Abstract
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice. The understanding of the pathophysiology of AF has changed during the last several decades, and a significant role of inflammation and of the renin–angiotensin–aldosterone system has been postulated both experimentally and clinically. There is emerging evidence of an association between inflammation and AF, and mounting evidence links increased C-reactive protein levels not only to already existing AF but also to the risk of developing future AF. The beneficial effects of statins on AF have been reported in several studies. Several randomized clinical and large observational studies have shown similar result that show the beneficial effect of statins in AF. In clinical studies, statins were considered effective in preventing AF after electrical cardioversion, post-ablation, and after permanent pacemaker and implantable cardioverter defibrillator insertion. The antiarrhythmic mechanisms of statins regarding AF prevention in patients with heart failure are still not clear. Perioperative statin use has been associated with favorable postoperative outcome in both cardiovascular and noncardiovascular conditions. Despite a growing body of evidence that drugs with anti-inflammatory properties such as statins may prevent AF, the observed positive effects of statins on the burden of AF appeared to be independent of their cholesterol-reducing properties. However, further data from large-scale randomized trials are clearly needed.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice. The prevalence of AF is constantly increasing and affects not only the elderly, but also younger patient groups. AF is associated with increased morbidity and mortality, mostly due to stroke and heart failure. Maintaining sinus rhythm in AF patients is a difficult task, and in many cases the therapeutic goal lies in postponing permanent AF as long as possible. The vast majority of therapeutic approaches have been focused on the electrical problem of AF. Recently, there is increasing evidence indicating that AF is associated with inflammation.Citation1–Citation4
The understanding of the pathophysiology of AF has changed a lot during recent decades. A significant role of inflammation and of the renin–angiotensin system has been postulated experimentally and clinically.Citation5–Citation7
Inflammation (documented by higher levels of C-reactive protein [CRP]) appears to be involved in the early phase of electrical remodeling (even within 24 hours after AF initiation) and promotes persistence of AF. While inflammation may be a pathogenetic component of AF, the clinical role for anti-inflammatory drugs still remains unclear. Statins have been proven to be potent anti-inflammatory agents.Citation8–Citation10
In clinical studies, statins were considered effective in preventing AF after electrical cardioversion, after cardiac surgery, in patients with coronary artery disease (CAD), and in patients with left ventricular dysfunction. It would thus seem intuitive to suppose that if AF is indeed linked to inflammation, then statins would offer a potentially preventative role in AF. Therefore statin therapy may provide an effective treatment strategy for AF because of its potent anti-inflammatory and antioxidant properties.Citation11–Citation13
A systematic search in PubMed was done using the keywords ‘atrial fibrillation’, ‘statins’, and ‘inflammation’. All the recent studies and meta-analysis in English and the abstract of non-English papers were collected, analyzed, and summarized in this review in our attempt to reach a conclusion on the role of inflammation and statins in AF.
Atrial fibrillation at the cellular level
The structural changes of the atria that define structural remodeling in AF include left atrial dilatation and increasing atrial fibrosis occurs in parallel with the changes of electrical remodelling. Key to this fibrotic process is the deposition of increased amounts of connective tissue between individual cells and with the deposition of large amounts of collagen and fibronectin. This leads to separation of myocytes from one another and subsequent impairment of atrial conduction at the cellular level. All the above culminates in alterations in the biophysical properties of atrial tissue, allowing the initiation and perpetuation of AF.Citation14–Citation18
Results of atrial biopsies taken from patients in AF compared with controls have demonstrated evidence of inflammatory infiltrates and oxidative damage within the atrial tissue. In one study, abnormal atrial histology was uniformly found in multiple biopsy specimens of 12 patients with lone AF, compared with normal histology in all of the controls, with 66% of the AF group showing evidence of occult myocarditis. This strongly support the view that inflammation acts as an initiator rather than as a result of AF.Citation19
Recently, activation of the local renin–angiotensin system and mitogen-activated protein kinase pathways in atrial myocardium has been found to play an important role in atrial structural remodeling related to AF. Another important mediator of the angiotensin II (Ang II) effect is the Janus kinase/signal transducers and activators of transcription (STAT) pathway, which has never been characterized in the atrium. In cultured atrial myocytes and fibroblasts, Ang II induced tyrosine phosphorylation of STAT3 through a Rac1-dependent mechanism, which was inhibited by dominant-negative Rac1, losartan, and simvastatin. In atrial myocytes, activation of STAT3 by Rac1 was mediated by direct association of Rac1 with STAT3; however, in atrial fibroblasts, it was mediated by an indirect paracrine effect. Constitutively active STAT3 increased protein synthesis, and dominant-negative STAT3 abrogated Ang II-induced protein synthesis in atrial myocytes and fibroblasts. Rats infused long term with Ang II exhibited higher levels of activated Rac1, phospho-STAT3, collagen synthesis, and atrial fibrosis in the atria, all of which were attenuated by oral losartan and simvastatin. In human atrial tissues from patients with AF, Ang II and phospho-STAT3 levels were also elevated. The Ang II/Rac1/STAT3 pathway is an important signaling pathway in the atrial myocardium to mediate atrial structural remodeling, and losartan and statin may be able to reverse Ang II-induced atrial structural remodeling in AF.Citation20
Chronic AF acutely upregulates CD40 expression as well as platelet adhesion to the endocardium. Simvastatin is effective in modulating this expression, thus it may potentially contribute to reduction of the risk of intra-atrial thrombus formation. This issue has been explored in experiments on right atrial segments obtained before the onset of cardiopulmonary bypass, in either presence or absence of 5 μM simvastatin. AF was associated with a significant increase of endocardial CD40 expression (293.1 ± 55.1 pg/ml vs. 230.9 ± 53.3 pg/ml; p < 0.01), and platelet–endocardial adhesion compared with sinus rhythm atria (10.8 ± 2.2 vs. 5.2 ± 1.3 platelet CD41 AU; p < 0.01). At immunofluorescence, about 62% of fibrillating endocardium was covered by platelets, compared with 12% of sinus rhythm atria. Addition of simvastatin significantly reduced CD40 expression as well as platelet adhesion to fibrillating atria. Its efficacy was not reversed by the addition of mevalonic acid.Citation21
AF has been associated with myocardial oxidative stress, and antioxidant agents have demonstrated antiarrhythmic benefit in humans (). Serum markers of oxidative stress and inflammation were compared in a cross-sectional, case-control design studyCitation22 of 40 male individuals, with or without persistent or permanent AF, who were matched for age, sex, diabetes, and smoking status, known confounding variables for the measurement of oxidative stress. (used derivatives of reactive oxidative metabolites [DROMs] and ratios of oxidized to reduced glutathione [E(h) GSH] and cysteine [E(h) CySH] to quantify oxidative stress). Inflammatory markers, including high-sensitivity CRP (hs-CRP), interleukins-1β (IL-1β) and -6, and tumor necrosis factor-α (TNF-α) were also measured. Univariate, conditional logistical regression analysis showed that oxidative stress but not inflammatory markers were statistically associated with AF (p < 0.05). The increase in the odds ratio (OR) for AF for E(h) GSH, E(h) CySH, and DROMs were 6.1 (95% confidence intervals [CI], 1.3–28.3; p = 0.02), 13.6 (95% CI: 2.5–74.1; p = 0.01), and 15.9 (95% CI: 1.7–153.9; p = 0.02), respectively. There was a stronger correlation between E (h) GSH and E (h) CySH (r = 0.66) than between E(h) GSH and DROMs (r = 0.41). In multivariate analysis corrected for statins and other AF risk factors this association of AF and oxidative stress remained significant.
Atrial fibrillation and inflammation
Historical evidence to support an association between AF and inflammation can be extracted from the frequent association of AF with inflammatory conditions of the heart, such as myocarditis and pericarditis.Citation23,Citation24
Bruins and colleaguesCitation25 were the first to propose the inflammation–AF hypothesis, following their observations of an increased frequency of AF after coronary artery bypass surgery. They noted that the peak incidence of AF occurred on the second and third postoperative days, which coincided with the peak elevation of CRP levels. In an interesting study by Maixent and colleagues,Citation26 the authors demonstrated the presence of circulating autoantibodies against myosin heavy chain in a significant percentage of patients with idiopathic paroxysmal AF, which raises the possibility of an inflammatory autoimmune process in some patients with paroxysmal AF.
CRP, a biomarker of inflammation, has been reported to be elevated in some patients with AF. Statins may prevent AF through anti-inflammatory and/or antioxidant effects.Citation2,Citation3,Citation27–Citation31 The precise mechanism for the increased circulating hs-CRP in AF is uncertain, but might reflect active participation of CRP in the local inflammatory response within the atrial myocardium. In patients with AF, CRP may localize in atrial tissue, possibly binding to the membranes of myocardial cells in inflamed tissues and activating complement, leading to tissue damage.Citation32,Citation33 Levels of hs-CRP have been noted to be higher among patients with AF compared with controls in sinus rhythm.Citation3,Citation27,Citation34–Citation39 Also, persistent AF patients have higher hs-CRP levels than paroxysmal AF patients, and both have higher levels than controls.Citation27 In one study, the combination of microalbuminuria and an elevated hs-CRP increased the risk of subsequent AF development by up to four-fold.Citation40 Furthermore, a longer duration of AF is associated with higher hs-CRP levels and larger left atrial dimensions, supporting a link between the burden of AF, inflammation, and structural remodeling.Citation41 In both cross sectional and longitudinal studies, hs-CRP has remained a consistent and significant predictor of early AF relapse after successful cardioversion, even after adjustment for risk factors for AF, such as hypertension and CAD.Citation29,Citation34,Citation36,Citation41,Citation43 hs-CRP has also been shown to be predictive of subsequent future development of new cases of AF among a large cohort of patients in sinus rhythm.Citation3 Ablation induces an acute inflammatory upregulation reflected by an increase of CRP and fibrinogen levels and of the leukocyte count. The observed inflammatory response is consistent with histopathologic information on ablation-induced inflammatory activation and is supposed to contribute to the phenomenon of early AF recurrence.Citation44 Although it is not yet known whether inflammation acts as initiator or is just a consequence of AF, there is evidence that CRP-lowering therapies could prevent AF.Citation34,Citation43 Statins as well as angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs) have the potential to modulate inflammatory pathwaysCitation5,Citation6 and could therefore reduce the susceptibility to AF after ablation in general and attenuate the acute inflammatory response in the early post ablation period .
Furthermore, CRP-lowering with atorvastatin appears to be effective in eliminating paroxysmal atrial fibrillation (PAF) during daily life in a significant proportion of patients. In a prospective studyCitation45 on 80 patients with proven PAF, 40 patients were randomized to placebo (placebo group) and 40 to atorvastatin (treatment group). Plasma CRP levels and ambulatory monitoring were repeated after four to six months of therapy. The two groups were comparable with respect to baseline characteristics, number of episodes of PAF, and baseline plasma CRP levels. The treatment group had lower median CRP levels exhibited a highly significant reduction in PAF (p < 0.001). Paroxysmal AF was completely resolved in 26 (65%) of 40 patients in the treatment group versus four (10%) of 40 in the placebo group. By logistic regression, treatment with atorvastatin was an independent predictor of PAF resolution.
As another inflammatory marker associated with AF, IL-6 is a pleiotropic cytokine that has diverse physiological roles, including mediation of both pro-inflammatory responses and cyto-protective functions. There have been few studies that have investigated the relationship between IL-6 and AF and shown significant correlation.Citation47
TNF-α is a cytokine that plays a significant role in the initial activation of the immune system. Its release is stimulated by several factors, including IL-1β and bacterial endotoxin. Intra-arterial TNF-α causes an acute local vascular inflammation that is associated with impaired endothelium-dependent relaxation. So far, there has been few studies that have looked into the possible association between TNF-α and AF. These were very small studies and did not adjust for confounding factors; however, they demonstrated increased levels of TNF-α in patients with AF compared with healthy controls in sinus rhythm.Citation38,Citation41 Going into the details of these studies is beyond the scope of this article.
Finally, a relationship between elevated white blood cell count as a marker of inflammation and the development of AF after cardiac surgery has been shown in 181 consecutive patients undergoing coronary bypass or cardiac valve surgery.Citation48
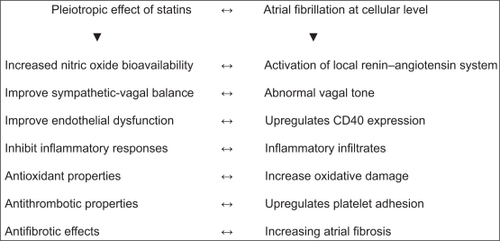
Pleiotropic effects of statins
The beneficial effects of statins on AF, beyond cholesterol lowering, have been reported in several studies, including improvement of endothelial dysfunction, increased nitric oxide (NO) bioavailability, antioxidant properties, inhibition of inflammatory responses, stabilization of atherosclerotic plaques, and antithrombotic properties ().Citation13,Citation49–Citation52 Statins are known to lower the levels of acute-phase proteins independently of their effects on cholesterol.Citation8,Citation53,Citation54 Also the antifibrotic effects, modulation of matrix metalloproteinases, interaction with peroxisome receptors that regulate proliferation, and endothelial NO synthase protect atrial myocardium during ischemia. For these reasons, statins may be able to decelerate or even reverse structural remodeling in patients with AF. Additionally, pre-treatment with high-dose atorvastatin prevented electrical remodeling in a pericarditis dog model and high-dose simvastatin attenuated down regulation of calcium channel subunits, preventing shortening of atrial refractoriness, a central element of electrical remodeling in AF. However, this sounds familiar: positive effects on intracellular calcium handling and atrial electrical remodeling have also been reported in animal studies with verapamil, but, in clinical trials this drug was unable to prevent atrial electrical remodeling and recurrence of AF.Citation13,Citation28,Citation49–Citation51,Citation55–Citation60
A recent study by Marín and colleaguesCitation13 found statin use was significantly associated with a decreased incidence of postoperative AF and an increased tissue inhibitor matrix metalloproteinase-1/matrix metalloproteinase-1 ratio in patients undergoing coronary artery bypass grafting (CABG). Recent data have suggested that statins can improve the sympathetic vagal balance in heart failure. One study showed that simvastatin could normalize sympathetic outflow and cardiovascular reflex regulation and restore the sympathovagal balance in rabbits with pacing-induced congestive heart failure (CHF). Furthermore another study demonstrated its beneficial effect on autonomic function in CHF by downregulating central Ang II and superoxide mechanisms. Therefore, the autonomic modulation effects of statins may favorably prevent AF after CABG.Citation61,Citation62
More recently the effect of statins on collagen type I degradation and CRP in patients with CAD and AF has been shown in a study of 106 patients with CAD and AF: 40 (36 men, mean age 72 ± 8 years) treated with a statin and 66 (48 men, mean age 74 ± 9 years) not treated with a statin. Serum concentrations of carboxy-terminal telopeptide of collagen type I, an index of collagen type I degradation, and hs-CRP were measured. Carboxy-terminal telopeptide of collagen type I levels were significantly higher (p < 0.001) in statin-treated patients (0.64 ng/ml, 95% CI: 0.57–0.71) compared with nonstatin-treated patients (0.38 ng/ml, 95% CI: 0.31–0.44). These changes were independent of cholesterol levels (before or after therapy). Statin-treated patients had significantly lower (p < 0.001) CRP levels (0.25 mg/dl, 95% CI: 0.23–0.28) compared to statin nonusers (1.1 mg/dl, 95% CI: 0.92–1.25). Thus therapy with statins in patients with CAD and AF is associated with an increase in collagen degradation and an attenuation of inflammation, independently of cholesterol lowering.Citation63
The effect of statins on endothelial function has been demonstrated in several studies which concluded that cholesterol levels even in the normal range may be inversely related to endothelium-dependent vasodilation, and this finding has important clinical implications. This suggests that lowering cholesterol levels even when it is within the normal range may improve the production and release of endothelium-dependent NO and hence improve endothelial function This idea is supported by recent reports that lowering cholesterol levels enhances endothelium-dependent vasodilation not only in subjects with massively elevated cholesterol levels but also in those with normal cholesterol levels. It is worth noting that lowering of average cholesterol levels in patients with documented CAD leads to decreased rates of myocardial infarction, and this protective effect may in part be due to improvement in endothelial function.Citation52 The beneficial effects of statins are now recognized to extend well beyond their lipid-lowering properties. Through a combination of both distinct and interdependent effects on endothelial cell Rho GTPase regulation, NAPDH oxidase activity, NO bioavailability, and differential gene expression statins confer significant protection of the vasculature. Abundant in vitro data in addition to myriad reports relying on a range of animal models now firmly support the idea that these drugs may serve as novel and effective therapeutic agents in a variety of disease states characterized by vascular dysfunction.Citation64
Statins and AF in the experimental studies
Supportive data from two studies demonstrate the efficacy of statins to reduce the burden of AF in animal models (). Kumagai and colleaguesCitation59 operatively induced sterile pericarditis in 20 dogs randomized to treatment with or without atorvastatin 2 mg/(kg/day) (commenced one week prior to operation). Atorvastatin reduces both the incidence of AF and the levels of hs-CRP compared with the control group, which suggests that atorvastatin reduced the burden of AF by reducing the inflammatory substrate. In addition, these findings were shown to correlate with a lower percentage of fibrosis in all atrial regions in the atorvastatin group compared with the placebo group. It was particularly interesting that the authors noted the greater difficulty of inducing AF before versus after the induction of pericarditis, which would support the influence of inflammation in AF generation. Another studyCitation65 showed that atorvastatin attenuates atrial oxidative stress and prevents atrial electrical and structural remodeling in rat hypertensive heart failure (HF) induced by chronic inhibition of NO synthesis.
Table 1 Experimental studies on the effect of statins on atrial fibrillation
Statin-induced inhibition of profibrotic atrial fibroblast responses and attenuation of left-ventricular dysfunction may contribute to preventing the CHF-induced fibrotic AF substrate, simvastatin, but not fenofibrate attenuated CHF-induced atrial structural remodeling and AF promotion. This had been found in an animal study done on dogs. Simvastatin prevented ventricular tachypacing-induced (VTP) pacing-induced AF (DAF) increases (147 ± 37 and 84 ± 37 s at 20 and 80 mg/day, respectively), but fenofibrate did not (1018 ± 352 s). Simvastatin also attenuated CHF-induced conduction abnormalities (heterogeneity-index reduced from 1.5 ± 0.1 to 1.1 ± 0.1 and 1.0 ± 0.1 at 20 and 80 mg/day; p < 0.01) and atrial fibrosis (from 19.4 ± 1.3% to 10.8 ± 0.8% and 9.9 ± 0.8% at 20 and 80 mg/day; p < 0.01), while fenofibrate did not. Simvastatin (but not fenofibrate) also attenuated VTP-induced left-ventricular nitric-oxide synthase and nitrotyrosine increases, along with hemodynamic dysfunction. Atrial fibroblast proliferation increased with 24-h fetal bovine serum (FBS) stimulation from 654 ± 153 to 7264 ± 1636 DPM (p < 0.001). Simvastatin, but not fenofibrate, suppressed fibroblast proliferation (664 ± 192 DPM; p < 0.001). Simvastatin also significantly attenuated transforming growth factor-β1-stimulated α-smooth muscle actin (α-SMA) expression (indicating myofibroblast differentiation) from 1.3 ± 0.1 to 1.0 ± 0.1 times baseline (p < 0.05).Citation66 In another study by the same authors. 39 dogs subjected to rapid atrial tachypacing in the absence and presence of treatment with simvastatin (and vitamins C and E) had no effect. The investigators were able to demonstrate that, compared with controls, simvastatin reduced the promotion of AF following tachypacing.Citation28
Statins and AF in the randomized trials and meta-analyses
Several randomized clinical trials (RCTs) and large observational studies have shown similar results on the beneficial effect of statins in AF, but no benefit was found in six RCTs and 10 observational studies with 7041 patients. The analysis of RCTs showed no significant effect of statins on AF development (relative risk [RR] 0.76, 95% CI: 0.55–1.05; p = 0.09), with significant heterogeneity between individual studies (p = 0.0008, I(2) = 74.0%). Subgroup analysis revealed that differences in AF detection methodology may be the cause of heterogeneity. The analysis of observational studies demonstrated that statin use reduced the relative risk for AF by 23% (95% CI: 0.70–0.85, Z = 4.95; p < 0.00001) without significant differences between the trials (p = 0.08). This favorable effect was greatest in the postoperative patients (RR 0.61, 95% CI: 0.49–0.76, Z = 4.30; p < 0.0001).Citation67
In six studies with 3,557 patients, three investigated the use of statins in patients with paroxysmal AF (one) or persistent AF undergoing electrical cardioversion (two), and three investigated the use of statins in primary prevention of AF in patients undergoing cardiac surgery or after acute coronary syndrome (ACS). Statins were significantly associated with a decreased risk of AF compared with control (OR 0.39, 95% CI: 0.18–0.85; p = 0.02). The benefit of statin therapy seemed more marked in secondary prevention of AF (OR 0.33, 95% CI: 0.10–1.03; p = 0.06) than for new-onset or postoperative AF (OR 0.60, 95% CI: 0.27–1.37; p = 0.23).Citation68
In a randomized comparison of 14 trials reporting the results of 15 unique analyses (n = 7402), there was a 20% incidence rate for any AF with varying rates depending on AF type (new-onset [11%], recurrent [56%], recurrent after cardioversion [54%], or postoperative [22%]). The use of a statin reduced the odds of developing any AF by 45% (OR 0.55; 95% CI: 0.43–0.70); Q statistic; p = 0.001). Statins reduced the odds of developing new-onset AF by 32% (OR 0.68; 95% CI: 0.51–0.90), recurrent AF by 57% (OR 0.43; 95% CI: 0.24–0.79), recurrent AF after cardioversion by 42% (OR 0.58; 95% CI: 0.32–1.05) and postoperative AF by 58% (OR 0.42; 95% CI: 0.27–0.65).Citation69 Randomized comparison among 8659 patients in two large, randomized trials, PROVE IT-TIMI 22 and phase Z of the A to Z trial found that higher-dose statin therapy did not reduce the short term incidence of AF among patients after ACS when compared with standard dose statin treatment, during the two years of follow-up. Neither study showed a decreased AF risk with higher-dose statin. In PROVE IT-TIMI 22, 2.9% versus 3.3% in the high-versus standard-dose statin therapy, respectively, experienced the onset of AF over two years (OR 0.86, 95% CI: 0.61–1.23; p = 0.41). In A to Z, rates were 1.6% versus 0.99%, respectively (OR 1.58, 95% CI: 0.92–2.70; p = 0.096). In both trials, CRP levels (plasma or serum) tended to be higher among patients experiencing the onset of AF.Citation70
Another large cohort included 13,783 patients.Citation71 The primary outcome was time to development of AF. Propensity scores were used to balance statin-treated and untreated patients with respect to baseline characteristics. Time from the initial visit to development of AF was analyzed with a Cox regression model, using statin treatment as a time-varying covariate. Among the 13,783 patients, 5417 (39%) received statin treatment. Statin-treated patients were younger with fewer co morbid conditions. After propensity adjustment, the baseline characteristics of the statin-treated and untreated patients were similar. During an average follow-up of 4.8 years, 1979 (14%) patients developed AF. In the overall study population there was no difference in AF incidence with statin treatment (hazard ratio [HR] 1.0, 95% CI: 0.88–1.14; p = 0.09). However, AF was less common among statin-treated patients with CHF (HR 0.57, 95% CI: 0.33–1.00; p = 0.04). No effects of statin treatment on AF incidence was found in patients with CAD.Citation71 In the largest study so far,Citation72 performed a cross-sectional analysis of 25,000 patients enrolled in the multicenter Guidant-sponsored Advancement Heart Failure Registry; of these patients, 7027 patients (27%) developed AF, and statin therapy led to a 23% reduction in AF, when compared with those not treated, even after multivariate analysis (OR for AF 0.685; p = 0.001) ().
Table 2 Result from major randomized trials and meta-analyses
Role of statins in primary AF- and CAD-associated AF
The role of statins and inflammation in primary AF are summarized in and others have been analyzed above. The effect of statins on the incidence of new-onset AF in patients presenting with suspicion of ACS has been explored in 1,526 patients. 164 (10.8%) had new-onset AF and 601 (39.4%) were on a statin on admission. In univariate analysis and after correcting for age, race, diabetes mellitus, chest pain, and use of ACE-I, patients on statins were significantly less likely to have new-onset AF (OR 0.40, 95% CI: 0.33–0.69; p < 0.01). This relation persisted in the multivariate model (OR 0.57, 95% CI: 0.39–0.83; p < 0.01). In conclusion, patients presenting with suspicion of ACS were much less likely to have new onset AF if they were on a statin at time of presentation.Citation73
Table 3 Most recent studies on the relation of atrial fibrillation, inflammation, and the role of statins
Use of statins in patients with chronic stable CAD appears to be protective against AF. The underlying mechanism for this effect is unknown but appears to be independent of the reduction in serum cholesterol levels.Citation11 This association between statin use and the risk of developing AF were examined univariately and with adjustment for potential confounding factors in 449 patients with CAD between the ages of 40 and 87 years who were followed for an average of five years. 52 patients (12%) developed AF during follow-up. Statin therapy was used by 59% of the patients during the study period and was associated with a significantly reduced risk of developing AF (crude OR 0.48, 95% CI: 0.28–0.83). This association remained significant after adjustment for potential confounders, including age, hypertension, left ventricular systolic function, occurrence of heart failure or acute ischemic events, and baseline cholesterol and changes in cholesterol levels (adjusted OR 0.37, 95% CI: 0.18–0.76). β-blocker use was significantly higher in the statin user group (80% vs. 67%; p = 0.02), but was not included in the final multivariable model. Again, the possibility of an interaction between statins and β-blockers was not reported in this observational study, thus it is possible that the observed reduction in AF may have been mediated by the higher β-blocker use in the statin group.
AF after cardioversion
In clinical studies, statins were considered effective in preventing AF after electrical cardioversion. Several studies have evaluated this association. Nevertheless, controversial results have been published concerning the protective role of statins after electrical cardioversion. Some investigators found a significant decrease in arrhythmia recurrence with statins,Citation12 and others did not.Citation74 The number of studies on this subject is small and their sample sizes were limited; thus, data are insufficient, in our opinion, to recommend the use of statins before electrical cardioversion, and larger randomized studies are needed to clarify this important topic. Siu and colleaguesCitation12 were the first to study the prevention of AF with statins. They retrospectively studied 62 patients with lone persistent AF lasting three months who underwent direct current cardioversion, and observed that statin-treated patients (n = 10) had less recurrent AF than the control group of 52 patients (40 vs 84%; p = 0.0007); however, this was a very small and under-powered observational study, In another study on 106 patients with a history of symptomatic AF lasting less than one day (age 63 ± 14 years, mean ± standard deviation [SD]) hs-CRP level determined prior to cardioversion represented an independent predictor of both successful cardioversion for AF and the maintenance of SR after conversion ().Citation75
In a retrospective review of 625 patients with new onset AF after successful cardioversion who were followed prospectively in the multicenter Canadian Registry of AF (CARAF) trial, logistic regression was used to model the effect of statin use on the recurrence of AF at one year while adjusting for potential confounders including concurrent medications. In a predominantly male population (62%) with median age 63 years, 12.3% were on statins at baseline. Overall, 32.5% had documented recurrence of AF at one year; 23.4% in patients on statins compared to 33.8% in those not on statins (p = 0.07). After adjustment for baseline differences and concomitant β-blocker use, statin use was associated with a 74% reduction in AF recurrence, but only in statin users on β-blockers (OR 0.26, 95% CI: 0.10–0.66); statin users not on β-blockers (OR 1.07, 95% CI: 0.44–2.58). While CARAF has limitations, it was not designed primarily to evaluate the effect of statins on recurrence of AF. Statin use was based on physician/patient choice, not random assignment, making confounding by indication a concern. Nevertheless, it is unlikely that physicians recommended statin therapy based on likelihood of AF recurrence. Careful analyses showed that CARAF did not document the exact time of AF recurrence; instead, the study required electrocardiography-documented evidence of recurrence between scheduled annual visits. Therefore, Kaplan–Meier curves comparing the time course of recurrence could not be constructed. Baseline characteristics varied significantly between the statin and nonstatin users, and it is possible that there were other unmeasured confounders. In statin users, older age, diabetes, hypertension, and larger left atrial size would be expected to increase the likelihood of AF recurrence. Conversely, statin users were more likely to be on rate- and rhythm-control medications, a bias that would tend to decrease the likelihood of recurrence. The study also had several strengths. First, the rigorous ascertainment of AF recurrence is unlikely to be biased by statin use because statin use was not a focus of the CARAF study, nor was the association between statin use and AF known at the time CARAF was enrolling patients. Second, the cohort comprises patients with newly diagnosed AF. By definition, these patients would not have been exposed to any prior rate- or rhythm-control strategies that might affect the likelihood of AF recurrence. Indeed, this is a unique feature and strength of the study.Citation76
A prospective studyCitation77 assessed the role of CRP in predicting long-term risk of AF recurrence after electrical cardioversion in 102 patients (age 67 ± 11 years; 58 men) with nonvalvular persistent AF who underwent successful biphasic electrical cardioversion, had hs-CRP measured immediately before cardioversion, and were followed-up for one year. Patients were divided into four groups according to CRP quartiles. The four groups were similar in age, gender, ejection fraction, and left atrial size. Patients in the lowest CRP quartile (<1.9 mg/L) had significantly lower rates of AF recurrence (4% vs. 33% at three months in the other three groups combined [p = 0.007] and 28% vs. 60% at one year [p = 0.01]). Survival analysis confirmed that patients in the lowest CRP quartile had a lower recurrence rate (p = 0.02). Cox regression analyses using age, gender, hypertension, diabetes, ejection fraction, left atrial diameter, use of antiarrhythmic drugs, ACE-Is or Ang II antagonists, and statins, and CRP quartiles as covariates showed that only CRP was independently associated with AF recurrence during follow-up (HR 4.98, 95% CI: 1.75–14.26; p = 0.003).
Additionally, atorvastatin was associated with a significantly reduced risk of developing AF (unadjusted RR 0.23, 95% CI: 0.064–0.82; p = 0.024). This association remained significant after adjustment for these predictors (adjusted RR 0.19, 95% CI: 0.052–0.72; p = 0.01). At baseline, hs-CRP levels were not different between the two groups (p = 0.92). Although the hs-CRP levels decreased significantly 48 hours after electrical cardioversion compared with the baseline levels in group I (2.82 ± 1.46 vs. 2.56 ± 1.3 mg/dl; p = 0.02), no significant change occurred in group II (2.87 ± 0.8 vs. 2.84 ± 0.8 mg/dl; p = 0.09).Citation78 In contradiction, pravastatin did not reduce the recurrence rate of AF after electrical cardioversion in an open, controlled multicenter study. Patients (n = 114) who had AF > 48 hours and who were scheduled for electrical cardioversion were randomized to receive 40 mg of pravastatin once daily for thee weeks before and six weeks after electrical cardioversion or no drug in addition to standard therapy.Citation74
Role of statins in postablation AF
Ablation-induced inflammatory activation and is supposed to contribute to the phenomenon of early AF recurrence.Citation44 Studies in this area are limited and have significant limitations. In a retrospective study of 177 consecutive patients (mean age = 56 ± 11 yrs, 69% males) who underwent ablation for paroxysmal (132 patients) or persistent AF (45 patients), patients were treated with ACE-I (31 patients) or ARB (18 patients) or statins (50 patients) prior to ablation and follow-up of 13.8 ± 8.6 months. 72% of these patients were free of AF. Thirty-three of 50 (60%) of patients taking statins were free of AF, 17 of 31 (55%) were free from AF in the ACE-I-treated group, while 17 of 18 (94%) were free from AF in the ARB group. Using Cox regression analysis to correct for baseline variables, treatment with statins did not decrease the recurrence rate (HR 1.10, 95% CI: 0.55–2.27; p = 0.79) nor did treatment with renin–angiotensin system blockers (HR 0.94, 95% CI: 0.46–1.93; p = 0.87). However, subgroup analysis showed that treatment with ARB was associated with a trend towards lower AF recurrence (HR 0.17, 95% CI: 0.02–1.34; p = 0.09). However this is a retrospective and not randomized and there may be other genetic and environmental factors that were not corrected for by multivariate analysis.Citation79 The long duration of AF prior to ablation might account for the lack of efficacy of these medications since the patients are more likely to have established fibrosis and scarring, and thus less likely to respond to medications that inhibit inflammation and initial collagen deposition.
A study by Richter and colleaguesCitation80 included 234 patients (23–80 years; 71.8% men) with drug-resistant paroxysmal (n = 165) or persistent AF (n = 69) who either underwent a Lasso-guided segmental pulmonary vein isolation (n = 83) or a CARTO-guided left atrial circumferential ablation (n = 151), which definitely resulted in inflammation. Treatment with statins (n = 113), ACE-Is, or ARBs (n = 124), or a combination of a statin and an ACE-I or ARB (n = 75) was started three months before ablation and was continued during follow-up of 12.7 months, 64% of patients with paroxysmal and 45% of patients with persistent AF were free of AF. Statin use (HR 1.06; p = 0.79), ACE-I or ARB use (HR 1.12; p = 0.59), and their combined use (statin + ACE-I/ARB; HR 1.17; p = 0.54) did not significantly influence ablation outcome as assessed by Cox regression analysis. In addition, after multivariate adjustment for potential confounders, the examined drugs did not significantly affect ablation outcome. Ablation induced an acute upregulation of CRP levels (preablation vs. 48 hours postablation, 5.9 F 8.1 vs. 33.7 F 30 mg/L; p = −0.001) and other inflammatory markers. The examined drugs did not significantly alter baseline levels or ablation-induced upregulation of inflammatory markers. However this study was a retrospective analysis of prospectively gathered data and hence is subject to the limitations inherent in any retrospective study. A possible limitation of this study is that AF-free follow up included a proportion of patients continued on previously ineffective antiarrhythmic drugs reflecting a rather conservative strategy of withdrawal of antiarrhythmic drugs after ablation. So far further studies are needed to clarify this aspect.
Role of statins in AF after pacemaker implantation
Recently and till the time of writing this review, few studies have evaluated the role of statins in AF after pacemaker implantation. The predictors of atrial tachyarrhythmia (AT)/AF recurrence in pacemaker implanted patients with sinus node disease were evaluated in prospective observational cohort study on 185 patients.Citation81 The time to first AT/AF recurrence and AT/AF burden (h/day) was retrieved at each follow-up visit by pacemaker interrogation. AT/AF recurred following pacemaker implantation in 157 (85%) patients. At one year of follow-up, patients without recurrence were more likely to be on statin therapy (54%) when compared with patients without statin therapy (25%, x = 12.31; p = 0.0004). Statin therapy was the only significant predictor of AT/AF recurrence in a multivariate logistic regression model (adjusted OR 0.33, 95% CI: 0.14–0.74; p = 0.007). AT/AF burden was significantly lower in the group on statin therapy (median 0.10 h/day) when compared with the no statin group (median 0.39 h/day; p = 0.0059).
The Atorvastatin Trial for Atrial High Rate Episodes in Patients with Bradycardia (ATAHEB) was a prospective, randomized, and open-label study designed to test the efficacy of atorvastatin in preventing the occurrence of atrial high-rate episodes (AHE) or AF episodes in patients with a pacemaker. Fifty-two patients (23 males, 70 ± 13 years old) were randomized to the statin group (atorvastatin 20 mg/d) and 54 (25 males, 72 ± 13 years old) to the nonstatin group. Event-free survivals from AHE ≥ 1 minute were not significantly different between the two groups (log-rank P = 0.410). However, patients in the nonstatin group were more likely to develop AHE ≥ 10 minutes than those in the statin group (log-rank p = 0.028). AHE ≥ 10 minutes occurred in three (5.8%) of 51 patients in the statin group after one year of follow-up, and 10 (19.2%) of 52 patients (OR 0.26; p = 0.041) in the nonstatin group. Interestingly the mean left atrial volume of the statin group was significantly lower than that of the nonstatin group at the end of follow-up (39.7 ± 1.7 vs. 43.7 ± 1.9 mL; p = 0.0001). However there are limitations to this study. First, this is a small randomized, but not placebo-controlled, study. The sample size is relatively small. But the pacemaker interrogation is more sensitive than the Holter ECG monitoring or symptom-based screening and thus substantially decreased the case number needed to find a significant result.Citation82
In a cohort of 264 patients (49% women, mean age [± SD] 71 ± 12 years) with permanent pacemakers who are at high risk for AF. 36% had CAD. AF developed in 70 patients (26%) at a median of 359 days post-pacemaker implantation. The incidence rate for the first occurrence of AF post-pacemaker implantation among patients treated and not treated with statins was 10.5 versus 9.8 events per 100 patient-years, respectively (p = 0.81). Even after controlling for baseline differences, the HR for developing AF among statin users did not achieve statistical significance (HR 0.59, 95% CI: 0.31–1.12). Concluding that statin therapy had no protective effect against the risk of AF in patients with permanent pacemaker. The low prevalence of CAD in these patients may partly explain the results of this study.Citation83
Another interesting study, in a large heterogeneous implantable cardioverter defibrillators (ICD) cohort, prospectively followed, 1,445 patients receiving an ICD for the primary (n = 833) or secondary (n = 612) prevention. with follow-up (mean ± SD) 874 ± 805 days. Overall, 745 patients received statin therapy and 700 did not. Outcome measures include incidence of AF/atrial flutter (AFL) that initiated ICD therapy or was detected during ICD interrogation. Cox hazard regression analyses conducted to determine the predictors of AF/AFL with and without inappropriate shock delivery excluding inappropriate shocks resulting from lead dysfunction or other exogenous factors. There were 563 episodes of AF/AFL detected, with 200 episodes resulting in inappropriate shock therapy. The use of statin therapy was associated with an adjusted HR of 0.472 (95% CI: 0.349–0.638; p < 0.001] for the development of AF/AFL with shock therapy and 0.613 (95% CI: 0.496–0.758; p < 0.001) without shock therapy when compared with the group without statin use ().Citation84
AF in left ventricular dysfunction
The antiarrhythmic mechanisms of statins regarding AF prevention in patients with HF are still not clear. One possible pathway involves inflammation and oxidative stress. Recent studies have demonstrated the implication of inflammation and oxidative stress in the pathophysiology of AFCitation11,Citation67,Citation85,Citation86 and HF.Citation87 Several pharmacological approaches with nonchannel blocking drugs that have anti-inflammatory and antioxidant effects have shown beneficial effects on AF.Citation88,Citation89 Furthermore, statin treatment has been associated with a significant clinical improvement in patients with HF along with a reduction in inflammatory biomarkers, statin use was associated with reduced mortality and hospitalization in patients with HF of ischemic and nonischemic HF patients equally.Citation90,Citation91 Besides the presumed anti-inflammatory and antioxidant effects of statins in AFCitation92 some additional mechanisms have also been suggested, these include anti-ischemic effects, attenuation of atrial remodeling, ion channel stabilization, improvement in endothelial function, and modulation of the autonomic nervous system.Citation60,Citation67,Citation93,Citation94
As mentioned above, two experimental studies have evaluated the role of statin treatment in animal models of HF. Firstly, Shiroshita-Takeshita and colleaguesCitation66 have demonstrated that simvastatin effectively attenuates atrial structural remodeling and AF promotion in a dog model of tachy-cardiomyopathy. Furthermore, Okazaki and colleaguesCitation65 showed that atorvastatin attenuates atrial oxidative stress and prevents atrial electrical and structural remodeling in rat hypertensive HF induced by chronic inhibition of NO synthesis.
Very recently, clinical observational study added new evidence to this issue. In a large prospective cohort of 13,783 patients with coronary heart disease, there was no difference in AF incidence between statin-treated and untreated patients during a follow-up of 4.8 years. Interestingly, subgroup analysis of patients with HF showed that recent statin treatment was associated with a 43% reduction in AF incidenceCitation71 .
In analysis of another two retrospective studies which evaluated the role of statins on AF prevention in patients with heart failure, the larger study was a cross-sectional study by Hanna and colleaguesCitation95 of 25,268 patients with left ventricular systolic dysfunction, enrolled in a multicenter registry (ADVANCENT). Only 25.3% of patients with prevalent AF used statins compared to 33.1% without AF (p = 0.001). Even after adjustment for concomitant drug use and comorbid conditions, the OR for AF in patients on statins was 0.69 (0.64–0.74) compared to those who had not used statins. β-Blocker use in this cohort was quite high (79.1%) and the prevalence of AF was lower in those on β-blockers (26.5%) versus in those who were not (33.5%), but the authors did not report on the effect of concomitant statin and β-blocker use. Post hoc analysis from the Sudden Cardiac Death in Heart Failure Trial (SCDHeFT)Citation96 demonstrated that, after adjusting for several confounding factors, statin use was independently associated with a significant reduction (28%) in the relative risk of AF or atrial flutter during a follow-up period of 45.5 months ().
GISSI-HF, a randomized, double-blind, placebo-controlled trial in 326 cardiology and 31 internal medicine centers in Italy, enrolled patients with chronic heart failure of New York Heart Association class II–IV irrespective of cause and left ventricular ejection fraction, and randomly assigned to n-3 poly unsaturated fatty acids (PUFA) 1 g daily (n = 3494) or placebo (n = 3481) and followed-up for a median of 3·9 years. Primary endpoints were time to death, and time to death or admission to hospital for cardiovascular reasons. GISSI-HF concluded that a simple and safe treatment with n-3 PUFA can provide a small beneficial advantage in terms of mortality and admission to hospital for cardiovascular reasons in patients with heart failure. In the GISSI-HF sub-analyses of patients randomly assigned to rosuvastatin 10 mg daily (n = 2285) or placebo (n = 2289) using primary endpoints of time to death, and time to death or admission to hospital for cardiovascular reasons, 657 (29%) patients died from any cause in the rosuvastatin group and 644 (28%) in the placebo group (adjusted HR 1.00, 95.5% CI: 0.898–1.122; p = 0.943), with a final conclusion that rosuvastatin 10 mg daily did not affect clinical outcomes in patients with chronic heart failure of any cause.Citation97 Whether higher doses have different effects need to be clarified.
Role of statins in perioperative AF
AF is the most common postoperative arrhythmia with significant consequences on patient health. Postoperative AF complicates up to 8% of all noncardiac surgeries, between 3% and 30% of thoracic surgeries, and between 16% and 46% of cardiac surgeries. It increases morbidity and prolongs hospital stay. Advanced age is associated with degenerative and inflammatory modifications in atrial anatomy (dilation, fibrosis), which cause alterations in atrial electrophysiological properties (shortness of effective refractory period, dispersion of refractoriness and conduction, abnormal automaticity, and anisotropic conduction). which act as potential substrates for postoperative AF.Citation98,Citation99 Inflammation associated with cardiosurgical procedures, together with catecholamine release, was suggested as having a pivotal role in postoperative AF reported that patients who developed AF after major thoracic surgery had a nearly two fold increase in postoperative CRP levels in comparison to control subjects.Citation13,Citation100,Citation101 Statins exert several actions in cardiothoracic surgical procedures in addition to lipid-lowering. In patients undergoing CABG, statins improve bypass graft potency, perioperative as well as long-term mortality rates. In addition, statins reduce the number of postoperative complications and clinical events, revascularization rates and postoperative hospital stay. Furthermore, they are protective against de novo AF and renal dysfunction following CABG. The preoperative use of statins has been related to a three-fold decrease in the odds of AF after noncardiac major thoracic surgery.Citation51 A recent meta-analysis of 14 statin trials, with 7,402 patients, found that statins reduced the odds of developing postoperative AF by 58% ().Citation69
During the past few years, the association between statin use and development of AF has been examined in different clinical settings. Recent meta-analysis on this issue showed different results between RCTs and observational studies, suggesting that statins may be effective in prevention of postoperative AF.Citation102,Citation67 In a prospective study which examines the relation between inflammation associated parameters to post-cardiac surgery. A single post-CABG cTnI measurement was assessed in 156 patients. Cardiac troponin-I levels were significantly higher in patients not preoperatively treated with statins (21.6 ± 4.1 vs. 13.3 ± 0.9; p = 0.05), thus preoperative treatment with statins may be beneficial in reducing postoperative inflammatory response.Citation103
A retrospective studyCitation104 of 680 consecutive patients undergoing CABG surgery and/or aortic valve replacement, Preoperative statin treatment and occurrence of postoperative AF were examined in a cohort comprised 623 patients. The statin-treated patients had a 27.1% incidence of postoperative AF vs. 38.3% in the nonstatin group (adjusted OR 2.00; 95% CI: 1.24 to 3.24; p = 0.004). Simvastatin (40 mg) and atorvastatin (40 mg) demonstrated the greatest effect on postoperative AF at 15.6% and 21.2%, respectively, versus nonstatins (respective adjusted ORs, 3.89 [p < 0.0001] and 2.76 [p = 0.012]). Intermediate-dose (20 mg) statins were also effective against AF, at 24.4% for simvastatin (adjusted OR, 2.32; p = 0.004) and 26.4% for atorvastatin (adjusted OR, 1.99, p = 0.047). Low-dose statins, simvastatin or atorvastatin (10 mg), did not influence postoperative AF ().
The beneficial effect of statins in CABG has been confirmed in two recent studies. In an observational cohort studyCitation105 of 2,497 adult patients who underwent isolated CABG. Similar perioperative mortality was found between matched pairs with statin therapy vs. nonstatin therapy, five (0.76%) and eight (1.2%) (p = 0.40), respectively. Cardiac, neurologic, renal, and respiratory morbidity, occurrence of AF, and length of hospital stay were similar between the matched pairs and among quintiles of propensity scores. But different result were obtained in another study with over a two-year period follow up, 405 consecutive patients underwent isolated CABG procedures. Preoperative statins were associated with a 42% reduction in risk of AF development after CABG surgery (OR 0.58, 95% CI: 0.37 to 0.91; p = 0.017, while stratifying on the propensity score). No different effect of statins on AF was observed with respect to age groups (< or = 70 and >70 years) (interaction, p = 0.711).Citation106 Previous AF and nonuse of statins are significantly associated with AF after CABG. This was explored in 234 consecutive patients underwent CABG (173 men; 65 ± 9 years of age) in whom the occurrence of postoperative AF was monitored. In a subgroup of 66 patients, plasma levels of matrix metalloproteinase-1 (MMP-1), its inhibitor, tissue inhibitor matrix metalloproteinase-1 (TIMP-1; as indexes of extracellular matrix remodeling), and N-terminus pro-BNP (related to left ventricular function) at baseline and at 24 hours after surgery were measured, 66 (28.2%) developed postoperative AF. In multivariate analysis, previous AF was related to an increase in the development of AF (OR; 11.92, 95%, CI; 2.37 to 59.98, p = 0.026), whereas statin use was related to a decrease in arrhythmia (OR 0.52, 95%; CI: 0.28 to 0.96; p = 0.038). A higher TIMP-1/MMP-1 ratio at 24 hours after surgery was present in those who did not develop postoperative AF (p = 0.043). Statin use was associated with increased TIMP-1 levels and TIMP-1/MMP-1 ratio (p = 0.027 and 0.036, respectively)Citation13 ().
In a population with appreciable β-blocker and amiodarone use, adjunctive preoperative statin use was still associated with a 40% reduction in patients’ odds of developing post-cardiothoracic surgery AF. Patients undergoing cardiothoracic surgery from the randomized, controlled Atrial Fibrillation Suppression Trials I, II, and III were evaluated in nested cohort evaluation. Overall, 331 patients (59.6%) received a statin preoperatively and 224 patients (40.4%) did not. The study population had an average age of 67.8 ± 8.6 years, 77.1% were male, 14.6% had valve surgery, 6.1% had a history of AF, 12.6% had a history of heart failure, In total, 174 patients (31.4%) developed post-cardiothoracic surgery AF. Upon multivariate logistic regression, statin use was associated with a reduction in post-cardiothoracic surgery AF (adjusted OR 0.60; 95% CI: 0.37–0.99). Higher intensity statin dosing (40 mg of atorvastatin or more) seemed to be associated with the greatest reductions in post-cardiothoracic surgery AF (OR 0.45; 95% CI: 0.21–0.99) ().Citation107 Again, in an observational study of 362 consecutive patients (267 on and 95 not on statin). Postoperative AF was less frequent and its duration was shorter in patients with preoperative statin treatment compared to control (p = 0.03 and 0.0001, respectively). The Kaplan–Meier analysis showed the protective effect of statins against the risk of developing AF again (p = 0.01).Citation108 This study is an observational study with a limitation. Meta-analysis of over 30,000 patients undergoing cardiac surgery including 19 studies (three randomized prospective clinical trials), 16 observational studies) with (n = 17 201; 54%) or without (n = 14 524; 46%) preoperative statin therapy. Outcomes analyzed included early all-cause mortality (30-day mortality), myocardial infarction (MI), AF, stroke and renal failure. Preoperative statin therapy resulted in a 1.5% absolute risk reduction (2.2 vs. 3.7%; p < 0.0001) and 43% odds reduction for early all-cause mortality (OR 0.57; 95% CI: 0.49–0.67). A significant reduction (p < 0.01) in statin pretreated patients was also observed for AF (24.9 vs. 29.3%; OR 0.67, 95% CI: 0.51–0.88), stroke (2.1 vs. 2.9%, OR 0.74, 95% CI: 0.60–0.91) ().Citation109
Recently, a prospective randomized study ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) has demonstrated that treatment with atorvastatin 40 mg/day started seven days before elective cardiac surgery under cardiopulmonary bypass and continued in the postoperative period significantly reduced the occurrence of postoperative AF by 61%. This benefit was more evident in the patients undergoing CABG (OR 0.24). However, this study did not include patients undergoing off-pump cardiac surgery, which is currently the preferred method of surgery in many centers.Citation110 However, there was a greater use of β-blockers in the treatment group. Though this is not significant in the formal statistical sense (p = 0.08), it is notably higher (72% vs. 60%). The authors do refer to the established role of β-blockade in the reduction of postoperative AF, and include it in their multivariate analysis, which is consider of particular relevance given the size of the study population (n = 200). Additionally, a nonsignificant excess of patients undergoing valve surgery in the placebo group (25% vs. 16%), which compared with nonvalvular surgery confers a greater likelihood of postoperative AF. whether this excess in valve surgery has been taken into account by the multivariate analysis is not clear.
The predisposing determinants of the development of AF after off-pump CABG surgery remain unclear. A prospectively designed study of patients undergoing elective off-pump CABG surgery showed that pretreatment with atorvastatin 20 mg/d, started three days before surgery, in patients undergoing elective off-pump CABG was beneficial in reducing the incidence of postoperative AF. The incidence of AF was significantly lower in the atorvastatin group than in the control group (13% vs. 27%; p = 0.04). Postoperative peak N-terminal pro-brain natriuretic peptide levels were significantly higher in the patients with AF (p = 0.03). Multivariate analysis identified pretreatment with atorvastatin as an independent factor associated with a significant reduction in postoperative AF (OR 0.34; p = 0.04). Higher postoperative peak N-terminus pro-B–type natriuretic peptide levels were associated with the development of postoperative AF (OR 1.02 per 100 pg/mL; p = 0.03).Citation111 this study demonstrate that a short-term pretreatment with atorvastatin results in a 66% risk reduction of postoperative AF that persists in patients undergoing off-pump CABG.
There is now an increasing body of evidence that inflammation have pivotal role in the pathogenesis of postoperative AF. Two recent studies have shown that inflammation can alter atrial conduction, facilitating re-entry and then predisposing to the development of postoperative AF.Citation112,Citation113 It is well known that extracorporeal circulation is associated with systemic inflammatory response, which might be in part responsible for the occurrence of postoperative AF. Interestingly, it has been reported recently that the leukocytosis, which is usually encountered in the days after cardiopulmonary bypass, is an independent predictor for the occurrence of postoperative AF.Citation48,Citation114 Perioperative statin use has been associated with favorable postoperative outcome in both cardiovascular and noncardiovascular conditions, and has particular advantage in combination with β-blocker therapy. However, elucidating the impact of statin therapy on a specific clinical end-point would require a larger study population in order to generate a robust conclusion.
Conclusion
Despite a growing body of evidence to support a protective role of drugs with anti-inflammatory properties in AF as illustrated above, it should not be overlooked that there are also numerous studies that could not find a preventive effect on AF occurrence at all. Such conflicting data may in part be related to different study populations as well as differences in AF history and predisposing diseases. Furthermore, there was a great variance in study protocols that could contribute to controversial results. Among others, the sample size, follow-up duration, duration of drug application, drug dose used, and method to assess occurrence of AF varied widely. Moreover, a wide range of different substances have been examined. Especially in the case of statins, this could account for inconsistent results. Thus, there are preliminary data to support the potential utility of statins in the primary and secondary prevention of AF. These observed positive effects of statins on the burden of AF appeared to be independent of their cholesterol reducing properties. However, further data from large-scale randomized trials are clearly needed.
Disclosure
The authors report no conflicts of interest in this work. This article has been written for interest and independently without support from industry or nonindustry.
References
- MihmMJYuFCarnesCAImpaired myofibrillar energetics and oxidative injury during human atrial fibrillationCirculation200110417418011447082
- CarnesCAChungMKNakayamaTAscorbate attenuates atrial pacing–induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillationCirc Res200189e32e3811557745
- AvilesRJMartinDOApperson-HansenCInflammation as a risk factor for atrial fibrillationCirculation20031083006301014623805
- GaudinoMAndreottiFZamparelliRThe −174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication?Circulation2003108Suppl 1II195II19912970232
- EngelmannMDSvendsenJHInflammation in the genesis and perpetuation of atrial fibrillationEur Heart J2005262083209215975993
- BoosCJAndersonRALipGYIs atrial fibrillation an inflammatory disorderEur Heart J20062713614916278230
- EhrlichJRHohnloserSHNattelSRole of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidenceEur Heart J20062751251816311236
- ChanAWBhattDLChewDPRelation of inflammation and benefit of statins after percutaneous coronary interventionsCirculation20031071750175612665489
- HorneBDMuhlesteinJBCarlquistJFStatin therapy interacts with cytomegalovirus seropositivity and high C-reactive protein in reducing mortality among patients with angiographically significant coronary diseaseCirculation200310725826312538425
- ShishehborMHBrennanMLAvilesRJStatins promote potent systemic antioxidant effects through specific inflammatory pathwaysCirculation200310842643112860913
- Young-XuYJabbourSGoldbergRUsefulness of statin drugs in protecting against atrial fibrillation in patients with coronary artery diseaseAm J Cardiol2003921379198314675569
- SiuCWLauCPTseHFPrevention of atrial fibrillation recurrence by statin therapy in patients with lone atrial fibrillation after successful cardioversionAm J Cardiol2003921343134514636918
- MarínFPascualDARoldánVStatins and postoperative risk of atrial fibrillation following coronary artery bypass graftingAm J Cardiol2006971556016377284
- SpachMSDolberPCRelating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle: evidence for electrical uncoupling of side-to-side fiber connections with increasing ageCardiovasc Res198658356371
- SchottenUAusmaJStellbrinkCHanrathPAllessieMACellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillationCirculation200110369169811156881
- KuceraJPRudyYMechanistic insights into very slow conduction in branching cardiac tissue: a model studyCirc Res20018979980611679410
- KostinSKleinGSzalayZStructural correlate of atrial fibrillation in human patientsCardiovasc Res20025436137912062341
- BoldtAWetzelULauschkeJFibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve diseaseHeart20049040040515020515
- FrustaciAChimentiCBellocciFHistological substrate of atrial biopsies in patients with lone atrial fibrillationCirculation199796118011849286947
- TsaiCTLaiLPKuoKTAngiotensin II activates signal transducer and activators of transcription 3 via Rac1 in atrial myocytes and fibroblasts: implication for the therapeutic effect of statin in atrial structural remodelingCirculation2008b117334435518172037
- ChelloMSpadaccioCPattiGSimvastatin reduces platelet-endocardium adhesion in atrial fibrillationAtherosclerosis2008197258859517904146
- NeumanRBBloomHLShukrullahIOxidative stress markers are associated with persistent atrial fibrillationClin Chem20075391652165717599958
- SpodickDHArrhythmias during acute pericarditis: a prospective study of 100 consecutive casesJAMA19762353941945999
- MorgeraTDi LenardaADreasLElectrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changesAm Heart J19921244554671636589
- BruinsPVelthuisHYazdanbakhshAPActivation of the complement system during and after cardiopulmonary bypass surgery: post surgery activation involves C-reactive protein and is associated with postoperative arrhythmiaCirculation199796354235489396453
- MaixentJMPaganelliFScaglioneJAntibodies against myosin in sera of patients with idiopathic paroxysmal atrial fibrillationJ Cardiovasc Electrophysiol199896126179654226
- ChungMKMartinDOSprecherDC-Reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillationCirculation20011042886289111739301
- Shiroshita-TakeshitaASchramGLavoieJEffect of Simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrialtachycardia remodeling in dogsCirculation20041102313231915477401
- KorantzopoulosPKolettisTMKountourisEOral vitamin C administration reduces early recurrence rates after electrical cardioversion of persistent atrial fibrillation and attenuates associated inflammationInt J Cardiol2005a10232132615982504
- KorantzopoulosPKolettisTMKountourisEVariation of inflammatory indexes after electrical cardioversion of persistent atrial fibrillation: Is there an association with early recurrence ratesInt J Clin Pract2005b5988188516033606
- DudleySCJrHochNEMcCannLAAtrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidasesCirculation20051121266127316129811
- KaplanMHVolanakisJEInteraction of C-reactive protein complexes with the complement system. I Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelinJ Immunol1974112213521474151108
- VolanakisJEWirtzKWInteraction of C-reactive protein with artificial phosphatidylcholine bilayersNature1979281155157471064
- DernellisJPanaretouMC-reactive protein and paroxysmal atrial fibrillation: evidence of the implication of an inflammatory process in paroxysmal atrial fibrillationActa Cardiol20015637538011791805
- BlakeGJRidkerPMC-reactive protein and other inflammatory risk markers in acute coronary syndromesJ Am Coll Cardiol200341Suppl 437S42S12644339
- ConwayDSBugginsPHughesEPredictive value of indexes of inflammation and hypercoagulability on success of cardioversion of persistent atrial fibrillationAm J Cardiol2004a4508510
- ConwayDSBugginsPHughesERelationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillationJ Am Coll Cardiol2004b432075208215172416
- SataNHamadaNHorinouchiTC-reactive protein and atrial fibrillation. Is inflammation a consequence or a cause of atrial fibrillationJpn Heart J20044544144515240964
- PsychariSNApostolouTSSinosLRelation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillationAm J Cardiol20059576476715757607
- AsselbergsFWvan den BergMPDiercksGFC-reactive protein and microalbuminuria are associated with atrial fibrillationInt J Cardiol200598737715676170
- WatanabeTTakeishiYHironoOC-reactive protein elevation predicts the occurrence of atrial structural remodeling in patients with paroxysmal atrial fibrillationHeart Vessels200520454915772777
- AndersonJLAllen MaycockCALappeDLIntermountain Heart Collaborative Study Group. Frequency of elevation of C-reactive protein in atrial fibrillationAm J Cardiol2004941255125915541240
- DernellisJPanaretouMRelationship between C-reactive protein concentrations during glucocorticoid therapy and recurrent atrial fibrillationEur Heart J2004251100110715231367
- OralHKnightBPOzaydinMClinical significance of early recurrences of atrial fibrillation after pulmonary vein isolationJ Am Coll Cardiol20024010010412103262
- DernellisJPanaretouMEffect of C-reactive protein reduction on paroxysmal atrial fibrillationAm Heart J200550106416290998
- NakaTNishimotoNKishimotoTThe paradigm of IL-6: from basic science to medicineArthritis Res20024Suppl 3S233S24212110143
- MarcusGMWhooleyMAGliddenDVInterleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul StudyAm Heart J2008155230330918215601
- AbdelhadiRHGurmHSVan WagonerDRRelation of an exaggerated rise in white blood cells after coronary bypass or cardiac valve surgery to development of atrial fibrillation postoperativelyAm J Cardiol2004931176117815110218
- LaufsUEndresMCustodisFSuppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcriptionCirculation20001023104311011120702
- AlbertMADanielsonERifaiNEffect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort studyJAMA2001286647011434828
- AmarDZhangHHeerdtPMStatin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive proteinChest20051283421342716304294
- HadiHACarrCSAl SuwaidiJEndothelial dysfunction: cardiovascular risk factors, therapy, and outcomeVasc Health Risk Manag20051318319817319104
- PlengeJKHernandezTLWeilKMSimvastatin lowers C-reactive protein within 14 days: an effect independent of cholesterol reductionCirculation20021061447145212234946
- Blanco-ColioLMTunonJMartin-VenturaJLAnti-inflammatory and immunomodulatory effects of statinsKidney Int200363122312472764
- GoetteAHoneycuttCLangbergJJElectrical remodeling in atrial fibrillation. Time course and mechanismsCirculation199694296829748941128
- TielemanRGDe LangenCVan GelderICVerapamil reduces tachycardia-induced electrical remodeling of the atriaCirculation199795194519539107184
- LeeSHYuWCChengJJEffect of verapamil on long-term tachycardia-induced atrial electrical remodelingCirculation200010120020610637209
- Van NoordTVan GelderICTielemanRGVERDICT: the Verapamil versus Digoxin Cardioversion Trial: a randomized study on the role of calcium lowering for maintenance of sinus rhythm after cardioversion of persistent atrial fibrillationJ Cardiovasc Electrophysiol20011276676911469424
- KumagaiKNakashimaHSakuKThe HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis modelCardiovasc Res20046210511115023557
- SavelievaICammJStatins and polyunsaturated fatty acids for treatment of atrial fibrillationNat Clin Pract Cardiovasc Med20085304118094671
- PliquettRUCornishKGPeulerJDSimvastatin normalizes autonomic neural control in experimental heart failureCirculation20031072493249812695293
- GaoLWangWLiYLSimvastatin therapy normalizes sympathetic neural control in experimental heart failure: roles of angiotensin II type 1 receptors and NAD (P) H oxidaseCirculation20051121763177016157767
- TziakasDNChalikiasGKStakosDAEffect of statins on collagen type I degradation in patients with coronary artery disease and atrial fibrillationAm J Cardiol2008101219920218178406
- JacobsonJStatins in endothelial signaling and activationAntioxid Redox Signal2008922 [Epub ahead of print]
- OkazakiHMinaminoTWakenoMStatin prevents structural and electrical atrial remodeling in rat hypertensive heart failure induced by chronic inhibition of NO synthesisCirculation2007116Suppl II140
- Shiroshita-TakeshitaABrundelBJBursteinBEffects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failureCardiovasc Res2007741758417270161
- LiuTLiLKorantzopoulosPStatin use and development of atrial fibrillation: A systematic review and meta-analysis of randomized clinical trials and observational studiesInt J Cardiol2008126216017018031847
- FauchierLPierreBde LabriolleAAntiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trialsJ Am Coll Cardiol200851882883518294568
- PatelAAWhiteCMShahSAThe relationship between statin use and atrial fibrillationCurr Med Res Opin2007231177118517519085
- McLeanDSRavidSBlazingMEffect of statin dose on incidence of atrial fibrillation: data from the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) and Aggrastat to Zocor (A to Z) trialsAm Heart J2008155229830218215600
- AdabagASNelsonDBBloomfieldHEEffects of statin therapy on preventing atrial fibrillation in coronary disease and heart failureAm Heart J20071541140114518035087
- HannaIRDudleySCJrKing-HagemanDLipid-lowering therapy reduces the prevalence of atrial fibrillation (Abstract)New Orleans, LA2005 Heart Rhythm Society Scientific SessionsMay 4–72005134
- RamaniGZahidMGoodCBComparison of frequency of new-onset atrial fibrillation or flutter in patients on statins versus not on statins presenting with suspected acute coronary syndromeAm J Cardiol2007100340440517659917
- TveitAGrundtvigMGundersenTAnalysis of pravastatin to prevent recurrence of atrial fibrillation after electrical cardioversionAm J Cardiol200493678078215019894
- WatanabeEArakawaTUchiyamaTHigh sensitivity C-reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversionInt J Cardiol2006108334635315964643
- HumphriesKHLeeMSheldonRCARAF InvestigatorsStatin use and recurrence of atrial fibrillation after successful cardioversionAm Heart J2007154590891317967597
- LoricchioMLCianfroccaCPasceriVRelation of C-reactive protein to long-term risk of recurrence of atrial fibrillation after electrical cardioversionAm J Cardiol200799101421142417493472
- OzaydinMVarolEAslanSMEffect of atorvastatin on the recurrence rates of atrial fibrillation after electrical cardioversionAm J Cardiol2006971490149316679090
- Al ChekakieMOAkarJGWangFThe effects of statins and renin-angiotensin system blockers on atrial fibrillation recurrence following antral pulmonary vein isolationJ Cardiovasc Electrophysiol200718994294617593228
- RichterBDerntlMMarxMTherapy with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and statins: no effect on ablation outcome after ablation of atrial fibrillationAm Heart J2007153111311917174648
- GillisAMMorckMExnerDVBeneficial effects of statin therapy for prevention of atrial fibrillation following DDDR pacemaker implantationEur Heart J2008291873188018477727
- TsaiCTLaiLPHwangJJAtorvastatin prevents atrial fibrillation in patients with brady-arrhythmias and implantation of an atrial-based or dual-chamber pacemaker: a prospective randomized trialAm Heart J2008a1561657018585498
- AmitGKatzABar-OnSAssociation of statin therapy and the risk of atrial fibrillation in patients with a permanent pacemakerClin Cardiol200629624925216796074
- BhavnaniSPColemanCIWhiteCMAssociation between statin therapy and reductions in atrial fibrillation or flutter and inappropriate shock therapyEuropace200810785485918495672
- KorantzopoulosPGalarisDPapaioannidesDC-reactive protein and oxidative stress in atrial fibrillationInt J Cardiol2003b8810310412659994
- KorantzopoulosPKolettisTMGalarisDThe role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillationInt J Cardiol200711513514316764958
- KorantzopoulosPGalarisDPapaioannidesDThe possible role of oxidative stress in heart failure and the potential of antioxidant interventionMed Sci Monit2003a9RA12012512824962
- LiuTLiGLiLAssociation between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysisJ Am Coll Cardiol2007491642164817433956
- MuscoSConwayELKoweyPRDrug therapy for atrial fibrillationMed Clin North Am20089212114118061001
- ShanesJGMinadeoKNMoretAStatin therapy in heart failure: prognostic effects and potential mechanismsAm Heart J200715461762317892981
- TousoulisDCharakidaMStefanadiEStatins in heart failure. Beyond the lipid lowering effectInt J Cardiol200711514415017175040
- KorantzopoulosPKokkorisSThe antioxidant effects of statins may extend beyond atherosclerosis: potential benefits for atrial fibrillation and heart failureAtherosclerosis2004175118715186966
- HadiHASuwaidiJAEndothelial dysfunction in diabetes mellitusVasc Health Risk Manag20073685387618200806
- KostapanosMSLiberopoulosENGoudevenosJADo statins have an antiarrhythmic activityCardiovasc Res200775102017383620
- HannaIRHeekeBBushHLipid-lowering drug use is associated with reduced prevalence of atrial fibrillation in patients with left ventricular systolic dysfunctionHeart Rhythm2006388188616876733
- DickinsonMGHellkampASIpJHStatin therapy was associated with reduced atrial fibrillation and flutter in heart failure patients in SCD-HEFTHeart Rhythm20063SupplS49
- ColettaAPCullingtonDClarkALClinical trials update from European Society of Cardiology meeting 2008: TIME-CHF, BACH, BEAUTIFUL, GISSI-HF, and HOME-HFEur J Heart Fail200810121264126719008149
- SpachMSDolberPCHeidlageJFInfluence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagationCirc Res1988628118322450697
- AllessieMABoydenPACammAJPathophysiology and prevention of atrial fibrillationCirculation20011037697711156892
- BruinsPVelthuisHYazdanbakhshAPActivation of the complement system during and after cardiopulmonary bypass surgery: post surgery activation involves C-reactive protein and is associated with postoperative arrhythmiaCirculation199796354235489396453
- AmarDFleisherMZhangHElevated C-reactive protein but not troponin is associated with postoperative atrial fibrillationAnesth Analg200294S67
- LiuTLiGPHuangTGAnti-inflammatory therapies in atrial fibrillationInt J Cardiol200510435936016087254
- KnayzerBAbramovDNataliaBAtrial fibrillation and plasma troponin I elevation after cardiac surgery: relation to inflammation-associated parametersJ Card Surg200722211712317338744
- KourliourosADe SouzaARobertsNDose-related effect of statins on atrial fibrillation after cardiac surgeryAnn Thorac Surg20088551515152018442529
- SubramaniamKKochCGBashourAPreoperative statin intake and morbid events after isolated coronary artery bypass graftingJ Clin Anesth200820141118346602
- MariscalcoGLorussoRKlersyCObservational study on the beneficial effect of preoperative statins in reducing atrial fibrillation after coronary surgeryAnn Thorac Surg20078441158116417888963
- LertsburapaKWhiteCMKlugerJPreoperative statins for the prevention of atrial fibrillation after cardiothoracic surgeryJ Thorac Cardiovasc Surg2008135240541118242276
- OzaydinMDoganAVarolEStatin use before by-pass surgery decreases the incidence and shortens the duration of postoperative atrial fibrillationCardiology2007107211712116864965
- LiakopoulosOJChoiYHHaldenwangPLImpact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta-analysis of over 30,000 patientsEur Heart J200829121548155918506053
- PattiGChelloMCanduraDRandomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) studyCirculation20061141455146117000910
- SongYBOnYKKimJHThe effects of atorvastatin on the occurrence of postoperative atrial fibrillation after off-pump coronary artery bypass grafting surgeryAm Heart J20081562373,e9e1618657672
- IshiiYSchuesslerRBGaynorSLInflammation of atrium after cardiac surgery is associated with in homogeneity of atrial conduction and atrial fibrillationCirculation20051112881288815927979
- TselentakisEVWoodfordEChandyJInflammation effects on the electrical properties of atrial tissue and inducibility of postoperative atrial fibrillationJ Surg Res2006135687516650868
- LammGAuerJWeberTPost-operative white blood cell count predicts atrial fibrillation after cardiac surgeryJ Cardiothorac Vasc Anesth200620515616458214
- ViraniSSNambiVRazaviMPreoperative statin therapy is not associated with a decrease in the incidence of postoperative atrial fibrillation in patients undergoing cardiac surgeryAm Heart J2008155354154618294494