Abstract
Pulmonary arterial hypertension (PAH) is a devastating disease characterized by progressive elevation of pulmonary arterial pressure and vascular resistance due to pulmonary vasoconstriction and vessel remodeling as well as inflammation. Rho-kinases (ROCKs) are one of the best-described effectors of the small G-protein RhoA, and ROCKs are involved in a variety of cellular functions including muscle cell contraction, proliferation and vascular inflammation through inhibition of myosin light chain phosphatase and activation of downstream mediators. A plethora of evidence in animal models suggests that heightened RhoA/ROCK signaling is important in the pathogenesis of pulmonary hypertension by causing enhanced constriction and remodeling of the pulmonary vasculature. Both animal and clinical studies suggest that ROCK inhibitors are effective for treatment of severe PAH with minimal risk, which supports the premise that ROCKs are important therapeutic targets in pulmonary hypertension and that ROCK inhibitors are a promising new class of drugs for this devastating disease.
Pulmonary hypertension
Pulmonary arterial hypertension (PAH), characterized by an elevated, sustained increase in pulmonary artery pressure greater than 25 mmHg at rest or 30 mmHg upon exertion, is a progressive disease with poor prognosis and death usually occurring within 5 years if left untreated.Citation1 Further, primary or idiopathic pulmonary hypertension (IPAH) can result in death within a median of 3 years from right ventricular failure without treatment, with a 15% 1-year mortality rate despite current therapy.Citation2 Factors contributing to PAH include prolonged vasoconstriction, vascular remodeling, inflammatory cell migration, and in situ thrombosis which result in the formation of vascular lesions.Citation3,Citation4 It is currently thought that the primary cause of the elevated pulmonary vascular resistance that occurs in PAH is due to mechanical obstruction from vascular remodeling.Citation5,Citation6 In addition, pathologic findings show that PAH is associated with intimal and/or medial hypertrophy, intimal fibrosis, and plexiform lesions.Citation7
Animal models of pulmonary hypertension
Although the long-term prognosis for patients with PAH is rather poor, recent advances in the understanding of pathophysiological mechanisms underlying the progression of PAH have been made possible through the use of experimental animal models. The monocrotaline model of PAH, initially used over 40 years ago, is induced via a single injection of 60 mg/kg monocrotaline either intraperitoneally or subcutaneously.Citation8 Rapid and severe pulmonary vascular disease usually occurs within a few days (independent of any cardiac or lung parenchymal disorders), suggesting that this model is an excellent choice to study IPAH. Although the basic underlying mechanism of monocrotaline-induced PAH is not well understood, it is known that the parent compound is not toxic, and must be activated to the reactive monocrotaline pyrrole by hepatic cytochrome P450 3A, which targets the pulmonary vascular endothelium.Citation9–Citation12 A limitation of this experimental model is that differences exist in monocrotaline sensitivity between rat strains as well as individual variances in the pharmacokinetics of monocrotaline involving degradation and hepatic formation of the pyrrole or conjugation and excretion.Citation13
A second widely employed model of PAH is the use of chronic hypoxia. Studies show that decreasing the alveolar oxygen pressure to <70 mmHg elicits a strong pulmonary vasoconstrictor response; however, the hypoxic-induced effect varies among animal species.Citation14 For example, rabbits show very little response to alveolar hypoxia, but cattle exhibit the greatest vasoconstriction, and hypoxic pulmonary vasoconstriction is milder in humans than in rats.Citation15 Further, the hypoxic pulmonary vasoconstrictor response varies among humans.Citation16 The time of exposure to hypoxia appears to be critical as short exposure causes acute pulmonary vasoconstriction, while prolonged hypoxia results in remodeling of the distal pulmonary arterial branches. It has also been observed that endothelial and smooth muscle hyperplasia occurs in the walls of pulmonary arteries in rats during the first days of hypoxic exposure.Citation17,Citation18 In animal models, intermittent severe hypoxia leads to the development of PAH, independent of the duration of the hypoxia to normoxia intervals. However, in humans, intermittent hypoxia elicits only a small clinically irrelevant effect on pulmonary hemodynamics.Citation19 Thus, caution must be exercised when extrapolating animal models of chronic hypoxic-induced PAH to the human setting. Another documented animal model of PAH involves the formation of chronic emboli in pulmonary vessels. Shelub et al induced chronic embolic PAH through repeated microembolizations with the injection of Sephadex® microspheres.Citation20 The utilization of this approach allows different-sized vessels to be targeted depending on the diameter size of the microspheres that are injected, and vascular obstruction and vasoconstriction are the primary mechanisms of the high pulmonary vascular resistance that occurs.Citation20,Citation21 More recently, repeated embolizations with poly-dextran microspheres were used in pigs to elicit a sustained elevation in pulmonary arterial pressure.Citation22
A recent rat animal model of experimental PAH described by Taraseviciene-Stewart and colleagues involves the combination of vascular endothelial growth factor receptor blockade with SUGEN (SU) 5416 and chronic hypoxia exposure.Citation23 A severe, progressive PAH occurs which is accompanied by precapillary arterial occlusion by proliferating factor VIII-positive endothelial cells. In addition, the PAH in these animals is resistant to treatment with drugs that are commonly used to treat human PAH.Citation24 Thus, it appears that this particular model of PAH more closely mimics human severe PAH than the monocrotaline and chronic hypoxia models of PAH which can be successfully treated with a variety of agents.Citation25 Finally, the newest animal models of PAH involve the use of genetically modified animals as genetic screening has identified a number of potentially important gene variants that may contribute towards the development of PAH. For example, since a heterozygous mutation of the BMPR2 gene which encodes for the bone morphogenetic protein receptor-II is present in a large portion of patients with IPAH, heterozygous BMPR2-deficient mice have been used to mimic the human condition with limited success.Citation26–Citation28 Further, serotonin (5-HT), and its plasma membrane transporter (5-TTT) has been reported to be involved in the pathogenesis of PAH in humans, and genetically engineered mice lacking the 5-HTTT exhibit attenuated hypoxia-induced PAH, and mice overexpressing 5-HTTT can cause PAH.Citation29,Citation30
Current therapies for pulmonary hypertension
Although there is a variety of drugs that are currently used or under investigation for the treatment of PAH, there is no specific drug class that is completely efficacious in reversing the deleterious effects of this disease. Nitric oxide (NO) has been used with mixed success as a therapy for PAH. Exogenous administration of the inhaled preparation of NO reduces pulmonary vascular resistance without having an effect on systemic vascular resistance or cardiac function.Citation31,Citation32 Inhaled NO has been used to treat primary pulmonary hypertension, secondary pulmonary hypertension in association with congenital or acquired heart disease, chronic obstructive pulmonary disease (COPD), and Adult Respiratory Distress Syndrome (ARDS).Citation33–Citation35 Evidence suggests that the efficacy of NO is dependent on the inhaled concentration. For example, it has been observed that 40 ppm inhaled NO will reverse hypoxic pulmonary vasoconstriction in healthy individuals independent of systemic effects, and initial results showed improvement in ARDS patients.Citation36,Citation37 However, it was also reported that inhaled NO in doses greater than 10 ppm worsen arterial oxygenation and therefore, it was suggested that lower doses be used to treat ARDS.Citation38 The primary limiting factor in the global use of inhaled NO is the potential toxicity that may occur if high concentrations are used. Chemical reactions with oxygen and reactive oxygen species yield toxic nitrogen oxides and hydroxyl radicals. Subsequently, nitrogen oxide reacts with superoxide to form peroxynitrite, which can elicit pulmonary cellular injury.Citation39
The prostanoids are another class of agents used to treat PAH as these substances have been successfully tested in animal models of PAH, and prostacyclin and its analogs have been extensively studied in the treatment of human PAH.Citation40,Citation41 Mechanistically, prostacyclin exerts vasoprotective effects through pulmonary vasodilatation, inhibition of platelet aggregation, and pulmonary arterial smooth muscle proliferation.Citation42 Because of prostacyclin’s short half-life, several analogs have been developed which appear to exhibit long-term beneficial vasodilatory and antithrombotic effects in patients with PAH when given by daily inhalation.Citation43 Specifically, aerosolized iloprost causes selective pulmonary vasodilation in patients with either primary or secondary pulmonary hypertension.Citation43 In contrast, beraprost has limited effectiveness in treating both primary and secondary PAH.Citation44,Citation45 Most recently, it was found that intravenous epoprostenol improves survival in patients with PAH.Citation46
Endothelin (ET) receptor antagonists represent some of the newest class of agents available for treatment of PAH. The selective ETA receptor antagonist BQ-123 was the first ET receptor antagonist to exhibit beneficial effects in animal models of PAH, and bosentan, an antagonist of both ETA and ETB receptors reduces the medial thickening and neomuscularization of pulmonary arteries as well as lowering pulmonary arterial pressure in rat animal models of PAH.Citation47–Citation49 Studies also show that bosentan is clinically effective in patients with PAH, which included significant improvement in exercise capacity, functional class, and pulmonary hemodynamics.Citation50,Citation51
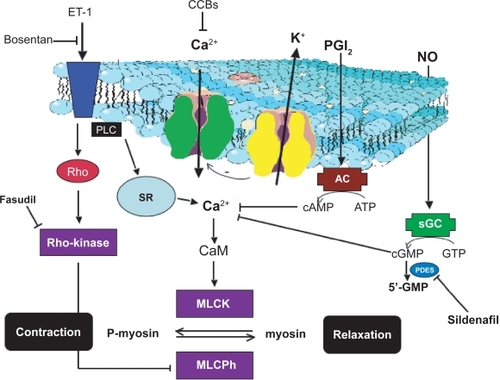
The newest and most potentially efficacious therapeutic agents to date are the phosphodiesterase (PDE) 5 inhibitors, which were discovered incidentally to have pulmonary vasodilating effects in addition to being used to treat erectile dysfunction. Sildenafil attenuates the acute pulmonary vasoconstrictor response to hypoxia in humans as well as lowering high-altitude-induced temporary PAH in healthy volunteers.Citation52,Citation53 In addition, sildenafil decreases RV mass in patients with PAH, suggesting that PDE5 inhibitors may be able to reverse the RV remodeling that occurs in PAH.Citation54 Further, the combination of sildenafil and besaprost may be efficacious in treating PAH.Citation55 Other therapies include adrenomedullin, an endogenously produced vasodilator originally discovered in human pheochromocytoma, and levosimendan, a dual calcium-sensitizing positive inotropic agent and potassium channel activator. Inhaled adrenomedullin may improve exercise capacity and selectively decrease pulmonary vascular pressure in patients with primary pulmonary hypertension, and intravenous levosimendan significantly lowers pulmonary vascular resistance after heart transplant procedure.Citation56,Citation57 Finally, calcium channel blockers have been used with limited success as less than 10% of patients with PAH have a beneficial acute pulmonary vasodilatory response to long-term treatment.Citation58 Mechanisms by which currently used drugs elicit pulmonary vasodilatation for treatment of pulmonary hypertension are shown in , although the limited success of these current therapeutic regimens has been the impetus for investigating the feasibility of therapeutically targeting Rho-kinase signaling mechanisms in PAH.
Figure 1 Mechanisms through which current drugs elicit pulmonary vasodilatation to treat pulmonary hypertension.
Rho-kinase
There is convincing evidence that Rho-kinases are involved in a variety of cardiovascular diseases including pulmonary hypertension.Citation59–Citation64 Approximately seventeen years ago it was revealed that a small monomeric GTPase called Rho induced the formation of stress fibers and focal adhesions in 3T3 cells.Citation65 Subsequently, a number of laboratories demonstrated that Rho was expressed in smooth muscle and could be activated by a plethora of contractile agonists.Citation66 Although Rho was shown to increase Ca2+ sensitivity and phosphorylate myosin light chain in intact smooth muscle, these events could not occur in permeabilized smooth muscle cells, suggesting that activation required interaction of Rho with a second component of the plasma membrane.Citation67,Citation68
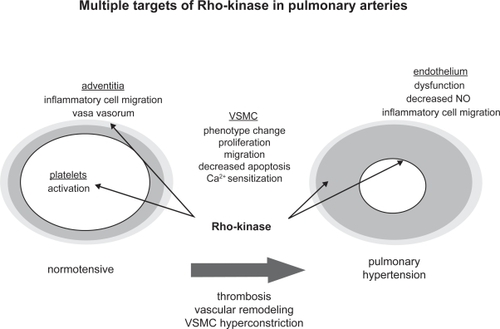
In the mid 1990s a number of investigators working independently identified one of the effectors of Rho and termed it Rho-kinase, and it was postulated that this was the component necessary for Rho activation.Citation69,Citation70 In 1996, it was reported that Rho-kinase (ROCK) was activated by Rho, which subsequently phosphorylated and inhibited myosin light chain phosphatase.Citation71 One year later it was found that translocation of Rho to the plasma membrane was necessary for increasing Ca2+ sensitivity.Citation72 Further, it was widely documented that Rho and/or ROCK antagonists inhibited the effect of contractile agonists indicating that both Rho and ROCK were major effectors of agonist-induced Ca2+ sensitization in smooth muscle.Citation67,Citation73–Citation75 ROCKs are serine/threonine kinases with a molecular mass of approximately 160 kDa.Citation1,Citation61 These kinases are expressed in invertebrates such as Caenorhabditis elegans, Drosophila, and mosquito, and in vertebrates such as zebrafish, Xenopus, chicken, mouse, rat, and human.Citation61 ROCK’s catalytic site is adjacent to its NH2 terminus, whereas the Rho binding site is located at the COOH-terminal portion of its coiled-coil domain.Citation66 ROCKs regulate a variety of cellular functions including motility, proliferation, apoptosis, contraction, and gene expression, and are believed to be the most important regulators of Ca2+ sensitivity in smooth muscle.Citation61,Citation66,Citation76,Citation77 ROCK-mediated pathophysiological mechanisms that may contribute to the development of pulmonary hypertension are summarized in .
Figure 2 Schematic illustration of possible Rho-kinase mediated pathophysiological mechanisms that may contribute to the development of pulmonary hypertension.
Currently, two isoforms (ROCK-1 and ROCK-2) have been identified, which are expressed in vascular smooth muscle.Citation61 ROCK activation increases the Ca2+ sensitivity of contraction in vascular smooth muscle via inhibition of myosin light chain phosphatase, which increases the phosphorylation of myosin light chain and augments contraction at any given level of cytosolic Ca2+ and activity of myosin light chain kinase.Citation78 ROCK inhibits myosin light chain phosphatase by phosphorylating the 130 kDa myosin-binding subunit myosin light chain phosphatase (MYPT-1) and/or the myosin light chain phosphatase inhibitor protein CPI-17.Citation66 Further, ROCKs target other substrates that are important for smooth muscle contraction such as calponin.Citation61
RhoA activates ROCK after extracellular G-protein coupled receptor binding.Citation72 Depending on the agonist stimulation, ROCK may increase Ca2+ sensitivity of the contractile apparatus via phosphorylation of MYPT-1 at threonine (Thr)-696 and Thr-853, and phosphorylation of CPI-17 at Thr-38.Citation66 More recently, it was shown that ROCK also phosphorylates Thr-855 on MYPT-1.Citation79,Citation80
Rho-kinases as targets for pulmonary hypertension
Evidence strongly suggests that RhoA/ROCK is an attractive target for the development of therapeutics to treat PAH as many studies have shown that ROCK signaling is involved in the sustained vasoconstriction and vascular remodeling and inflammation that occurs in this disease. Oka et al reported that ROCK signaling mediated vasoconstriction in severe occlusive pulmonary hypertension in rats, and other studies have shown that ROCKs are involved in hypoxic pulmonary vasoconstriction, hypoxic pulmonary hypertension, and monocrotaline-induced pulmonary hypertension.Citation3,Citation81–Citation83 More recently, studies confirmed that ROCK-mediated prolonged vasoconstriction was inherently involved in chronic hypoxic pulmonary hypertension in both neonatal and adult rats as well as in bleomycin-induced pulmonary hypertension.Citation84,Citation85 Endothelin-1 increases pulmonary vasoconstriction via ROCK signaling, and small pulmonary arteries exhibit ROCK-dependent increases in myogenic tone in chronic hypoxic pulmonary hypertension.Citation86–Citation88 Further, ROCKs have been implicated in the vascular remodeling associated with experimental models of PAH, and a recent report by Guilluy and colleagues suggests that transglutaminase-mediated activation of RhoA by serotonin may be involved in pulmonary vascular remodeling induced by chronic hypoxia.Citation78,Citation89–Citation91 In humans, preliminary studies show that the pulmonary vasodilatory response in hypertensive pulmonary arteries and isolated perfused lung lobes removed from lung transplantation patients for severe PAH is associated with high RhoA/ROCK activity.Citation92
Studies involving ROCK inhibitors have been primarily done with either fasudil or Y-27632. Both compounds are cell-permeable and potently inhibit ROCK in vascular smooth muscle.Citation93,Citation94 Fasudil, initially described as an intracellular calcium antagonist, is a selective inhibitor of ROCK.Citation61,Citation74,Citation95 Specifically, fasudil is metabolized in the liver to the active compound hydroxyfasudil, which is a specific ROCK inhibitor because its efficacy for ROCK is 100-fold higher than for protein kinase C (PKC), and 1000-fold higher than for myosin light chain kinase.Citation93,Citation96 Similarly, Y-27632, a pyridine derivative, binds to and inhibits p160ROCK up to 200-fold higher than PKC or PKA.Citation74
The mechanisms by which ROCK antagonists attenuate PAH are widespread. Evidence from in vivo studies suggests that the effect of ROCK inhibitors is associated with decreased pulmonary artery expression of growth factors and markers of cell proliferation, matrix protein production, and inflammatory cell infiltration as well as an increase in signals for apoptosis.Citation97 In vitro studies also suggest a negating effect of ROCK inhibitors on pulmonary vascular cell growth. Chapados and colleagues reported that Y-27632 prevented stress fiber formation and reduced nuclear extracellular signal-regulated kinase and tenascin-C expression.Citation98 In animal models of PAH, fasudil or Y-27632 decreases the sustained vasoconstrictor response and vascular remodeling that occurs during exposure to monocrotaline or hypoxia.Citation78,Citation99 Further, inhibition of ROCK attenuates pulmonary arterial pressure and reverses pulmonary vasoconstriction caused by NO synthase (NOS) antagonists in chronically hypoxic lungs, and ROCK antagonists also lower acute hypoxia-mediated contraction of isolated rat pulmonary artery segments and isolated mouse lungs.Citation77,Citation78,Citation83 Oka et al also showed that this same compound acutely lowered right ventricular systolic pressure in SU5416/hypoxia-exposed rat lungs.Citation3 In mouse models of PAH, treatment with Y-27632 decreased the muscularization of distal pulmonary arteries and upregulated eNOS expression, and Abe and colleagues observed that inhibiting chronic hypoxic PAH in mice increased lung eNOS expression and Akt phosphorylation.Citation78,Citation100
Finally, evidence suggests that statins may also be effective inhibitors of ROCK. Statins (HMG-CoA reductase inhibitors) block the synthesis of mevalonate and its isoprenoid intermediate compound geranylgeranylpyrophosphate, which subsequently inhibits isoprenylation of RhoA and its translocation to the plasma membrane.Citation101 Statins ameliorate PAH in a variety of rat models, and a recent report by Girgis et al provides evidence that reversal of hypoxic PAH by simvastatin is coupled to decreased lung expression and activity of both ROCK I and II.Citation24,Citation102–Citation107 In addition, Li et al observed that atorvastatin blocked serotonergic-mediated pulmonary artery smooth muscle proliferation and migration by inhibiting membrane translocation of RhoA.Citation108
Rho-kinase inhibitors as therapies for pulmonary hypertension
ROCK inhibitors have been used for approximately 15 years to treat cardiovascular disorders including vasospasm after subarachnoid hemorrhage and stable effort angina pectoris with no adverse effects.Citation61,Citation95 Although relatively few, clinical studies with ROCK inhibitors also show that fasudil is an effective treatment for severe pulmonary hypertension. In PAH patients that did not respond to oxygen inhalation, NO inhalation, or nifedipine, 30-minute intravenous fasudil treatment significantly decreased elevated pulmonary vascular resistance without causing systemic hypotension.Citation63 Further, Ishikura et al confirmed that fasudil had acute beneficial pulmonary hemodynamic effects in patients with PAH.Citation109 Collectively, these clinical studies suggest that ROCK signaling is a viable therapeutic target as a treatment for pulmonary hypertension with ROCK inhibitors (). However, although fasudil appears to be a promising agent to use for PAH, long-term effects of fasudil administration in patients with severe pulmonary hypertension need to be evaluated before more definitive conclusions can be made as to the efficacy of this type of agent. Studies also suggest that this drug can be used for other cardiovascular diseases with minimal side effects. Oral treatment with fasudil for at least 4 weeks significantly lengthened maximum exercise time without any effect on blood pressure and heart rate during the exercise independent of any serious unwanted side effects from the drug.Citation61 In addition, fasudil improved myocardial ischemia in patients with microvascular angina caused by coronary microvascular vasoconstriction.Citation110
Conclusions
One of the biggest obstacles in treating pulmonary hypertension is identifying effective therapeutic agents that selectively target the pulmonary vasculature with minimal side effects. The RhoA/ROCK signaling pathway is an important regulator of pulmonary vascular function and animal studies have shown that this pathway is important in the pathogenesis of major disease states such as pulmonary hypertension. Further studies should be done to determine how ROCKs mediate pulmonary vascular smooth muscle cell physiology and how these moieties are involved in vascular disease states. Recent clinical studies in humans show promise in that acute treatment with ROCK inhibitors in patients with severe pulmonary hypertension exhibited signs of improved pulmonary vascular function by attenuating the ROCK-mediated increase in pulmonary vascular resistance. Towards this end, large clinical trials are the logical next step in demonstrating that long-term treatment with ROCK inhibitors is both safe and efficacious in patients with pulmonary hypertension.
Acknowledgments and disclosures
This review was supported by NIH HL68026 (SA Barman), AHA-Grant-in-Aid Award 0555149B (SA Barman), and NIH HL73890 (RE White). The authors report no conflicts of interest.
References
- CogolludoAMorenoLVillamorEMechanisms controlling vascular tone in pulmonary arterial hypertension: implications for vasodilator therapyPharmacology200779657517148943
- ThenappanTShahSJRichSGomberg-MaitlandMA USA-based registry for pulmonary arterial hypertension: 1982–2006Eur Respir J200730225S230S
- OkaMHommaNTaraseviciene-StewartLRho-kinase mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in ratsCirc Res200710092392917332430
- ShimokawaHTakeshitaARho-kinase is an important therapeutic target in cardiovascular medicineArterioscler Thromb Vasc Biol2005251767177516002741
- HumbertMSitbonOSimonneauGTreatment of pulmonary hypertensionN Engl J Med20043511425143615459304
- CoolCDGroshongSDOakeyJVoelkelNFPulmonary hypertension: cellular and molecular mechanismsChest2005128565S571S16373828
- RunoJRLoydJEPrimary pulmonary hypertensionLancet20033611533154412737878
- LalichJJMerkowLPulmonary arteritis produced in rats by feeding Crotolaria spectabilisLab Invest19611074475013758395
- MattocksARToxicity of pirrolizidine alkaloidsNature19982177237285641123
- ReidMJLameMWMorinDWilsonDWSegallHJInvolvement of cytochrome P450 3A in the metabolism and covalent bonding of 14C-monocrotaline in rat liver microsomesJ Biochem Toxicol199812157166
- LoscalzoJEndothelial dysfunction in pulmonary hypertensionN Engl J Med1992271171191603118
- TuderRMGrovesBBadeschDBVoelkelNFExuberant endothelial cell growth and elements of inflammation are present in plexiform lesion of pulmonary hypertensionAm J Pathol19941442752857508683
- MattocksARDriverHEBarbourRHRobinsDJMetabolism and toxicity of synthetic analogues of macrocyclic diester pyrolizidine alkaloidsChem Biol Interact198658951083708724
- CampianMEHardziyenkaMMichelMCTanHLHow valid are animal models to evaluate treatments for pulmonary hypertension?Naunyn-Schmeideberg’s Arch Pharmacol2006373391400
- ReeveJTWagnerWWJrMcMurtryIFGroverRFPhysiological effects of high altitude on the pulmonary circulationInt Rev Physiol197920289310387636
- NaejieRMelotCHMoisPHallemansREffects of vasodilators on hypoxic pulmonary vasoconstriction in normal manChest1982824044106811216
- MeyrickBReadLThe effect of continuous hypoxia on rat pulmonary arterial circulation: an ultra structural studyLab Invest197838188200146763
- FungYCLinQChanges of zero-stress state of rat pulmonary arteries in hypoxic hypertensionJ Appl Physiol199170245524701885439
- WeitzenblumEChauatAHypoxic pulmonary hypertension in man: what minimum daily duration of hypoxaemia is required?Eur Resp J200118251253
- ShelubIvan GrondelleAMcCulloughRHofmeisterSReevesJTA model of chronic embolic pulmonary hypertension in dogJ Appl Physiol1984568108156706785
- DantzkerDRBowerJSPartial reversibility of chronic pulmonary hypertension caused by thromboembolic diseaseAnn Rev Respir Dis1981124129131
- WeimannJZinkWSchnabelPASelective vasodilatation by nitric oxide inhalation during sustained pulmonary hypertension following recurrent microembolism in pigsJ Crit Care19991413314010527251
- Taraseviciene-StewartLKasaharaYAlgerLInhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertensionFASEB J20011542743811156958
- Taraseviciene-StewartLScerbaviciusRChoeKHSimvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertensionAm J Physiol Lung Cell Mol Physiol2006291L668L67616698853
- VoelkelNFTuderRMHypoxia-induced pulmonary vascular remodeling: a model for what human disease?J Clin Invest200010673373810995781
- LaneKBMachadoRDPauciuloMWHeterozygous germline mutation in BMPR2, encoding a TGF-beta receptor, causing familial primary pulmonary hypertension. The international PPH ConsortiumNat Genet200026818410973254
- DengZMorseJHSlagerSLFamilial primary pulmonary hypertension (gene PPH1) is caused by mutation in the bone morphogenetic protein receptor-II geneAm J Hum Genet20046773774410903931
- BeppuHKawabataMHamamotoTBMP type II receptor is required for gastrulation and early development of mouse embryosDev Biol200022124925810772805
- EddahibiSHanounNLanfumeyLAttenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine B11 transporter geneJ Clin Invest20001051555156210841514
- EddahibiSHumbertMFadelESerotonin transporter over-expression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertensionJ Clin Invest20011081141115011602621
- FrostellCFratacciMDWainJCJonesRZapolWMInhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstrictionCirculation199183203820472040056
- Pepke-ZabaJHiggenbottamTWDinh-XuanATStoneDWallworkJInhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertensionLancet1991338117311741682593
- AdatiaIThompsonJLandzbergMWesselDLInhaled nitric oxide in chronic obstructive lung diseaseLancet19933413073088093943
- RossaintRFalkeKJLopezFSlamaKPisonUZapolWMInhaled nitric oxide for the adult respiratory distress syndromeN Engl J Med19933283994058357359
- McIntyreRCJrMooreFAMooreEEPiedalueFHaenalJSFullerttonDAInhaled nitric oxide variably improves oxygenation and pulmonary hypertension in patients with acute respiratory distress syndromeJ Trauma1995394184257473902
- FrostellCGBlomqvistHHedenstiernaGLundbergJZapolWMInhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilationAnesthesiology1993784274358457043
- WalmrathDSchneiderTSchermulyROlschewskiHGrimmingerFSeegerWDirect comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndromeAm J Respir Crit Care Med19961569919968630585
- GerlachHRossaintRPappertDFalkeKJTime-course and dose-response of nitric oxide inhalation for systemic oxygenation and pulmonary hypertension in patients with adult respiratory distress syndromeEur J Clin Invest1993234995028405003
- GowAJThomSRIschiropoulosHNitric oxide and peroxynitrite-mediated pulmonary cell deathAm J Physiol1998274L112L1189458808
- McLaughlinVVRichSPulmonary hypertension-advances in medical and surgical interventionJ Heart Lung Transplant1998177397439730421
- WanstallJCJeffreyTKRecognition and management of pulmonary hypertensionDrugs19985698910079878988
- RichSMcLaughlinVVThe effect of chronic prostacyclin therapy on cardiac output and symptoms in primary pulmonary hypertensionJ Am Coll Cardiol1999341184118710520810
- WittWMullerBAntithrombotic profile of iloprost in experimental models of in vivo platelet aggregation and thrombosisAdv Prostaglandin Thromboxane Leuktriene Res198717A279284
- SajiTOzawaYIshikitaTMatsuuraHMatsuoNShort-term hemodynamic effect of a new oral PGI2 analogue, beraprost, in primary and secondary pulmonary hypertensionAm J Cardiol1996782442478712155
- HashidaHHamadaMShigematsuYBeneficial hemodynamic effect of oral prostacyclin (PGI2) analogue, beraprost sodium, on a patient with primary pulmonary hypertension: a case reportAngiology1998491611649482517
- ArcherSJMichelakisEDAn evidence-based approach to the management of pulmonary arterial hypertensionCurr Opin Cardiol20062138539216755209
- MiyauchiTYorikaneRSakaiSContribution of endogenous endothelin-1 to the progression of cardiopulmonary alteration in rats with monocrotaline-induced pulmonary hypertensionCirc Res1993738878978403258
- ChenSChenYMengQCDurandJDicarloVSOparilSEndothelin-receptor antagonist bosentan prevents and reverses hypoxic pulmonary hypertension in ratsJ Appl Physiol199579212221318847282
- HillNSWarburtonRRPietrasLKlingerJRNon-specific endothelin-receptor antagonists blunt monocrotaline-induced pulmonary hypertension in ratsJ Appl Physiol199783120912159338430
- ChannickRNSimonneauGSitbonOEffects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomized placebo-controlled studyLancet20013581119112311597664
- RubinLJBadeschDBBarstRJBosentan therapy for pulmonary arterial hypertensionN Engl J Med200234689690311907289
- ZhaoLMasonNAMorrellNWSildenafil inhibits hypoxia-induced pulmonary hypertensionCirculation200110442442811468204
- RichaletJPGratadourPRobachPSildenafil inhibits altitude-induced hypoxemia and pulmonary hypertensionAm J Respir Crit Care Med200517127528115516532
- WilkinsMRPaulGAStrangeJWSildenafil versus endothelin receptor antagonist for pulmonary hypertension (SERAPH) studyAm J Respir Crit Care Med20051711292129715750042
- IkedaDTsujimoIOhiraHAddition of oral sildenafil to beraprost is a safe and effective therapeutic option for patients with pulmonary hypertensionJ Cardiovasc Pharmacol20054528628915772514
- NagayaNYokoyamaCKyotaniSGene transfer of human prostacyclin synthase ameliorates monocrotaline-induced pulmonary hypertension in ratsCirculation20001022005201211034952
- Schulze-NieckILutherYCEwertPLehmkuhlHBHetzerRLangePEEnd-stage heart failure with pulmonary hypertension: levosimendan to evaluate for heart transplantation alone versus combined heart-lung transplantationTransplantation2004781237123815502728
- SitbonOHumbertMJaisXLong-term response to calcium channel blockers in idiopathic pulmonary arterial hypertensionCirculation20051113105311115939821
- ShimokawaHRho-kinase as a novel therapeutic target in treatment of cardiovascular diseasesJ Cardiovasc Pharmacol20023931932711862109
- FukomotoYTawaraSShimokawaHRecent progress in the treatment of pulmonary arterial hypertension: expectation for rho-kinase inhibitorsTohuku J Exp Med2007211309320
- LoirandGGuerinPPacaudPRho kinases in cardiovascular physiology and pathophysiologyCirc Res20069832233416484628
- LiFXiaWLiAZhaoCSunRLong-term inhibition of Rho kinase fasudil attenuates high flow induced pulmonary artery remodeling in ratsPharmacol Res200755647117127075
- FukomotoYMatobaTItoAAcute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertensionHeart20059139139215710736
- ShimokawaHTakeshitaARho-kinase is an important therapeutic target in cardiovascular medicineArterioscler Thromb Vasc Biol2005251767177516002741
- RidleyAJHallAThe small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factorsCell1992703893991643657
- SomlyoAPSomlyoAVCa2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatasePhysiol Rev2003831325133814506307
- GongMCLizukaKNixonGRole of guanine nucleotide-binding proteins-ras-family or trimeric proteins or both-in Ca2+ sensitization of smooth muscleProc Soc Natl Sci USA19969313401345
- HirataKKikuchiASasakiTInvolvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contractionJ Biol Chem1992267871987221577714
- IshizakiTMaekawaMFujikawaKThe small GTP-binding protein Rho binds to activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinaseEMBO J199615188518938617235
- KaibuchiKKurodaSAmanoMRegulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cellsAnn Rev Biochem19996845948610872457
- KimuraKItoMAmanoMRegulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase)Science19962732452488662509
- GongMCFujiharaHSomlyoAVSomlyoAPTranslocation of rhoA associated with Ca2+ sensitization of smooth muscleJ Biol Chem199727210704107099099720
- OttoBSteusloffAJustIAktoriesKPfitzerGRole of Rho proteins in carbachol-induced contractions in intact and permeabilized guinea-pig intestinal smooth muscleJ Physiol19964963173298910218
- UehataMIshizakiTSatohHCalcium sensitization of smooth smooth muscle mediated by a Rho-associated protein kinase in hypertensionNature19973899909949353125
- YoshiiALizukaKDobashiKRelaxation of contracted rabbit tracheal and human bronchial smooth muscle by Y-27632 through inhibition of Ca2+ sensitizationAm J Respir Cell Moll Biol19992011901200
- MuellerBKMackHTeuschNRho kinase, a promising drug target for neurological disordersNat Rev20054387398
- NagaokaTMorioYCasanovaNRho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic ratsAm J Physiol Lung Cell Mol Physiol2004287L665L67212959926
- FaganKAOkaMBauerNRAttenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinaseAm J Physiol Lung Cell Mol Physiol2004287L656L66414977625
- KnockGASnetkovVAShaiftaYSuperoxide constricts rat pulmonary arteries via Rho-kinase-mediated Ca2+ sensitizationFree Rad Biol Med20094663364219103285
- WilsonDPSusnjarMKissESutherlandCWalshMPThromboxane A2 –induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697Biochem J200538976377415823093
- WangZJinNGanguliSSwartzDRLiLRhoadesRARho-kinase activation is involved in hypoxia-induced pulmonary vasoconstrictionAm J Respir Cell Mol Biol20012562863511713106
- BaillyKRidleyAJHallSMHaworthSGRhoA activation by hypoxia in pulmonary arterial smooth muscle cells is age and site specificCirc Res2004941383139115087418
- RobertsonTPDippMWardJPAaronsonPIEvansAMInhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intra-pulmonary arteries and perfused lung of the ratBr J Pharmacol20001315910960061
- HyvelinJMHowellKNicholACostelloCMPrestonRJMcLoughlinPInhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulationCirc Res20059718519115961717
- McNamaraPJMurthyPKantoresCAcute vasodilator effects of Rho-kinase inhibitors in neonatal rats with pulmonary hypertension unresponsive to nitric oxideAm J Physiol Lung Cell Mol Physiol2008294L205L21318032699
- BarmanSAVasoconstrictor effect of endothelin-1 in hypertensive pulmonary arterial smooth muscle involves Rho kinase and protein kinase CAm J Physiol2007293L472L479
- WeigandLSylvesterJTShimodaLAMechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic ratsAm J Physiol Lung Cell Mol Physiol2006290L284L29016155085
- BroughtonWalkerBRRestaTCChronic hypoxia induces Rho-kinase dependent myogenic tone in small pulmonary arteriesAm J Physiol Lung Cell Mol Physiol2008294797806
- NagaokaTFaganKAGebbSAInhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertensionAm J Respir Crit Care Med200517149449915563635
- GuilluyCRolli-DerkinderenMTharauxP-LMelinoGPacaudPLoirandGTransglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cellsJ Biol Chem20072822918292817142836
- WaltherDJPeterJUWinterSSerotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule releaseCell200311585186214697203
- HemnesARWigleyFRodriguesFWPulmonary hypertension is associated with increased expression and activity of phosphodiesterase type 5ACirculation2005112II-221II-222
- ShimokawaHSetoMKatsumataNRho-kinase-mediated pathway induces enhanced myosin light chain phosphorylation in a swine model of coronary artery spasmCardiovasc Res1999431029103910615430
- KatsumataNShimokawaHSetoMEnhanced myosin light chain phosphorylations as a central mechanism for coronary artery spasm in a swine model with interleukin-1betãCirculation199796435743639416904
- ShimokawaHHiramoriKLinumaHAntianginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter studyJ Cardiovasc Pharmacol20023931932711862109
- ShimokawaHRashidMDevelopment of Rho-kinase inhibitors for cardiovascular medicineTrends Pharmacol Sci20072829630217482681
- OkaMFaganKAJonesPLMcMurtryTherapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertensionBr J Pharmacol200815544445418536743
- ChapadosRAbeKIheda-StansburyKROCK controls matrix synthesis in vascular smooth muscle cells: coupling vasoconstriction to vascular remodelingCirc Res20069983784416990566
- AbeKShimokawaHMorikawaKLong-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertensionCirc Res20049438539314670839
- AbeKTawaraSOiKLong-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in miceJ Cardiovasc Pharmacol20064828028517204906
- NomaKOyamaNLiaoJKPhysiological role of ROCKs in the cardiovascular systemAm J Physiol Cell Physiol2006290C661C66816469861
- NishimuraTVaszarLTFaulJLSimvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cellsCirculation20031081640164512963647
- LeeJHLeeDSKimEKSimvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungsAm J Resp Crit Care Med200517298799316002570
- MurataTKinoshitaKHoriMStatin protects endothelial nitric oxide synthase activity in hypoxia-induced pulmonary hypertensionArterioscler Thromb Vasc Biol2005252335234216166567
- GuerardPRakotoniainaZGoirandFThe HMG-CoA reductase inhibitor, pravastatin, prevents the development of monocrotaline-induced pulmonary hypertension in the rat through reduction of endothelial cell apoptosis and overexpression of eNOSNaunyn Schmeidebergs Arch Pharmacol2006373401414
- LaudiSTrumpSSchmitzVSerotonin transporter protein in pulmonary hypertensive rats treated with atorvastatinAm J Lung Cell Mol Physiol2007293L630L638
- GirgisREMozammelSChampionHCRegression of chronic hypoxic pulmonary hypertension by simvastatinAm J Lung Cell Mol Physiol2007292L1105L1110
- LiMLiuYDuttPFanburgBLToksozDInhibition of serotonin-induced mitogenesis, migration, and ERK MAPK nuclear translocation in vascular smooth muscle cells by atorvastatinAm J Lung Cell Mol Physiol2007293L463L471
- IshikuraKYamadaNItoMBeneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertensionCirc J20067017417816434811
- MohriMShimokawaHHirakawaYMasumotoATakeshitaARho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasmJ Am Coll Cardiol200341151912570938