Abstract
Insulin resistance and islet (beta and alpha) cell dysfunction are major pathophysiologic abnormalities in type 2 diabetes mellitus (T2DM). Pioglitazone is a potent insulin sensitizer, improves pancreatic beta cell function and has been shown in several outcome trials to lower the risk of atherosclerotic and cardiovascular events. Glucagon-like peptide-1 deficiency/resistance contributes to islet cell dysfunction by impairing insulin secretion and increasing glucagon secretion. Dipeptidyl peptidase-4 (DPP-4) inhibitors improve pancreatic islet function by augmenting glucose-dependent insulin secretion and decreasing elevated plasma glucagon levels. Alogliptin is a new DPP-4 inhibitor that reduces glycosylated hemoglobin (HbA1c), is weight neutral, has an excellent safety profile, and can be used in combination with oral agents and insulin. Alogliptin has a low risk of hypoglycemia, and serious adverse events are uncommon. An alogliptin–pioglitazone combination is advantageous because it addresses both insulin resistance and islet dysfunction in T2DM. HbA1c reductions are significantly greater than with either monotherapy. This once-daily oral combination medication does not increase the risk of hypoglycemia, and tolerability and discontinuation rates do not differ significantly from either monotherapy. Importantly, measures of beta cell function and health are improved beyond that observed with either monotherapy, potentially improving durability of HbA1c reduction. The alogliptin–pioglitazone combination represents a pathophysiologically sound treatment of T2DM.
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) has reached epidemic proportions and continues to rise.Citation1 Currently, T2DM has been diagnosed in nearly 24 million Americans and is projected to affect nearly 50 million individuals by 2050. Increases in T2DM are paralleled by a robust increase in people at high risk for the development of diabetes. Prediabetes, as of 2007, may be present in up to 57 million individuals through the diagnosis of impaired fasting glucose (100–125 mg/dL) or impaired glucose tolerance (IGT, two-hour value on a 75 g oral glucose tolerance test [OGTT] of 140–199 mg/dL).Citation1 It is imperative to understand that development of diabetes is not inevitable with prediabetes. Lifestyle interventions and medications, which will be discussed, may be appropriate in select patients to prevent progression to diabetes. In T2DM, hyperglycemia is the key determinant of microvascular complications,Citation2 and the evidence that improving glycemic control lowers the risk of microvascular complications is unequivocal. Hyperglycemia also contributes to macrovascular complications,Citation3 although to a lesser extent. As demonstrated in the United Kingdom Prospective Diabetes Study, glycemic control deteriorates progressively over time in T2DM patients treated with sulfonlyureas, metformin, and/or insulin.Citation2,Citation4 In ADOPT (A Diabetes Outcome Progression Trial), the thiazolidinedione (TZD) rosiglitazone markedly slowed the rise in glycosylated hemoglobin (HbA1c) in newly diagnosed T2DM patients versus sulfonylureas or metformin, but even monotherapy with the TZD could not completely arrest the deterioration of glycemic control over the five years of follow-up.Citation5 Although the progressive worsening of glycemic control can be controlled with lifestyle intervention combined with aggressive stepwise addition of multiple hypoglycemic agents,Citation3,Citation4 clinicians often intervene with additional antihyperglycemic agents only when the HbA1c has risen to values that are well above target.Citation6 To overcome this problem of clinical inertia and to achieve optimal HbA1c goals, early combination therapy with agents that minimize the risk of hypoglycemia and address the multiple underlying pathophysiologic abnormalities has been advocated to assist clinicians in attaining and maintaining glycemic goals.Citation7
In the US, most clinicians initiate therapy with metformin, especially if the patient is overweight. Because metformin improves glucose control, reduces cardiovascular complications in obese patients with T2DM, and is generic, this biguanide represents a logical choice as first-line therapy in diabetic patients.Citation4 Addition of a TZD or a sulfonylurea is commonly employed as the next step by most clinicians. Sulfonylureas are generic and inexpensive, but are inferior to metformin and TZDs with respect to durability of HbA1c reduction,Citation5 may cause hypoglycemia, and impart no other nonglycemic advantages to the T2DM patient. Most importantly, sulfonylureas, like metformin, do not preserve beta cell function.
The core pathophysiologic disturbances (insulin resistance and impaired insulin secretion) that are present in T2DM can be ameliorated by improving muscle/hepatic insulin sensitivity with the addition of a TZD and correction of glucagon-like peptide-1 (GLP-1) deficiency. GLP-1 agonists (exenatide and liraglutide) and dipeptidyl peptidase-4 (DPP-4) inhibitors (sitagliptin, saxagliptin, vildagliptin, and alogliptin) improve insulin secretion by pancreatic beta cells, and decrease the elevated rate of glucagon secretion by alpha cells. GLP-1 receptors have been identified in the pancreas (beta and alpha cells), kidney, heart, stomach, lung, and brain.Citation8,Citation9 GLP-1 enhances glucose-dependent insulin secretion, causes glucose-dependent suppression of elevated glucagon secretion, slows gastric emptying, and reduces food intake. Because the effects of GLP-1 on insulin and glucagon secretion wane as the fasting glucose level returns to normal, hypoglycemia is minimized in T2DM patients treated with GLP-1-based therapy. The glucoregulatory mechanisms by which GLP-1 and exenatide/liraglutide act are similar, but GLP-1 suppresses gastric acid secretion, whereas exenatide and liraglutide do not.Citation10 DPP-4 inhibitors augment insulin secretion and inhibit glucagon release, but do not slow gastric emptying and are weight neutral.Citation11
Given that approximately 50% of T2DM patients have HbA1c levels greater than 7% despite currently available therapies to control glycemia,Citation12–Citation14 adverse metabolic effects are often cited as therapeutically limiting by clinicians, and clinical inertia remains a major problem, combination therapy can help to overcome these multiple barriers. In practice, the combination of an insulin sensitizer (metformin or a TZD) with a GLP-1 analog or a DPP-4 inhibitor minimizes the risk of hypoglycemia and weight gain, and can help to achieve and maintain glycemic goals long term. In this review, we briefly examine the pathophysiology of T2DM, with an emphasis on the role of the TZD pioglitazone, incretin analogs, and specifically the DPP-4 inhibitors, with a special emphasis on alogliptin and the combination of pioglitazone-alogliptin.
Abnormal glucose homeostasis in type 2 diabetes
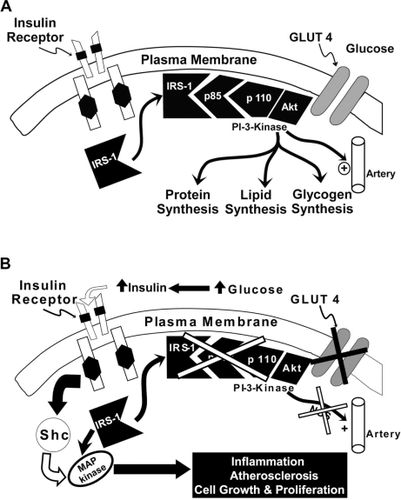
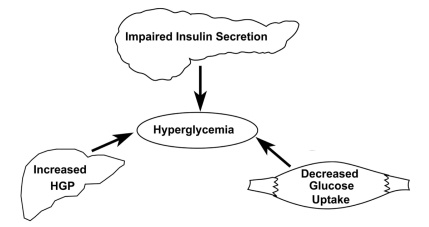
Insulin resistance and beta cell failure represent the two cornerstone pathophysiologic abnormalities in T2DM.Citation7,Citation15–Citation19 Liver, muscle, and adipose tissue are resistant to the actions of insulin. Basal hepatic glucose production (HGP) is increased despite elevated fasting plasma insulin concentrations, indicating the presence of hepatic insulin resistance. The increase in basal HGP is the primary disturbance responsible for the elevation in fasting plasma glucose (FPG) concentration, and impaired suppression of HGP by insulin contributes to postprandial hyperglycemia. The ability of insulin to increase glucose uptake by peripheral tissues (primarily muscle) is markedly reduced and this peripheral insulin resistance plays a major role in postprandial hyperglycemia.Citation7,Citation15 Insulin binds to the insulin receptor, resulting in tyrosine phosphorylation both of the insulin receptor and insulin receptor substrate-1 with subsequent activation of phosphoinositol 3 kinase and Akt (). Activation of the insulin signaling pathway leads to increased glucose transport into the cell, enhanced glucose phosphorylation (hexokinase II), and stimulation of glycogen synthesis (glycogen synthase) and glucose oxidation (pyruvate dehydrogenase).
The adipocyte is also resistant to insulin, and the accelerated rate of lipolysis contributes to day-long elevation in the plasma free fatty acid (FFA) concentration.Citation20 Elevated plasma FFA levels aggravate insulin resistance in both liver and muscle.Citation21 FFA metabolites, such as long-chain FACoAs, impair insulin signaling and inhibit glycogen synthesis and glucose oxidation.Citation22 In addition, FFAs increase HGP in the liverCitation7,Citation16,Citation23 and impair insulin signaling.Citation24 Muscle and hepatic insulin resistance, in combination with impaired insulin secretion, are responsible for postprandial hyperglycemia ().
Figure 2 The triumvirate: insulin resistance in liver and muscle with impaired insulin secretion represent the three core defects in T2DM. Reproduced with permission from DeFronzo RA. Lilly lecture. The triumvirate: Beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1998;37:667–687.Citation16 Copyright © 1998 American Diabetes Association.
Prior to the development of T2DM, the insulin resistance in liver and muscle is compensated by enhanced insulin secretion.Citation7 With time, however, pancreatic beta cell function declines (both because of reduced beta cell sensitivity to glucose and decreased beta cell mass) and the plasma glucose concentration rises. Both “glucotoxicity”Citation25 and “lipotoxicity”Citation26 contribute to the decline in beta cell function. Even small increases in the mean plasma glucose concentration, if present on a chronic basis, can impair insulin secretion by beta cells.Citation27 Additionally, elevated plasma FFA concentrations impair insulin secretion and promote beta cell failure.Citation28 Beta cell dysfunction can be identified during the OGTT long before the diagnosis of T2DM. At the time of diagnosis of IGT, about 50%–60% of beta cell function has already been lost, while individuals in the upper tertile of IGT (two-hour postprandial glucose 180–199 mg/dL) have lost approximately 70%–80% of their beta cell function.Citation29 Thiazolidinediones,Citation30–Citation33 exenatide,Citation34,Citation35 and possibly the DPP-4 inhibitors,Citation36,Citation37 can slow or prevent the decline in beta cell function.
In addition to impaired insulin secretion and moderate to severe insulin resistance, T2DM patients have elevated fasting plasma glucagon levels that fail to suppress normally after a mixed meal and may even rise paradoxically.Citation38–Citation40 Evidence for hepatic hypersensitivity to glucagon has also been provided.Citation41 The elevated plasma glucagon levels stimulate HGP and contribute to fasting and postprandial hyperglycemia. During hyperglycemia, the rate of gastric emptying is normally slowed, resulting in a better match between glucose appearance and glucose disappearance from the circulation. In contrast, patients with newly diagnosed T2DM, despite hyperglycemia, often have an abnormally accelerated gastric emptying rate.Citation42 In this review, we first explore the use of pioglitazone for the treatment of T2DM, and then examine therapies designed to augment plasma GLP-1 levels.
Pioglitazone
Insulin sensitivity and metabolic effects
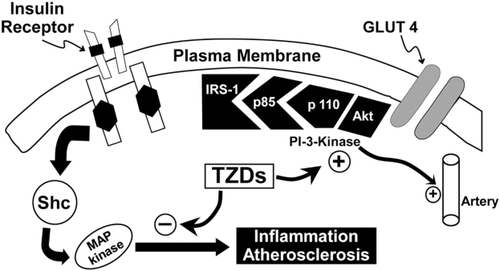
Pioglitazone is a potent insulin sensitizer, which binds to the peroxisome-proliferator activated receptor-gamma, resulting in enhanced muscle, liver, and adipose tissue sensitivity to insulin, with a resultant decline in fasting and postprandial plasma glucose levels.Citation43–Citation45 Pioglitazone also augments beta cell functionCitation46 (), reduces inflammation,Citation47 improves endothelial dysfunction,Citation48,Citation49 corrects diabetic dyslipidemia,Citation50 and improves the defect in insulin signaling in muscle, leading to impaired glucose transport/metabolism results in increased generation of nitric oxide (NO). NO is a potent vasodilator and antiatherogenic molecule,Citation51,Citation52 and deficiency of NO contributes to the markedly increased rate of atherogenesis in T2DM individuals. The compensatory increase in insulin secretion that occurs as the beta cell tries to compensate for the insulin resistance leads to hyperinsulinemia, causing excessive stimulation of the mitogen-activated protein (MAP) kinase pathway which retains normal sensitivity to insulin in T2DM patients. Activation of MAP kinase stimulates multiple intracellular pathways involved in inflammation and augments vascular smooth muscle cell growth and proliferation, thereby promoting atherosclerosis.Citation53 TZDs, including pioglitazone, improve insulin signaling and insulin sensitivity in muscle,Citation43,Citation44 augment NO generation, and simultaneously inhibit the MAP kinase pathway, thus reducing the risk of atherosclerosis in T2DM ().
Hepatic glucose metabolism
In the liver, pioglitazone increases splanchnic glucose uptake, reduces HGP via inhibition of gluconeogenesis, and decreases hepatic fat content.Citation54 Belfort et alCitation22 studied 55 subjects with T2DM or IGT and biopsy-confirmed nonalcoholic steatohepatitis. Subjects were randomized to a hypocaloric diet ± pioglitazone 45 mg/day. After six months of pioglitazone treatment, muscle/hepatic insulin sensitivity improved, liver fat content (measured by magnetic resonance spectroscopy) decreased by 54%, and liver aminotransferase levels were normalized. Liver biopsy demonstrated histologic improvements in steatosis, inflammation, ballooning necrosis, and fibrosis. Pioglitazone also reduced inflammation, as manifested by reductions in C-reactive protein (CRP), tumor necrosis factor alpha, and transforming growth factor-beta, and increased plasma adiponectin levels.
Adipose tissue
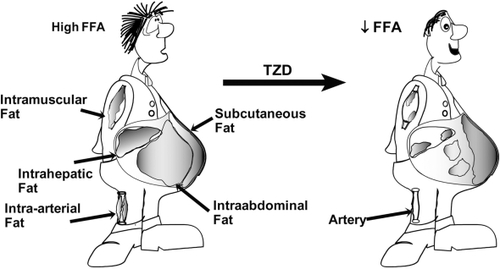
Pioglitazone also exerts positive effects on adipose tissue metabolism. By improving adipocyte sensitivity to the anti-lipolytic effects of insulin, pioglitazone reduces plasma FFA levels,Citation54,Citation55 leading to enhanced insulin sensitivity in muscle/liver and improved insulin secretion.Citation55–Citation57 Pioglitazone also causes a redistribution of fat from highly metabolically active visceral fat (which is associated with accelerated atherogenesis) to subcutaneous fat stores ().
Lipids
Pioglitazone also improves diabetic dyslipidemia, increasing high-density lipoprotein (HDL) cholesterol, reducing plasma triglycerides, and causing a shift from small dense low-density lipoprotein (LDL) to larger more buoyant LDL. Pioglitazone has a neutral effect on LDL cholesterol. In contrast, rosiglitazone increases both LDL and triglyceride levels.Citation50,Citation58 Goldberg et al compared the metabolic effects of pioglitazone and rosiglitazone in lipid-lowering agent-naïve subjects over 24 weeks. Pioglitazone significantly increased HDL and lowered triglycerides compared with rosiglitazone.Citation50 These differences in plasma lipids may, in part, explain the adverse cardiovascular signal that has been reported with rosiglitazone.Citation59,Citation60
Pioglitazone dose-response effect
Miyazaki et al examined the effect of placebo and pioglitazone 7.5, 15, 30, and 45 mg/day daily for 26 weeks in subjects poorly controlled on diet alone. Patients taking previous antidiabetic therapy underwent a 6–8 week washout period. Compared with placebo, HbA1c was significantly reduced in the 15 mg (−1.3%), 30 mg (−2.0%), and 45 mg (−3.0%) groups versus placebo (1.2%). During the OGTT, the insulinogenic index (change in the area under the plasma-concentration time curve [ΔAUC] insulin/ΔAUC glucose) in the 30 mg/day and 45 mg/day groups increased significantly versus placebo. Insulin sensitivity, measured by the Matsuda index of whole-body insulin sensitivity, improved with all doses of pioglitazone, and was greatest at the 45 mg/day dose. The hepatic insulin sensitivity index (k/FPG × fasting plasma insulin) was also significantly improved.Citation61
In summary, pioglitazone improves insulin sensitivity in liver, muscle, and adipose tissue, resulting in improvements in glucose and lipid metabolism.
Beta cell effects and impaired glucose tolerance
TZDs, along with the GLP-1 analogs, are the only classes of drugs that have been shown to enhance and preserve beta cell function.Citation30–Citation33,Citation62–Citation64 It is not widely recognized that individuals with IGT are already maximally/near maximally insulin resistant and have lost as much as 70%–80% of their beta cell function. At baseline in the ACT NOW (Actos Now for Prevention of Diabetes) trial (see subsequent discussion) subjects with IGT had a 48% reduction in insulin sensitivity, as measured by the Matsuda index, and a 78% reduction in ability of pancreatic beta cells to respond to an oral glucose load versus normal glucose tolerant individuals.Citation65 Similar observations have been reported in the VAGES (Veterans Administration Genetic Epidemiology Study) and SAM (San Antonio Metabolism) studies.Citation29,Citation66
Buchanan et al first reported on the use of troglitazone 400 mg daily versus placebo in Hispanic women with a previous history of gestational diabetes and IGT. Over a 30-month follow-up period, troglitazone reduced the risk of diabetes by 55%, and this protective effect persisted eight months after discontinuation of troglitazone therapy.Citation31 Subjects who completed the study without diabetes were asked to continue in an open-label observational study using pioglitazone 45 mg daily for up to three years.Citation32 The annual incidence of diabetes remained low (about 5%), similar to the rate observed during troglitazone treatment. The best predictor of reduced risk of progression to diabetes was a reduction in early insulin output, as measured by the frequently sampled intravenous glucose tolerance test. Subjects who failed to reduce insulin output during TZD therapy did not have a significant reduction in the risk for T2DM. Thus, “off-loading” the pancreatic beta cells was the best predictor for preventing the progression of IGT to T2DM.
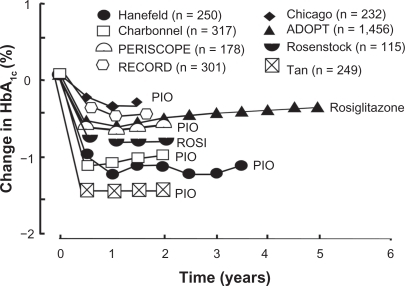
Most recently, pioglitazone has been evaluated in a randomized, double-blind, placebo-controlled trial in 602 subjects confirmed by OGTT to have IGT. Over a mean follow-up of 2.6 years, the risk of progression of IGT to T2DM was reduced by 70% (P < 0.000001). Pioglitazone significantly improved both insulin sensitivity (measured by the Matsuda index and frequently sampled intravenous glucose tolerance test) and pancreatic beta cell function (measured by the insulin secretion/insulin resistance [disposition] index).Citation33 In a double-blind, placebo-controlled, four-month study carried out in poorly controlled, drug-naïve, and sulfonylurea-treated T2DM patients, both pioglitazone and rosiglitazone significantly improved beta cell function ().Citation57 In eight of eight long-term (>1.5 years), double-blind, placebo-controlled or active comparator studies, pioglitazone, as well as rosiglitazone, caused a durable reduction in HbA1cCitation64–Citation73 (). Such a durable reduction in HbA1c can only be explained by preservation of beta cell function.Citation17
Cardiovascular effects
Both pioglitazone and rosiglitazone improve endothelial dysfunction, decrease high-sensitivity CRP, reduce elevated levels of prothrombotic and inflammatory cytokines, increase plasma adiponectin, and reduce blood pressure.Citation47,Citation74 Pioglitazone also lowers plasma triglycerides, raises HDL cholesterol, and converts small dense LDL particles to larger, more buoyant, less atherogenic particles. Both TZDs reduce restenosis after coronary stent placement, and decrease the need for revascularization when given up to six months after stent placement.Citation75
Pioglitazone has also been associated with a reduced risk of cardiovascular disease. In a meta-analysis of pioglitazone studies, Lincoff et alCitation76 reported that the combined endpoint of death, myocardial infarction (MI), and stroke was significantly reduced (hazards ratio [HR] 0.82, 95% confidence interval [CI] 0.72–0.94; P = 0.005]). The PROactive (Prospective Pioglitazone Clinical Trial in Macrovascular Events) trial was designed to explore the cardiovascular benefits of pioglitazone in T2DM subjects at high cardiovascular risk. Entry criteria included history of a prior cardiovascular event. In this double-blind, randomized, placebo-controlled study, eligible subjects were assigned to pioglitazone 45 mg/day or placebo for three years. The primary endpoint (composite of death, MI, stroke, leg amputation, acute coronary syndrome, cardiac bypass, or leg revascularization) was reduced by 10% but this did not reach statistical significance because of an increase in leg revascularization (HR 0.90, 95% CI 0.80–1.02; P = 0.095). There were 195 events in the pioglitazone group versus 240 in the placebo group. The principal secondary endpoint (Kaplan-Meier time to death, non-fatal MI, or stroke) was reduced by 16% and did reach statistical significance (HR 0.84, 95% CI 0.72–0.98; P = 0.027).Citation77 In conclusion, pioglitazone was effective in reducing cerebral and cardiac events, but did not decrease peripheral arterial events. Interestingly, only subjects with baseline peripheral artery disease had an increased risk of leg revascularization (HR 1.68, 95% CI 1.15–2.47; P = 0.008). Subjects without peripheral artery disease at baseline had no higher risk of leg revascularization. Overall, pioglitazone tended to reduce the primary composite endpoint and significantly reduced the principal secondary endpoint of time to death, MI, and stroke.
In addition to the Lincoff meta-analysisCitation76 and PROactive,Citation77 two ultrasound studies have provided evidence of anatomic regression of atherosclerotic disease. In the CHICAGO (Carotid Intima-Media Thickness in Atherosclerosis Using Pioglitazone) study, T2DM subjects were randomized to pioglitazone or glimepiride for 18 months and carotid intima-media thickness was measured before and after randomization. In pioglitazone-treated subjects, carotid intima-media thickness did not progress (−0.001 mm), whereas subjects receiving glimepiride had significant atherosclerosis progression (+0.012 mm). The absolute difference between treatment groups was −0.013mm (95% CI −0.024 to −0.002; P = 0.02).Citation69
PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) was a prospective, randomized, double-blind study comparing the effect of 18 months of pioglitazone versus glimepiride on coronary atheroma volume, quantitated with intravascular ultrasound. After 18 months pioglitazone reduced the percentage atheroma volume from baseline (−0.16%), whereas glimepiride significantly increased the percentage atheroma volume by 0.73% (95% CI 0.33%–1.12%; P < 0.001), resulting in a significant difference between treatment groups (P = 0.002).Citation67
Side effects
Side effects encountered with all TZDs, including pioglitazone, include fat weight gain, fluid retention, and bone fractures. Paradoxically, the greater the fat weight gain, the greater is the decrease in HbA1c and the greater are the increases in insulin sensitivity and beta cell function.Citation78,Citation79 Thus, the fat weight gain is purely a cosmetic, not a metabolic, issue. Fluid retention occurs in 5%–10% of TZD-treated T2DM patients who are inadequately controlled with sulfonylureas, metformin, and/or insulin and less than 1% of these individuals develop congestive heart failure (CHF).Citation80,Citation81 In PROactive, diabetic subjects who developed CHF on pioglitazone had no increase in mortality,Citation77 and in a study by Masoudi et alCitation82 TZD-treated diabetic individuals who developed CHF had a lower risk of mortality at one-year compared with individuals not treated with an insulin sensitizer. Because of occult diastolic dysfunction in T2DM subjects, fluid overload can lead to CHF. Therefore, pedal edema, an easily detected clinical sign for volume overload, should be treated promptly and aggressively with diuretics (triamterene, spironolactone, amiloride) that work in the collecting duct,Citation83,Citation84 and reduction in the pioglitazone dose if necessary to promote diuresis. If the pedal edema does not resolve, pioglitazone should be discontinued. This will minimize the risk of CHF. There is a small increase in the incidence (approximately one per 100 patient treatment years) of bone fractures in postmenopausal diabetic women treated with TZDs.Citation5 An increased incidence of fractures has not been seen in premenopausal women or men. The fractures most commonly are related to trauma and involve the distal portions of the long bones of the extremities. To negate the fracture risk completely, one simply could avoid the use of TZDs in postmenopausal women. Alternatively, one could consider obtaining a bone mineralization scan and, if bone density is reduced, avoid the use of TZDs.
Pharmacoeconomic considerations may play a role in the use of pioglitazone in some managed markets, although the previous discussion should clearly delineate TZDs as a unique class of medication for the treatment of T2DM. The cost-effectiveness of pioglitazone, using the CORE (Center for Outcomes Research) diabetes simulation model on the PROactive study data and discounting 3.5% per annum, was examined. Pioglitazone, using a 35-year time horizon of use, was shown to provide an incremental cost-effectiveness ratio (cost per quality-adjusted life year gained) of <$50,000 based on 2005 dollars, which is considered to be cost-effective.Citation85
Summary
In summary, because of the beneficial effects of pioglitazone on insulin sensitivity, beta cell function, durable HbA1c control, and cardiovascular disease, in conjunction with a low risk of hypoglycemia and manageable side effects, we feel that pioglitazone should be considered as first-line therapy in T2DM patients.
Incretinomimetic agents
Glucose-dependent insulin secretion and loss of incretin effect
The incretin effect accounts for approximately 70% of all insulin that is secreted during an OGTT in normal glucose tolerant subjects,Citation86 and GLP-1 and glucose-dependent insulinotrophic polypeptide (previously called gastric inhibitory polypeptide, GIP) account for over 90% of the incretin effect. GLP-1 is secreted from the L-cells in the distal small intestine/colon in response to mixed meals (glucose or fat). Circulating concentrations of GLP-1 rise rapidly within minutes after food ingestion indicating that neural signals, initiated by food entry in the proximal gastrointestinal tract, stimulate GLP-1 secretion via the L-cells.Citation87 Acutely, GLP-1 promotes normal glucose homeostasis by augmenting insulin secretion, inhibiting glucagon secretion and delaying gastric emptying.
GIP is secreted by the K-cells of the early small intestine in response to meal ingestion. However, unlike GLP-1, GIP does not inhibit glucagon secretion, does not slow gastric emptying, inhibit food intake, or promote weight loss.Citation88 Both GLP-1 and GIP are rapid degraded by the DPP-4, which is ubiquitously present in plasma and on all cell membranes. Thus, the secreted GLP-1 and GIP have a short half-life in the range of 2–3 minutes.
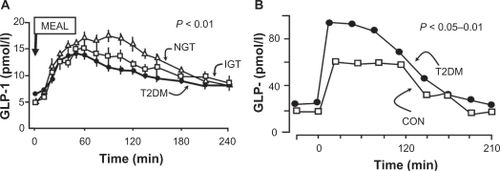
As individuals progress from normal glucose tolerance to IGT to T2DM, stimulated GLP-1 levels declineCitation89,Citation90 (), and there is beta cell resistance to the glucose-dependent stimulatory effect of both GLP-1 and GIP on insulin secretion.Citation91 In T2DM the contribution of incretin hormones to the insulin response has been estimated to be reduced to about 36% in T2DM subjects.Citation86,Citation92 From the therapeutic standpoint, one can increase circulating GLP-1 levels by administering a GLP-1 analog that is resistant to DPP-4 degradation or by giving a DPP-4 inhibitor.Citation7,Citation93,Citation94
Figure 7 GLP-1 levels decline as glucose tolerance deteriorates A), whereas GIP levels are normal or elevated in patients with type 2 diabetes mellitus B).Citation86–Citation88
The insulinotropic action of GLP-1 is glucose-dependent. In order for GLP-1 to enhance insulin secretion, the plasma glucose concentration must be higher than 90 mg/dL.Citation95–Citation99 In contrast, sulfonylureas stimulate insulin secretion irrespective of the ambient glucose concentration. Clinically, this results in an elevated risk of hypoglycemia with sulfonylureas. GLP-1 does not produce significant hypoglycemia. In addition to its stimulatory effect on insulin secretion, GLP-1 augments insulin biosynthesis and gene transcription, thereby restoring the cellular supplies of insulin for subsequent release.Citation100–Citation103 Of great interest, studies in animals have shown that GLP-1 stimulates islet neogenesis, causes beta cell replication, and inhibits beta cell apoptosis, leading to an increase in beta cell mass.Citation104 However, short-term washout studies with exenatide suggest that exenatide is unlikely to increase beta cell mass in humans.Citation64
Elevated plasma glucagon levels
For over 30 years, it has been known that fasting plasma glucagon levels are increased in T2DM and that glucagon secretion is not appropriately suppressed following a carbohydrate or mixed meal or may paradoxically increase.Citation105–Citation107 This abnormality is evident before the diagnosis of diabetes, and has been observed in subjects with IGT.Citation108–Citation110 Hyperglucagonemia in the fasting state results in excessive HGP and elevated FPG levels, while impaired suppression of plasma glucagon levels following a meal results in postprandial hyperglycemia. The main physiologic role of glucagon is to oppose the action of insulin on HGP in order to protect against hypoglycemia and restore normoglycemia.Citation111 GLP-1 inhibits the inappropriately high glucagon secretion after a meal, both directly through the GLP-1 receptor on the alpha cell and indirectly by stimulating insulin secretion, although the absolute contribution of each component is still debated.Citation112 This glucose-dependent inhibitory effect of GLP-1 on glucagon secretion reduces HGP and decreases postprandial plasma glucose levels.Citation113
Correction of accelerated gastric emptying
The rate of gastric emptying is a key determinant of postprandial glucose excursion.Citation114–Citation116 Mismatch between the rate of glucose appearance in the systemic circulation and the rate of glucose disappearance can account for as much as 34% of the variability in peak postprandial glucose concentrations following glucose ingestion in normal glucose tolerant subjects.Citation114,Citation117,Citation118 The normal physiologic response to hypoglycemia is to accelerate gastric emptying. This increases nutrient delivery into the systemic circulation and restores normal plasma glucose concentrations. During hyperglycemia, the rate of gastric emptying is slowed, resulting in a better match between glucose appearance and glucose disappearance from the circulation. Despite hyperglycemia, newly diagnosed T2DM patients often have an accelerated rate of gastric emptying that can exceed the rate of gastric emptying in NGT subjects by 25%–30%.Citation116–Citation119
GLP-1, which is deficient and to which the beta cell is resistant in T2DM, is a potent inhibitor of gastric emptying, and slows the rate of glucose appearance in the systemic circulation.Citation86 GLP-1 agonists, such as exenatide, delay gastric emptying in healthy, nondiabetic subjectsCitation93 and in individuals with T2DM.Citation113,Citation120 The effect of GLP-1 and exenatide on inhibition of gastric emptying is centrally mediated by vagal afferent nerves.Citation121
Reduction in food intake
GLP-1 administration reduces food intake and body weight in a dose-dependent manner. In animal models, the inhibitory effect on food intake is observed when GLP-1 is administered peripherallyCitation122 or intraventricularly.Citation123,Citation124 The inhibition of food intake by GLP-1 results from activation of GLP-1 receptors in the hypothalamus and the area postrema, which are accessible from the systemic circulation.Citation125 A meta-analysis of seven human studies has demonstrated that GLP-1 administration reduces energy intake and increases satiety in lean and overweight subjects.Citation126
Incretin formulations
Because the half-life of GLP-1 is extremely short (less than minutes), it is not practical for use in the treatment of T2DM patients. To overcome the rapid degradation of GLP-1 by DPP-4,Citation127 two approaches have been developed, ie, alteration of the peptide structure of GLP-1 to prevent its degradation by DPP-4, but allow GLP-1 receptor activation, and development of DPP-4 inhibitors, which block the degradation of GLP-1 by DPP-4, thus increasing the reduced concentrations of GLP-1 back to normal physiologic levels in T2DM.
In a mechanism of action study, DeFronzo et alCitation10 compared sitagliptin, a DPP-4 inhibitor, with exenatide, a GLP-1 agonist. T2DM subjects on a stable dose of metformin were randomized to sitagliptin 100 mg daily for two weeks or exenatide 5 μg bid for one week, then 10 μg bid for one week. Subjects were crossed over after two weeks and followed for an additional two weeks. At baseline and at the end of each two-week period, subjects received a meal tolerance test with acetaminophen to measure gastric emptying. After the initial two weeks of treatment, the mean plasma glucose concentration and the two-hour post-meal plasma glucose concentration were markedly reduced in the exenatide versus sitagliptin groups (133 versus 208 mg/dL, P < 0.001). The greater reduction in postprandial glucose excursion with exenatide was accounted for by a higher insulinogenic index, a greater inhibition of glucagon secretion, and delayed gastric emptying. Sitagliptin had no effect on gastric emptying, but did reduce plasma glucagon levels. The greater reduction in FPG and plasma glucagon concentrations and the greater increase in insulin secretion in the exenatide-treated group was explained by the pharmacologic exenatide levels achieved (64 pM) compared with the more physiologic GLP-1 concentrations (15 pM) achieved with sitagliptin.Citation10
Alogliptin
Alogliptin benzoate (formerly called SYR-322) is a non-covalent, selective inhibitor of DPP-4.Citation28 Active GLP-1 is rapidly converted to inactive GLP-1 (9–36 amide or 9–37 amide) by the serine protease DPP-4. Alogliptin prevents the degradation of endogenous GLP-1 (and GIP), thus extending its half-life and restoring endogenous GLP-1 to normal physiologic levels.
Animal data
Rats with streptozotocin-induced diabetes and maintained on glibenclamide 10 mg/kg per day for 27 days were divided into four groups at 20 weeks of age and treated with placebo, glibenclamide 10 mg/kg/day, nateglinide 50 mg/kg/day, or alogliptin 1 mg/kg/day prior to an oral glucose load (1 mg/kg). Alogliptin significantly increased the plasma insulin concentration at 10 minutes and decreased the glucose AUC from 0–120 minute glucose compared with rats receiving glibenclamide and nateglinide prior to the oral glucose load. In a separate group of diabetic rats, DPP-4 activity and plasma GLP-1 levels (GLP-1 [7–36 amide] and GLP-1 [7–37 amide]) were inversely related to the dose of alogliptin over the range 0.03–3.0 mg/kg.Citation129
Pertinent to the use of combined alogliptin-pioglitazone therapy, seven-week-old male Lepob/Lepob (ob/ob) mice and their nondiabetic male littermates received placebo, alogliptin 45.7 mg/kg/day, pioglitazone 4.0 mg/kg/day, or both (alogliptin-pioglitazone) for 33 days. In mice treated with alogliptin, plasma DPP-4 activity was inhibited by 80%, and plasma active GLP-1 levels were increased 3.5-fold and 4.1-fold in the alogliptin alone and alogliptin-pioglitazone groups, without a change in the pioglitazone alone group. Insulin levels were increased approximately 1.5-fold in alogliptin- and pioglitazone-treated mice, and 3.2-fold in alogliptin-pioglitazone mice. Glucagon levels were decreased by approximately 25% in alogliptin-treated and alogliptin-pioglitazone treated mice, whereas no change was seen in the pioglitazone or placebo groups. Adiponectin increased only in mice who received pioglitazone. HbA1c levels decreased by 1.0, 1.5, and 2.3 in the alogliptin-, pioglitazone- and alogliptin-pioglitazone-treated mice, respectively. Pancreatic insulin content increased by 1.3-, 1.5-, and 2.2-fold in mice treated with alogliptin, pioglitazone, and alogliptin-pioglitazone, respectively. In conclusion, the addition of alogliptin to pioglitazone produced completely additive metabolic and hormonal effects in ob/ob mice.Citation130
Human data
Pharmacokinetic and pharmacodynamic profile
Healthy males (n = 36) received a single dose of alogliptin (five subjects for each dose at 25, 50, 100, 200, 400, or 800 mg) or placebo (one subject per each dosing cohort) 30 minutes prior breakfast, and pharmacokinetic and pharmacodynamic parameters were measured over the next 24 hours. Alogliptin was rapidly absorbed and reached maximal concentrations in approximately two hours. Over the dosing range, the rise in plasma alogliptin concentration was linear, and the t1/2 was approximately 16–20 hours after the single dose. In these normal healthy subjects, plasma GLP-1 levels increased 2.5- to 4.0-fold versus placebo over the 24-hour period, with the highest levels achieved 60–120 minutes after ingestion of the meal.Citation131
In a second randomized, double-blind, placebo-controlled study, diet-treated T2DM subjects received alogliptin 25, 100, or 400 mg daily for 14 days. Alogliptin was rapidly absorbed, with a time to maximal concentration of about one hour. At 14 days, the half-life was consistent at approximately 20 hours, supporting daily dosing of alogliptin. Most of the alogliptin (nearly 60%) was recovered unchanged in the urine after 24 hours. The data suggest that alogliptin undergoes some renal secretion, and, similar to other marketed DPP-4 inhibitors, requires a dose reduction in patients with moderate to severe renal impairment.Citation132,Citation133 One active metabolite (N-demethylated alogliptin), which is as active as the parent compound, was identified, but it accounted for only 1% of the recovered drug. After 14 days, all three doses of alogliptin inhibited plasma DPP-4 by more than 80% at 24 hours. Consistent with other DPP-4 inhibitors, alogliptin reduced postprandial plasma glucose levels, but plasma insulin levels did not change significantly. However, the increment in insulin per increment in glucose clearly increased, indicating an effect on the beta cells to augment insulin secretion. Glucagon levels were not measured but, in previously reported animal studies, plasma glucagon was reduced by 25%.Citation128,Citation134
Clinical studies
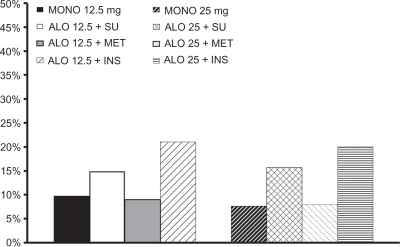
Alogliptin has been studied in T2DM subjects as monotherapy and in combination with metformin, sulfonylureas, pioglitazone, and insulin (see ). DeFronzo et alCitation135 in a randomized, double-blind, placebo-controlled trial, reported on drug-naïve, poorly controlled (HbA1c 7.9% ± 0.8%) T2DM patients (n = 329) treated with alogliptin 12.5 and 25 mg/day or placebo for 26 weeks. Baseline characteristics were similar () in all three groups. Alogliptin 12.5 mg/day (−0.56% and −10 ± 4 mg/dL; P < 0.001 for both) and 25 mg/day (−0.59% and −16 ± 4 mg/dL; P < 0.001 for both) similarly reduced HbA1c and FPG compared with placebo (−0.02% and +11 ± 5 mg/dL). More subjects were able to achieve an HbA1c ≤ 7.0% with alogliptin 12.5 mg/day (47.4%; P = 0.001) or 25 mg/day (44.3%; P = 0.008) versus placebo (23.4%). Approximately 50% of subjects on either dose of alogliptin had at least a ≥0.5% HbA1c reduction, and about 29% had a ≥ 1.0% reduction in HbA1c. Alogliptin was weight neutral and, at both doses, improved the proinsulin-to-insulin ratio. Alogliptin 25 mg/day resulted in a small, but significant reduction in plasma total cholesterol and triglyceride concentration.
Figure 8 Percentage (%) of subjects achieving select HbA1c targets with alogliptin in Phase 3 trials.Citation135–Citation138
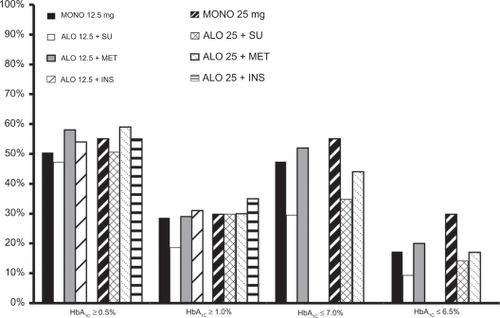
Table 1 Phase III alogliptin trials and alogliptin–pioglitazone combination studies
In a double-blind, randomized, placebo-controlled 26-week trial, Pratley et alCitation136 compared alogliptin 12.5 and 25 mg/day versus placebo in 500 poorly controlled (mean HbA1c 8.1%) T2DM subjects taking background sulfonylurea therapy. Subjects were required to be on sulfonylurea monotherapy for at least three months prior to screening and had to be without serious concomitant diabetic complications. Subjects were switched to glyburide at equivalent dose, if necessary, and completed a four-week glyburide run-in period (). HbA1c was reduced in the alogliptin 12.5 mg/day (−0.39%) and 25 mg/day (−0.53%) groups versus placebo (+0.1%; both P < 0.001). As with other antihyperglycemic agents, the HbA1c reduction was correlated positively with baseline HbA1c. In subjects with a baseline HbA1c ≥ 9.0%, the HbA1c reduction with alogliptin 12.5 mg/day (−0.82%) and 25 mg/day (−1.06%) were more robust. Weight increased slightly (0.6 kg) over the 26-week study, and there were no significant lipid changes.
In a randomized, double-blind, placebo-controlled trial of 26 weeks, Nauck et alCitation137 treated poorly controlled T2DM with alogliptin 12.5 mg/day (n = 213), 25 mg/day (n = 210), or placebo (n = 104). Prior to randomization, all subjects entered a four-week run-in period on a stable dose of metformin ≥ 1500 mg/day (mean dose = 1847 mg/day). Subjects could not have significant diabetes-related complications. At baseline, all three groups were well matched, with an HbA1c of 7.9%–8.0% and FPG of 168–180 mg/dL. HbA1c was significantly reduced by −0.6% on the 12.5 and 25 mg/day alogliptin doses versus placebo (−0.1%). The reduction from baseline in FPG was greater in the alogliptin 12.5 mg/day (−19 mg/dL) and 25 mg/day (−17 mg/dL) groups versus placebo (no change; both P < 0.001, ).
Rosenstock et alCitation138 explored the addition of alogliptin 12.5 and 25 mg/day versus placebo in T2DM subjects inadequately controlled on insulin (at least 15 U/day but not more than 100 U/day) ± metformin therapy in a 26-week, randomized, double-blind, placebo-controlled trial. Subjects were not allowed to have significant diabetes-related complications. The groups were well matched at baseline, with a mean HbA1c of 9.3%, FPG about 190 mg/dL, and diabetes duration of 12–13 years. At baseline all subjects were on insulin (64%, premix or insulin “combo”; 34%, long-acting insulin; 2%, short-acting insulin), and 60% of subjects were taking metformin (mean dose > 1500 mg daily). After 26 weeks, alogliptin 12.5 and 25 mg/day significantly reduced HbA1c (−0.63% and −0.71%, respectively) compared with placebo (0.13%). Only alogliptin 25 mg/day significantly reduced FPG (−11 mg/dL; P = 0.03). Insulin doses were fairly stable throughout the 26-week study. Weight changes were similar in the placebo and alogliptin groups, and no significant changes in the lipid profile were noted ().
In all four of these trials,Citation135–Citation138 the reduction in HbA1c was 0.5–1.0% on mean (). All four trials provided information on the number of subjects who required rescue therapy. For alogliptin in combination with metformin or glyburide, or as monotherapy, rescue therapy guidelines were FPG ≥ 275 mg/dL after more than one week of treatment, ≥ 250 mg/dL after week 4, and ≥225 mg/dL after week 8, or HbA1c ≥ 8.5% with a ≤ 0.5% reduction from baseline by week 12.Citation135–Citation137 In the Rosenstock et alCitation138 paper, the rescue criteria were FPG ≥ 300 mg/dL after more than one week of treatment, ≥ 275 mg/dL after week 4, and ≥250 mg/dL after week 8, or HbA1c ≥ 8.7% with a ≤ 0.5% reduction from baseline by week 12 (). The combination of metformin with alogliptin required fewer hyperglycemic rescues, although no statistical analysis was done on the differences.
Figure 9 Necessity for hyperglycemic rescue* in Phase III trials with alogliptin.Citation135–Citation138
*see text for definitions
Adverse events and tolerability in Phase III trials
DPP-4 inhibitors, including alogliptin, are very well tolerated.Citation135–Citation138 The incidence of all adverse events was, in general, similar to placebo, and not dose-dependent. Discontinuation rates were not different from placebo in any of the studies. The most common adverse events reported across studies were upper respiratory infection, urinary tract infection, nasopharyngitis, headache, diarrhea, arthralgia, and peripheral edema. Headache occurred more frequently than placebo in one study.Citation135 Alogliptin monotherapy had slightly higher gastrointestinal events (abdominal pain, nausea, diarrhea, and vomiting) versus placebo. Other adverse effects of special interest were skin lesions and pruritus, which were closely monitored. Skin lesions were very rare. With alogliptin monotherapy, at 25 mg/day, one case of subcorneal pustular dermatosis was reported. There were no cases of skin lesions with glyburide-alogliptin.Citation136 In the metformin-alogliptin study, skin lesions were observed in 7.7% of placebo versus 12% of alogliptin-treated subjects.Citation137 In the insulin ± metformin study, skin lesions occurred in about 12% of alogliptin-treated patients versus 10.9% of patients receiving placebo.Citation138 Pruritus occurred slightly more frequently with alogliptin. The severity of side effects was mild to moderate, and serious adverse events were not common and did not occur more frequently than placebo. Serious adverse events potentially related to alogliptin included one subject with cholecystitis and pancreatitis, one with CHF, one with pulmonary embolism, and one with severe hypoglycemia when alogliptin was combined with glyburide.Citation135–Citation138
Hypoglycemia rates were dependent upon concomitant therapy. In monotherapy, hypoglycemia rates were 1.5%–3.0% and similar to placebo.Citation135 When alogliptin was combined with metformin, hypoglycemia occurred in 3% of the placebo group, 1% of the alogliptin 12.5 mg/day group, and in none of the subjects in the alogliptin 25 mg/day group.Citation137 Hypoglycemia was defined as <60 mg/dL with symptoms or <50 mg/dL with or without symptoms. These clinical observations reinforce the mechanism of action of endogenously-secreted GLP-1 on insulin secretion as being glucose-dependent and demonstrate that, when the DPP-4 inhibitor alogliptin is administered with agents that do not augment insulin secretion, hypoglycemia is uncommon and does not occur more frequently than in the placebo group. Rates of hypoglycemia when alogliptin was combined with glyburide were 11.1% in the placebo group, 15.8% in the alogliptin 12.5 mg/day group, and 9.6% in the alogliptin 25 mg/day group.Citation136 When combined with insulin, 24%, 26.7%, and 27.1% experienced hypoglycemia in the placebo, alogliptin 12.5 mg/day, and alogliptin 25 mg/day groups, respectively.Citation138 As expected, the rates of hypoglycemia were higher in insulin-treated T2DM patients, but alogliptin did not significantly exacerbate the risk of hypoglycemia.
Alogliptin selectivity
Selectivity of alogliptin for DPP-4 inhibition is defined as a > 10,000 greater affinity for the DPP-4 enzyme than for competing DPP enzymes, such as DPP-2, 8, and 9. Activation of DPP-8 and DPP-9 have been associated with untoward side effects, including thrombocytopenia, anemia, splenic enlargement, alopecia, and skin lesions. Therefore, selectivity for DPP-4 is desirable. DPP-4 inhibition can also prolong the action of endogenous peptides, such as pituitary adenylate cyclase-activating peptide, growth hormone-releasing hormone, peptide YY, neuropeptide Y, and substance P, as well as several other chemokines. However, to date, alogliptin has not been reported to cause an increase in side effects that may be related to inhibition of the degradation of the above peptides, and short-term studies with doses of alogliptin up to 400 mg/day for 14 days in T2DM subjects have reported no dose-limiting toxicities.Citation131
Drug–drug interactions
Alogliptin has not been associated with any significant drug-drug or drug-food interactions. Alogliptin may be taken without regard to meals.Citation139 Alogliptin has been studied in combination with pioglitazone, glyburide, metformin, cimetidine, cyclosporine, and digoxin. Pioglitazone increased the AUC of alogliptin by 10%, but this is considered to be of no clinical significance.Citation140
Alogliptin–pioglitazone combination therapy
The combination of two antihyperglycemic agents with different, but complementary, mechanisms of action, a low risk of hypoglycemia, and easy, once-daily dosing is a logical step in the treatment of T2DM. Several studies or abstracts have examined this combination.
Pratley et alCitation141 reported an international double-blind, randomized, placebo-controlled study in T2DM subjects randomized to alogliptin 12.5 mg/day (n = 197), alogliptin 25 mg/day (n = 199), or placebo (n = 97). Subjects were on a TZD at baseline and, during a four-week run-in period, were stabilized on pioglitazone at 35 mg/day on average. If subjects were on pioglitazone, the current daily dose was continued; if on rosiglitazone, the subject was switched to the equivalent dose of pioglitazone, at 30 mg or 45 mg daily, and subjects were continued on metformin or sulfonylurea if their dose was stable for at least one month. At baseline, subjects were well matched with respect to mean age (55 years), ethnicity (white, 74%), duration of diabetes (7.6 years), body mass index (BMI, 32.8 kg/m2), and baseline HbA1c (8.0%). Concomitant therapy was metformin in 55% (mean dose 1688 mg/day), sulfonylurea in 20% (mean dose 37 mg/day), and no concomitant therapy (25%) at baseline. After 26 weeks, HbA1c and FPG were significantly reduced from baseline versus placebo (alogliptin 12.5 mg/day: −20 mg/dL and −0.66%; alogliptin 25 mg/day: −0.8% and −20 mg/dL; placebo: −0.19% and −6 mg/dL). Subjects treated with either dose of alogliptin, 12.5 mg (44.2%) or 25 mg (49.2%), were more likely than placebo (34%) to reach the HbA1c goal of ≤7.0%, P = 0.01). The number of subjects achieving a HbA1c reduction ≥1.0% was two-fold greater in the alogliptin 12.5 mg/day group and three-fold higher in the alogliptin 25 mg/day group compared with placebo, and significantly fewer alogliptin subjects needed hyperglycemic rescue treatment (). Average weight gain was approximately 1 kg with no significant differences between any of the three groups. Both doses of alogliptin were well tolerated and similar numbers of subjects (3%–4%) compared with placebo discontinued therapy due to adverse events. The total number of adverse events was similar (18%–19%) between alogliptin and placebo groups. Adverse reactions possibly related to alogliptin included one subject each with palpitations, CHF, road traffic accident, hypokalemia, serum sickness, and sudden death (no autopsy was performed). Hypoglycemia rates were dependent on baseline therapy. Importantly, in subjects taking the sulfonylurea–pioglitazone combination, rates of hypoglycemia were about 20% versus about 3% in subjects taking pioglitazone–metformin. This substantiates our previous observations that combination therapy with medications, such as metformin, pioglitazone, and GLP-1-based incretinomimetic agents, are associated with a very low risk of adverse effects and hypoglycemia. This will increase the likelihood of patients continuing therapy and achieving glycemic goals.
Alogliptin–pioglitazone combination therapy
Combination therapy with alogliptin-pioglitazone has been examined in conjunction with various background therapies (). In a randomized, double-blind, placebo-controlled, 26-week study, DeFronzo et alCitation142 investigated the combination of alogliptin-pioglitazone in subjects inadequately controlled on metformin. Arms of the study included placebo, alogliptin 12.5 mg/day and 25 mg/day, pioglitazone 15 mg/day, 30 mg/day, and 45 mg/day, and alogliptin 12.5 mg/day or 25 mg/day combined with pioglitazone 15 mg/day, 30 mg/day, or 45 mg/day. For analysis, the authors pooled all doses of pioglitazone, alogliptin 12.5 mg/day plus any dose of pioglitazone, and alogliptin 25 mg/day plus any dose of pioglitazone. The mean change in HbA1c from baseline was −0.89% in the pioglitazone groups, −1.43% in the alogliptin 12.5 mg + pioglitazone groups, and −1.42% in the alogliptin 25 mg + pioglitazone groups (both alogliptin doses + pioglitazone were significant at P < 0.001 versus pioglitazone alone). The mean change in FPG was −28, −45, and −44 mg/dL for the pioglitazone, alogliptin 12.5 mg + pioglitazone, and alogliptin 25 mg + pioglitazone groups, respectively.Citation143 The combination of alogliptin–pioglitazone significantly improved beta cell function measures of the proinsulin to insulin ratio and HOMA-IR versus pioglitazone alone (−0.087 and 18.2; −0.076 and 22.2; −0.027 and 5.1, respectively). HOMA-IR improved in all groups, but did not reach statistical significance between groups ().Citation143
In a randomized, double-blind, 26-week study, 655 subjects (age 53 years, duration of diabetes 3.2 years, HbA1c 8.8%, FPG 191 mg/dL, BMI 21 kg/m2) inadequately controlled on diet and exercise were given an alogliptin–pioglitazone combination (alogliptin 12.5 mg + pioglitazone 30 mg or alogliptin 25 mg + pioglitazone 30 mg daily) or monotherapy with alogliptin 25 mg/day or pioglitazone 30 mg/day. At 26 weeks, the decrements in HbA1c and FPG in the four groups were 1.7% and 50 mg/dL, 1.56% and 48 mg/dL, 1.1% and 28 mg/dL, 0.96% and 26 mg/dL for the alogliptin 25 mg + pioglitazone 30 mg, alogliptin 12.5 mg + pioglitazone 30 mg, pioglitazone 30 mg/day, and alogliptin 25 mg/day groups, respectively. HbA1c reduction was superior with both combination therapies compared with alogliptin alone (P < 0.001) and alogliptin 25 mg + pioglitazone 30 mg was superior to pioglitazone monotherapy (P < 0.001). Weight changes were +3.1, +2.5, +2.2, and −0.3.0 kg in the four groups, respectively. Hypoglycemia was ≤3.0% in all groups.Citation144 Combination alogliptin-pioglitazone therapy improved beta cell function compared with alogliptin alone. Proinsulin to insulin ratio (30% versus 14%, P = 0.006), HOMA-β (67% versus 16%, P = 0.018), and HOMA-IR (46% versus 16%, P < 0.001) improved more in the alogliptin 25 mg + pioglitazone 30 mg group than in the alogliptin 25 mg/day alone groups. In addition, the increases in adiponectin and decrease in high-sensitivity CRP were significantly improved with alogliptin 25 mg + pioglitazone 30 mg versus alogliptin 25 mg/day monotherapyCitation145 ().
Conclusion
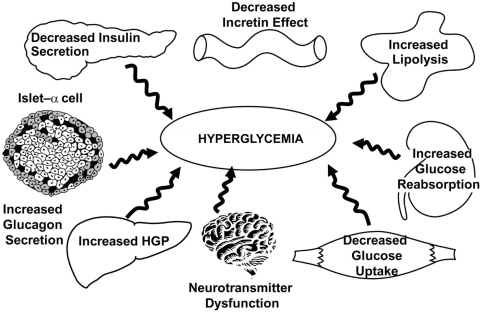
Type 2 diabetes is characterized by at least eight pathophysiologic abnormalities ().Citation17 The combination of alogliptin plus pioglitazone improves at least six of these pathophysiologic disturbances, including improved insulin resistance in skeletal muscle (→↑ muscle glucose uptake), liver (→↓ hepatic glucose production), and adipocytes (↓ lipolysis →↓ plasma FFA), increased incretin effect, enhanced insulin secretion, and decreased glucagon secretion (). Insulin resistance is an early manifestation in individuals with IGT and T2DM and increases beta cell stress, contributing to beta cell failure and the eventual development of overt T2DM. Insulin resistance can be improved with insulin-sensitizing drugs. Metformin is a weak peripheral (muscle) insulin sensitizer, but improves hepatic insulin sensitivity and reduces HGP. TZDs, such as pioglitazone, are potent insulin sensitizers in both peripheral tissues (muscle and adipocytes) and liver. Beta cell function is markedly impaired in T2DM, and alpha cell secretion of glucagon is increased. GLP-1 is deficient in T2DM, and beta cell responsiveness to GLP-1 is markedly impaired. On average, the incretin effect in T2DM individuals is reduced by approximately half compared with nondiabetic patients. GLP-1 increases insulin secretion, decreases glucagon, slows gastric emptying, and results in satiety and weight loss. The two methods of replacing GLP-1 include GLP-1 receptor agonists, which are effective in mimicking all the actions of GLP-1. Blocking the endogenous enzyme, DPP-4, which degrades active GLP-1, which is also effective in elevating to normal the reduced circulating GLP-1 levels that are present in T2DM. DPP-4 inhibitors augment beta cell function and simultaneously reduce elevated plasma glucagon levels in T2DM patients.
Figure 10 The ominous octet: pathophysiologic abnormalities in type 2 diabetes mellitus.Citation7
Alogliptin has been studied as monotherapy and in combination with metformin, sulfonylureas, TZDs, and insulin. Alogliptin significantly improves HbA1c, is weight neutral, does not cause hypoglycemia unless combined with an insulin secretagogue or insulin, has few associated side effects, and very few people discontinue the medication due to intolerance. Pruritic reactions appear to be slightly higher with alogliptin versus placebo, but no significant increase in skin lesions has been observed. The alogliptin–pioglitazone combination reverses multiple metabolic defects in T2DM (). With regard to the beta cell defect, pioglitazone decreases lipotoxicity and exerts direct effects via the peroxisome-proliferator activated receptor-gamma to augment insulin secretion, while alogliptin improves islet function by increasing insulin secretion and lowering glucagon secretion in response to elevated plasma glucose levels. Alogliptin–pioglitazone gives an additive effect to improve HbA1c and reduce FPG, while the risk of hypoglycemia is similar to that with placebo. Alogliptin is weight neutral, whereas alogliptin–pioglitazone combination therapy is usually associated with a 1–3 kg of weight gain during the first year of treatment. Combination therapy also reduces high-sensitivity CRP and increases adiponectin levels. CHICAGO, PERISCOPE, and PROActive demonstrate that pioglitazone has positive effects on vascular function and reduces cardiovascular events. The combination of pioglitazone–alogliptin addresses insulin resistance and islet cell dysfunction in T2DM patients in a once-daily medication that is well tolerated, effectively lowers HbA1c, and has a very low risk of hypoglycemia. In summary, alogliptin–pioglitazone combination therapy can reverse known several pathophysiologic processes in T2DM and is clinically effective.
Disclosure
The authors report no conflict of interest in this work.
References
- Statistics Clearinghouse, NIH/NIDDK. Available from: diabetes.niddk.nih.gov. Accseesed on Apr 14, 2010.
- UK Prospective Diabetes Study (UKPDS) GroupIntensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with Type 2 diabetes (UKPDS 33)Lancet19983528378539742976
- StrattonIMAdlerAINeilHAWAssociation of glycaemia with macrovascular and microvascular complications of Type 2 diabetes (UKPDS 35): Prospective observational studyBMJ200032140541210938048
- UK Prospective Diabetes Study (UKPDS) GroupEffect of intensive blood-glucose control with metformin on complications in overweight patients with Type 2 diabetes (UKPDS 34)Lancet19983528548659742977
- KahnSEHaffnerSMHeiseMAGlycemic durability of rosiglitazone, metformin, or glyburide monotherapyN Engl J Med20063552427244317145742
- BrownJBNicholsGAPerryAThe burden of treatment failure in Type 2 diabetesDiabetes Care2004271535134015220224
- DeFronzoRAFrom the triumvirate to the ominous octet: A new paradigm for the treatment of Type 2 diabetes mellitusDiabetes20095877379519336687
- BullockBPHellerRSHabenerJFTissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptorEndocrinology1996137296829788770921
- BrubakerPLDruckerDJStructure-function of the glucagon receptor family of G protein-coupled receptors: The glucagon, GIP, GLP-1, and GLP-2 receptorsReceptor Channels20028179188
- GedulinBLawlerRJodkaCYoungAAmylin inhibits pentagastrin-stimulated gastric acid secretion: Comparison with glucagon-like peptide-1 and exendin-4Diabetes199746 Abstr 188.
- DeFronzoRAOkersonTViswanathanPEffects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: A randomized, cross-over studyCurr Med Res Opin2008242943295218786299
- SaydahSHFradkinJCowieCCPoor control of risk factors for vascular disease among adults with previously diagnosed diabetesJAMA200429133534214734596
- KoroCEBowlinSJBourgeoisNFedderDOGlycemic control from 1988 to 2000 among US adults diagnosed with Type 2 diabetes: A preliminary reportDiabetes Care200427172014693960
- CheungBMYOngKLCherneySSDiabetes prevalence and therapeutic target achievement in the United States, 1999–2006Am J Med200912244345319375554
- DeFronzoRAPathogenesis of Type 2 diabetes: Metabolic and molecular implications for identifying diabetes genesDiabetes Reviews19975177269
- DeFronzoRALilly lecture. The triumvirate: Beta-cell, muscle, liver. A collusion responsible for NIDDMDiabetes1998376676873289989
- DeFronzoRAPathogenesis of Type 2 diabetes mellitusMed Clin N Am20048878783515308380
- KahnSEClinical Review 135. The importance of beta-cell failure in the development and progression of Type 2 diabetesJ Clin Endocrinol Metab2001864047405811549624
- BergmanRNFinegoodDTKahnSEThe evolution of beta-cell dysfunction and insulin resistance in Type 2 diabetesEur J Clin Invest200232354512028373
- ReavenGMHollenbeckCJengC-YWuMSChenY-DIMeasurement of plasma glucose, free fatty acid, lactate, and insulin for 24 hours in patients with NIDDMDiabetes199837102011043292322
- McGarryJDBanting Lecture 2001: Dysregulation of fatty acid metabolism in the etiology of Type 2 diabetesDiabetes20025171811756317
- BelfortRHarrisonSABrownKA placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitisN Engl J Med20063552297230717135584
- BodenGRole of fatty acids in the pathogenesis of insulin resistance and NIDDMDiabetes1997463108971073
- BelfortRMandarinoLKashyapSDose-response effect of elevated plasma free fatty acid on insulin signalingDiabetes2005541640164815919784
- RossettiLGiaccariADeFronzoRAGlucose toxicityDiabetes Care1990136106302192847
- BaysHMandarinoLDeFronzoRARole of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of Type 2 diabetes mellitus: Peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approachJ Clin Endocrinol Metab20048946347814764748
- RossettiLShulmanGIZawalichWDeFronzoRAEffect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized ratsJ Clin Invest198780103710443308956
- KashyapSBelfortRGastaldelliAA sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop Type 2 diabetesDiabetes2003522461247414514628
- GastaldelliAFerranniniEMiyazakiYMatsudaMDeFronzoRABeta-cell dysfunction and glucose intolerance: Results from the San Antonio Metabolism (SAM) studyDiabetologia200441313914666364
- Diabetes Prevention Research GroupReduction in the evidence of Type 2 diabetes with life-style intervention or metforminN Engl J Med200234639340311832527
- BuchananTAXiangAHPetersRKPreservation of pancreatic beta-cell function and prevention of Type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic womenDiabetes2002512796280312196473
- BuchananTAXiangAHKjosSLDiabetes rates and B-cell function in the pioglitazone and prevention of diabetes (PIPOD) studyDiabetes200554Suppl 1A39
- DeFronzoRABanerjiMBrayGAActos NOW for the prevention of diabetes (ACT NOW) studyBMC Endocr Disord20099172519640291
- WangQBrubakerPLGlucagon-like peptide-1 treatment delays the onset of diabetes in 8 week old db/db miceDiabetologia2002451263127312242459
- GedulinBRNikoulinaSESmithPAExenatide (exendin-4) improves insulin sensitivity and beta-cell mass in insulin resistant obese fa/fa Zucker rats independent of glycemia and body weightEndocrinology20051462069207615618356
- AhrenBPaciniGFoleyJESchweizerAImproved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with Type 2 diabetes over 1 yearDiabetes Care2005281936194016043735
- XuLManCDCharbonnelBEffect of sitagliptin, a dipeptidyl-peptidase 4 inhibitor, on beta-cell function in patients with Type 2 diabetes, a model-based approachDiabetes Obes Metab2008101212122018476982
- MullerWAFaloonaGRAguilar-ParadaEUngerRHAbnormal alpha-cell function in diabetes: Response to carbohydrate and protein ingestionN Engl J Med19702831091154912452
- UngerRHAguilar-ParadaEMullerWAEisentrautAMStudies of pancreatic alpha cell function in normal and diabetic subjectsJ Clin Invest1970498378484986215
- Aguilar-ParadaEEisentrautAMUngerRHPancreatic glucagon secretion in normal and diabetic subjectsAm J Med Sci19692574154194893149
- MatsudaMDeFronzoRAConsoliABresslerPDel PratoSDose-response curve relation plasma glucagon to hepatic glucose production and glucose disposal in Type 2 diabetes mellitusMetabolism2002511111111912200754
- SchwartzJGGreenGMGuanDMcMahanCAPhillipsWTRapid gastric emptying of a solid pancake meal in Type II diabetic patientsDiabetes Care1996194684718732711
- MiyazakiYMahankaliAMatsudaMEffect of pioglitazone on abdominal fat distribution and insulin sensitivity in Type 2 diabetic patientsJ Clin Endocrinol Metab2002872784279112050251
- MiyazakiYDeFronzoRARosiglitazone and pioglitazone similarly improve insulin sensitivity and secretion, glucose tolerance and adipocytokines in Type 2 diabetic patientsDiabetes Obes Metab2008101204121118476983
- MatthewsDRCharbonnelBHHanefeldMBrunettiPSchernthanerGLong-term therapy with addition of pioglitazone to metformin compared with the addition of gliclazide to metformin in patients with Type 2 diabetes: A randomized, comparative studyDiabetes Metab Res Rev20052116717415386821
- GastaldelliAFerranniniEMiyazakiYMatsudaMMariADeFronzoRAThiazolidinediones improve beta-cell function in Type 2 diabetic patientsAm J Physiol Endocrinol Metab2007292E871E88317106061
- HanefeldMMarxNPfutznerAAnti-inflammatory effects of pioglitazone and/or simvastatin in high cardiovascular-risk patients with elevated high sensitivity c-reactive protein: The PIOSTAT studyJ Am Coll Cardiol20074929029717239709
- FernandezMTriplittCWajcbergEAddition of pioglitazone and ramipril to intensive insulin therapy in Type 2 diabetes patients improves vascular dysfunction by different mechanismsDiabetes Care20083112112717909084
- WajcbergESriwijitkamolAMusiNDeFronzoRACersosimoERelationship between vascular reactivity and lipids in Mexican-Americans with Type 2 diabetes treated with pioglitazoneJ Clin Endocrinol Metab2007921256126217244785
- GoldbergRBKendallDMDeegMAA comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with Type 2 diabetes and dyslipidemiaDiabetes Care2005281547155415983299
- Lloyd-JonesDMBlochKDThe vascular biology of nitric oxide and its role in atherogenesisAnnu Rev Med1996473653758712788
- de CaterinaRLibbyPPengHBNitric oxide decreases cytokine-induced endothelial activation: Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokinesJ Clin Invest19959660687542286
- DefronzoRAInsulin resistance, lipotoxicity, Type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009Diabetologia2010531270128720361178
- BajajMSuraamornkulSHardiesLJPratipanawatrTDeFronzoRAPlasma resistin concentration, hepatic fat content, and hepatic and peripheral insulin resistance in pioglitazone-treated Type 2 diabetic patientsInt J Obes200428783789
- MiyazakiYMahankaliAMatsudaMImproved glycemic control and enhanced insulin sensitivity in liver and muscle in Type 2 diabetic subjects treated with pioglitazoneDiabetes Care20012471071911315836
- GastaldelliAMiyazakiYMahankaliAThe effect of pioglitazone on the liverDiabetes Care2006292275228117003306
- GastaldelliAFerranniniEMiyazakiYMatsudaMMariADeFronzoRAThiazolidinediones improve beta-cell function in Type 2 diabetes patientsAm J Physiol Endocrinol Metab2007292E871E88317106061
- ChiquetteERamirezGDeFronzoRA meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factorsArch Intern Med20041642097210415505122
- NissenSEWolskiKEffect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causesN Engl J Med20073562457247117517853
- SinghSLokeUKFurbergCDLong-term risk of cardiovascular events with rosiglitazoneJAMA20072981189119517848653
- MiyazakiYMatsudaMDefronzoRADose-response effect of pioglitazone on insulin sensitivity and insulin secretionin Type 2 diabetesDiabetes Care20022551752311874940
- KlonoffDCBuseJBNielsenLLExenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with Type 2 diabetes treated for at least 3 yearsCurr Med Res Opin20082427528618053320
- The Dream (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial InvestigatorsEffect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomized controlled trialLancet20063681096110516997664
- BunckMCDiamontMCornerAOne-year treatment with exenatide improves β-cell function, compared with insulin glargine, in metformin-treated Type 2 diabetic patients: A randomized, controlled trialDiabetes Care20093276276819196887
- DeFronzoRABanerjiMABrayGAfor the ACT NOW Study GroupDeterminants of glucose tolerance in impaired glucose tolerance at baseline in the Actos Now for Prevention of Diabetes (ACT NOW)Diabetologia20105343544520012012
- Abdul-GhaniMAJenkinsonCPRichardsonDKTripathyDDeFronzoRAInsulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: Results from the Veterans Administration Genetic Epidemiology StudyDiabetes2006551430143516644701
- NissenSENichollsSJWolskiKPERISCOPE InvestigatorsComparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with Type 2 diabetes: The PERISCOPE randomized controlled trialJAMA20082991561157318378631
- MazzoneTMeyerPMFeinsteinSBEffect of pioglitazone compared with glimepiride on carotid intima-media thickness in Type 2 diabetes: A randomized trialJAMA20062962572258117101640
- CharbonnelBSchernthanerGBrunettiPLong-term efficacy and tolerability of add-on pioglitazone therapy to failing monotherapy compared with addition of gliclazide or metformin in patients with Type 2 diabetesDiabetologia2005481093110415889234
- HanefeldMPfutznerAForstTLubbenGGlycemic control and treatment failure with pioglitazone versus glibenclamide in Type 2 diabetes mellitus: A 42-month, open-label, observational, primary care studyCurr Med Res Opin2006221211121516846554
- TanMHBaksiAGLAL Study GroupComparison of pioglitazone and gliclazide in sustaining glycemic control over 2 years in patients with Type 2 diabetesDiabetes Care20052854455015735185
- RosenstockJGoldsteinBJVinikARESULT Study GroupEffect of early addition of rosiglitazone to sulphonylurea therapy in older Type 2 diabetes patients (60 years): The Rosiglitazone Early vs SULphonylurea Titration (RESULT) studyDiab Obes Metab200684957
- HomePDJonesNPPocockSJRECORD Study GroupRosiglitazone RECORD study: Glucose control outcomes at 18 monthsDiabet Med20072462663417517066
- MiyazakiYDeFronzoRARosiglitazone and pioglitazone similarly improve insulin sensitivity and secretion, glucose tolerance and adipocytokines in Type 2 diabetic patientsDiabetes Obes Metab2008101204121118476983
- RosmarakisESFalagasMEEffect of thiazolidinedione therapy on restenosis after coronary stent implantation: A meta-analysis of randomized controlled trialsAm Heart J200715414415017584567
- LincoffAMWolskiKNichollsSJNissenSEPioglitazone and risk of cardiovascular events in patients with Type 2 diabetes mellitus: A meta-analysis of randomized trialsJAMA2007 122981180118817848652
- DormandyJACharbonnelBEcklandDJASecondary prevention of macrovascular events in patients with Type 2 diabetes in the proactive study (PROspective pioglitAzone Clincal Trial In macroVascular Events): A randomized controlled trialLancet20053661279128916214598
- MiyazakiYGlassLTriplittCAbdominal fat distribution and peripheral and hepatic insulin resistance in Type 2 diabetes mellitusAm J Physiol Endocrinol Metab200246E1135E1481E114312424102
- PetersenKFDufourSShulmanGIDecreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of Type 2 diabetic parentsPLoS Medicine20052879884
- NestoRWBellDBonowROThiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and the American Diabetes Association: Oct 7, 2003Circulation20031082941294814662691
- KaulSBolgerAHerringtonDGiuglianoREckelRThiazolidinedione drugs and cardiovascular risks: A science advisory from the American Heart Association and American College of Cardiology FoundationCirculation20101211868187720179252
- MasoudiFAInzucchiSEWangYHavranekEPFoodyJMKrumholzHMThiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure; an observational studyCirculation200511158359015699279
- PuschettJBPharmacological classification and renal actions of diureticsCardiology199484Suppl 24137954544
- KarallieddeJBuckinghamRStarkieMEffect of various diuretic treatments on rosiglitazone-induced fluid retentionJ Am Soc Nephrol2006173482349017093067
- ValentineWJBottomleyJMPalmerAJPROactive Study GroupPROactive 06: Cost-effectiveness of pioglitazone in Type 2 diabetes in the UKDiabet Med200724982100217593245
- NauckMStockmannFEbertRCreutzfeldtWReduced incretin effect in Type 2 (non-insulin-dependent) diabetesDiabetologia19862946523514343
- VilsbollTHolstJJIncretins, insulin secretion and Type 2 diabetes mellitusDiabetologia20044735736614968296
- MeierJJNauckMASchmidtWEGallwitzBGastric inhibitory polypeptide: The neglected incretin revisitedRegul Pept200210711312137960
- Toft-NielsenMBDamholtMBMadsbadSDeterminants of the impaired secretion of glucagon-like peptide-1 in Type 2 diabetic patientsJ Clin Endocrinol Metab2001863717372311502801
- DruckerDJGlucagon-like peptidesDiabetes1998471591699519708
- JonesIROwensDRLuzioSWilliamsSHayesTMThe glucose dependent insulinotropic polypeptide response to oral glucose and mixed meals is increased in patients with Type 2 (non-insulin-dependent) diabetes mellitusDiabetologia1989326686772676668
- NauckMAHeimesaatMMOrskovCPreserved incretin activity of glucagon-like peptide-1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with Type-2 diabetes mellitusJ Clin Invest1993913013078423228
- DruckerDJNauckMAThe incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in Type 2 diabetesLancet20063681696170517098089
- DeaconCFIncretin-based treatment of Type 2 diabetes: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitorsDiabetes Obes Metab20079Suppl 1233117877544
- FerranniniEBjorkmanOReichardGAPiloAOlsonMThe disposal of an oral glucose load in healthy subjects. A quantitative studyDiabetes1985345805883891471
- AhrenBGut peptides and Type 2 diabetes mellitus treatmentCurr Diab Rep2003336537212975025
- AhrenBInsulinotropic action of truncated glucagon-like peptide-1 in miceActa Physiol Scand19951532052067778462
- DruckerDJPhilippeJMojsovSChickWLHabenerJFGlucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell lineProc Natl Acad Sci U S A19878434343033647
- KashimaYMikiTShibasakiTCritical role of cAMP-GEFII – Rim2 complex in incretin-potentiated insulin secretionJ Biol Chem2001276460464605311598134
- FehmannHCGokeRGokeBGlucagon-like peptide-1(7–37)/(7–36) amide is a new incretinMol Cell Endocrinol199285C39C441382025
- WangYEganJMRaygadaMNadivORothJMontrose-RafizadehCGlucagon-like peptide-1 affects gene transcription and messenger ribonucleic acid stability of components of the insulin secretory system in RIN 1046-38 cellsEndocrinology1995136491049177588224
- FehmannHCHabenerJFInsulinotropic hormone glucagon-like peptide-I(7–37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cellsEndocrinology19921301591661309325
- FehmannHCHabenerJFGalanin inhibits proinsulin gene expression stimulated by the insulinotropic hormone glucagon-like peptide-I(7–37) in mouse insulinoma beta TC-1 cellsEndocrinology1992130289028961374016
- DruckerDJGlucagon-like peptides: Regulators of cell proliferation, differentiation, and apoptosisMol Endocrinol20031716117112554744
- MullerWAFaloonaGRAguilar-ParadaEUngerRHAbnormal alpha-cell function in diabetes: Response to carbohydrate and protein ingestionN Engl J Med19702831091154912452
- UngerRHAguilar-ParadaEMullerWAEisentrautAMStudies of pancreatic alpha cell function in normal and diabetic subjectsJ Clin Invest1970498378484986215
- Aguilar-ParadaEEisentrautAMUngerRHPancreatic glucagon secretion in normal and diabetic subjectsAm J Med Sci19692574154194893149
- LarssonHBerglundGAhrenBGlucose modulation of insulin and glucagon secretion is altered in impaired glucose toleranceJ Clin Endocrinol Metab199580177817827775622
- AhrenBLarssonHImpaired glucose tolerance (IGT) is associated with reduced insulin-induced suppression of glucagon concentrationsDiabetologia2001441998200311719830
- LarssonHAhrenBGlucose intolerance is predicted by low insulin secretion and high glucagon secretion: Outcome of a prospective study in postmenopausal Caucasian womenDiabetologia20004319420210753041
- UngerRHGlucagon physiology and pathophysiologyN Engl J Med19712854434494997492
- HellerRSKiefferTJHabenerJFInsulinotropic glucagon-like peptide I receptor expression in glucagon-producing alpha-cells of the rat endocrine pancreasDiabetes1997467857919133545
- KoltermanOGBuseJBFinemanMSSynthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with Type 2 diabetesJ Clin Endocrinol Metab2003883082308912843147
- HorowitzMDentJFraserRSunWHebbardGRole and integration of mechanisms controlling gastric emptyingDig Dis Sci199439Suppl 127S13S7995220
- PhillipsWTSchwartzJGMcMahanCARapid gastric emptying of an oral glucose solution in Type 2 diabetic patientsJ Nucl Med199233149615001634941
- HorowitzMFraserRDisordered gastric motor function in diabetes mellitusDiabetologia1994375435517926337
- SchwartzJGGreenGMGuanDMcMahanCAPhillipsWTRapid gastric emptying of a solid pancake meal in Type II diabetic patientsDiabetes Care1996194684718732711
- HorowitzMEdelbroekMAWishartJMStraathofJWRelationship between oral glucose tolerance and gastric emptying in normal healthy subjectsDiabetologia1993368578628405758
- PhillipsWTSchwartzJGMcMahanCARapid gastric emptying of an oral glucose solution in Type 2 diabetic patientsJ Nucl Med19923314965001634941
- KoltermanOGKimDDShenLPharmacokinetics, pharmacodynamics, and safety of exenatide in patients with Type 2 diabetes mellitusAm J Health Syst Pharm20056217318115700891
- ImeryuzNYegenBCBozkurtACoskunTVillanueva-PenacarrilloMLUlusoyNBGlucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanismsAm J Physiol1997273G920G9279357836
- OrskovCPoulsenSSMollerMHolstJJGlucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide IDiabetes1996458328358635662
- Tang-ChristensenMLarsenPJGokeRCentral administration of GLP-1-(7–36) amide inhibits food and water intake in ratsAm J Physiol1996271R848R8568897973
- TurtonMDO’SheaDGunnIA role for glucagon-like peptide-1 in the central regulation of feedingNature199637969728538742
- AhrenBGlucagon-like peptide-1 (GLP-1): A gut hormone of potential interest in the treatment of diabetesBioessays1998206426519780839
- VerdichCFlintAGutzwillerJPA meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humansJ Clin Endocrinol Metab2001864382438911549680
- DeaconCFNauckMAToft-NielsenMPridalLWillmsBHolstJJBoth subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in Type II diabetic patients and in healthy subjectsDiabetes199544112611317657039
- FengJZhangZWallaceMBDiscovery of alogliptin: A potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IVJ Med Chem2007502297230017441705
- AsakawaTMoritohYKataokaOSuzukiNTakeuchiKOdakaHA novel dipeptidyl peptidase-4 inhibitor, alogliptin (SYR-322), is effective in diabetic rats with sulfonylurea-induced secondary failureLife Sci20098512212619427871
- MoritohYTakeuchiKAsakawaTKataokaOdakaHChronic administration of alogliptin, a novel, potent, and highly selective dipeptidyl peptidase-4 inhibitor, improves glycemic control and beta-cell function in obese diabetic ob/ob miceEur J Pharmacol200858832533218499100
- ChristopherRCovingtonPDavenportMPharmacokinetics, pharmacodynamics, and tolerability of single increasing doses of the dipeptidyl peptidase-4 inhibitor alogliptin in health male subjectsClin Ther20083051352718405789
- Onglyza® [package insert]Princeton, NJBristol-Myers Squibb2009
- Januvia® [package insert]Whitehouse Station, NJMerck and Company, Inc.2010
- CovingtonPChristopherRDavenportMPharmacokinetic, pharmacodynamic, and tolerability prof iles of the dipeptidyl peptidase-4 inhibitor alogliptin: A randomized, double-blind placebo-controlled multiple-dose study in adult patients with Type 2 diabetesClin Ther20083049951218405788
- DeFronzoRAFleckPRWilsonCAMekkiQEfficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with Type 2 diabetes and inadequate glycemic controlDiabetes Care2008312315231718809631
- PratleyREKipnesMSFleckPRWilsonCMekkiQEfficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with Type 2 diabetes inadequately controlled by glyburide monotherapyDiabetes Obes Metab20091116717619125778
- NauckMAEllisGCFleckPRWilsonCAMekkiQEfficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with Type 2 diabetes inadequately controlled with metformin monotherapy: A multicenter, randomized, double-blind, placebo-controlled studyInt J Clin Pract200963465519125992
- RosenstockJRendellMSGrossJLFleckPRWilsonCAMekkiQAloglitpin added to insulin therapy in patients with Type 2 diabetes reduces HbA1c without causing weight gain or increased hypoglycaemiaDiabetes Obes Metab2009111145115219758359
- KarimACovingtonPChristopherRPharmacokinetics of alogliptin when administered with food, metformin, or cimetidine: A two-phase, crossover study in health subjectsInt J Clin Pharmacol Ther201048465820040339
- KarimALaurentAMunsakaMWannEFleckPMekkiQCoadministration of pioglitazone or glyburide and alogliptin: Pharmacokinetic drug interaction assessment in healthy participantsJ Clin Pharmacol2009491210121919622714
- PratleyRReuschJFleckPWilsonCMekkiQEfficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with Type 2 diabetes: A randomized, double-blind, placebo-controlled studyCurr Med Res Opin2009252361237119650752
- DeFronzoRABurantCFFleckPWilsonCMekkiQPratleyREEffect of alogliptin combined with pioglitazone in glycemic control in metformin-treated patients with Type 2 diabetesDiabetes200958Suppl 1A519 Abstr 2023.
- DefronzoRABurantCFFleckPWilsonCMekkiQPratleyREEffect of alogliptin combined with pioglitazone on glycemic control in metformin-treated patients with Type 2 diabetesDiabetes200958Suppl 1A519A520 Abstr 2024.
- RosenstockJInzucchiSSeufertJFleckPWilsonCMekkiQEfficacy and safety of alogliptin combined with pioglitazone in patients with Type 2 diabetes inadequately controlled with diet and exerciseDiabetes200958Suppl 1A523 Abstr 2036.
- InzucchiSERosenstockJSeufertJFleckPWilsonCMekkiQEffect of combination therapy with alogliptin and pioglitazone in drug-naïve patients with Type 2 diabetes on b-cell function and insulin resistanceDiabetes200958Suppl 1A520 Abstr 2026.