused heartCirculation199999793494110027818
- GrangerDNBenoitJNSuzukiMGrishamMBLeukocyte adherence to venular endothelium during ischemia-reperfusionAm J Physiol1989257(5 Pt 1):G683G6882596604
- KittlesonMMHareJMXanthine oxidase inhibitors: an emerging class of drugs for heart failureEur Heart J200526151458146015917281
- EkelundUEHarrisonRWShokekOThakkarRNTuninRSSenzakiHIntravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failureCirc Res199985543744510473673
- SaavedraWFPaolocciNSt JohnMESkafMWStewartGCXieJSImbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heartCirc Res2002 22;90329730411861418
- NaumovaAVChackoVPOuwerkerkRStullLMarbanEWeissRGXanthine oxidase inhibitors improve energetics and function after infarction in failing mouse heartsAm J Physiol Heart Circ Physiol20062902H837H84316183726
- StullLBLeppoMKSzwedaLGaoWDMarbanEChronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathyCirc Res200495101005101115499028
- KhanSALeeKMinhasKMGonzalezDRRajuSVTejaniADNeuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction couplingProc Natl Acad Sci U S A200410145159441594815486091
- PerezNGGaoWDMarbanENovel myofilament Ca2+-sensitizing property of xanthine oxidase inhibitorsCirc Res19988344234309721699
- CappolaTPKassDANelsonGSBergerRDRosasGOKobeissiZAAllopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathyCirculation2001104202407241111705816