Abstract
Investigational product:
Rosuvastatin (Crestor®; Astra Zeneca).
Active ingredients:
Rosuvastatin (5 mg).
Study title:
Prevention of Atherosclerosis in Patients Living with HIV.
Phase of study:
Phase III.
Aims:
Primary aim:
To assess whether rosuvastatin therapy could slow the progression of the carotid intima-media thickness (C-IMT; as measured by the change in the mean IMT of the near and far walls of the distal common carotid arteries) over 2 years in HIV-infected patients (HIV-IP).
Secondary aims:
To assess whether rosuvastatin therapy could reduce highly sensitive C reactive protein (hs-CRP) inflammatory marker that is increased in HIV-IP.
To assess the effect of rosuvastatin therapy on serum lipid levels (total cholesterol [TC], low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol and triglycerides [TG]) and apolipoproteins (APO A1, APO B and APO B/A1).
To assess the safety of rosuvastatin in HIV-IP through the evaluation of clinical laboratory analyses (liver function tests and creatine kinase) and adverse events (AEs).
Study design:
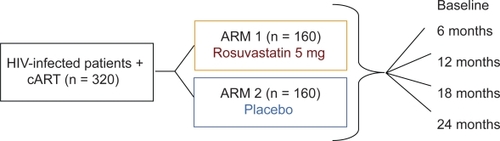
Two-year randomized, double-blind, placebo-controlled, parallel group study.
Planned sample size:
320 HIV-IP.
Summary of eligibility criteria:
HIV-IP who are aged between 30 and 60 years, with a CD4 count. greater than 200 cells/mm3. Patients must be stable on combination antiretroviral therapy (cART) for at least 12 months and have a 10-year CVD risk of less than 20% (using the Framingham risk score).
Number of study centers:
One.
Duration of treatment:
Two years (5 mg rosuvastatin or placebo once daily).
Dose and route of administration:
Oral rosuvastatin (5 mg) once daily.
The incidence of cardiovascular disease (CVD) in HIV-IP is at least three times higher than in the general population and further increases each year with combination anti-retroviral therapy (cART). The carotid atherosclerosis progression rate is 10 times higher in HIV-IP than in uninfected individuals. The aim of this study is to assess whether therapy with 5 mg rosuvastatin could:
1) Slow the progression in the mean IMT of the distal common carotid arteries over two years in HIV-IP.
2) Change the concentration in the inflammatory marker – hs-CRP, which is increased in HIV-IP.
3) Change the concentrations of TC, LDL cholesterol, HDL cholesterol, TG, apolipoproteins (APO) B, APO A1 and APO B/A1.
4) Be administered safely in the study population.
Pharmacological intervention with rosuvastatin will be evaluated in a double-blind, placebo-controlled, randomized clinical trial in HIV-IP treated with cART not matching the published selection criteria for lipid-lowering therapy. For the first time, this study will investigate anti-inflammatory and anti-atherogenic effects of a pharmacological lipid-lowering agent in HIV-IP that may lead to the reduction of CVD.
Background information
Introduction
HIV infection and cardiovascular disease risk
Clinicians who treat HIV-infected patients (HIV-IP) are increasingly concerned in regard to cardiovascular (CV) risk associated with combination antiretroviral therapy (cART).Citation1,Citation2 HIV-IP have significant risk factors for the development of cardiovascular disease (CVD):
HIV infection and its treatment affect endothelial function, induce vascular inflammation and carotid atherosclerosis.Citation3
In HIV-IP high-sensitivity C-reactive (hs-CRP) protein concentration is increased and has been shown to predict mortality.Citation2
HIV and its treatment are associated with pro-atherogenic dyslipidemia,Citation1 which is an important risk factor for the development of CVD.Citation4
The Framingham risk equation underestimates the risk of developing CVD in HIV-IP.Citation5
HIV infection is associated with a state of chronic immune activationCitation6 that persists in patients on cART who achieve suppression of viral replication.Citation7
CVD is the fourth cause of death in HIV-IP.Citation1
The best evidence linking HIV infection and its treatment to increased CV risk comes from the D:A:D cohort, a large epidemiological study that combined data from several primary care clinics in Europe and the United States. The D:A:D study demonstrated an increased incidence of myocardial infarction and CV events in HIV-IP who were receiving cART.Citation8,Citation9 The incidence of coronary heart disease in HIV-IP is at least three times the incidence in the general population and increases with increasing exposure to cART.Citation10 Lipid-lowering therapy has been shown to reduce CV events in a large number of studies;Citation11–Citation13 however there are no data available in HIV-IP.
Atherosclerosis in patients living with HIV – State of Science Conference in 2007
Scientists and healthcare providers convened in June 2007 for the ‘State of the Science Conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS’, a joint effort of the American Heart Association (AHA) and the American Academy of HIV Medicine. The conference proceedings are published online in Circulation, the Journal of the American Heart Association and the Journal of Acquired Immune Deficiency Syndrome.Citation14 Among the group’s key findings:
Clinical trials focusing on atherosclerosis prevention that specifically target HIV patients are much needed. In order to achieve progress in the prevention of CV complications of HIV, important gap areas in our knowledge need to be clearly spelled out and targeted for further research.
The rate of progression of carotid intima-media thickness (C-IMT) over time is valuable in determining cardiovascular risk in HIV-IP.
The risk for heart attack is 70% to 80% higher among people with HIV compared to those who do not have HIV.
Use of agents for lipid lowering may improve individual CV risk in the HIV population.
Newer inflammatory biomarkers, such as hs-CRP may prove useful for identifying infected patients at risk for CVD (of the biomarkers only hs-CRP has been recommended for use in clinical practice by CDC and prevention and the AHA).
HIV antiretroviral therapy and cardiovascular disease risk
The current cART regimens include two nucleoside reverse transcriptase inhibitors (NRTI) in combination with either a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a boosted protease inhibitor (PI).Citation15 Antiretroviral therapy has been associated with lipid metabolism disorders and insulin resistance increasing the risk of CV disease.Citation16 Results from clinical trials suggest that most PIs increase both triglycerides (TG) and total cholesterol (TC). Approximately 30% to 40% of HIV-IP starting boosted PI-containing regimens show an increase in TG and TC at 48 weeks.Citation16 The mechanisms by which HIV infection and cART cause lipid dysregulation are not fully elucidated and many investigations are currently underway. However, the association between cART and the risk of myocardial infarction is obvious.Citation17–Citation21 The International Forum for Collaborative HIV research has thus highlighted the need to identify and manage HIV-IP at increased risk of developing CVD.Citation22
Progression of atherosclerosis as assessed by C-IMT in HIV-IP
Measurement of C-IMT with high-resolution B-mode ultrasound is a well-accepted, noninvasive method of assessing atherosclerosis and tracking its progression. C-IMT measurements correlate well with pathological measurementsCitation23 and are potent predictors of myocardial infarction and stroke, even after adjustment for other risk factors.Citation24–Citation26 Progression of C-IMT can be measured reliably over timeCitation27 and has been used as an endpoint in clinical trials in which treatment reduced both C-IMT progression and CV events.Citation28
Carotid B-mode ultrasound has been used in several studies to assess atherosclerosis in HIV-IP. Maggi et al reported that carotid plaques were more common in 55 patients receiving PI than in 47 PI-naïve patients.Citation29 In a larger series, investigators from the Swiss HIV Cohort Study found that carotid and femoral artery plaques were associated with classic coronary risk factors and not with PI use.Citation30 Seminari et al found that carotid IMT was increased in 28 HIV-IP receiving PI compared with measurements in 15 PI-naïve patients and 16 HIV-negative subjects.Citation31 Chironi et al reported that 36 HIV-IP receiving PI had C-IMT equal to that of non-HIV subjects with similar lipid and glucose disturbances;Citation32 both groups had increased C-IMT compared with control subjects without lipid and glucose abnormalities. In another study of 423 HIV-IP, conventional risk factors but not lipodystrophy or cART were independent predictors of increased C-IMT.Citation33
The findings of Hsue et al indicated that HIV-IP have greater C-IMT compared with age- and sex-matched control subjects.Citation5,Citation34,Citation35 Furthermore, the rate of progression of C-IMT in HIV-IP of ∼0.074 mm/year was several-fold higher than the rate of ∼0.006 mm/year reported in non–HIV-IP. Of interest, in 45 of the 121 HIV-infected patients (or HIV-IP) (37%), mean carotid C-IMT progressed by ≥0.10 mm/year.Citation5
Because increasing C-IMT is an independent predictor of stroke and myocardial infarction in other populations,Citation24–Citation26 the findings of Hsue et al study suggest that the rate of vascular events is likely to increase substantially in HIV-IP.Citation5 The pathogenesis of accelerated atherosclerosis in HIV-IP has not been adequately studied, and the degree to which classic coronary risk factors are responsible, as opposed to HIV-related factors and factors related to treatment, is not known. Additional studies with longer follow-up are needed to determine the cause of accelerated atherosclerosis in HIV-IP.
Impact of statins on structural and functional changes of the vasculature in HIV-IP
C-IMT is considered to be an objective marker of early atherosclerosis.Citation5 It has been demonstrated that C-IMT is a reliable indicator for studying the long-term effects of anti-retroviral treatment, but systematic longitudinal observations are scarce. The only longitudinal study in HIV-IP found C-IMT progression rate to be 10 times higher in the treatment group than the control group,Citation5 suggesting that both traditional risk factors and the PI therapy were affecting atherosclerosis. The mean C-IMT was 0.90 ± 0.27 mm higher than expected from a large population study of similarly aged individuals. The mean progression rate was 0.1 ± 0.1 mm/year, which was greatly accelerated compared to 0.01 ± mm/year from published report of non-HIV-IP. Recently Charakida et al have demonstrated that HIV infection in childhood is associated with adverse structural and functional vascular changes that are most pronounced in children exposed to PI therapy.Citation36 Yu et al documented that statin-therapy significantly reduced C-IMT and inflammatory markers in patients with CVD in 26 weeks.Citation37 The Meteor study demonstrated the efficacy of rosuvastatin in reducing progression of C-IMT (over 2 years) in a randomized clinical trial in low risk individuals with subclinical atherosclerosis.Citation38
Rosuvastatin and C-IMT changes
A 19% reduction in coronary mortality has been recorded per 1.0 mmol/L (38.7 mg/dL) decrease in LDL cholesterol.Citation39 Statins also have been shown to slow the progression of and even regress atherosclerosis.Citation40 This has been demonstrated in coronary atherosclerosis by quantitative coronary angiography, intravascular ultrasound and carotid atherosclerosis by B-mode ultrasound measurement of C-IMT.Citation41–Citation45 Most clinical trials of lipid-lowering efficacy have shown that lowering lipid levels is beneficial irrespective of baseline LDL cholesterol level. However, the majority of such trials have been performed in high-risk populations, in individuals with high lipid concentrations or in patients with existing CVD but not in those with HIV/AIDS. Rosuvastatin is effective in lowering LDL cholesterol concentrations.Citation46,Citation47 Rosuvastatin also has favorable effects on other components of the lipid profile, raising HDL cholesterol and reducing TC and TG.Citation48
Involvement of hs-CRP in atherogenesis
A number of biomarkers that appear to be linked to inflammation and atherogenesis have been identified and hs-CRP has attracted particular attention. Plasma hs-CRP has a long half-life, exhibits stable concentrations in individuals, has negligible circadian variation and can be easily measured. As a downstream biomarker, hs-CRP provides a functional integration of overall upstream cytokine activation and exhibits activities that may initiate and stimulate progression of vascular disease, including the binding and activation of complement. It has also been shown that high concentration of plasma hs-CRP is associated with elevated levels of cell adhesion molecules (CAMs) and tissue factor, LDL cholesterol uptake by endothelial macrophages. Moreover, it induces the recruitment of monocytes into blood vessel walls and increases the levels of monocyte chemoattractant protein-122. hs-CRP has been widely considered as a predictor of CVD.Citation49,Citation50 The currently recommended plasma hs-CRP cut points are <1.0 mg/L for low risk, 1.0 to 3.0 mg/L for average risk, and >3.0 mg/L for high risk.Citation50 Accumulating evidence suggests that inflammatory markers like hs-CRP may provide an adjunctive method for global assessment of CV risk. Recent data suggest several biological effects are mediated through hs-CRP activation of nuclear factor-kappa B (NF-κB):Citation51
Up-regulation of chemokines that promotes neutrophil and monocyte endothelial cell adhesion (IL-8), monocyte chemotaxis and recruitment (MCP-1).
Up-regulation of key regulator of fibrinolysis by inhibiting tissue plasminogen activator (PAI-1).
Up-regulation of soluble CAMs that activate monocyte adhesion to endothelium (ICAM-1, VCAM-1).
Up-regulation of the primary initiator of the serine protease cascade of the coagulation system promoting thrombosis (Tissue Factor). Although these effects are important in atherogenesis, they clearly contribute to the genesis of acute coronary syndrome by recruiting leukocytes and promoting thrombosis.
hs-CRP in HIV-IP
Hsue et al reported that HIV-IP have higher hs-CRP levels than controls.Citation52 The median hs-CRP level was 1.6 mg/L in HIV-IP compared to 0.7 mg/L in controls (p = 0.001). Approximately 10% of HIV-IP had hs-CRP greater than 10 mg/L.Citation52,Citation53 Noursadeghi suggested that the increased hs-CRP is the consequence of HIV infection per se.Citation54 Feldman et al reported that hs-CRP may be a useful and inexpensive predictor of HIV disease mortality in women.Citation55 Lau et al obtained a single measurement of hs-CRP from 513 HIV-infected men in a multicenter AIDS Cohort Study to examine the association between hs-CRP and immune suppression and progression to AIDS.Citation56 In this study, concentrations of hs-CRP were associated with HIV disease progression independent of CD4 lymphocyte counts and HIV RNA levels. In addition, regardless of progression to AIDS, HIV-IP had a significant increase in hs-CRP over time. hs-CRP levels tended to be quite high in HIV-IP, even with virological suppression to <50 copies/mL over 3 years.Citation7 Therefore, increased hs-CRP has significant implications for CVD in HIV-IP.Citation56 On the basis of the available evidence, the Centers for Disease Control (CDC)/AHA suggest that patients with moderate risk (10% to 20% risk of CVD over 10 years) may benefit from measurements of hs-CRP to identify individuals who should be considered for medical therapy.Citation14
Table 1 Detailed breakdown of screening visits
Pharmacological anti-inflammatory effect of statins
Evidence from clinical trials: Large clinical trials, such as 4S, WOSCOPS, CARE, LIPID, AFCAPS/TexCAPS, ASCOTT-LLA and HPS, provided unequivocal evidence that statins reduce CV mortality and non-fatal vascular events.Citation57–Citation59 The most compelling clinical data relating statin therapy to anti-inflammatory mechanisms is derived from studies of inflammatory biomarkers, such as C reactive protein (CRP).Citation59 Individuals with elevated CRP (>3 mg/L) are at increased risk for first, as well as recurrent, CV events independent of lipid levels and disease severity as determined by global risk-assessment tools such as the Framingham Risk Score (FRS).Citation60 Multiple studies have demonstrated that statins as a class lower median CRP level by 15% to 35% in a manner largely independent of plasma lipid reduction.Citation57,Citation58 Patients who have low CRP levels after statin therapy have better clinical outcomes than those with higher CRP levels, regardless of the resultant level of LDL cholesterol.Citation59 The effect of statin therapy may result from:Citation61,Citation62
Actions dependent on lipid alterations.
Actions independent of lipid alterations but dependent on inhibition of HMG-CoA reductase, such as that resulting from cellular mevalonate depletion.
Distinct combinations of the above actions.
At present, there are no data available on the efficacy of CVD primary prevention with lipid-lowering therapy (eg, statins) in HIV-IP. In addition, no clinical trials have yet been designed to evaluate the possibility that lipid-lowering agents may interfere with inflammatory activity and early vascular remodelling taking place in HIV-IP. HIV-IP (at increased risk of developing CVD) with lipid metabolism disorders are, therefore, still assessed and treated in accordance to the global risk-assessment tools, such as the FRS used for the general population.Citation60 There is, therefore, an urgent clinical need to identify the strategy for a beneficial primary prevention (lipid-lowering-agent therapy) in HIV-IP.
Mechanisms of action of statins
Depletion of intracellular mevalonate inhibits the formation of isoprenoids, farnesyl-pyrophosphate and geranylgeranyl-pyrophosphate, and thus prenylation of many cell-signaling proteins.Citation63 Prenylation is the attachment of isoprenoids to proteins, a process which is necessary for insertion and anchorage of proteins to cell membranes for the full biological function. Rho is a small GTPase protein that mediates activation of the pro-inflammatory transcription factor NF-κB required for monocyte adhesion to endothelial cells.Citation64 Statins, through mevalonate depletion and prevention of prenylation, ultimately prevent monocyte and endothelial interaction and reverse the above inflammatory processes associated with atherosclerosis.Citation64 Plaque rupture and stabilization has also been related to mechanisms that include modulation of NF-κB and decrease prenylation of small signaling Rho proteins.Citation65,Citation66 NF-κB normally resides in the cytoplasm bound to its inhibitor (IκB). In response to inflam-matory stimuli, IκB is phosphorylated and degraded thereby liberating NF-κB to translocate to the nucleus and induce the expression of target genes.Citation65 Statins have been shown to limit NF-κB nuclear accumulation and DNA binding, perhaps via an increase in the expression of IκB.Citation50 NF-κB regulates expression of both adhesion molecules (eg, VCAM-1) and selectins (eg, E-selectin)Citation58 being responsible for up-regulation of the inflammatory cytokines. Recent studies suggest that a second mechanism by which statins confer favorable properties to the endothelium is through the induction of nuclear factors, such as Kruppel-like factor 2 (KLF2) and peroxi-some proliferators-activated receptors (PPARs).Citation67,Citation68 It has been found that sustained expression of KLF2 induces key factors, such as thrombomodulin, and inhibits the cytokine-mediated activation of pro-adhesive, prothrombotic factors (for example VCAM-1).Citation69,Citation70 The ability to inhibit endothelial pro-inflammatory activation by KLF2 is due to inhibition of NF-κB transcriptional activity.Citation70 The mutual antagonism between NF-κB and KLF2 might, therefore, serve as key mechanism by which endothelial health is regulated. Of interest, multiple statins can induce KLF2 expression.Citation71 PPARγ and PPARα – that have been identified in vascular cells and shown to possess potent anti-inflammatory properties (in part via inhibition of NF-κB) – can be augmented by statin treatment.Citation72,Citation73 The early clinical benefits from lipid-lowering therapy are attributed to the plaque-stabilizing properties of statins, mediated through a combined reduction in lipids and macrophages.Citation57
Rationale and need for the current study
In view of the present situation, strategies to estimate the association between HIV disease, cART and CV risk could take a direct or indirect approach. A direct approach would compare CVD rates or CVD markers within or between large populations. For example a study to determine whether statin therapy will lower the risk of CVD in people with antiretroviral-induced lipid elevations would require several thousand people followed for several years and would cost US$200 million or more, according to the International Forum for Collaborative HIV research. Indirect strategies (recommended by the Forum for Collaborative HIV research) to estimate the association between cART and CVD risk could estimate the effect of anti-retrovirals on risk factors and surrogate markers for CVD. As long as the questions asked by a surrogate marker study are clear, the markers to be included and the biology behind these markers have to be considered carefully. CVD markers should measure endothelial function, inflammation and macrophage activation. This study will produce evidence for the need of a more aggressive primary prevention approach in HIV-IP. The current identification of patients, in need of treatment, is not satisfactory in view of the fact that within the same age range the HIV-IP are at higher risk of developing CVD compared to the general population.
Our study has been carefully designed to address a crucial unanswered question regarding HIV infection, cART, progression of carotid atherosclerosis, statins and CVD, as follows:
Will statin therapy prevent progression of C-IMT over 2 years in HIV-IP? This issue is of exceptional clinical importance in accordance to the International Forum for Collabortaive HIV Research and the recent statements from a group of experts in HIV/CVD published by Circulation in June 2008.Citation2,Citation22 In fact, progression of C-IMT in HIV-IP is 10 times higher than in uninfected individuals. Thus, a strong positive finding from this study will dramatically affect prevention and would provide a clear rationale for much broader use of statin therapy for the primary prevention of CVD events than currently endorsed. On the other hand, a negative finding would also be of great importance, as it would direct the use of scarce prevention resources to other non-statin methods for CVD reduction. By using rosuvastatin, this study will also be addressing whether LDL-cholesterol reduction has efficacy in primary prevention among those individuals that do not match the Joint British Societies’ Guidelines on Prevention of Cardiovascular Disease in Clinical Practice (JBS2) criteria for lipid-lowering therapy.Citation74
Rationale for the use of rosuvastatin
Clinically relevant drug-drug interactions have been reported between commonly used NNRTIs (inducers of cytochrome P450 [CYP450] – mainly the isoforms 3A4 and 2B6) or ritonavir-boosted PIs (inhibitors of CYP450 – mainly 3A4). Rosuvastatin, however, is not extensively metabolized; approximately 10% of a radiolabelled dose is recoverable as a metabolite. The major metabolite is N-desmethyl rosuvastatin, which is formed principally by CYP450 2C9. Nevertheless, unexpected drug-drug interactions have been observed between lopinavir/ritonavir in both HIV-IPCitation75 and healthy volunteers.Citation76 While lopinavir/ritonavir concentrations are not affected by rosuvastatin co-administration, an approximate 1.6- to 4-fold increase in rosuvastatin exposure has been observed in the presence of lopinavir/ritonavir. Rosuvastatin is safe when administered at 5 mg once daily to patients on boosted PI and higher doses should be avoided. Studies are needed to elucidate the mechanism for this interaction.
AIM(S)
“Rosuvastatin slows progression of carotid atherosclerosis (C-IMT) by down-regulating inflammation in plasma (as assayed by plasma hs-CRP).”
Primary aim:
To assess whether rosuvastatin therapy could slow the progression of the C-IMT (as measured by the change in the mean IMT of the near and far walls of the distal common carotid arteries) over two years in HIV-IP.
Secondary aims:
To assess whether rosuvastatin therapy could reduce hs-CRP inflammatory marker that is increased in HIV-IP
To assess the effect of rosuvastatin therapy on serum lipid levels (TC, LDL cholesterol, HDL and TG) and apolipoproteins (APO A1, APO B and APO B/A1)
To assess the safety of rosuvastatin in HIV-IP through the evaluation of clinical laboratory analyses (liver function tests and creatine kinase) and adverse events.
Expected value of the results
The number of people living with HIV worldwide has been estimated to be 40 million in 2006 (of these 740,000 are in Western and Central Europe) – 8000 die and 12,000 become infected every day.Citation77 In 2006, 4.3 million (22,000 in Western and Central Europe) people were newly infected with HIV. cART has altered the nature of HIV disease, converting a lethal illness into a chronic and stable condition. Despite the benefits of cART, its use is complicated by a number of factors, including the risk for CVD, side effects, drug-drug interactions and the selection of drug-resistant virus. In 2003 the international Forum for Collaborative HIV research issued guidelines to develop new strategies with the goal to prevent CVD. Members of the Forum are well recognized clinical and research leaders in HIV medicine. Their work has clearly identified the urgent need to respond, in the first instance to the increased risk of CVD in HIV-IP, and to the concern expressed regarding increasing CVD risk with the aging of the HIV-IP. This study should demonstrate for the first time the:
Pharmacological anti-atherogenic and anti-inflammatory effects of statin therapy in HIV-IP.
Evidence to reconsider the lipid-lowering therapeutic criteria presently used (JBS2) in HIV-IP.
Trial design
Trial overview
Design of the proposed clinical investigation is as follows. This randomized, double-blind, placebo-controlled, parallel group study is aimed at investigating the effect of rosuvastatin on vascular inflammation/dysfunction in HIV-IP (n = 320). At present, no large trials have presented data on the impact of reducing LDL cholesterol in HIV-IP; therefore the lipid-lowering recommendations for the general population are applied to the HIV-IP. We propose to include in the C-IMT study HIV-IP that do not already match published selection criteria for lipid-lowering therapy.Citation74 Following written consent and screening procedures, eligible patients will be randomized on study day 1 either to receive rosuvastatin 5 mg once daily (ARM 1) or placebo once daily (ARM 2). Rosuvastatin or placebo will be administered for 2 years.Citation38 Carotid ultrasound to measure the change in C-IMT, safety laboratory tests, lipid profiles, apolipoproteins, immunological and virological response markers and an inflammatory marker (hs-CRP) will be assessed at baseline (before rosuvastatin or placebo initiation), following 6 months, 12 months (study day 365), 18 months (study day 547) and 24 months (study day 730) of therapy. On study day 730, ARM 1 and ARM 2 subjects will stop rosuvastatin/placebo intake. The rationale for the 24-month duration of rosuvastatin therapy is in accordance with the International Guidelines for the Treatment of HIV Infection,Citation10 which report that hyperlipidemia occurs weeks to months after beginning of CART.
Inclusion criteria
Documented HIV infection.
HIV-IP with a CD4 count ≥ 200 cells/mm3, who, according to the International Guidelines for the Treatment of HIV Infection, are stable on cART for at least 12 months.
Viral load ≤50 copies/mL.
Aged 30 to 60 years.
Asymptomatic for any atherosclerosis-related disease.
10-year CVD risk < 20% (using the JBS 2 criteria).Citation74
Exclusion criteria
Pharmacological lipid-lowering therapies (eg, statins, fibrates.) in the 12 months before the first visit.
Clinical evidence of coronary heart disease, angina, myocardial infarction, stroke or other peripheral athero-sclerotic disease.
Previous revascularization procedures.
10-year CVD risk ≥ 20% (JBS 2 criteria).
Diabetes mellitus, uncontrolled hypertension or familial hypercholesterolemia.
Serum creatinine levels of >177 pmol/L during screening.
Active liver disease or elevated liver enzymes (ALT > 3 times upper limit of normal [ULN]).
Creatine kinase >3 times ULN.
History of cancer.
Chronic inflammatory conditions, such as severe arthritis, lupus or inflammatory bowel disease.
Alcohol or drug abuse within the past year.
Serious medical or psychological conditions that may, in the opinion of the investigator, compromise successful study participation.
Uncontrolled hypothyroidism.
Current use of immunosuppressants.
Current use of post-menopausal oral hormone therapy.
Current use of cART containing less than two different classes of antiretroviral agents.
Evidence of focal plaques (carotid ultrasound).
Duration of involvement
The total duration of involvement in the study is 730 days, with a screening visit within 28 days prior to rosuvastatin/placebo commencement at day 1 and follow-up visits at 2, 6, 12, 18 and 24 months.
Withdrawal of patients and discontinuation criteria
Criteria for premature withdrawal
Subjects have the right to withdraw from the study at any time and for any reason. The investigator may also withdraw subjects from the study or decide to discontinue medication dosing in the event of inter-current illness, adverse events, protocol violations, administrative reasons or other reasons. An excessive rate of withdrawals can render the study uninterpretable. As a result, the unnecessary withdrawal of subjects should be avoided.
In all cases of withdrawal, the date and reasons for the withdrawal or discontinuation of medication will be clearly stated on the subject’s case report form (CRF). If the reason for removal of a subject from the study is an adverse event or an abnormal laboratory test result, the principal specific event or test will be recorded on the CRF.
Withdrawn subjects may be replaced at the discretion of the investigator.
Follow up of abnormal laboratory test values
In the event of unexplained abnormal laboratory test values, the tests will be repeated and followed up until they have returned to the normal range and/or an adequate explanation of the abnormality is found. If a clear explanation is established it should be recorded on the CRF.
Intervention(s) or method
Outlined below is the experimental work for the four different clinical trial aims:
Aim 1: C-IMT at different time points over 2-years treatment
B-mode ultrasound method: Standardized longitudinal B-mode images will be obtained of the near and far walls of the distal common carotid arteries and reported as the average value for the bilateral measurements. This location was chosen a priori because of its demonstrated reproducibility campared with C-IMT at other sites.Citation25,Citation78,Citation79 A commercially available ultrasound system with comprehensive vascular applications will be used. All images will be acquired and stored digitally, and analyzed off-line by an independent sonographer who is blinded from patient’s information. Color duplex ultrasound scanning will be performed with a 7 to 10 MHz linear-array transducers and settings adjusted to get an optimal picture of the carotid walls. The carotid arteries will be scanned transversely and longitudinally. Color flow mapping (“Angio”) will be used to identify the bifurcation, the flow divider and localized small plaques along the course of the common carotid artery, carotid bulb and internal carotid artery on both sides.
The IMT of both far and near walls will be measured with magnified pictures frozen at the R wave on the ECG. The IMT is defined as the distance between the leading edge of the luminal echo to the leading edge of the media/adventitia echo. It will be measured over a length of 10 mm immediately distal to the flow divider. This will be accomplished by the use of the calipers and the trace function of the ultrasound system and calculation of the mean IMT over this length. The distal common carotid arteries will be assessed in the segment extending from 10 to 20 mm proximal the carotid bifurcation. All ultrasound scans will be carried out with standardized settings. The image boundaries will be marked manually. For C-IMT measurements, trailing edges will be traced on the near wall boundaries and leading edges on the far wall boundaries. Measurements will be performed on images from selected angles, 60°, 90°, 120°, 150° and 180° for the right carotid artery and 300°, 270°, 240°, 210° and 180° for the left carotid artery. Measurements will be made of both the mean and maximum C-IMT of each wall at all selected angles. Both mean and maximum IMT will be calculated and analyzed. Any plaques will be excluded from the measurements. Intraoperator and interoperator variability will be assessed by means of coefficients of variation. The mean length of the C-IMT will be evaluated in both the rosuvastatin and placebo groups. The precision and reliability of the ultrasound method will be tested in a randomly selected subgroup of 32 arteries with paired images obtained on the same day. Carotid ultrasound to measure C-IMT will be performed at baseline (before therapy with lipid lowering) and after 6, 12, 18 and 24 months.
Aim 2: Vascular inflammation by measuring plasma hs-CRP in HIV-IP treated with cART
There are currently no definitive data on the effect of statins on hs-CRP in patients HIV-IP. HIV-IP will be advised not to take any platelet inhibiting drugs for a week before blood sample collection, which is taken before breakfast. Venous blood drawn in a Vacutainer (no more than 30 minute wait) is processed and divided into aliquots (plasma or serum if not anticoagulated) and frozen at −70 °C for later analysis. hs-CRP will be assessed in stored frozen blood samples from baseline, 6, 12, 18 and 24 months according to methods described by the manufacturer of CRP Latex HS (Roche).
Aim 3: Serum lipid levels and apolipoproteins in HIV-IP treated with cART
Blood samples will be taken at specific visits to monitor serum lipid and apolipoproteins levels (TC, HDL cholesterol, LDL cholesterol, TG, HDL/TC ratio, APO B, APO A1, APO B/A1).
Aim 4: Safety
Safety will be assessed by the evaluation of clinical laboratory analysis (such as creatine kinase, creatinine, alanine/aspartate aminotransferase, alkaline phosphatase, total bilirubin, albumin and fasting blood glucose), hematology and adverse events (AEs).
How subjects are randomized and how allocation is concealed
The randomization list of the study will be prepared in sealed envelopes and handed to a pharmacist. In addition, further emergency un-blinding envelopes will be prepared in sealed envelopes and given to a lead pharmacist in the unit where the trial is conducted who would keep these secure from study PI, study co-investigators and the study pharmacist.
The emergency un-blinding sealed envelopes would be kept securely and these envelopes would only be opened in the event of an emergency to reveal details of the study arm of individual subjects recruited to the trial.
In addition once the sealed envelope is opened a strip inside the envelop will contain details of ‘Subject Number’ and the study group the subject should be allocated. This ‘Subject Number’ will, therefore, be assigned in the study CRF pages from baseline visit onwards, including allocating this number in any additional data for the study, such as printed copies of laboratory results. This number will, therefore, serve and be used to track patient data during the study.
Primary and secondary endpoints
Primary endpoint
To assess whether rosuvastatin therapy could slow the progression of the C-IMT (as measured by the change in the mean IMT of the near and far walls of the distal common carotid arteries) over 2 years in HIV-IP.
Secondary endpoints
To assess whether rosuvastatin therapy could reduce hs-CRP inflammatory marker that is increased in HIV-IP.
To assess the effect of rosuvastatin therapy on serum lipid levels (TC, LDL cholesterol, HDL and TG) and apolipoproteins (APO A1, APO B and APO B/A1).
Safety variables
Safety and tolerability will be monitored by evaluating the incidence and severity of AEs and abnormal laboratory values (hematology and clinical chemistry).
Statistical analysis plan
Sample size and power calculations
The findings of Hsue et al indicated that HIV-IP have greater C-IMT compared with age- and sex-matched control subjects.Citation5,Citation34,Citation35 Furthermore, the rate of progression of C-IMT in HIV-IP of 0.074 mm/year was several-fold higher than the rate of 0.006 mm/year reported in non-HIV-infected subjects. Of interest, in 45 of the 121 HIV-IP (37%) the mean carotid C-IMT progressed by 0.10 mm/year.Citation5
Based on published data,Citation80 assuming an overall progression rate between the control and the active arms at year 1 since entry to study are 0.045 and 0.030 mm, respectively. Assuming the rate of progression rate will remain constant at year 2 after entry to the study, the mean progression rate in IMT is expected to be 0.09 (SD 0.08) mm in the control arm while that in the active arm it is expected to be 0.0054 mm. This assumes that a 40% difference will be observed between arms at 2 years post study, which allows for greater variations in the active arm at year 2 compared to year 1 and standard deviation is estimated at 0.072. Based on these assumptions and assuming 30% of the subjects recruited to the study will drop out by year 2, n = 160 subjects per arm (total number of study subjects n = 320) will need to be recruited in order to show the effect size of this magnitude to be significantly different at 1% level of significance with 90% power using two-sided test. Type I error rate is set at 1% since one interim analyses is planned at year 1.
Statistical tests
The primary objective of this study is to assess the effect of lipid-lowering drug on the change in the C-IMT from baseline to 2 years post entry to study by two study arms. The primary endpoint is to assess the change in C-IMT from baseline to study time points in the rosuvastatin and placebo arms. All patients who meet the inclusion criteria at baseline will form part of the statistical analysis. Data will be analyzed and presented as both on treatment and intent-to-treat (ITT) methods.
All summary statistics will be presented with point estimates, which are mean changes from baseline to study time point and indication of the variability in data, such as the standard deviation or the standard error. All missing data at each study visits will be presented and categorized as: patients off trial, patients who did not attend, and data missing even though patient had attended for their scheduled study visit. Where data are missing for such patients these will be grouped into missing data.
The ITT method approach that will be undertaken when summarizing the data will be the last observation of C-IMT carried forward (LOCF) if data are unavailable at the time point. The ITT method will be used to describe the data and presented with relevant point estimates and 95% CI. This will be presented for each study visits from baseline to 2 years or end of study.
Although this method is frequently used in data analyses of clinical trials, subject drop out rates and patterns (reasons for missing data) in clinical trials may be affected by many factors, such as the disease, study population, efficacy of treatment, side effects and length of trial. Often the missing data are completely random. Carrying forward observations may confound treatment with time. This confounding can bias estimates of differences between treatment groups in mean change from baseline to endpoint and the associated SE leading to inaccurate assessment of type I and type II error rates. MIXED model analyses are more robust to potential bias from missing data than LOCF. Estimates of effects based on MIXED models assume that any missing data are random.
In addition to the ITT method described above, longitudinal data of the C-IMT will be available and linear mixed models will be used between treatment group comparisons to calculate the difference in averages (DAVG), which will represent the time weighted difference in the C-IMT from baseline to each study time point and where deemed necessary data will be transformed to stabilise the variance.
MIXED procedure in SAS will be used by fitting values of the C-IMT from all study time points as a dependent variable. Independent variables will include the fixed effects of the intervention arms rosuvastatin and placebo, study visit time points and intervention by study time points interaction. A covariance matrix will be used to model the within patient errors.
Estimates of change in C-IMT from baseline will be obtained from intervention by study time points interaction.
In addition, this method will be used to analyze the effects of changes in antiretroviral treatment prescription patterns over time, which will be added as time varying covariate in the MIXED model.
Trends over time will be presented as point estimates with 95% CI. Further multivariable analyses will be adjusted for other times varying co-variables assumed to have potential confounding and or residual effect on trend of outcome variable over time.
Further exploratory analyses will be undertaken to look at factors predicting time to ‘optimal’ change in C-IMT estimated as ‘getting worse.’
Survival plots will be used to estimate time since entry into the study until ‘optimal’ change in C-IMT estimated as ‘getting worse’ within two years since entry into study. This will, however, need to be defined using clinically meaningful change.
Between study group comparisons in time to ‘optimal’ change in C-IMT will be made using the log rank cCitation2 test. Univariate and multivariable Cox’s Proportional hazards regression models will be used to identify factors that predict ‘optimal’ change in C-IMT estimated as ‘getting worse.’
Demographic characteristics will be summarized by randomized groups and p-values will be presented describing associations with these. In addition, all serious adverse events will be reported by study arms together with number of patients and number of events.
Secondary endpoint is to assess the change in:
hs-CRP
To avoid the potential for misclassification because of an exogenous acute-phase stimulus, data from patients with either a baseline or two years hs-CRP value > 3 SD above the mean value will be analyzed and data will be presented both including and excluding this from analysis on a priori basis.Citation81
Lipid parameters
TC
HDL cholesterol
LDL cholesterol
TG
HDL cholesterol:TC
Apolipoproteins
APO B
APO A1
APO B/APO A1
Safety variables
All these secondary endpoints will be assessed from baseline to study time points by rosuvastatin and the placebo arms.
All patients who meet the inclusion criteria at baseline will form part of the statistical analysis. Data will be analyzed and presented as both on treatment and intent to treat (ITT) methods.
All statistical analysis will be performed using SAS statistical software and all p-values presented will be two sided.
One interim analysis is planned during the study duration and the sample size estimate has taken account of this. Where further unplanned interim analyses are performed, however, p-values will be appropriately adjusted for unplanned repeated data analyses.
Planned subgroup analyses
No subgroup analyses are planned for this study.
Interim analyses and stopping rules
An independent data safety monitoring board (DSMB) will be established, which will meet initially, after a predetermined number of subjects have completed two months of randomized therapy and at every study visit – at 6, 12, 18 and 24 months. The DSMB will be made up of three external, independent experts within the field of HIV and lipid metabolism disorders; two physicians and one statistician.
Stopping rules will be agreed at the first meeting and will include the following.
Elevation of creatine kinase >3 times ULN
ALT >3 times ULN
Intolerable myalgia or other intolerable side effects (see ACTG grading; Appendix 2).
Criteria for termination of the trial
The sponsor or investigator may terminate either part of, or the entire study for safety or administrative reasons. A written statement fully documenting the reasons for such a termination will be provided to the Ethics Committee and the Regulatory Authority as appropriate.
Adverse events
Definitions
An adverse event is any untoward medical occurrence in a subject administered a pharmaceutical product and which does not necessarily have a casual relationship with the study treatments. An adverse event can, therefore, be any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal (investigational) product, whether or not considered related to the medicinal (investigational) product.
Adverse events observed by the investigator, or reported by the subject, and any remedial action taken, will be recorded in the subject’s CRF and should be verifiable in the subject’s notes throughout the study. The nature of each event, time of onset after drug administration, duration and severity will be documented together with the investigator’s opinion of the causal relationship to the treatment (unrelated, unlikely, possible, probable and definite).
All subjects experiencing adverse events, whether considered associated with the use of the study medication or not, must be monitored until the symptoms subside and any clinically relevant changes in laboratory values have returned to baseline, or until there is a satisfactory explanation for the changes observed.
Procedures such as surgery should not be reported as adverse events. However, the medical condition for which the procedure was performed should be reported if it meets the definition of an adverse event. For example, an acute appendicitis that begins during the adverse event reporting period should be reported as the adverse event and the resulting appendectomy noted on the CRF. Planned procedures, such as surgery planned prior to the subject’s enrolment into the study, need not be reported as adverse events if these are documented as planned at the screening visit.
Clinically significant changes in physical examination and blood safety profiles should also be recorded as adverse events.
Serious adverse events (SAEs)
An SAE is any untoward medical occurrence that at any dose:
Results in death
Is life threatening
Requires in patient hospitalization or prolongation of existing hospitalization
Results in persistent or significant disability/incapacity
Is a congenital anomaly/birth defect.
All SAEs must be reported immediately, except for those SAEs which the protocol or other document (eg, Investigator Brochure) identified as do no need reporting.
The SAEs should be reported immediately to the principal investigator (within 24 hours of a member of the study team becoming aware of the event). An SSAT SAE form should be completed, and an assessment of whether the SAE is a Suspected Unexpected Serious Adverse Reaction (SUSAR) conducted.
The principal investigator is responsible for determining whether the SAE is a SUSAR and for reporting this in accordance with Good Clinical Practice (GCP) and applicable regulatory requirements.
Administrative procedures
Ethics approval
The study protocol, subject information and consent form, the Investigator Brochure, available safety information, subject recruitment procedures (eg, advertisements), information about payments and compensation available to the subjects and documentation evidencing the investigator’s qualifications should be submitted to the Ethics Committee for ethical review and approval according to local regulations, prior to the study start. Any changes, which may need to be made, will be submitted in the form of numbered and dated protocol amendments in accordance with local regulations.
Regulatory notification
As required by local regulations, approval of the appropriate regulatory bodes will be obtained, prior to study initiation.
Indemnities
The sponsor (St Stephen’s AIDS Trust) has taken out appropriate insurance for non-negligence harm for the trial. Negligence harm is covered by Chelsea and Westminster Hospital NHS Foundation Trust.
Publication policy
A whole or part of this study data will be communicated, orally presented and/or published in appropriate scientific journals. Full anonymity of subject’s details will be maintained throughout. Subjects wanting to see the results of the trial can request a copy of the article from the investigators once it has been published.
Abbreviations
| AEs | = | adverse events; |
| AHA | = | American Heart Association; |
| APO | = | apolipoprotein; |
| C-IMT | = | carotid intima-media thickness; |
| CAMs | = | cell adhesion molecules; |
| cART | = | combined antiretroviral therapy; |
| CDC | = | Centers for Disease Control; |
| CRF | = | case report form; |
| CRP | = | C reactive protein; |
| CV | = | cardiovascular; |
| CVD | = | cardiovascular disease; |
| FRS | = | Framingham risk score; |
| HDL | = | high-density lipoprotein; |
| HIV | = | human immunodeficiency virus; |
| HIV-IP | = | HIV-infected patients; |
| hs-CRP | = | highly sensitive C reactive protein; |
| JBS | = | Joint British Societies; |
| KLF2 | = | Kruppel-like factor 2; |
| LDL | = | low-density lipoprotein; |
| LOCF | = | last observation carried forward; |
| NNRTI | = | non-nucleoside reverse transcriptase inhibitor; |
| NRTI | = | nucleoside reverse transcriptase inhibitor; |
| PI | = | protease inhibitors; |
| PPARs | = | peroxisome proliferator-activated receptors; |
| SAE | = | serious adverse event; |
| TC | = | total cholesterol; |
| TG | = | triglycerides; |
| ULN | = | upper limit of normal. |
Disclosures
The authors have no conflicts of interest to disclose.
References
- GrinspoonSCarrACardiovascular risk and body-fat abnormalities in HIV-infected adultsN Engl J Med2005352486215635112
- GrinspoonSKGrunfeldCKotlerDPState of the Science Conference. Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS. Executivve summaryCirculation200811819821018566320
- SteinJHKleinMABellehumeurJLUse of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunctionCirculation200110425726211457741
- DressmanJKincerJMatveevSVHIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophagesJ Clin Invest200311138939712569165
- HsuePYLoJCFranklinAProgression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infectionCirculation20041091603160815023877
- HazenbergMDHamannDSchuitemakerHT cell depletion in HIV-1 infection: how CD4+ T cells go out of stockNat Immunol2000128528911017098
- HuntPWMartinJNSinclairET cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapyJ Infect Dis20031871534154312721933
- Friis-MollerNSabinCAWeberRCombination antiretroviral therapy and the risk of myocardial infarctionN Engl J Med20033491993200314627784
- Friis-MollerNReissPSabinCAClass of antiretroviral drugs and the risk of myocardial infarctionN Engl J Med20073561723173517460226
- VittecoqDEscautLChironiGCoronary heart disease in HIV-infected patients in the highly active antiretroviral treatment eraAIDS200317Suppl 1S70S7612870533
- LaRosaJCGrundySMWatersDDIntensive lipid lowering with atorvastatin in patients with stable coronary diseaseN Engl J Med20053521425143515755765
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trialLancet200236072212114036
- ColhounHMBetteridgeDJDurringtonPNPrimary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trialLancet200436468569615325833
- GrinspoonSKGrunfeldCKotlerDPState of the science conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: executive summaryCirculation20081182198210Erratum in: Circulation. 2008;118(6):e109.18566320
- GazzardBBernardAJBoffitoMBritish HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy (2006)HIV Med2006748750317105508
- EronJJrYeniPGatheJJrThe KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: A randomised non-inferiority trialLancet200636847648216890834
- CurrierJSTaylorABoydFCoronary heart disease in HIV-infected individualsJ Acquir Immune Defic Syndr20033350651212869840
- El-SadrWNeatonJEpisodic CD4-guided use of ART is inferior to continuous therapy: Results of the SMART StudyPaper presented at: Conference on Retroviruses and Opportunistic Infections (CROI)2006Denver
- KleinDHurleyLQuesenberryCHospitalizations for CHD and MI among Northern California HIV+ and HIV− men: changes in practice and Framingham Risk ScoresPaper presented at: Conference on Retroviruses and Opportunistic Infections (CROI)2006Denver
- MooreRKerulyJIncreasing Incidence of Cardiovascular Disease in HIV-infected Persons in CarePaper presented at: Conference on Retroviruses and Opportunistic Infections (CROI)2003Boston
- RickertsVBrodtHStaszewskiSIncidence of myocardial infarctions in HIV-infected patients between 1983 and 1998: the Frankfurt HIV-cohort studyEur J Med Res2000532933310958765
- Available at: http://www.hivforum.org/publications/CVD%20risk%20aug%2019%20w%20logo.pdf
- PignoliPTremoliEPoliAIntimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imagingCirculation198674139914063536154
- ChamblessLEHeissGFolsomARAssociation of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993Am J Epidemiol19971464834949290509
- BotsMLHoesAWKoudstaalPCommon carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam StudyCirculation199796143214379315528
- O’LearyDHPolakJFKronmalRAfor the Cardiovascular Health Study Collaborative Research GroupCarotid-artery intima media thickness as a risk factor for myocardial infarction and stroke in older adultsN Engl J Med199934014229878640
- EspelandMACravenTARileyWAReliability of longitudinal ultrasonic measurements of carotid intima-medial thicknessStroke1996274804858610317
- FurbergCDAdamsHPJrApplegateWBfor the Asymptomatic Carotid Artery Progression (ACAPS) Study Research GroupEffects of lovastatin on early carotid atherosclerosis and cardiovascular eventsCirculation199490167916877734010
- MaggiPSerioGEpifaniGPremature lesions of the carotid vessels in HIV-1-infected patients treated with protease inhibitorsAIDS20001412312810708282
- DepaironMChessexSSudrePwith the Swiss HIV Cohort StudyPremature atherosclerosis in HIV-infected individuals: focus on protease inhibitor therapyAIDS20011532933411273212
- SeminariEPanAVoltiniGAssessment of atherosclerosis using carotid ultrasonography in a cohort of HIV-positive patients treated with protease inhibitorsAtherosclerosis200216243342811996964
- ChironiGEscautLGariepyJCarotid intima-media thickness in heavily pretreated HIV-infected patientsJ Acquir Immune Defic Syndr20033249049312679699
- MerciePThiébautRLavignolleVEvaluation of cardiovascular risk factors in HIV-1 infected patients using carotid intima-media thickness measurementAnn Med200234556312014436
- HowardGSharrettARHeissGCarotid artery intimal-media thickness distribution in general populations as evaluated by B-mode ultrasoundStroke199324129713048362421
- AminbakhshAManciniGBJCarotid intima-media thickness measurements: what defines an abnormality? A systematic reviewClin Invest Med19992214915710497713
- CharakidaMDonaldAEGreenHEarly structural and functional changes of the vasculature in HIV-infected children: impact of disease and antiretroviral therapyCirculation200511210310915983247
- YuCMZhangQLamLComparison of intensive and low-dose atorvastatin therapy in the reduction of carotid intimal-medial thickness in patients with coronary heart diseaseHeart20079393393917344325
- CrouseJR3rdRaichlenJSRileyWAEffect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR TrialJAMA20072971344135317384434
- BaigentCKeechAKearneyPMEfficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statinsLancet20053661267127816214597
- CrouseJR3rdThematic review series: patient-oriented research. Imaging atherosclerosis: state of the artJ Lipid Res2006471677169916705212
- NissenSENichollsSJSipahiIEffect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trialJAMA20062951556156516533939
- Rodriguez-GranilloGAAgostoniPGarcia-GarciaHMMeta-analysis of the studies assessing temporal changes in coronary plaque volume using intravascular ultrasoundAm J Cardiol20079951017196453
- de GrootEJukemaJWMontauban van SwijndregtADB-mode ultrasound assessment of pravastatin treatment effect on carotid and femoral artery walls and its correlations with coronary arteriographic findings: a report of the Regression Growth Evaluation Statin Study (REGRESS)J Am Coll Cardiol199831156115679626835
- SalonenRNyyssonenKPorkkalaEKuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteriesCirculation199592175817647671358
- AmarencoPLabreucheJLavalleePStatins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysisStroke2004352902290915514180
- JonesPHDavidsonMHSteinEAComparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial)Am J Cardiol20039215216012860216
- SchusterHBarterPJStenderSEffects of switching statins on achievement of lipid goals: Measuring Effective Reductions in Cholesterol Using Rosuvastatin Therapy (MERCURY I) studyAm Heart J200414770571315077101
- JonesPHHunninghakeDBFerdinandKCEffects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trialClin Ther2004261388139915531001
- LibbyPRidkerPMInflammation and atherosclerosis: role of C-reactive protein in risk assessmentAm J Med2004116Suppl 6A9S16S15050187
- RidkerPMHigh-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular diseaseCirculation20011031813181811282915
- JialalIDevarajSThe role of C-reactive protein activation of nuclear factor kappa-B in the pathogenesis of unstable anginaJ Am Coll Cardiol20074919519717222730
- HsuePLoJFranklinAC-reactive protein levels in patients with HIV: a marker of cardiovascular risk or chronic infection?Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI)2005Boston
- HenryKZackinRDubeMACTG 5056: C-Reactive protein (CRP) levels and cardiovascular risk status for a cohort of HIV-1-infected persons Durably suppressed on an indinavir (IDV)-containing regimenPaper presented at: Conference on Retroviruses and Opportunistic Infections (CROI)2002Seattle
- NoursadeghiMMillerRFClinical value of C-reactive protein measurements in HIV-positive patientsInt J STD AIDS20051643844115969780
- FeldmanJGGoldwasserPHolmanSC-reactive protein is an independent predictor of mortality in women with HIV-1 infectionJ Acquir Immune Defic Syndr20033221021412571532
- LauBSharrettARKingsleyLAC-reactive protein is a marker for human immunodeficiency virus disease progressionArch Intern Med2006166647016401812
- de LorenzoFFeherMMartinJStatin therapy-evidence beyond lipid lowering contributing to plaque stabilityCurr Med Chem2006133385339317168712
- JainMKRidkerPMAnti-inflammatory effects of statins: clinical evidence and basic mechanismsNat Rev Drug Discov2005497798716341063
- RidkerPMCannonCPMorrowDC-reactive protein levels and outcomes after statin therapyN Engl J Med2005352202815635109
- RidkerPMWilsonPWGrundySMShould C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk?Circulation20041092818282515197153
- MassyZAKeaneWFKasiskeBLInhibition of the mevalonate pathway: benefits beyond cholesterol reduction?Lancet19963471021038538301
- SpositoACChapmanMJStatin therapy in acute coronary syndromes: mechanistic insight into clinical benefitArterioscler Thromb Vasc Biol2002221524153412377727
- LiaoJKIsoprenoids as mediators of the biological effects of statinsJ Clin Invest200211028528812163444
- SmithDAGalinIStatin therapy for native and peri-interventional coronary heart diseaseCurr Mol Med2006658960216918378
- HaydenMSGhoshSSignaling to NF-kappa BGenes Dev2004182195222415371334
- WongBLummaWCSmithAMStatins suppress THP-1 cell migration and secretion of matrix metalloproteinase 9 by inhibiting geranylgeranylationJ Leukoc Biol2001699596211404382
- FeinbergMWLinZFischSAn emerging role for Kruppel-like factors in vascular biologyTrends Cardiovasc Med20041424124615451516
- PlutzkyJPeroxisome proliferator-activated receptors as therapeutic targets in inflammationJ Am Coll Cardiol2003421764176614642685
- LinZKumarASenBanerjeeSKruppel-like factor 2 (KLF2) regulates endothelial thrombotic functionCirc Res200596e48e5715718498
- SenBanerjeeSLinZAtkinsGBKLF2 Is a novel transcriptional regulator of endothelial proinflammatory activationJ Exp Med20041991305131515136591
- ParmarKMNambudiriVDaiGStatins exert endothelial atheroprotective effects via the KLF2 transcription factorJ Biol Chem2005280267142671915878865
- LandrierJFThomasCGroberJStatin induction of liver fatty acid-binding protein (L-FABP) gene expression is peroxisome proliferator-activated receptor-alpha-dependentJ Biol Chem2004279455124551815337740
- ZelvyteIDominaitieneRCrisbyMModulation of inflammatory mediators and PPARgamma and NFkappa B expression by pravastatin in response to lipoproteins in human monocytes in vitroPharmacol Res20024514715411846628
- JBS 2: Joint British Societies’ guidelines on the prevention of cardiovascular disease in clinical practiceHeart200591Suppl 5v1v5216365341
- Van Der LeeMVogelMSchippersEPharmacokinetics and pharmacodynamics of combined use of lopinavir/ritonavir and rosuvastatin in HIV-infected patientsPaper presented at: Conference on Retroviruses and Opportunistic Infections (CROI)2006Denver
- KiserJJGerberJGPredhommeJADrug/drug interaction between lopinavir/ritonavir and rosuvastatin in healthy volunteersJ Acquir Immune Defic Syndr20084757057818176327
- SmithJMuch about HIVBMJ200733417303861
- TaylorAJKentSMFlahertyAJARBITER: Arterial biology for the investigation of the treatment effects of reducing cholesterol: A randomized trial comparing the effects of aforvastatin and pravastatin on carotid intima media thicknessCirculation20021062055206012379573
- SmildeTJWollersheimHvan LangenHReproducibility of ultrasonographic measurements of different carotid and femoral artery segments in healthy subjects and in patients with increase intima-media thicknessClin Sci (Lond)1997933173249404223
- MarkwoodTTKentsSCoyleLCDesign and rationale of the ARBITER trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol) – a randomized trial comparing the effects of atorvastatin and pravastatin on carotid artery intima-media thicknessAm Heart J200114134234711231429
- RidkerPMRifaiNPfefferMALong-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) InvestigatorsCirulcation1999100230235