Abstract
Pulmonary embolism (PE) represents a common disease in emergency medicine and guidelines for diagnosis and treatment have had wide diffusion. However, PE morbidity and mortality remain high, especially when associated to hemodynamic instability or right ventricular dysfunction. Prognostic stratification to identify high risk patients needing to receive more aggressive pharmacological and closer monitoring is of utmost importance. Modern guidelines for management of acute PE are based on risk stratification using either clinical, radiological, or laboratory findings. This article reviews the modern treatment of acute PE, which is customized upon patient prognosis. Accordingly the current risk stratification tools described in the literature such as clinical scores, echocardiography, helical computer tomography, and biomarkers will be reviewed.
Introduction
Pulmonary embolism (PE) remains one of the leading causes of morbidity and mortality in the emergency and cardiovascular setting, especially when associated to hemodynamic instability. In the United States, about 150.000 patients per year are diagnosed with acute PE, resulting in thousands of recognized deaths annually from massive PE. Mortality for PE is 2% in normotensive patients without evidence of right ventricular dysfunction (RVD), but rises up to 30% in patients with shock and up to 65% in patients with cardiac arrest at presentation.Citation1
Guidelines on diagnosis have had wide diffusion in the last years with strategies based on pre-test clinical probability, D-Dimers levels, ultrasonography of the legs, lung scan, and more recently computer tomography pulmonary angiography (CTPA).Citation2–Citation9 Concomitantly modern concepts about mortality risk evaluation, prognostic stratification and consequent treatment have also emerged. Therefore the aim of the present literature review is to summarize the concept of PE risk stratification focusing on emerging stratification tools and discuss its consequences in clinical practice.
Pathophysiology, clinical classification, and modern concepts of treatment of acute PE
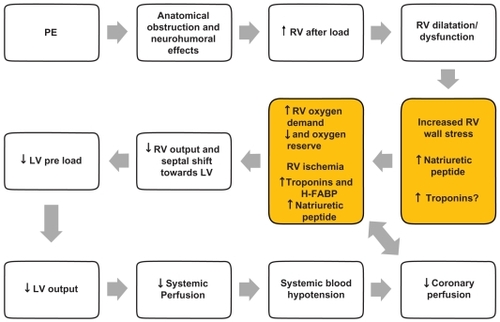
The pathophysiological response to acute PE is of utmost importance to understand its hemodynamic consequences, which in turn will affect patient prognosis. Patient prognosis depends on the extent to which pulmonary artery blood flow is obstructed, pre-existing cardiopulmonary disease, and the release of vasoactive humoral factors from clots.Citation1,Citation6 The mechanical obstruction formed by the clot, together with the pulmonary artery vasoconstriction stimulated by neurohumoral substances (such as serotonin from platelets, thrombin from plasma, and histamine from tissue) and hypoxemia, could cause increased pulmonary vascular resistance and right side cardiac afterload, which in turn can result in cardiac dilatation, hypokinesis, and myocardial ischemia. In some patients, a rapid progression in systemic arterial hypotension and cardiogenic shock may occur. Cardiac arrest and death could be the fatal evolution.Citation1,Citation6,Citation10 This cascade could explain some important consequences in biomarkers increase; myocardial damage represented by micro-infarcts leads to increased levels of cardiac troponins (cTn) and heart type fatty acid-binding proteins (H-FABP), whereas wall stress caused by higher right heart after-load leads to increased levels of natriuretic peptides (NP). summarizes the hemodynamic consequences of PE.
Figure 1 Pathophysiology of hemodynamic instability due to PE and mechanism of biomarkers increase.
Classically PE has been subdivided in massive, hemodynamically unstable (hypotension is defined as arterial blood pressure less than 90 mmHg, shock, or cardiac arrest), submassive (normotensive PE with evidence of RVD) or nonmassive (normotensive PE without RVD), which are both hemodynamically stable.Citation2–Citation5 About 5% of acute PE are represented by massive PE. About 50% of normotensive patients have transthoracic echocardiographic (TTE) pattern of RVD, and around 10% will die.Citation11
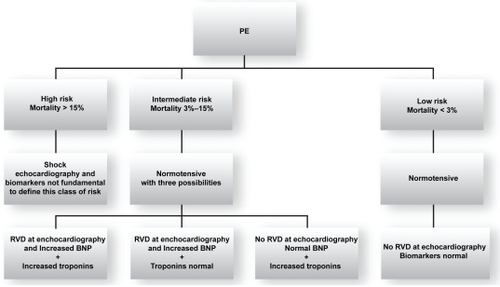
The European Society of Cardiology (ESC) recommends classifying PE according to classes of risk for adverse prognosis.Citation12 Therefore PE is divided into high risk (corresponding to massive PE with short term mortality >15%) and nonhigh risk. Nonhigh risk patients are normotensive. The presence of increased NP and/or troponins is currently not mandatory for defining the high risk class. As many normotensive patients often present with RVD and potential adverse outcomes, nonhigh risk PE has been further divided into intermediate risk (corresponding to submassive PE: normotensive plus signs of RVD and/or signs of myocardial damage, short-term mortality being 3%–15%) and in low risk (corresponding to nonmassive PE: normotensive without signs of RVD and myocardial damage, short-term mortality < 3%).Citation12 shows ESC criteria for risk assessment.
Figure 2 ESC criteria for identifing the risk of adverse prognosis in acute PE
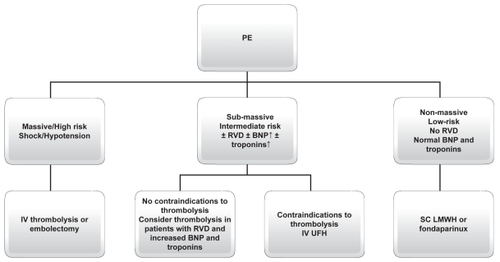
Acute treatment of PE is customized by mortality risk based upon prognostic stratification. In their new guidelines, the ESC and American College of Chest Physicians (ACCP) suggest treatment of PE according to clinical risk.Citation12,Citation13 In massive-highrisk PE, thrombolysis with alteplase (rtPA), streptokinase, or urokinase is the recommended therapy. Embolectomy could represent an alternative therapy for patients with shock in the acute setting when thrombolysis is contraindicated or when it has been unsuccessful. In submassive-intermediate risk PE, thrombolysis has been proposed in selected patients at high risk for adverse prognosis without contraindications (grade IIB of ESC and ACCP VIII Edition),Citation13 where as intravenous unfractioned heparin (UFH) should be reserved to conditions in which thrombolysis is contraindicated (grade IA ESC and ACCP VIII Edition). In nonmassive-low risk PE, subcutaneous low-molecular weight heparins (LMWH) or fondaparinux are recommended (grade IA ESC and ACCP VIII Edition). As this subgroup of patients represent the majority of PE patients and that ambulatory treatment has been reported to be safe, early identification of such patients at admission could potentially lead to a substantial decrease in hospitalization rates and PE-related costs.Citation14,Citation15 Vitamin Kantagonists (VKA) should be started in the first day and should be overlapped with UFH and LMWH or fondaparinux for at least five days (grade I A ESC and ACCP VIII Edition).Citation12,Citation13
summarizes the choice treatment in different class of risk for patients with acute PE. Thus, PE risk stratification will become fundamental not only to select appropriate treatment strategy, but also to potentially reduce the costs of PE management. For both purposes, several risk stratification algorithms have been reported in the literature, including clinical, radiological, and laboratory parameters.
Risk evaluation and prognostic stratification
Clinical parameters and clinical scores
Shock or systemic blood hypotension at presentation represent the most important clinical sign of poor prognosis in patients with acute PE.Citation1,Citation11 In the ICOPER Study, the mortality rate was 58.3% in patients who were hemodynamically unstable at the time of presentation and 15.1% for those who were hemodynamically stable.Citation11 Other clinical variables, easily available at admission, associated to poor prognosis are represented by age over 70 years, history of bed rest over five days, cancer, chronic obstructive pulmonary disease, renal failure, heart failure, cardiovascular diseases, and tachycardia.Citation10
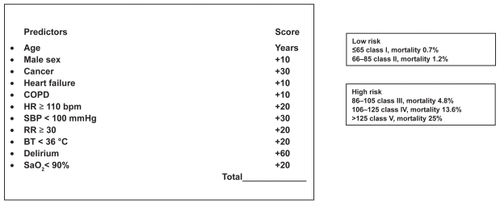
The Pulmonary Embolism Prognostic Index (PESI), and Geneva Prediction Rule represent two clinical scores identifying classes of patients with increased risk of adverse outcomes.Citation16,Citation17 These scores reliably identify low-risk patients with PE (patients classified as PESI classes I and II) who could be candidates for less costly ambulatory treatment. The major strength of these score is their easy use in all clinical setting; the disadvantage is that they have not been compared to more recent prognostic factors (such as biomarkers and imaging findings). In fact, PESI seems to be more accurate for predicting low risk patients than the Geneva Prediction Rule.Citation18 The PESI score tool is displayed in .
Figure 4 Pulmonary embolism severity index (P.E.S.I). Modified from Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med. 2007;261:597–604.
The shock index, heart rate (beats for minute)/systolic blood pressure (mmHg) ratio, is a simple method to predict high risk patients for adverse outcome, when its value is over 1. This ratio has been shown to be related to in-hospital mortality and it is sensitive to predict poor prognosis alone or in combination with trans-thoracic echocardiogram (TTE).Citation19,Citation20 12-lead ECG findings of poor prognosis are represented by presence and number of T waves inversion in precordial leads and QR in V1,Citation21–Citation23 but overall, ECG does not seem to be a reliable marker of severity of PE.
Echocardiographic and radiological parameters
TTE represents the most useful tool in everyday clinical practice to show RVD because of its noninvasive nature and relative low cost. Hence, RVD assessed on TTE has been described as one of the strongest predictor of early mortality in nonmassive PE.Citation24–Citation26
The main TTE findings detectable in PE are represented by right ventricle hypokinesis (mild, moderate, severe), right ventricle dilatation (especially represented by four chambers end-diastolic RV/left ventricular [LV] ratio > 1), and signs of pulmonary hypertension. The presence of RVD is related to poor prognosis in PE with hemodynamic instability.Citation11,Citation25 Furthermore, TTE detects RVD in about 30%–40% of normotensive patients (systolic blood pressure > 90 mmHg) at presentation.Citation24,Citation25 Thirty-day mortality of normotensive patients with RVD is two-fold compared to normotensive patients without RVD.Citation27 RVD at presentation seems also to predict poor pulmonary clot resolution six months after the initial event and a higher incidence of VTE recurrence.Citation28 Fremont and colleagues reported data from a monocentric study enrolling more than 1400 patients. The authors found that an TTE RV/LV diameter ratio > 0.9 was an independent risk factor for hospital mortality in normotensive patients with PE.Citation29 A recent review has shown that in-hospital mortality of normotensive patients without RVD was 0%–9.6% compared to 11.8%–23% for patients with RVD.Citation30 However other studies which evaluated the prognostic value of TTE in normotensive PE patients were less convincing.Citation31,Citation32 The limit of TTE examination is that the test is operator-dependent and not necessarily available around the clock in all institutions. Moreover TTE criteria of RVD are not definitely established.
Currently, CTPA represents the diagnostic gold standard for PE, and is widely integrated in validated diagnostic strategies.Citation6–Citation8,Citation33 Recently several studies focused on the correlation between findings of CTPA, presence of RVD, and prognosis of PE.Citation34–Citation42 The Computer Tomography Pulmonary Embolism (CTPE) index combines distribution and severity of vascular obstruction of clots in pulmonary circulation; PE severity seems to be linearly related to CTPE index values.Citation40 Ghanima and colleagues have proposed to divide the pulmonary vascular tree in four groups of arteries: sub-segmental, segmental, lobar, and main pulmonary artery with its (left and right) branches (respectively named 1, 2, 3, 4).Citation41 These authors showed that the pulmonary artery obstruction index was correlated to troponin T levels, CTPA RV/LV diameter ratio and partial pressure of oxygen in arterial blood (PaO2).Citation41 It seems that the Ghanima index could be related also to TTE RVD.Citation43 A RV/LV diameter ratio is also easily determined by CTPA and the thirty-day mortality rate is 15.6% in patients with CTPA RV/LV > 0.9 compared to 7.7% in patients without RV enlargement.Citation42 However these results have not been confirmed in the PIOPED II trial.Citation44 Finally, CTPA seems to be a promising method for PE prognosis stratification, but the sample size of these previous studies precludes any firm conclusion. Thus, further studies are warranted to assess CTPA as a prognostic tool, because it could be very useful for clinicians to have at the same time a validated diagnostic tool, with an additional prognostic value.
Pulmonary real-time magnetic resonance (rtMR) and magnetic resonance angiography (MRA) represent a safe alternative and/or complementary examination compared with CTPA both as diagnostic imaging and for detecting RV enlargement or dysfunction.Citation45–Citation49
Laboratory parameters
Arterial blood gas analysis (BGA) remains a first-line examination in patients with suspected PE for evaluation of gas exchange and acid-base status. The role of BGA as prognosticator has been studied with discordant results between younger and elderly patients. Much recently hypoxemia was found as an independent negative predictor of three-month all-cause mortality in PE patients (hazard ratio [HR] 5.7, 95% confidence interval [CI]: 2.1–15.1).Citation50 PaO2 values have been demonstrated to be linearly associated to CTPA parameters such as proximal extension of pulmonary clots and RV/LV diameter.Citation41 Parameters derived from BGA, such as alveolar-arterial oxygen gradient (values > 50 mmHg) and arterial–alveolar oxygen tension ratio (values < 0.50) have been demonstrated to be associated to poor prognosis in nonelderly patients with PE.Citation51,Citation52 Moreover, alveolar dead space measured from volumetric capnography and BGA seems to correlate with embolic burden of PE.Citation53 In elderly PE patients, only lower arterial oxygen saturation seems to predict short-term mortality; neither PaO2 nor alveolar–arterial oxygen gradient seem to identify high risk patients.Citation54
D-Dimer values seems to be linearly related to the extend of the clot and the severity of PE.Citation43,Citation55–Citation57 One study demonstrated that patients who had D-Dimer levels below 1500 μg/L have a very low mortality.Citation56
As detailed below, cardiac biomarkers, such as cardiac troponins, (cTnI and cTnT), NP, H-FABP, myoglobin, and growth differentiation factor-15 have been extensively evaluated as risk stratification tools in PE.
Troponins are released in the bloodstream in presence of myocardial damage secondary to microinfarction.Citation58 The increase of troponins is correlated with TTE RVD and CTPA findings and its elevation has a strong negative prognostic value.Citation59 Many studies have demonstrated the negative prognostic values of increased troponins in PE.Citation60–Citation64 Becattini and colleagues have published a meta-analysis on the relation between troponins and mortality and morbidity in acute PE.Citation65 They confirmed that the increase of troponins I and/or T was associated with a higher mortality (17.9% in patients with elevated troponin levels and 2.3%in patients with normal troponin levels), even in the subgroup of hemodynamically stable patients (odds ratio, 4.12).Citation65,Citation66 Jimenez and colleagues also confirmed in a large prospective study that elevated cTnI predicted fatal PE in hemodynamically stable patients; the negative predictive value of a negative cTnI for mortality was 93%.Citation67
NP secretion is due to RV wall strech and therefore due to RVD.Citation68 The increase of the B (B type) NP biomarker (BNP and its amino terminal portion, NT-proBNP) is highly sensitive but poorly specific for detecting RVD and patients at risk of severe adverse events such as cardiac arrest, shock, need for thrombolysis or vasopressors or mechanical ventilation, or need for intensive care units.Citation69–Citation83 Two studies suggested that NT-proBNP correlated better with prognosis when compared to troponins,Citation84,Citation85 especially in combination with TTE.Citation84 Several recent reviews and meta-analyses strongly confirmed the prognostic usefulness of NP.Citation86–Citation89
H-FABP, a small cytosolic protein released earlier than troponins into circulation when the myocardium is injured, has been evaluated as a prognostic tool and small studies suggested that this cardiac biomarker could also identify the patients with poorer outcomes when compared to cTns and BNP/NT-proBNP.Citation90,Citation91 The negative prognostic role of myoglobin and growth-differentiation factor-15 (gdf-15), a cytokine induced in the heart after ischemia or pressure overload, in acute PE have also been reported.Citation92,Citation93 However, H-FABP and gdf-15 measurements are not yet widely available.
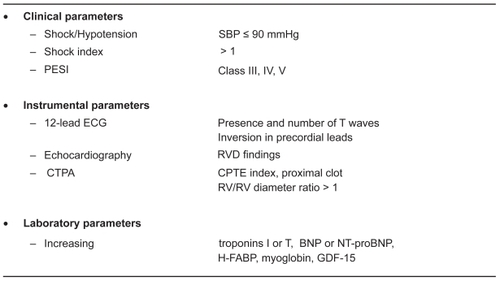
summarizes the results of studies which evaluated various clinical, instrumental, and laboratory indexes for predicting poor outcomes in acute PE. This figure suggests that cardiac biomarkers have all been shown to correlate with RV dysfunction/dilatation and prognosis in PE.
Figure 5 Clinical, instrumental and laboratory parameters associated with adverse outcomes in patients with acute Pe.
However, because most of those studies did not perform an extensive comparison between all the available biomarkers, knowing which one will yield the best prognostic value still remains debated. Among other limitations worth to be mentioned are that different biomarkers thresholds were used (and often determined retrospectively), and that various outcome definitions were used in the aforementioned studies. In order to compare their respective prognostic value, it will be necessary to use uniform pre-defined cut-offs. One possibility would be to use the cut-offs validated in their respective initial context. To this respect, a small systematic prospective comparative study using the pre-defined and validated thresholds (either in acute coronary syndrome or heart failure) for NT-proBNP, BNP, H-FABP, and myoglobin showed that only NPs (BNP or NT-proBNP) were significantly correlated with RV dilatation on CTPA.Citation94 Those results are corroborated by a recent multicentre study showing that NT-proBNP appeared as the most effective biomarker for rule-out purposes in non-massive PE.Citation95 Using the commonly defined cut-off of 300 pg/ml validated for heart failure,Citation96 this test had a negative predictive value of 100% (95% CI: 91–100).Citation95
Defining which of those biomarkers should be used as rule-out or rule-in test needs further clarification, but based upon the aforementioned observations, it appears that BNP/NT-proBNP and cTnI could potentially be used as rule-out tests. Finally, determining whether a biomarker alone or in combination with other clinical or radiological or ECG features would add incremental prognostic value deserves further study. Overall, NP (BNP or NT-proBNP) and cTns, with their 24-hour availability in most of emergency laboratories, could represent very convenient prognostic tools for PE risk stratification in an acute setting, especially in the institutions where echocardiography is not widely available.
Concerning the identification of patients, two major points are under investigation. First, it becomes of utmost importance to select patients who may be safely treated on an outpatient basis, and both clinical scoresCitation97 or probably biomarkers like NT-pro-BNPCitation95 may fulfil this request. This hypothesis is being currently tested in a prospective study (the OTPE trial).Citation98 Second, there is a need to better define which patient may benefit from fibrinolysis, and at least one randomized study is comparing anticoagulation against fibrinolysis in patients with no hemodynamic failure and RVD on TTE.Citation99 These currently ongoing studies should allow improvements in the care of PE patients in the near future.
Conclusions
Risk evaluation and prognostic stratification are the cornerstones of modern acute PE management. The use of either clinical, ECG, or biochemical parameters will probably be crucial to appropriately select stable patients for fibrinolyis, which currently represents one of the utmost therapeutic challenges of PE. For the time being, the remaining questions are: 1) which treatment should be reserved to submassive PE patients? 2) What is the best modalityto identify such patients: TTE, CTPA, biomarkers, or clinical scores? 3) Could a combination of such stratification tools add incremental value to one modality alone?
Intend-to-treat and noninferiority trials are now requested to resolve those matters. In this respect, the results of ongoing randomized multicenter trials, such as the European Pulmonary Embolism Thrombolysis (PEITHO) trial,Citation99 are eagerly awaited.
Disclosure
The authors report no conflicts of interest in this work.
References
- WhiteRHThe epidemiology of venous thromboembolismCirculation2003107Suppl 1I4812814979
- British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development GroupBritish Thoracic Society guidelines for the management of suspected acute pulmonary embolismThorax20035847048412775856
- ESC Task ForceGuidelines on diagnosis and management of acute pulmonary embolismEur Heart J2000211301133610952823
- TapsonVFCarrollBADavidsonBLThe diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic SocietyAm J Respir Crit Care Med19991601043106610471639
- American College of Emergency Physicians Clinical Policies Committee; Clinical Policies Committee Subcommittee on Suspected Pulmonary EmbolismClinical policy: critical issues in the evaluation and management of adult patients presenting with suspected pulmonary embolismAnn Emerg Med20034125727012548278
- GoldhaberSZElliottCGAcute pulmonary embolism: part I. Epidemiology, pathophysiology, and diagnosisCirculation20031082726272914656907
- SteinPDWoodardPKWegJGDiagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigatorsAm J Med20061191048105517145249
- Writing Group for Christopher Study InvestigatorsEffectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computer tomographyJAMA200629517217916403929
- Le GalGRighiniMRoyPMPrediction of pulmonary embolism in the emergency department: the revised Geneva scoreAnn Intern Med200614416517116461960
- BecattiniCAgnelliGAcute pulmonary embolism: risk stratification in the emergency departmentIntern Emerg Med2007211912917619833
- GoldhaberSZVisaniLDe RosaMAcute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER)Lancet19993531386138910227218
- TorbickiAPerrierAKonstantinidesSGuidelines on the diagnosis and management of acute pulmonary embolism of the European Society of CardiologyEur Heart J2008292276231518757870
- KearonCKahnSRAgnelliGGoldhaberSZRaskobGEComerotaAJAntithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition)Chest2008133454S545S18574272
- KovacsMJAndersonDMorrowBOutpatient treatment of pulmonary embolism with dalteparinThromb Haemost20008320921110739374
- BeerJHBurgerMGretenerSBernard BagattiniSBounameauxHOutpatients treatment of pulmonary embolism is feasible and safe in a substantial proportion of patientsJ Thromb Haemost2003118618712871557
- AujeskyDPerrierARoyPMValidation of a clinical prognostic model to identify low-risk patients with pulmonary embolismJ Intern Med200726159760417547715
- WickiJPerrierAPernegerTVPredicting adverse outcome in patients with acute pulmonary embolism: a risk scoreThromb Haemost20008454855211057848
- JimenezDYusenRGOteroRPrognostic models for selecting patients with acute pulmonary embolism for initial outpatient therapyChest2007132243017625081
- ToosiMSMerlinoJDLeeperKVPrognostic value oft he shock index along with thransthoracic echocardiography in risk stratification of patients with pulmonary embolismAm J Cardiol200810170070518308025
- OteroRTrujillo-SantosJCayuelaARegistro Informatizado de la Enfermedad Tromboembolica (RIETE) Investigators. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock indexEur Respir J2007301111111617804446
- JimenezDECG for risk stratification in patients with pulmonary embolismEur Respir J20052636636716055892
- ToosiMSMerlinoJDLeeperKVElectrocardiographic score and short term outcomes of acute pulmonary embolismAm J Cardiol20071001172117617884383
- KucherNWalpothNWustmannKNoveanuMGertschMQR in V1– an ECG sign associated with right ventricular strain and adverse clinical outcome in pulmonary embolismEur Heart J2003241113111912804925
- GoldhaberSZEchocardiography in the management of pulmonary embolismAnn Intern Med200213669170011992305
- KreitJWThe impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolismChest20041251539154515078772
- Vieillard-BaronAPageBAugardeRAcute cor pulmonale in massive pulmonary embolism: incidence, echocardiographic pattern, clinical implications and recovery rateIntensive Care Med2001271481148611685341
- KucherNRossiEDe RosaMGoldhaberSZPrognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mmHg or higherArch Intern Med20051651777178116087827
- KaczynskaAKostrubiecMPachoRKunikowskaJPruszczykPElevated D-Dimer concentration identifies patients withincomplete recanalization of pulmonary artery thromboemboli despite 6 months after the first episode of acute pulmonary embolismThromb Res2008122212517931694
- FrémontBPacouretGJacobiDPuglisiRCharbonnierBdeLabriolleAPrognostic value of echocardiographic right/left ventricular end-diastolic diameter ratio in patients with acute pulmonary embolism: results from a monocenter registry of 1,416 patientsChest200813335836217951624
- GibsonNSohneMBullerHPrognostic value of echocardiography and spiral computer tomography in patients with pulmonary embolismCurr Opin Pulm Med20061138038416093809
- LualdiJCGoldhaberSZRight ventricular dysfunction after acute pulmonary embolism: pathophysiologic factors, detection, and therapeutic implicationsAm Heart J199530127612827484782
- ten WoldeMSöhneMQuakEMac GillavryMRBüllerHRPrognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolismArch Intern Med20041641685168915302640
- GhayeBGhuysenABruyerePJD’OrioVDondelingerRFCan CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to knowRadiographics200626234016418240
- AraozPAGotwayMBHarringtonJRHarmsenWSMandrekarJNPulmonary embolism: prognostic CT findingsRadiology200724288989717325073
- SchoepfUJCastelloPCT angiography for diagnosis of pulmonary embolism: state of the artRadiology200423032933714752178
- van der MeerRWPattynamaPMvan StrijenMJRight ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolismRadiology200523579880315845793
- GhuysenAGhayeBWillemsVComputed tomographic pulmonary angiography and prognostic significance in patients with acute pulmonary embolismThorax20056095696116131526
- MansencalNJosephTVieillard-BaronADiagnosis of right ventricular dysfunction in acute pulmonary embolism using helical computed tomographyAm J Cardiol2005951260126315878009
- SchoepfUJKucherNKipfmuellerFQuirozRCostelloPGoldhaberSZRight ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolismCirculation20041103276328015533868
- QanadliSDEl HajjamMViellard-BaronANew CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiographyAJR Am J Roentgenol20011761415142011373204
- GhanimaWAbdelnoorMHolmenLONielssenBESandsetPMThe association between the proximal extension of the clot and the severity of pulmonary embolism (PE): a proposal for a new radiological score for PEJ Intern Med2007261748117222170
- WuASPezzulloJACronanJJHouDDMayo-SmithWWCT pulmonary angiography: quantification of pulmonary embolus as a predictor of patient outcome-initial experienceRadiology200423083183514739314
- MasottiLAntonelliFVenturiniELandiniGCCardiac troponin I and plasma D-dimer are related to proximal and bilateral extension of clots and right cardiac dysfunction in patients with pulmonary embolismJ Intern Med200726258858917949367
- SteinPDBeemathAMattaFEnlarged right ventricle without shock in acute pulmonary embolism: prognosisAm J Med2008121344218187071
- SteinPDWoodardPKHullRDGdolinium-enhanced magnetic resonance angiography for detection of acute pulmonary embolismChest20031242324232814665516
- HaagePPirothWKrombachGPulmonary embolism. Comparison of angiography with spiral computer tomography, magnetic resonance angiography, and real-time magnetic resonance imagingAm J Respir Crit Care Med200316772973412446272
- PleszewskiBChartrand-LefebvreCQanadliSDGadolinium-enhanced pulmonary magnetic resonance angiography in the diagnosis of acute pulmonary embolism: a prospective study on 48 patientsClin Imaging20063016617216632150
- BlumABellouAGuilleminFPerformance of magnetic resonance angiography in suspected acute pulmonary embolismThromb Haemost20059350351115735802
- KlugeAMullerCHanselJGerrietsTBachmannGReal-time MR with TrueFISP for the detection of acute pulmonary embolism: initial clinical experienceEur Radiol20041470971814658001
- BovaCPesaventoRMarchioriARisk stratification and outcomes in hemodinamically stable patients with acute pulmonary embolism. A prospective, multicentre, cohort study with three months of follow-upJ Thromb Hemost2009Mar 19 [Epub ahead of print]
- HsuJTChuCMChangSTPrognostic value of arterial/alveolar oxygen tension ratio (a/APO2) in acute pulmonary embolismCirc J2007711560156617895552
- HsuJTChuCMChangSTPrognostic role of alveolar-arterial oxygen pressure difference in acute pulmonary embolismCirc J2006701611161617127809
- KlineJAKubinAKPatelMMEastonEJSeupalRAAlveolar dead space as a predictor of severity of pulmonary embolismAcad Emerg Med2000761161710905639
- MasottiLOCeccarelliECappelliRBarabesiLForconiSArterial blood gas analysis and alveolar-arterial oxygen gradient in diagnosis and prognosis of elderly patients with suspected pulmonary embolismJ Gerontol A Biol Sci Med Sci.200055AM760764
- GhanimaWAbdelnoorMHolmenLONielsenBERossSSandsetPMD-Dimer level is associated with the extent of pulmonary embolismThromb Res200712028128817030057
- AujeskyDRoyPMGuyMCornuzJSanchezOPerrierAPrognostic value of D-Dimer in patients with pulmonary embolismThromb Haemost20069647848217003925
- DeMonyèWSansonBJMacGillavryMREmbolus location affects the sensitivity of a rapid quantitative D-Dimer assay in the diagnosis of pulmonary embolismAm J Respir Crit Care Med200216534534811818319
- BecattiniCVedovatiMCAgnelliGDiagnosis and prognosis of acute pulmonary embolism: focus on troponinsExpert Rev Mol Diagn2008833934918598112
- Muller-BardorffMWeidtmannBGiannitsisERelease kinetics of cardiac troponin T in survivors of confirmed severe pulmonary embolismClin Chem20024867367511901075
- GiannitsisEMuller-BardorffMKurowskiVIndependent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolismCirculation200010221121710889133
- KonstantinidesSGeibelAOlschewskiMImportance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolismCirculation20021061263126812208803
- PruszczykPBochowiczATorbickiACardiac troponin T monitoring identifies high risk group of normotensive patients with acute pulmonary embolismChest20031231947195212796172
- JanataKHolzerMLaggnerANMullnerMCardiac troponin T in the severity assessment of patients with pulmonary embolism: cohort studyBMJ200332631231312574045
- KucherNGoldhaberSZCardiac biomarkers for risk stratification of patients with acute pulmonary embolismCirculation20031082191219414597581
- BecattiniCVedovatiMCAgnelliGPrognostic value of troponins in acute pulmonary embolism: a meta-analysisCirculation200711642743317606843
- BecattiniCAgnelliGPredictors of mortality from pulmonary embolism and their influence on clinical managementThromb Haemost200810074775118989514
- JimènezDDiazGMolinaJTroponin I and risk stratification of patients with acute non massive pulmonary embolismEur Respir J20083184785318094010
- RayPDelermeSJourdainPChenevier-GobeauxCDifferential diagnosis of acute dyspnea: the value of B natriuretic peptides in the emergency departmentQJM200810183184318664534
- ten WoldeMTulevskiIIMulderJWBrain natriuretic peptide as a predictor of adverse outcome in patients with pulmonary embolismCirculation20031072082208412707233
- KucherNPrintzenGGoldhaberSZPrognostic role of brain natriuretic peptide in acute pulmonary embolismCirculation20031072545254712742987
- KrugerSGrafJMerxMWBrain natriuretic peptide predicts right heart failure in patients with acute pulmonary embolismAm Heart J2004147606514691420
- KielyDGKennebyNSPirzadaOBatchelorSAStruthersSALipworthBJElevated levels of natriuretic peptides in patients with pulmonary thromboembolismRespir Med2005991286129116099151
- SöhneMTen WoldeMBoomsmaFReitsmaJBDouketisJDBüllerHRBrain natriuretic peptide in hemodinamically stable acute pulmonary embolismJ Thromb Haemost2006455255616405522
- PieralliFOlivottoIVanniSUsefulness of bedside testing for brain natriuretic peptide to identify right ventricular dysfunction and outcome in normotensive patients with acute pulmonary embolismAm J Cardiol2006971386139016635617
- RayPMaziereFMedimaghSEvaluation of B-type natriuretic peptide to predict complicated pulmonary embolism in patients aged 65 years and older: a brief reportAm J Emerg Med20062660360716938601
- LogeartDLecuyerLThabutGBiomarker-based strategy for screening right ventricular dysfunction in patients with non-massive pulmonary embolismIntensive Care Med20073328629217165016
- TulevskiIIten WoldeMvan VeldhuisenDJCombined utility of brain natriuretic peptide and cardiac troponin T may improve rapid triage and risk stratification in normotensive patients with pulmonary embolismInt J Cardiol200711616116616814410
- VuilleumierNRighiniMPerrierACorrelation between cardiac biomarkers and right ventricular enlargement on chest CT in non massive pulmonary embolismThromb Res200812161762417716710
- YardanTAltintopLBaydinAYilmazOGuvenHB-type natriuretic peptide as an indicator of right ventricular dysfunction in acute pulmonary embolismInt J Clin Pract2008621177118217537186
- PruszczykPKostrubiecMBochowiczAN-terminal natriuretic peptide in patients with acute pulmonary embolismEur Repir J200322649653
- KostrubiecMPruszczykPBochowiczABiomarker-based strategy risk assessment model in acute pulmonary embolismEur Heart J2005262166217215911566
- KostrubiecMPruszczykPKaczynskaAKucherNPersistent NT-proBNP elevation in acute pulmonary embolism predicts early deathClin Chim Acta200738212412817507005
- KlineJAZeitouniRMarchickMRHernandez-NinoJRoseGAComparison of 8 biomarkers for prediction of right ventricular hypokinesis 6 months after submassive pulmonary embolismAm Heart J200815630831418657661
- BinderLPieskeBOlschewskiMN-terminal pro-brain natriuretic peptide or troponin testing followed by echocardiography for risk stratification of acute pulmonary embolismCirculation20051121573157916144990
- MaziereFBirolleauSMedimaghSComparison of troponin I and N-terminal-pro B-type natriuretic peptide for risk stratification in patients with pulmonary embolismEur J Emerg Med20071420721117620911
- KlokFAMosICHuismanMVBrain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysisAm J Respir Crit Care Med200817842543018556626
- CoutanceGLe PageOLoTHamonMPrognostic value of brain natriuretic peptide in acute pulmonary embolismCrit Care200812R10918721456
- CavallazziRNairAVasuTMarikPENatriuretic peptides in acute pulmonary embolism: a systematic reviewIntensive Care Med2008342147215618626627
- SanchezOTringuartLColombetIPrognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic reviewEur Heart J2008291569157718495689
- PulsMDellasCLankeitMHeart-type fatty acid-binding protein permits early risk stratification of pulmonary embolismEur Heart J20072822422917127709
- KaczynskaAPelsersMMBochowiczAKostrubiecMGlatzJFPruszczykPPlasma heart-type fatty acid binding protein is superior to troponin and myoglobin for rapid risk stratification in acute pulmonary embolismClin Chim Acta200637111712316698008
- PruszczykPBochowiczAKostrubiecMMyoglobin stratifies short-term risk in acute major pulmonary embolismClin Chim Acta2003338535614637265
- LankeitMKempfTDellasCGrowth differentiation factor-15 for prognostic assessment of patients with acute pulmonary embolismAm J Respir Crit Care Med20081771018102518263797
- VuilleumierNRighiniMPerrierACorrelation between cardiac biomarkers and right ventricular enlargement on chest CT in non massive pulmonary embolismThromb Res200812161762417716710
- VuilleumierNLe GalGVerschurenFCardiac biomarkers for risk stratification in non massive pulmonary embolism: a multicenter prospective studyJ Thromb Haemost2009739139819087222
- JanuzziJLJrCamargoCAAnwaruddinSThe N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) studyAm J Cardiol20059594895415820160
- DonzéJLe GalGFineMProspective validation of the Pulmonary Embolism Severity Index. A clinical prognostic model for pulmonary embolismThromb Haemost200810094394818989542
- Safety Study of Outpatient Treatment for Pulmonary Embolism (OTPE). NCT00425542January 152009Accessed on March 1, 2009 Available from http://www.clinicaltrial.gov/
- PEITHO Pulmonary Embolism Thrombolysis Trial. NCT00639743May 262008Accessed on March 1, 2009 Available from http://www.clinicaltrial.gov/