Abstract
As the risk factors for thrombosis are becoming better understood, so is the need for anticoagulation. The inherent difficulties with warfarin are such that a low-molecular-weight heparin (LMWH) is often the key therapeutic. However, there are several different species of LMWH available to the practitioner, which leads to the need for an objective guide. New agents are coming onto the marketplace, and these may supersede both warfarin and the heparins. The current report will review the biochemistry and pharmacology of different LWMHs and identify which are more suitable for the different presentations of venous thromboembolism. It will conclude with a brief synopsis of new agents which may supersede warfarin and heparin.
Keywords:
Introduction
The major common endpoint in most human disease is thrombosis and the major causes of arterial thrombosis (such as diabetes) have been established for decades. The risk factors for venous thromboembolism (VTE) (such as obesity and orthopedic surgery) are also now becoming well established and the penetrance of these factors in the pathophysiology of thrombosis is so strong that anticoagulation is often mandatory. Indeed, VTE is a frequent complication among hospital in-patients and contributes to longer hospital stays, morbidity, and mortality.Citation1–Citation4 Some VTEs may be subclinical and patients presenting with sudden pulmonary embolus (PE) or deep vein thrombosis (DVT) are common in acute medicine. Using ultrasonic Doppler and venographic techniques, DVT of the lower limb has been documented in half of all major lower limb orthopedic operations performed without antithrombotic prophylaxis, a quarter of patients with acute myocardial infarction (MI) and more than half of patients with acute ischemic stroke. Indeed, some risk factors are more likely to evoke a VTE than others ().
Table 1 Stratification of the risk factor for VTE
Accordingly, anticoagulation is demanded. However, the perfect anticoagulant (rapid mode of action and cessation, oral availability, minimal side effects, etc) does not exist. Instead, we have a crude poison with a long time to action and long half-life with a wide range of effect in different patients leading to difficulties in management (that is, warfarin), and an injectable agent with poor pharmacokinetics such that regular blood testing is required, and a risk of thrombocytopenia (that is, heparin).Citation5 More recently, some of the disadvantages of heparin have been reduced with the introduction of an improved preparation: low-molecular-weight heparin (LMWH).Citation6,Citation7 However, there are several different species of LMWH available for the prevention of VTE, and all have slightly different properties and licenses for different risk situations. Fortunately, new anticoagulants are coming on to the market and it is conceivable that they will slowly take over from both warfarin and the heparins over the coming decade.
The objective of this communication is to review the role of the different LMWHs in the prevention of VTE. In order to do this the position of (unfractionated) heparin will be first be reviewed, following by an in-depth examination of LMWH. It will conclude with an examination of new anticoagulants.
Heparin
The modern era of heparin can be traced to 1880 with the description of anticoagulant preparations that evolved into heparinCitation8–Citation11 that ultimately became clinically useful.Citation12–Citation14 Brinkhous and colleaguesCitation15 subsequently demonstrated the requirement of heparin for another substance in order for anticoagulation to be effected, a factor that we now recognize as antithrombin (formerly antithrombin III). Commercial heparin was thus ready for industrial/pharmacological scale production. By 1979, its value in prophylaxis for venous thrombosis, PE, and other possible problems were established.Citation5 The laboratory potential of a more efficacious fraction of whole, unfractionated heparin, ie, LMWH, was recognized in 1979Citation16,Citation17 and clinical note was made shortly afterwards.Citation18,Citation19
Mode of action
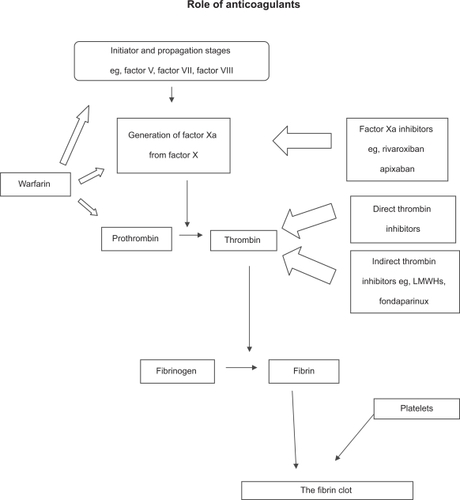
Heparin is a natural product present on the cell membrane, but when provided as a therapeutic drug, it is a mixture of glycosaminoglycans and polysaccharides composed of long chains of repeated disaccharide units (hexosamine and glucuronic or iduronic acid), although the composition of different macromolecules vary markedly. In its whole, unfractionated form, with species of molecular weights ranging from 3 to 30 kDa (although most is in the range 12–15 kDa), perhaps only one-third of a standard heparin preparation has anticoagulant activity.Citation5 Heparin by itself is not an anticoagulant: it is a co-factor in the activity of antithrombin, a 58 kDa single chain polypeptide synthesized in the liver.Citation20
Heparin binding to specific sites on antithrombin induces a conformational change in the latter, exposing a site that binds serine proteases factor Xa, thrombin (IIa) (in approximately equal proportions) and, with less affinity, factors IXa, XIa, XIIa, kallikrein, plasmin, and C1-esterase, although almost all of its effects are against thrombin and factor Xa.Citation5 However, heparin fractions less than 18 monosaccharide units (3–4 kDa) do not enhance the inhibition of thrombin by antithrombin and probably act via factor Xa inhibition. The major inhibitor of coagulation, the effects of antithrombin are accelerated some 1000-fold in the presence of heparin. It follows, therefore, that special measures are required in subjects deficient in antithrombin (whether by genetics or excess consumption). In high doses, heparin also prolongs the bleeding time by inhibiting platelet aggregation in vitroCitation21 and may exert some effect on the endothelium,Citation22 although it is unclear (and possibly academic) whether or not these influence clinical efficacy.
Clinical effectiveness
Due to variability of effect in different individuals, mostly due to complex pharmacokinetics (such as degree of binding to numerous proteins including, platelet factor 4, vitronectin, fibronectin and von Willebrand factor),Citation23,Citation24 laboratory monitoring of the effects of heparin is necessary and can be followed with the relatively straightforward activated partial thromboplastin time (APTT), thrombin time, and to lesser extent, the prothrombin time. The APTT derives from the ability of citrated plasma to clot an artificial ‘platelet’ substitute of phospholipids and other substances to which calcium has to be added: quality control is crucial as the quality of reagents can vary markedly.Citation25 In practice, most clinicians aim for a degree of anticoagulation that prolongs the APTT by anything from 1.5 to 3 times that of normal, uncoagulated blood, ie, an APTT patient/control ratio of 1.5:3.0.Citation4,Citation26
The primary use of heparin has been, and continues to be, in the treatment and prophylaxis of VTE.Citation5,Citation26,Citation27 As with aspirin, early crucial work was performed in the 1960’s with studies looking at the effect of heparin on PE and DVT.Citation28,Citation29 However, heparin is not absorbed from the gastrointestinal tract and so must be given intravenously or subcutaneously, with the former providing a more rapid effect. Subsequent trials not only confirmed the efficacy of this agent, but also showed that continuous intravenous infusion was frequently preferable to subcutaneous or intermittent infusions.Citation30,Citation31 One ‘typical’ trial reported a frequency of asymptomatic extension of DVT or PE of 8.2% of patients with proximal DVT taking heparin and oral anticoagulation compared to a frequency of 39.6% in those taking oral anticoagulants alone.Citation32 There was also a reduction in the report of symptomatic events (6.7% versus 20%), but no difference in hemorrhagic complication (3% versus 5%). In practice, until recently, hospital treatment of, and prophylaxis against, VTE, generally began with heparin, moving oral anticoagulants following discharge, for varying periods up to six months.Citation33
Clinical side effects
As with aspirin, the major problem with heparin is in dose-dependent excess bleeding. Early commentaries reported a rate of hemorrhagic complications of up to 30%.Citation34 However, a later review reported an incidence of 6.8% in patients given continuous infusion and 14.2% in patients given intermittent intravenous injections,Citation5 emphasizing four important variables: dose, patient’s anticoagulant response, method of administration, and other patient-related factors such as renal failure or chronic alcohol use. Gallus and colleaguesCitation35 reported an incidence of major bleeding of 11% in high-risk patients, compared to 1% in low-risk patients.
Surprisingly, heparin-induced thrombocytopenia (HIT; generally platelet count <140,000 cells/μL, and reversible) has been described for 50 years,Citation36 with two forms (nonimmune and immune [often an immunoglobulin G [IgG] targeting a heparin/platelet factor 4 complex]) being recognized.Citation37 When the platelet count falls, especially if it falls from normal to less than <100,000 cells/μL, cessation is mandatory and alternate anticoagulant cover (eg, argatroban)Citation26,Citation38 is necessary. Curiously, 0.4% of patients with HIT suffer arterial thrombosis (that may follow platelet aggregation in vivo) or venous thrombosis (that may result from heparin resistance caused by the neutralizing effect of the heparin-induced release of platelet factor 4). These events may be related to the generation of thrombin,Citation39 and also to platelet activation by heparin.Citation21,Citation40 If present, HIT is likely to be apparent 3 to 15 days after initiation (such that a platelet count is advised on days 3–5), but may also occur very rarely within hours, with the platelet count returning to normal within four days of discontinuation.Citation41 Rates of HIT vary between <0.1% and 3%.Citation26,Citation37,Citation42
Other uncommon side effects of heparin (especially in long-term and high-dose use) include fractures and osteopenia, hyperkalemia, elevations in liver enzymes, skin necrosis at the site of administration, alopecia, hypersensitivity, priapism, and hypoaldosteronism.Citation5,Citation26,Citation43–Citation48 Thus, despite the proven safety and effectiveness of continuous infusions of heparin, its limitations and adverse side effect profile, unpredictable pharmacokinetic response, daily laboratory monitoring with dose adjustments, and requirement for hospitalization leaves room for alternatives. One such alternative is a low-molecular-weight variant of the standard (unfractionated) heparin preparation.
Low-molecular-weight heparin
The laboratory demonstration of a more efficacious fraction of whole, unfractionated heparin, ie, LMWH,Citation16–Citation19 has ushered in a new era of anticoagulation. LMWH can be manufactured from unfractionated heparin by diverse routes, such as benzylation with alkaline hydrolysis, nitrous acid depolymerization or digestion, heparinase digestion, and isoamyl nitrate digestion. Each provides a different LMWH with mean molecular weights varying from 4371 Da to 5866 Da, so that each has different pharmacokinetics and activities.Citation49–Citation51 However, there are common class advantages over unfractionated heparin (), most of which address mode of action and side effects. Broadly speaking, compared to unfractionated heparin, LMWH has a longer subcutaneous half-life and therefore has potential for outpatient use, a more predictable anticoagulant response requiring less monitoring, and has better antifactor Xa effect.
Table 2 Differences between low-molecular-weight heparin and unfractionated heparinCitation48–Citation51
Building on early trials,Citation18,Citation19 several large studies have unequivocally demonstrated the value of LMWH in the initial treatment of DVT.Citation52–Citation54 Lensing and colleaguesCitation52 described a recurrence rate of 3.1% in patients on LWMH compared to 6.6% (p < 0.01) in those on standard heparin, with 0.8% and 2.8% (p < 0.005) suffering a major bleed, respectively, which was confirmed by other analyses. However, later trialsCitation55,Citation56 failed to find a difference in efficacy between the two types of heparin with equivalence of adverse events. Interestingly, these meta-analyses also reported a lower mortality with LWMH due to causes unrelated to VTE in the patients particularly with cancer. A subsequent more formal meta-analysis of 629 cancer patients did indeed demonstrate a benefit in three-month mortality for LMWH versus unfractionated heparin.Citation57 While this is an unsought for endpoint and therefore of questionable value, there is nevertheless good mechanistic evidence for this benefit.Citation58 The value of LMWH compared to unfractionated heparin now extends to prevention of thrombosis in general surgery, orthopedic surgery, hip fracture, multiple trauma, and neurosurgery,Citation51 whilst efficacy and safety issues in medical patients have been addressed.Citation59
Apart from its clinical effectiveness and better side effect profile, a further advantage of LMWH is in home use by appropriate patients with DVT who are not severely ill.Citation60,Citation61 In both studies, LMWH administered subcutaneously twice-daily was compared with standard continuous infusion in hospital. Both reported no significant difference in recurrence of thromboembolism with similar rates of bleeding. Those on LMWH in the study of Levine and colleaguesCitation60 remained in hospital for a mean of 1.1 days, suffering 13 embolic events, compared to 6.5 days for those on unfractionated heparin, with 17 events, of which two were fatal. The health economics of these figures is apparent, even after adjustment for the higher costs of the LMWH. The study of Koopman and colleaguesCitation61 included quality of life data: overall, there was no difference between the LMWH and unfractionated heparin groups, but those on LMWH reported better physical activity and social functioning at weeks 1–2. Data demonstrating the superiority of LMWH compared to unfractionated heparin (eg, shorter time to effective anticoagulation and more days of effective anticoagulation) continues to be published.Citation62
Whilst the weight of literature focuses on (unprovoked) DVT and PE, LMWH is also effective in those with conditions predisposing to VTE, such as cancer,Citation63–Citation65 although more data on other groups, such as the obese and those in renal failure or with thrombophiliaCitation66–Citation69 is needed and these are slowly becoming available. However, despite the weight of literature on LMWH, questions and doubts remain. For example, Knight and colleagues conclude that heparins may, in some circumstances, be pro-thrombotic.Citation70 Thus there remains scope for more efficacious, yet safe, anticoagulants.Citation71
Which LMWHs are available?
In the United Kingdom, pharmaceutical practice in NHS hospitals is dominated by the British National Formulary (BNF).Citation72 This book, also available online, is updated each six months, and lists all government-approved pharmaceutical agents and their licensed indications. It follows that doses and guidelines may differ in other countries, and that doses in prefilled syringes may also differ. Four types of LMWH are described In issue 57 of the BNF. These are (in alphabetic order) bemiparin, dalteparin, enoxaparin, and tinzaparin. All are given subcutaneously and regimes are markedly different for prophylaxis of VTE versus treatment of VTE. Furthermore, all have numerous cautions and contraindications. Principal among these is the need to refer to renal function because several agents are cleared by this organ so that defective clearance effectively leads to higher biologically active levels and thus a risk of overanticoagulation, which may lead to hemorrhage. An additional concern is the liver, as failure of this organ to synthesize key coagulation proteins may also lead to a risk of hemorrhage. Other LMWHs are available and include nadroparin and certoparin.
Bemiparin
This agent is licensed for the prophylaxis of DVT. For patients at moderate risk (for the definition of risk, see and and the discussion to come), the recommended dose is 2500 units two hours before or six hours after surgery, then 2500 units every 24 hours for 7–10 days. High-risk patients should receive 3500 units two hours before to six hours after surgery, then 3500 units every 24 hours for 7–10 days. Treatment of DVT (with or without PE) is likely to be 115 units/kg every 24 hours for 5–9 days and until adequate oral anticoagulation (inevitably warfarin) is established.
Table 4 Risk factors for VTE
Table 5 Additional risk factors for surgical inpatients
Dalteparin
This agent is licensed for additional indications. For prophylaxis of DVT in surgical patients at moderate risk, 2500 units 1–2 hours are given before surgery, then 2500 units every 24 hours for 5–7 days or longer. High-risk patients are also likely to require 2500 units 1–2 hours before surgery, but then 2500 units 8–12 hours later (or 5000 units on the evening before surgery, then 5000 units on the following evening), with 5000 units every 24 hours for 5–7 days or longer (five weeks in hip replacement). For prophylaxis of DVT in medical patients, 5000 units are given every 24 hours. Treatment of DVT and PE in adults is dose-adjusted to body weight less than 46 kg, 7500 units/day, 46–56 kg 10,000 units/day, 57–68 kg, 12,500 units/day, 69–82 kg, 15,000 units/day, and body weight over 83 kg 18,000 units/day, with oral anticoagulant until the international normalized ratio (INR) is in the therapeutic range (which may take up to seven days). Monitoring of the effect of the LMWH is not usually needed but if so is by antifactor Xa assay.
There is an unlicensed indication for dalteparin for the treatment of VTE in pregnancy. This calls for dose-adjusted injections based on early pregnancy body weight: under 50 kg, 5000 units twice daily; 50–70 kg, 6000 units twice daily; 70–90 kg 8000 units twice daily, and body weight over 90 kg, 10,000 units twice daily. If called for, blood should be taken for monitoring 3–4 hours after a dose, and ideally the result will be in the range or 0.5 to 1 unit/mL of antifactor Xa activity. Monitoring is generally not required for once-daily treatments. Notably, this desired range is higher than that for prophylaxis of a VTE, which is 0.1–0.3 antifactor Xa units/mL. Daltaparin is also licensed for use in acute coronary syndromes, which are effectively unstable coronary artery disease of ST and non-ST elevation MI. The weight-adjusted dose is 120 units/kg every 12 hours (up to 10,000 units twice daily) for up to eight days. Beyond this period, such as if awaiting invasive procedures, then the dose may change. Dose also varies by sex. For women weighing less than 80 kg and men less than 70 kg, the recommended dose is 5000 units every 12 hours. Above this weight the dose is 7500 units every 12 hours. This proceeds until the day of the procedure for a maximum of 45 days.
Enoxparin
This agent also has multiple indications. For prophylaxis of DVT in surgical patients at moderate risk, the recommended dose is 20 mg (2000 units) about two hours before surgery then 20 mg (2000 units) every 24 hours. High-risk patients (such as those undergoing orthopedic surgery) will need 40 mg (4000 units) 12 hours before surgery and repeated every 24 hours for 7–10 days. Medical patients have the same regime but for at least six days and until ambulant to a maximum of 14 days. Treatment of either forms of VTE calls for 1.5 mg/kg (150 units/kg) every 24 hours, usually for at least five days and until oral anticoagulation is effective (generally INR 2–3). In unstable angina and non-ST-segment elevation MI, 1 mg/kg (100 units/kg) every 12 hours usually for 2–8 days (minimum two days) is recommended, and there is also an unlicensed indication for the treatment of VTE in pregnancy: early pregnancy body weight under 50 kg, 40 mg (4000 units) twice daily; body weight 50–70 kg, 60 mg (6000 units) twice daily; body weight 70–90 kg, 80 mg (8000 units) twice daily; body weight over 90 kg, 100 mg (10 000 units) twice daily.
Tinzaparin
For the prophylaxis of DVT in general surgery, the recommended dose is 3500 units two hours before surgery, then 3500 units every 24 hours for 7–10 days. In orthopedic surgery, it may be dose-adjusted: 50 units/kg two hours before surgery, then 50 units/kg every 24 hours for 7–10 days or 4500 units 12 hours before surgery, then 4500 units every 24 hours for 7–10 days. The treatment of DVT and PE calls for 175 units/kg once daily for at least six days and until adequate oral anticoagulation, eg, with warfarin, is established. Once more, there is an unlicensed indication of VTE in pregnancy, which is 175 units/kg once daily.
Choice of LMWH
The previous section listed the different clinical scenarios that each LMWH may be used for. Leaving aside economic considerations, the practical level issues of ease of prescribing, dose adjustment, and timing of dose seem relevant. The practitioner must also be fully aware of cautions, contraindications, and other caveats of each particular LMWH which may be relevant in a particular patient. Irritatingly, there are few (if any) good, well powered trials comparing one LMWH with another. In almost all such trials the (generally inferior) comparator is unfractionated heparin.Citation52–Citation59 All other factors being equal, due to pressures on individual hospital pharmacies, many opt for a LMWH that has the broadest range of clinical applications ().
Table 3 Indication for the use of low-molecular-weight heparins
Use of LMWHs in practice
Fortunately, there are numerous guidelines for the use of LMWHs for prophylaxis in various settings such as in obstetrics and gynecology, and after surgery, and many are available free and online.Citation73–Citation79 Practice in surgery in the UK is dominated by guidelines from the National Institute for Health and Clinical Excellent (NICE).Citation79 However, other comprehensive guidelines with more international appeal such as those of the American College of Chest PhysiciansCitation73 are widely used. Unsurprisingly, there is often considerable agreement between these guidelines. The NICE document recommends that all patients about to undergo surgery should be assessed to identify their risk factors for developing VTE, which can be assessed by a scoring system. Once a particular patient’s risk of VTE has been assessed (), then the risk afforded by their surgery must also be assessed (). Clearly, some operations are more likely to promote VTE than other, and the most risky include orthopedic surgery. Once any cautions and contraindications have been addressed (such as allergy, HIT, hepatic or renal impairment), then treatment may be given (). The duration of treatment varies, bearing in mind that some patients may transfer to warfarin, especially if they have concurrent long-standing risk factors such as cancer. Notably, NICE recommends treatment for four weeks after certain orthopedic procedures.Citation79 The section above applies to the prevention of a VTE. However, once a patient actually has a DVT or PE, then a different dosing regime is called for, which is effectively more anticoagulants ().
Table 6 Application of risk assessment for the use of low-molecular-weight heparin (LMWH)
Table 7 Use of low-molecular-weight heparins in prophylaxis versus treatment in an 80 kg patient
Fondaparinux
Technically, this agent may not be a ‘heparin’ or even a ‘LMWH’, but it certainly does inhibit the coagulation pathway in the same manner. Marketed as Arixtra® by GlaxoSmithKline, fondaparinux is a novel, selective and reversible Xa-inhibitor, although based on the structure of heparin, is different from both heparin and LMWH. Accordingly, unlike heparin and LMWH, it has minimal, if any, activity against thrombin.Citation80,Citation81 Pharmacokinetics are characterized by a 100% bioavailability by subcutaneous route, lack of biometabolism, urinary excretion and a relatively long plasma half-life of 14–21 hours which permits once-daily injection. There is a rapid onset of action, with a peak activity reached in two hours. No interactions with aspirin, warfarin, or digitoxin have been noted. Fondaparinux, like LMWHs, does not affect the prothrombin time (PT) and has very weak effects on APPT but its activity can be determined by specific anti-Xa assays, if necessary. Thrombocytopenia (platelet count <100 × 109/L) occurs even less commonly than with LMWH.
Use of fondaparinux has been approved by NICE as an alternative to LMWH in certain types of surgery, such as orthopedic.Citation79 In this setting the prophylaxis regime recommended by the BNFCitation72 calls for 2.5 mg six hours before surgery then 2.5 mg daily for 5–9 days (longer after hip surgery), and the same dose for thromboprophylaxis in medical patients usually for 6–19 days. For treatment of VTE, the recommended weight-adjusted dose is 5 mg (if weight under 50 kg), 7.5 mg (50–100 kg), or 10 mg (over 100 kg) every 24 hours and generally for five days and until oral anticoagulation is adequate.
New anticoagulants
Although LMWHs are very effective agents, they still carry some problems such as a residual risk of heparin-induced thrombocytopenia and reliance of parenteral introduction. These, and other problems, are being overcome with the introduction of new agents. Some operate directly against thrombin, others target coagulation factor Xa.Citation82–Citation86
Direct thrombin inhibitors
Dabigatran etexilate (marketed as Pradaxa® by Boehringer Ingelheim GmbH) is the first in a new class of anticoagulant that works by directly inhibiting thrombin. It possesses various qualities which make it potentially an attractive and promising novel oral anticoagulant with its predictable pharmacokinetics and pharmacodynamics.Citation87 The drug has rapid absorption (within two hours) and distribution with estimated half-lives of 8–10 hours and 14–17 hours for single and multiple dose administrations, respectively. Nearly 80% of dabigatran etexilate is excreted unchanged by the kidneys with average bioavailability of 6.5%, hence high doses are required to maintain adequate plasma concentrations. Of note, the drug absorption is reduced by 20%–25% if patients are concurrently on proton pump inhibitors. Phase II and III clinical trials have been conducted assessing dosages, efficacy, and tolerability in numerous indications such as prevention of secondary VTE, DVT, stroke, and embolism due to atrial fibrillation (AF).Citation88–Citation93
In the BISTRO I study,Citation88 dabigatran etexilate demonstrated an acceptable safety therapeutic profile with doses ranging between 12.5 mg and 300 mg twice daily in patients undergoing elective total hip replacement. Historically, the latter is one type of surgery where more than 50% of patients develop VTE in the absence of thromboprophylaxis. Subsequently, the BISTRO II studyCitation89 compared four different doses of dabigatran etexilate with enoxaparin, with a significant reduction of VTE events in total hip or knee replacement patients receiving 150 mg twice daily, 300 mg once daily, and 225 mg twice daily. There is also data on stroke prevention in AF. The PETRO studyCitation90 was conducted in 502 participants randomized to receive different dozes of dabigatran alone or in combination with aspirin (81 mg or 325 mg) or open-label warfarin. This clinical study found no serious liver toxicity with only a small fraction of patients (0.9%) observed to have raised aminotransferase levels and also justified dose selection for subsequent trials.
In March 2008, the regulatory authorities of the European Union approved the use of dabigatran etexilate for DVT prevention postelective total hip or knee replacement surgery. This was followed by approvals from Health Canada in June 2008. Dabigatran etexilate has been available in UK since April 2008 and was subsequently included in the DVT prevention guidance issued by NICE five months later.Citation94,Citation95 The BNF recommended daily doses for VTE prevention in total knee replacement surgery is 110 mg (elderly over 75 years, 75 mg) 1–4 hours after surgery, then 220 mg daily (elderly over 75, 150 mg) for nine days. For total hip replacement surgery, the same dose is recommended but it should continue for 27–34 days. As is common practice with almost all anticoagulants, patients with moderate renal impairment (glomerular filtration rate 30–50 mL/min) require reduced dosages.
Factor Xa inhibitors
These agents reversibly block free factor Xa and that factor Xa that is bound to platelets within the prothrombinase complex. Two oral factor Xa inhibitors are currently under various stages of clinical development and are close to potential clinical application. Rivaroxaban (marketed as Xarelto® by Bayer Schering Pharma AG) is the first selective oral direct factor Xa inhibitor advanced to phase III clinical trials. It has favorable pharmacokinetic characteristics with a bioavailability of 60%–80%. Rivaroxaban achieves peak plasma levels in three hours and has a half-life of nine hours in healthy, young subjects and about 12 hours in elderly subjects. However, the drug has been tested for clinical use only on a once-daily basis. Rivaroxaban is metabolized by the liver via CYP3A4 with up to two-thirds of the drug being eliminated by the kidneys. The use of rivaroxaban in patients with renal impairment has to be cautious because of its renal clearance. It does not significantly interact with platelet function in preclinical studies, where it demonstrated an excellent correlation between its plasma levels and achieved clotting times, while the bleeding risk was comparable to that of enoxaparin.Citation96,Citation97
The phase III clinical trials, the RECORD Studies, were designed to look at the effectiveness of rivaroxaban in the prevention of VTE in patients undergoing hip or knee surgery. The RECORD I and RECORD III trials revealed higher effectiveness of rivaroxaban compared to enoxaparin in VTE prevention following orthopedic surgery surgery, whilst the RECORD II study provided evidence that prolonged administration of rivaroxaban had additional clinical benefits compared to short-term therapy.Citation98–Citation100 The utility of rivaroxaban for VTE treatment was assessed in two phase II studies which showed that rivaroxiban had similar efficacy when compared with enoxaparin/warfarin in the treatment of VTE.
In the ODIXa-DVT study, the incidence of major and minor bleeding increased with escalating doses of rivaroxiban (range of 5.0% to 11.6%) as compared with patients treated with enoxaparin/warfarin (6.3%).Citation101 In the EINSTEIN-DVT study, the incidence of clinically relevant bleeding occurred in 2.2%–6.0% of patients in rivaroxaban groups and in 8.8% of patients in heparin/warfarin group, with no significant dose response for bleeding.Citation102 In these two studies, no dose-efficacy relationship with rivaroxaban treatment was observed. However, the dose-dependent increase in the risk of bleeding was reported in the ODIXa-DVT study indicating that a twice-daily regimen for rivaroxaban might increase risk of bleeding. Nonetheless, the results of these two studies had supported the safety and efficacy of rivaroxaban in the treatment of DVT. Based on these results, further phase III randomized trials of rivaroxaban for the treatment of DVT and for the prevention of stroke in AF (ROCKET-AF) with 20 mg once-daily are undergoing. Rivaroxaban was been assessed by NICE,Citation103 who have recommended it as an option for the prevention of VTE in adults having elective total hip replacement surgery or elective total knee replacement surgery. The initial dose, subject to normal hemostasis and reflective of additional risk factors, should be 10 mg within 6–10 hours after surgery, then for two weeks in knee surgery or five weeks in major hip surgery.
Apixaban is a highly selective and potent inhibitor of factor Xa at advanced clinical development. It has a small molecular weight, with a bioavailability of more than 50% and half-life of between nine and 14 hours. Apixaban has fixed twice-daily dosing and is metabolized in the liver via CYP3A4, with about 25% excreted by the kidneys and the remainder by intestinal excretion.Citation104 It has been shown to be safe and well tolerated in initial testing in volunteers and its anticoagulant effects closely correlated to plasma concentration of the drug. In the phase II APROPOS trial apixaban prevented more cases of VTE and death after total knee replacement than enoxaparin, but the risk of bleeding correlated with drug dose.Citation105 In a relatively small Botticelli-DVT trial (520 patients with acute symptomatic DVT), apixaban demonstrated effectiveness in the prevention of recurrent VTE similar to conventional therapy with LMWH followed by warfarin (target INR 2.0–3.0). The principal safety outcome (composite of major and clinically relevant, nonmajor bleeding) occurred in 7.3% of the apixaban-treated patients versus 7.9% of patients with reference treatment.Citation106
Another direct thrombin inhibitor, betrixaban, is being trialed. It has a small molecular weight, with an oral bioavailability of 47% and half-life of 19 hours, and is eliminated via intestinal excretion. A randomized phase II clinical trial, the EXPERT study, investigated the safety and efficacy of betrixaban in comparison with enoxaparin for VTE prevention in patients undergoing total knee replacement surgery. More than 200 patients were randomized to either betrixaban (15 or 40 mg twice daily) or enoxaparin (30 mg twice daily). Betrixaban demonstrated antithrombotic activity and appeared well tolerated in knee replacement patients at the doses studied.Citation107
Conclusions
In the UK, government initiatives have pushed anticoagulation center-stage in many aspects of medical and surgical care.Citation108,Citation109 The vast weight of prevention and treatment of VTE falls to the management of warfarin and LMWHs, with fondaparinux gaining use. However, the last few years has seen the development of new oral anticoagulants that may take over from currently established agents,Citation110 and for which government bodies are releasing guidelines.Citation78,Citation79,Citation94,Citation103 It seems likely that future practice will see the increasing use of novel oral anticoagulants.
Disclosures
ADB has taken hospitality, speaker fees and research funds from GlaxoSmithKline, Sanofi-Aventis and Boehringer Ingleheim. The University Department of Medicine at City Hospital recruits for anticoagulant trials.
References
- WhiteRHThe epidemiology of venous thromboembolismCirculation200310723 Suppl 11418
- HeitJAVenous thromboembolism: disease burden, outcomes and risk factorsJ Thromb Haemost200531611161716102026
- AndersonFASpencer FA. Risk factors for venous thromboembolismCirculation2003107I9I1612814980
- BlannADLipGYHVenous thromboembolismBr Med J200633221521916439400
- HeparinJHirsh HeparinN Engl J Med1991324156515742027360
- BullerHRSohneMMiddeldorpSTreatment of venous thromboembolismJ Thromb Haemost200531554156016102019
- TurpieAGChinBSLipGYVenous thromboembolism: pathophysiology, clinical features and preventionBr Med J200232588789012386044
- Schmidt-MulheimABeitrage zur Kenntnis des peptones under seiner physiologischen BedeutungArchiv fur Anatomie und Physiologie Physiologische Abteilung18803356
- DoyonMRapports du foie avec la coagulation du sangJournal de Physiologie et de Pathologie General191214229240
- McLeanJThe thromboplastinics action of cephalinAm J Physiol191641250257
- HowellWHHeparin and anticoagulationAm J Physiol192363434435
- JorpesJEThe chemistry of heparinBiochem J1935291817183016745848
- CrafoordCPreliminary report on post-operative treatment with heparin as a preventative of thrombosisActa Chir Scand193779407426
- BestCHPreparation of heparin and its use in the first clinical caseCirculation195919798613619024
- BrinkhousKMSmithHPWarnerEDSeegersWHThe inhibition of blood clotting: an unidentified substance which acts in conjunction with heparin to prevent the conversion of prothrombin to thrombinAm J Physiol1939125683687
- BeelerDRosenbergRJordanRFractionation of low molecular weight heparin species and the interaction with antithrombinJ Biol Chem197925429022913429327
- BarrowcliffeTWJohnsonEAEggletonCAKemball-CookGThorneDPAnticoagulant activities of high and low molecular weight heparin fractionsBr J Haematol197941573583435404
- KakkarVVDjazaeriBFokMScullyMFWestwickJLow molecular weight heparin and prevention of postoperative deep vein thrombosisBr Med J19822843753796800465
- HirshJOfosuFBuchananMRationale behind the development of low molecular weight heparin derivativesSemin Thromb Haemost1985111316
- RoemischJGrayEHoffmannJNWiedermannCJAntithrombin: a new look at the actions of a serine protease inhibitorBlood Coag Fibrinolysis200213657670
- SalzmanEWRosenbergRDSmithMHLindonJNFavreauLEffects of heparin and heparin fractions on platelet aggregationJ Clin Invest19806564736243142
- BlajchmanMAYoungEOfusuFAEffects of unfractionated heparin, dermatan sulphate and low molecular weight heparin on vessel wall permeability in rabbitsAnn NY Acad Sci19895562452542544126
- LindahlUHookMGlycosaminoglycans and their binding to biological macromoleculesAnn Rev Biochem197847385417354500
- CipolleRSeifertRNeilanBZaskeDEHasusEHeparin kinetics: variables related to disposition and dosageClin Pharmacol Ther1981293873937471609
- ShojaniaAMTetreaultJTurnbullJLThe variations between heparin sensitivity of different lots of activated partial thromboplastin time reagent produced by the same manufacturerAm J Clin Pathol19888919233337049
- HyersTMAgnelliGHullRDAntithrombotic therapy for venous thromboembolic diseaseChest2001119176S193S11157648
- TurpieAGGChinBSPLipGYHVenous thromboembolism: treatment strategiesBr Med J200232594895012399348
- BarrittDWJordanSCAnticoagulant drugs in the treatment of pulmonary embolus: a controlled clinical trialLancet1960171381309131213797091
- KernohanRJToddCHeparin therapy in thromboembolic diseaseLancet1966174386216234159671
- SalzmanEWDeykinDShaprioMRRosenbergRManagement of heparin therapy: controlled prospective trialN Engl J Med1975292104610501091856
- HullRDRaskobGEHirshJContinuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosisN Engl J Med1986315110911143531862
- BrandjesDPMHeijboerHBullerHRde RijkMJagtHten CateJWAcenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal vein thrombosisN Engl J Med1992327148514891406880
- SchulmanSRhedinASLindmarkerPA comparison of six weeks of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of anticoagulation trial study groupN Engl J Med1995332166116657760866
- TurpieAGHirschJProphylaxis and therapy of venous thromboembolismCRC Crit Rev Clin Lab Sci197910247274380902
- GallusAJackamanJTillettJMillsWWycherleyASafety and efficacy of warfarin started early after submassive proximal venous thrombosis or pulmonary embolusLancet198628519129312962878173
- WeismannRETobinRWArterial embolism occurring during systemic heparin therapyArch Surg195876219227
- ChongBHHeparin induced thrombocytopeniaJ Thromb Haemost200311471147812871282
- WarkentinTEHeparin induced thrombocytopenia. A ten year retrospectiveAnnu Rev Med19995012914710073268
- WarkentinTECurrent agents for the treatment of patients with heparin induced thrombocytopeniaCurr Opin Pulm Med2002840541212172444
- XiaoZTherouxPPlatelet activation with unfractionated heparin at therapeutic concentrations and comparisons with a low molecular weight heparin and with a direct thrombin inhibitorCirculation1998972512569462526
- YusufSMehtaSRChrolaviciusSComparison of fondaparinux and enoxaparin in acute coronary syndromesN Engl J Med20063541464147616537663
- WarkentinTELevineMNHirshJHeparin-induced thrombocytopenia in patients treated with low molecular weight heparin or unfractionated heparinN Engl J Med1995332133013357715641
- SquiresJWPinchLWHeparin induced spinal fracturesJAMA197924124172418439319
- SacklerJPLiuLHeparin induced osteoporosisBr J Radiol197346458460
- GinsbergJSKowalchukGHirschJHeparin effect on bone densityThromb Haemost1990642862892270535
- EdesTESunderrajanEVHeparin-induced hyperkalaemiaArch Intern Med1985145107010724004433
- DukesGEJrSandersSWRussoJJrTransaminase elevations in patients receiving bovine or porcine heparinAnn Intern Med19841006466506712030
- HirschJDalenJEDeykinDPollerLHeparin: Mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy, safetyChest1992102Suppl337S351S1327666
- TurpieAGGCan we differentiate the low molecular weight heparins?Clin Cardiol200023Suppl II4I710680037
- OffordRPerryDAnticoagulationLondon, UKScience Press2002
- HirshJWarkentinTEShaughnessySGHeparin and low molecular weight heparin. Mechanism of action, pharmacokinetics, dosing, monitoring, efficacy, safetyChest2001119Suppl64S94S11157643
- LensingAWAPrinsMHDavidsonBLHirshJTreatment of deep vein thrombosis with low molecular weight heparins. A meta-analysisArch Intern Med19951556016077887755
- LeizoroviczASimonneauGDecoususHBoisselJPComparison of efficacy and safety of low molecular weight heparins and unfractionated heparin in initial treatment of deep vein thrombosis: a meta analysisBr Med J19943096486518086990
- SiragusaSCosmiBPiovellaFHirschJGinsbergGLow molecular weight heparins an unfractionated in the treatment of patients with acute venous thromboembolism. Results of a meta-analysisAm J Med19961002692778629671
- Low-molecular-weight heparin in the treatment of patients with venous thromboembolism. The Columbus InvestigatorsN Engl J Med19973376576629280815
- SimonneauGSorsHCharbonnierBfor the THESEE study groupA comparison of low molecular weight heparin with unfractionated heparin for acute pulmonary embolismN Engl J Med19973376636699278462
- GouldMKDembitzerADDoyleRLLow molecular weight heparins compared with unfractionated heparin for the treatment of acute deep vein thrombosis: a meta-analysis of randomised controlled trialsAnn Intern Med199913080080910366369
- NorrbyKHeparin and angiogenesis: a low-molecular-weight fraction inhibits and a high-molecular-weight fraction stimulates angiogenesis systemicallyHaemostasis199323Suppl 11411497684350
- SjälanderAJanssonJHBergqvistDErikssonHCarlbergBSvenssonPEfficacy and safety of anticoagulant prophylaxis to prevent venous thromboembolism in acutely ill medical inpatients: a meta-analysisJ Intern Med2008263526018088252
- LevineMGentMHirschJA comparison of low molecular weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep vein thrombosisN Engl J Med19963346776818594425
- KoopmanMMWPrandoniPPiovellaFTreatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous LMWH administered at homeN Engl J Med19963346826878594426
- OmranHHammerstinglCSchmidtHvon der ReckeGPaarWDLüderitzBA prospective and randomised comparison off the safety and effects of therapeutic levels of enoxaparin versus unfractionated heparin in chronically anticoagulated patients undergoing elective cardiac catheterisationThromb Haemost20039026727112888874
- JacksonMRClagettGPAntithrombotic therapy in peripheral arterial occlusive diseaseChest2001119283S299S11157655
- LeeAYThe role of low molecular weight heparins in the prevention and treatment of venous thromboembolism in cancer patientsCurr Opin Pulm Med2003935135512904702
- LeeAYLevineMNBakerRILow-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancerN Engl J Med200334914615312853587
- KhoranaAAThe NCCN Clinical Practice Guidelines on Venous Thromboembolic Disease: strategies for improving VTE prophylaxis in hospitalized cancer patientsOncologist2007121361137018055857
- LevineMNLeeAYKakkarAKFrom Trousseau to targeted therapy: New insights and innovations in thrombosis and cancerJ Thromb Haemost200311456146312871280
- FolsomARCushmanMTsaiMYA prospective study of venous thromboembolism in relation to factor V Leiden and related factorsBlood2002992720272511929758
- SchulmanSUnresolved issues in anticoagulant therapyJ Thromb Haemost200311464147012871281
- KnightCJPanseartMWilsonDJIncreased platelet responsiveness following coronary stentingEur Heart J199819123912489740346
- The British National FormularyIssue 57. 2009. Available from http://www.bnf.org/ Accessed March 10, 2009.
- TurpieAGBauerKAErikssonBILassenMRFondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studiesArch Intern Med20021621833184012196081
- HirshJGuyattGAlbersGWAntithrombotic and thrombolytic therapy. American College of Chest Physicians. Evidence-Based Clinical Practice Guidelines (8th Edition)Chest20081336 Suppl71S109S Errata in: Chest. 2008;134:473 and Chest. 2008;134:892.18574259
- British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development GroupBritish Thoracic Society Standards of Care Committee Pulmonary embolism guidelineThorax20035847048312775856
- The Scottish Intercollegiate Guidelines Network (SIGN). Available from http://www.sign.ac.uk/ Accessed March 10, 2009.
- BaglinTBarrowcliffeTWCohenAGreavesMGuidelines on the use and monitoring of heparinBr J Haematol2006133193416512825
- Royal College of Obstetrics and Gynaecology. Guideline 28. Thromboembolic disease in pregnancy and the puerperium: Acute managementLondon UKRoyal College of Obstetrics and Gynaecology2001Available from http://www.rcog.org.uk/ Accessed March 10, 2009.
- Report of the Independent Expert Working Group on the prevention of venous thromboembolism in hospitalized patients. Department of Health. Available from http://www.doh.gov.uk/ Accessed March 10, 2009.
- National Institute for Health and Clinical ExcellenceGuideline 46. VTE: Reducing the risk of VTE (DVT and PE) in in-patients undergoing surgery. 2008. Available from http://www.nice.org.uk/ Accessed March 10, 2009.
- TanKTLipGYFondaparinuxCurr Pharm Des20051141541915725062
- GrossPLWeitzJINew anticoagulants for treatment of venous thromboembolismArterioscler Thromb Vasc Biol20082838038618296593
- CrowtherMAWarkentinTEBleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agentsBlood20081114871487918309033
- HirshJO’DonnellMEikelboomJWBeyond unfractionated heparin and warfarinCirculation200711655256017664384
- KhooCWTayKHShantsilaELipGYHNovel oral anticoagulantsInt J Clin Pract20096363064119222611
- ShantsilaELipGYApixaban, an oral, direct inhibitor of activated Factor XaCurr Opin Investig Drugs2008910201033
- KakarPWatsonTLipGYRivaroxabanDrugs Today20074312913617380210
- StangierJRathgenKStahleHGansserDRothWThe pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjectsBr J Clin Pharmacol20076429230317506785
- ErikssonBIDahlOEAhnfeltLDose escalating safety study of a new oral direct thrombin inhibitor, dabigatran etexilate, in patients undergoing total hip replacement: BISTRO IJ Thromb Haemost200421573158015333033
- ErikssonBIDahlOEBullerHRBISTRO II Study GroupA new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: The BISTRO II randomized trialJ Thromb Haemost2005310311115634273
- EzekowitzMDReillyPANehmizGDabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study)Am J Cardiol20071001419142617950801
- ErikssonBIDahlOERosencherNRE-NOVATE Study GroupDabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trialLancet200737094995617869635
- ErikssonBIDahlOERosencherNRE-MODEL Study GroupOral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trialJ Thromb Haemost200752178218517764540
- GinsbergJSDavidsonBLRE-MOBILIZE Writing Committee. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery200924119
- National Institute for Health and Clinical ExcellenceFinal appraisal determination. Dabigatran etexilate for the prevention of VTE after hip or knee replacement surgery in adults2008Available from http://www.nice.org.uk/ Accessed March 10, 2009.
- EikleboomJEWeitzJIDabigatran etexilate for prevention of venous thromboembolismThromb Haemost2009101doi:10.1160/TH08-10-0708.
- KubitzaDBeckaMVoithBZuehlsdorfMWensingGSafety, pharmacodynamics, and pharmacokinetics of single doses of BAY59-7939, an oral, direct factor Xa inhibitorClin Pharmacol Ther20057841242116198660
- PerzbornEStrassburgerJWilmenAIn vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939 – an oral, direct factor Xa inhibitorJ Thromb Haemost2005351452115748242
- ErikssonBIBorrisLCFriedmanRJRECORD1 Study GroupRivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplastyN Engl J Med20083582765277518579811
- KakkarAKBrennerBDahlOERECORD2 InvestigatorsExtended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled studyLancet20083719632313918582928
- LassenMRAgenoWBorrisLCRECORD3 InvestigatorsRivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplastyN Engl J Med20083582776278618579812
- AgnelliGGallusAGoldhaberSZthe ODIXa-DVT Study InvestigatorsTreatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT studyCirculation200711618018717576867
- BullerHRLensingAWAPrinsMHthe Einstein-DVT Dose-Ranging Study InvestigatorsA dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-Ranging StudyBlood20081122242224718621928
- National Institute for Health and Clinical ExcellenceFinal appraisal determination. Rivaroxaban for the prevention of VTE after hip or knee replacement in adultsMarch 5, 2009. Available from http://www.nice.org.uk/guidance/index.jsp?action=download&o=43417 Accessed March 10, 2009.
- WongPCCrainEJXinBApixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studiesJ Thromb Haemost2008682082918315548
- LassenMRDavidsonBLGallusAPineoGAnsellJDeitchmanDThe efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacementJ Thromb Haemost200752368237517868430
- BullerHDeitchmanDPrinsMSegersABotticelli InvestigatorsEfficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging studyJ Thromb Haemost200861313131818541000
- TurpieAGBauerKADavidsonBLEXPERT Study GroupA randomized evaluation of betrixaban, an oral factor Xa inhibitor, for prevention of thromboembolic events after total knee replacement (EXPERT)Thromb Haemost2009101687619132191
- National Patients Safety AgencyPatient Safety Alert 18. Actions that can make anticoagulant therapy safer2008. Available from: http://www.npsa.nhs.uk/health/alerts Accessed March 15, 2009.
- Department of Health. The VTE Implementation Working Group. September 18, 2008. Available from: http://www.dh.gov.uk/en/Publichealth/Healthprotection/Bloodsafety/DH_082132
- WeitzJIHirshJSamamaMMAmerican College of Chest PhysiciansNew antithrombotic drugs: American College of Chest Physicians Evidence-Based. Clinical Practice Guidelines (8th Edition)Chest20081336 Suppl234S256S Erratum in Chest. 2008;134:473.18574267