Abstract
Objectives
Sepsis remains a disease with a high mortality in neonates. Microcirculatory impairment plays a pivotal role in the development of multiorgan failure in septic newborns. The hemodynamic effects of recombinant activated protein C (rhAPC) were tested in an animal model of neonatal septic shock focusing on intestinal microcirculation.
Materials and methods
Endotoxic shock was triggered by intravenous application of Escherichia coli lipopolysaccarides in newborn piglets. Thereafter, five animals received a continuous infusion of 24 μg/kg/h rhAPC, and five received vehicle for control. Over the course of three hours, intestinal microcirculation was assessed by intravital microscopy every 30 min. Macrocirculation and blood counts were monitored simultaneously.
Results
After a short hypotensive period in all animals, the arterial blood pressure returned to baseline in the rhAPC-treated piglets, whereas the hypotension became increasingly severe in the controls. By 90 min, mean blood pressure in the controls was significantly lower than in the treatment group. Similar observations were made regaring microcirculation. After an early impairment in all study animals, functional capillary density and intestinal microcirculatory red blood cell velocity and red blood cell flow recovered in the rhAPC group, but deteriorated further in the control piglets.
Conclusion
Recombinant activated protein C protects macro- and microcirculation from endotoxic shock.
Introduction
Sepsis is one of the leading causes of death in hospitalized neonates and preterm infants.Citation1 Sepsis results from a systemic response to infection, which can be triggered by components of the microbial cell wall or microbial DNA among others. These nonself patterns are detected by pattern-recognition receptors (PRRs) such as Toll-like receptors (TLRs). Upon ligand binding, TLRs induce pro-inflammatory cytokines (eg, tumor necrosis factor-α [TNFα], interleukin-1 [IL-1], IL-6, IL-8), which in turn activate coagulation and suppress fibrinolysis by stimulating the surface expression of tissue factor on monocytes and endothelium. Subsequently, thrombin is generated which leads to deposition of fibrin, disruption of the endothelial coagulatory equilibrium, and ultimately in microvascular thrombosis.Citation2 On the basis of the molecular mechanisms involved in sepsis, it seems likely that therapeutic interventions and drugs targeting the interface of coagulation and inflammation may improve microcirculation and microvascular blood flow.
The protein C pathway is one of the major mechanisms reining hemostasis. The zymogen protein C is activated by the thrombin–thrombomodulin complex in combination with the endothelial protein C receptor (EPCR). Activated protein C (APC) exerts its anticoagulatory effect by proteolytically degrading factors Va and VIIIa, and via facilitation of fibrinolysis through inhibition of tissue plasminogen activator inhibitor (PAI). APC also has an important anti-inflammatory role as illustrated by its amelioration of the inflammatory activation that occurs upon intravenous infusion of Escherichia coli;Citation3 APC exerts this effect by inhibiting the liberation of TNFα and other pro-inflammatory cytokines through blocking nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinase activation.Citation4–Citation7
Protein C is commercially available as recombinant human APC (rhAPC; Xigris®, Eli Lilly, Indianapolis, IN, USA) or as the nonactivated zymogen human protein C (hPC; Ceprotin®, Baxter, Glendale, CA, USA). A large multicenter trial (PROWESS) showed a benefit for adult patients with severe sepsis who were treated with rhAPC.Citation8 However, a multicenter trial in which rhAPC was administered to septic children was terminated because of an increased incidence in intracranial hemorrhage, especially in the subgroup of children younger than 60 days.Citation9 As a result, the use of rhAPC in septic neonates is still limited and no multicenter trial data is available. Nonetheless, single-case reports describe the use of rhAPC in septic neonates.Citation10–Citation12 In addition, a beneficial effect of rhAPC in an animal model of neonatal ischemic brain injury has been reported.Citation13 Another publication by our group demonstrated that the nonactivated zymogen protein C in neonatal septic shock was effective.Citation14 Low protein C plasma levels have been identified as a predictor for increased mortality in the neonatal sepsis by Venkataseshan and colleagues.Citation15
Studies investigating the molecular basis of inflammation have demonstrated increased production of pro-inflammatory mediators such as prostaglandins, nitric oxide, IL-1, and TNFα. The release of these pro-inflammatory mediators can result in a vasodilatory/vasoconstrictory imbalance and subsequent microcirculatory impairment that improves after the application of rhAPC in an animal model of septic shock.Citation16 Since microcirculation is one of the aspects of sepsis that has received less attention than others, we designed a study which focused on the important pathophysiological problem of microcirculatory impairment during septic shock. We hypothesized that treatment with rhAPC at least partly reverses these deleterious effects of endotoxemia. To investigate this hypothesis, we measured several parameters of macro- and microcirculation in a model of neonatal endotoxic shock in which piglets were challenged with lipopolysaccharide (LPS).
Methods
Intravital microscopy
The use of orthogonal polarization spectral imaging (OPS imaging; Cytoscan®, Cytometrics, Philadelphia, PA, USA) as a noninvasive method of microcirculatory assessment in preterm and term infants has been described by Genzel-Boroviczeny.Citation17 OPS imaging is a microscopic approach based on epi-illumination with polarized light.Citation18 A virtual light source is created at a depth of about 1 mm within the tissue through the use of special optics. The light shining through the tissue is absorbed by hemoglobin, yielding an image of the illuminated vessel in negative contrast with a resolution of 1 pixel = 1 μm. Fluorescent dyes are not necessary. Capillary red blood cell velocity (RBCV) is directly measured, whereas functional capillary density (FCD) and capillary red blood cell flow (FRBC) are calculated. Functional capillary density (FCD [cm/cm2]) represents the fraction of capillaries that are perfused compared to all capillaries in a defined region of interest. Capillary red blood cell flow (FRBC [μm3/sec]) is calculated from the capillary diameter and red blood cell velocity (VRBC [μm/sec]) (FRBC = π/4 × D2 × RBCV).Citation19 Using OPS imaging, it is possible to investigate any accessible surface in animals or humans such as skin, mucous membranes or internal organs.Citation20 OPS has been validated for quantitative measurements of microcirculatory parameters in an animal model compared to common intravital microscopy with fluorescent dyes.Citation20 In humans, OPS has been validated in healthy volunteers before and after venous occlusion of the forearm, as well as in patients with sepsis.Citation21
Here, we captured and examined video sequences of an unbranched part of an arteriole and venule with a length of 100 μm and a maximal diameter of 50 μm for RBC flow (FRBC) and RBC velocity (VRBC). Of the three video films taken at each time point, the one with the best quality was further used for calculation of functional capillary density (FCD) with an integrated calculating program (Capyscope®; KK Technology, Brightham, UK).
OPS microscopy has a number of intrinsic limitations. In order to maximize the reproducibility within our study and for other researchers, we applied the following precautions: The piglets were paralyzed to reduce the spontaneous movements of the intestine and therefore potential errors of microcirculatory measurements. Analysis was performed off line in order to keep intervals between measurements identical. To avoid interindividual differences, one nonblinded investigator performed assessment and analysis of microcirculation data. Later, the video sequences were independently re-evaluated by four blinded investigators to prevent bias. The videos were categorized using the following semiquantitative scoring system:
Score 0: No capillaries visible.
Score 1: No or few capillaries visible, low velocity or no visible flow.
Score 2: Some capillaries visible, moderate velocity, visible flow.
Score 3: Many capillaries visible, high velocity, flow obvious.
Setting
Ten German Landrace neonatal piglets (mean bodyweight 2420 g, mean age 2 d) were used in this study. The animals received 0.02mg atropine (Atropin®, Braun, Melsungen, Germany) and were sedated with azaperon (Stresnil®, Janssen-Cilag, Baar, Switzerland, 8 mg/kg i.m.). The piglets received a continuous infusion of glucose 10% (10 ml/kg/h) via a peripheral venous access during the whole study period. Ketamine (10 mg/kg) was given i.m., followed by insertion of a 3.0 CH endotracheal tube via tracheotomy. For continuous monitoring of hemodynamic parameters and blood sampling, a catheter (4 F; Argyle®, Sherwood Medical, Tullamore, Ireland) was placed into the left carotid artery. Median laparotomy was performed to access the ileum. Analgesia and sedation were achieved with 0.3mg/kg/h midazolam (Dormicum®, Braun) and 1 μg/kg/h fentanyl (Fentanyl®, Janssen Cilag). The piglets were paralyzed with 0.1mg/kg/h pancuronium (Pancuronium Curamed®, Curamed, Karlsruhe, Germany). Rectal body temperature was recorded continuously and a temperature of 38.5 °C was maintained with a heated mattress. Hemodynamic measurements included continuous monitoring of heart rate as well as systolic, mean, and diastolic pressures (Monitor: Solar 8000®, Marquette Hellige Freiburg, Germany; Transducer: Medex System®, Medex, Carlsbad, CA, USA). Measurement of these parameters and assessment of microcirculation using OPS imaging (Cytoscan®) were performed simultaneously. At each time point, three different sites on the peritoneal surface of the ileum were recorded for 30 sec at 10 frames/second. After a stabilization period of 30 min after the surgical procedure, each piglet received an intravenous bolus of 500 μg/kg E. coli LPS (O 111 B 04, Sigma-Aldrich Chemie GmbH, Munich, Germany). Five piglets received 24 μg/kg/h rhAPC (Xigris®, Eli Lilly) as a continuous intravenous infusion starting 30 min after LPS. The infusion was continued to the end of the study period. Five piglets received saline and served as controls. Hemodynamic and microcirculatory parameters (at three sites as described above) were assessed prior to endotoxemia (baseline, 0 min) and every 30 min for a period of three hours (30, 60, 90, 120, 150, and 180 min). At the same time points, blood samples were taken in which red blood cells (RBC), white blood cells (WBC) and platelets (PLT) and the hematocrit (Hct) were measured. No intervention such as volume resuscitation or inotropic support was given. After the study period, the piglets were euthanized with T 61® (embrutamide/tetracaine, Hoechst, Frankfurt/Main, Germany). Laboratory evaluation was performed by blinded laboratory technicians. The Committee on Animal Research/State of Hesse approved the protocol of this study (Nr F. 133/04).
Statistics
Statistic analysis was performed with Sigma Stat® (SYSTAT Software Inc, Richmond, CA, USA) using a two-way repeated measure ANOVA with Bonferroni post-hoc pairwise comparison on the raw data. A p < 0.05 was considered significant. Microcirculatory parameters and mean arterial blood pressure (MAP) were assigned a value of 0 after subjects died from hypotension. All data are displayed as mean values and ± SEM.
Results
Survival and bleeding complications
Two animals in the control group died of septic shock-induced hypotension at 125 and 135 min. None of the animals treated with rhAPC died during the study period. None of the animals in either group suffered from bleeding events, defined as macroscopic bleeding or an obvious extravasation of RBC from arterioles or venules.
Hematology
Hematocrit and hemoglobin levels as well as RBC count did not change significantly (). There was a trend to reduced platelet and white blood counts after the initiation of endotoxic shock in all animals. The most pronounced decline was observed in the control group, although the difference to the rhAPC group was not statistically significant ().
Table 1 Blood cell counts
Macrocirculation
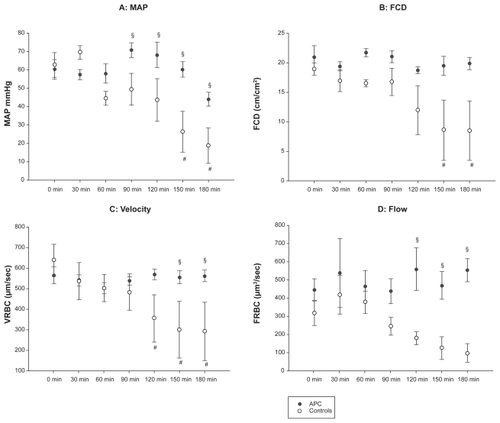
Whereas the MAP dropped dramatically in the control piglets (open circles in ), the decrease in animals which received rhAPC was not significant. From 90 min onwards, the difference between the treatment and the control groups was marked () and statistically significant despite a considerable interindividual variability.
Figure 1 Macro- and microcirculatory effects of recombinant activated protein C (rhAPC). Endotoxic shock was induced by injection of 500 μg/kg LPS in 10 piglets at t0 (0 min). A continuous infusion of 24 μg/kg/hrhAPC(five animals, black circles) or vehicle (five animals, open circles) was started at 30 min.
Microcirculation
We detected changes in the microcirculation as early as 30 min after LPS administration. The observed impairment of the microcirculation increased over time in the control group, whereas microcirculation was preserved in the animals receiving rhAPC (). Although the rhAPC-mediated protection as measured by functional capillary density (FDC), a parameter for perfusion, did not reach statistical significance due to inter-individual variability, the decrease in FDC in the control animals was considerable (). As shown in , the velocity of capillary red blood cell flow (VRBC) was significantly higher in rhAPC-treated animals compared to the control group at 150 and 180 min. In the late stages of the experiment, the VRBC was significantly reduced compared to baseline at 120 min and 180 min in control piglets.
Red blood cell flow (FRBC) was maintained over the complete study period of 180 min in the rhAPC group, whereas FRBC decreased in control animals. From 120 min onwards, FRBC was significantly higher in animals treated with rhAPC compared to controls.
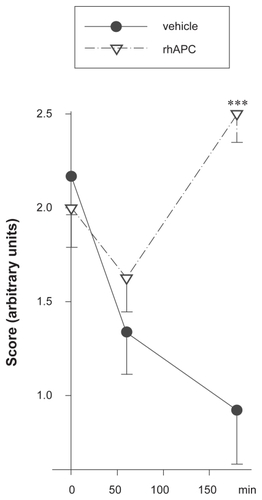
In addition, video sequences recorded at different time points and under different treatment conditions were scored by four independent, blinded investigators. These volunteers were provided with one exemplary video for the semi-quantitative scores 0 to 3 with 0 being no flow with rarified capillaries and 3 standing for unimpaired flow with normal capillary density. Thereafter, 18 video sequences were assessed by each volunteer. The blinded assessors confirmed the results documented by the nonblinded investigator. shows the LPS-induced deterioration of microcirculation over the course of the study period as well as the reversal of this effect by rhAPC as per the blinded volunteers. One representative video for each condition (Normal microcirculation before LPS injection [t0], impaired microcirculation [LPS alone], and improvement by treatment with rhAPC [LPS + rhAPC]) can be viewed online (see http://dovepress.com/submission_video_5863.php).
Figure 2 Amelioration of LPS-induced impairment of microcirculation. Microcirculation was assessed half-hourly by OPS-microscopy at three sites of the intestine in each of 10 piglets which had received 500 μg/kg LPS. The video sequences obtained during the three-hour study period were independently assessed by four blinded investigators using a semiquantitative scoring system. After having seen one representative video for each category, they applied the following scores to 18 sequences: score 0, no visible capillaries; score 1, very few visible capillaries, low velocity or no visible blood cell flow; score 2, some visible capillaries, moderate velocity, visible blood cell flow; score 3, many visible capillaries, high velocity, blood cell flow obvious.
Discussion
In newborn piglets, we demonstrate impairment of the intestinal microcirculation early after induction of endotoxic shock and show that this can be prevented by treatment with rhAPC. Our data concur with another study that provided an insight into the visceral endothelial response to a sepsis trigger and underlined the importance of the intestine as a primary shock organ.Citation21 Our study provides new affirmative data for the clinical observation that the use of protein C results in an improvement of sepsis-induced microcirculatory dysfunction.Citation14,Citation22
In accord with the findings of De Backer and colleagues, sepsis-induced alteration of the MAP was positively influenced by rhAPC.Citation22 The De Backer study demonstrated a rapid increase in MAP shortly after infusion of rhAPC in patients with septic shock. In addition, a recent study by Wang and colleagues pointed out the beneficial effects of rhAPC on arterial blood pressure and microcirculation.Citation23 Our study, which focused on the early phase of endotoxic shock, established that early intervention with rhAPC prevented a dramatic fall in blood pressure induced by injection of LPS. We did not measure the cardiac index, therefore we can only speculate that the preservation of the MAP may have partly been caused by a direct positive effect of rhAPC on cardiac output. However, it seems more likely that the improvement of the MAP was caused by an endothelial effect of rhAPC, for example by a reduction in vasodilatation in the treated animals compared to the controls or by a prevention of exaggerated endothelial inflammation and consequently reduced coagulability.
In fact, other groups as well as our own previously reported that both rhAPC and the zymogen human protein C concentrate (hPC) inhibit the induction of pro-inflammatory cytokines and thereby reduce sepsis-induced endothelial injury, resulting in a reduction in the incidence of microthrombosis.Citation5 This reduction in microvascular thrombosis may play a role in the overall protection conferred by rhAPC.
The major disadvantage in the clinical use of rhAPC in septic patients is the risk of life-threatening hemorrhage. In contrast with other publications in which hemorrhage following treatment with rhAPC was observed, there were no macro- or microscopic bleeding events in our study and Hb and Hct levels were maintained in treatment and control groups. Although our study does not allow any conclusions regarding the risk of hemorrhage and the safety of rhAPC in septic neonates because of a limited number of animals and a short observation period, we speculate that potential differences in bleeding events may have been due to the different APC dosing regimen employed in our study (infusion of 24 μg/kg/h) versus a bolus of 2 and 5 mg/kg used by Iba and colleagues.Citation16 This regimen has been described by Russo and colleaguesCitation24 who treated pediatric cancer patients with severe ongoing sepsis with 24 μg/kg/h. Although these patients had a low platelet count (mean 9000/nl), none of them experienced bleeding events during treatment. However, these patients were not coagulopathic and therefore do not necessarily represent the common pediatric sepsis patient.
Although we found no bleeding in our study, there is no doubt that rhAPC induces significant bleeding when administered to patients, thereby limiting its use in adults and causing extreme reluctance to employ rhAPC in infants and children.Citation8,Citation9,Citation25 As an alternative to rhAPC, there is strong evidence of sufficient endogenous activation of hPC in children with sepsis.Citation26 We and others have shown that hPC is effective in the treatment of neonates and children with sepsis-induced purpura fulminans without increasing the risk of hemorrhage.Citation14,Citation26–Citation28 In porcine animal models, however, activation of hPC is known to be less effective. Thus, a benefit was doubtful and we decided not to use it in this study.
Limitations of this study include the rather small number of subjects as well as the fact that the animal model requires the use of rhAPC as opposed to the clinically preferable zymogen hPC. In addition, data obtained by OPS microscopy have to be interpreted with caution, since although we employed an array of precautions (see Methods), it is impossible to eliminate all subjective elements from an OPS study. Nonetheless, our data present significant evidence for a beneficial effect of protein C on sepsis-induced deterioration of the macro- and microcirculation in an experimental model of neonatal septic shock. We show that microvascular perfusion is protected in the treatment group as measured by microcirculatory blood flow and functional capillary density. The findings of our study may encourage researchers to further explore protein C in neonatal diseases in which intestinal microcirculation is impaired.
Acknowledgments
We would like to thank Prof Philip Berger and Elizabeth Skuza for their help in data analysis and review of the manuscript. Dr Alex Veldman is a member of the Baxter Advisory Board and as such receives an honorarium. All other authors declare that there are no financial or other conflicts of interest. This study was not supported by any industrial grant.
References
- MeadowWFrainLRenYLeeGSonejiSLantosJSerial assessment of mortality in the neonatal intensive care unit by algorithm and intuition: certainty, uncertainty, and informed consentPediatrics2002109587888611986450
- AsakaSShibayamaYNakataKPathogenesis of focal and random hepatocellular necrosis in endotoxemia: microscopic observation in vivoLiver2006161831878873005
- TaylorFBJrChangAEsmonCTD’AngeloAVigano-D’AngeloSBlickKEProtein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboonJ Clin Invest1987799189253102560
- KurosawaSEsmonCTStearns-KurosawaDJThe soluble endothelial protein C receptor binds to activated neutrophils: involvement of proteinase-3 and CD11b/CD18J Immunol20001654697470311035113
- NoldMFNold-PetryCAFischerDActivated protein C down-regulates p38 mitogen-activated protein kinase and improves clinical parameters in an in-vivo model of septic shockThromb Haemost2007981118112618000619
- TaylorFBJrStearns-KurosawaDJKurosawaSThe endothelial cell protein C receptor aids in host defense against Escherichia coli sepsisBlood2000951680168610688824
- WhiteBSchmidtMMurphyCLivingstoneWActivated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kappaB (NF-kappaB) and tumour necrosis factor alpha (TNF-alpha) production in the THP-1 monocytic cell lineBr J Haematol200011013013410930989
- BernardGRVincentJLLaterrePFEfficacy and safety of recombinant human activated protein C for severe sepsisN Engl J Med200134469970911236773
- NadelSGoldsteinBWilliamsMDDrotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trialLancet200736983684317350452
- AlbualiWJSinghRNFraserDDScottLAKorneckiADrotrecogin alfa activated treatment in a neonate with sepsis and multi-organ failureSaudi Med J2005261289129216127532
- RawiczMSitkowskaBRudzinskaIKornackaMKBochenskiPRecombinant human activated protein C for severe sepsis in a neonateMed Sci Monit20028CS90CS9412444386
- De CarolisMPPolimeniVPapacciPLacerenzaSRomagnoliCSevere sepsis in a premature baby. protein c replacement therapyTurk J Pediatr200850440540819014060
- YesilirmakDCKumralATugyanKEffects of activated protein C on neonatal hypoxic ischemic brain injuryBrain Res20081210566218420181
- KreuzWVeldmannAFischerDSchlosserRVolkWREttingshausenCENeonatal sepsis: a challenge in hemostaseologySemin Thromb Hemost19992553153510632474
- VenkataseshanSDuttaSAhluwaliaJNarangALow plasma protein C vaules predict mortality in low birth weight neonates with septicemiaPediatr Infect Dis J20072668468817848878
- IbaTKidokoroAFukunagaMNagakariKShirahamaAIdaYActivated protein C improves the visceral microcirculation by attenuating the leukocyte-endothelial interaction in a rat lipopolysaccharide modelCrit Care Med20053336837215699841
- Genzel-BoroviczenyOStrotgenJHarrisAGMessmerKChristFOrthogonal polarization spectral imaging (OPS): a novel method to measure the microcirculation in term and preterm infants transcutaneouslyPediatr Res20035138639111861946
- SlaafDWTangelderGJRenemanRSJagerKBollingerAA versatile incident illuminator for intravital microscopyInt J Microcirc Clin Exp198763913973429145
- GrossJFAroestyJMathematical models of capillary flow: a critical reviewBiorheology197292252644579396
- LangerSBiberthalerPHarrisAGSteinauHUMessmerKIn vivo monitoring of microvessels in skin flaps: introduction of a novel techniqueMicrosurgery20012131732411754431
- BiberthalerPLangerSLuchtingBKhandogaAMessmerKIn vivo assessment of colon microcirculation: comparison of the new OPS imaging technique with intravital microscopyEur J Med Res2001652553411772540
- BoermaECMathuraKRvan der VoortPHSpronkPEInceCQuantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: a prospective validation studyCrit Care20059R601R60616280059
- De BackerVerdantCChieregoMKochMGulloAVincentJLEffects of drotrecogin alfa activated on microcirculatory alterations in patients with severe sepsisCrit Care Med2006341918192416715034
- WangZSuFRogiersPVincentJLBeneficial effects of recombinant activated protein C in a ewe model of septic shockCrit Care2007351125942600
- RussoGLa SpinaMDismaNAstutoMDrotrecogin alfa activated as a treatment for severe sepsis in pediatric cancerBone Marrow Transplant20063857557616921401
- KylatRIOhlssonARecombinant human activated protein C for severe sepsis in neonatesCochrane Database Syst Rev20062CD00538516625638
- de KleijnEDde GrootRHackCEActivation of protein C following infusion of protein C concentrate in children with severe meningococcal sepsis and purpura fulminans: a randomized, double-blinded, placebo-controlled, dose-finding studyCrit Care Med2003311839184712794428
- WhiteBLivingstoneWMurphyCHodgsonARaffertyMSmithOPAn open-label study of the role of adjuvant hemostatic support with protein C replacement therapy in purpura fulminans-associated meningococcemiaBlood2000963719372411090052