Abstract
Stroke is a leading cause of death and disability worldwide. The importance of lowering blood pressure for reducing the risk of stroke is well established. However, not all the benefits of antihypertensive treatments in stroke can be accounted for by reductions in BP and there may be differences between antihypertensive classes as to which provides optimal protection. Dihydropyridine calcium channel blockers, such as amlodipine, and angiotensin receptor blockers, such as valsartan, represent the two antihypertensive drug classes with the strongest supportive data for the prevention of stroke. Therefore, when combination therapy is required, a combination of these two antihypertensive classes represents a logical approach.
Introduction
Stroke is a leading cause of death and disability worldwide.Citation1 It has been estimated that 15 million people worldwide suffer a stroke each year and one-third of these individuals will die.Citation2 Moreover, one-third of these stroke victims will be left permanently disabled, profoundly affecting their quality of life and placing a large burden on their families, communities and society.Citation2 The total incidence of stroke is expected to increase considerably over the next two decades.Citation1 In the European Union, for example, the World Health Organization-estimated number of stroke events is expected to increase from 1.1 million in 2000 to 1.5 million by 2025.Citation3 In other, less developed regions of the world, stroke is reaching pandemic proportions as a result of rapid urbanization and industrialization.Citation4
Risk factors for stroke and the importance of blood pressure lowering
Risk factors for stroke are classified according to whether they are modifiable or not. Nonmodifiable risk factors include old age, male gender, Asian and Black ethnicities, and strong family history. Among the well documented modifiable risk factors for stroke are: hypertension, cigarette smoking, diabetes, dyslipidemia, obesity, atrial fibrillation (AF), carotid artery stenosis, and a previous stroke, transient ischemic attack (TIA) or heart attack.Citation5 In addition, left ventricular hypertrophy (LVH) and abnormal left ventricular geometry have been shown to be associated with increased risk of stroke in a multi-ethnic population.Citation6
A prior stroke or TIA places patients at very high risk of a recurrent cerebrovascular event.Citation7 Indeed, in a population-based study of early risk of stroke after a TIA or minor stroke, the estimated risk of recurrence at 3 months post event was 17.3% and 18.5%, respectively.Citation8 In a Chinese patient population with ischemic stroke who were registered in the Nanjing Stroke Registry Program, a first-year recurrence rate of 11.2% was reported. This is of interest because data on stroke occurrence and recurrence are very limited in ChinaCitation9 and much of Asia.Citation10 Given the global burden of stroke, effective therapeutic interventions aimed at primary and secondary prevention are needed.
Of the modifiable risk factors for stroke, hypertension serves as the most prevalent and powerful of risks,Citation11 regardless of geographic location and ethnicity. Approximately 54% of strokes worldwide can be attributed to elevated blood pressure (BP).Citation12 Such is the association that people with hypertension are 3 to 4 times more likely to suffer a stroke than those without hypertension.Citation13 The relationship between BP and risk of first stroke is direct, continuous and independent, with the risk increasing continuously above a BP of 115/75 mmHg.Citation11 Hypertension also increases the risk of stroke recurrence and it has been shown that approximately 25% to 30% of patients recovering from a stroke have raised BP at the time of discharge from hospital.Citation14
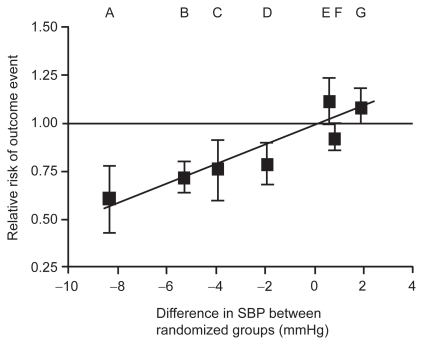
There is strong and consistent evidence that lowering elevated BP is an important therapeutic target in the primary and secondary prevention of stroke, regardless of age, gender or ethnicity (Asian or White).Citation15 A meta-analysis of nine randomized comparative trials found that a reduction in systolic blood pressure (SBP) of just 1 to 3 mmHg led to a reduction in risk of stroke of 20% to 30%.Citation16 Moreover, in age-specific analyses from two cohort study overviews (the Prospective Studies Collaboration and the Asia Pacific Cohort Studies Collaboration),Citation17 a 10 mmHg reduction in SBP was associated with a 35% reduction in the risk of stroke in subjects aged 60 to 69 years ().Citation18 Similar benefits have also been shown for stroke survivors. In a meta-analysis including 6752 patients with a previous history of cerebrovascular disease (stroke or TIA), antihypertensive therapy resulted in a 28% reduction in risk for stroke recurrence.Citation19 Antihypertensive treatment that effectively reduces BP to target levels may therefore be one of the most important approaches for reducing the risk of stroke. Indeed, the importance of treatment has been demonstrated in a study where early discontinuation with antihypertensive therapy was associated with a 28% increase in the risk of stroke.Citation20
Table 1 Reductions in the risk of stroke related to systolic blood pressure (SBP) predicted from cohort studies and observed in clinical trials
This review will examine the evidence available for the use of calcium channel blockers (CCBs) and renin angiotensin system (RAS) blockers – with focus on angiotensin receptor blockers (ARBs) – in the primary and secondary prevention of stroke, and explore whether there is potential in this regard for dual-mechanism therapy with a CCB/ARB.
Antihypertensive therapy in the primary and secondary prevention of stroke
What evidence is available with CCBs?
Numerous studies have compared the effects of CCBs with placebo or an active treatment for preventing cerebrovascular events (). Two placebo controlled trials, the Prospective Randomized Evaluation of the Vascular Effects of Norvasc Trial (PREVENT) and Systolic Hypertension in Europe (Syst-Eur) study have assessed the effects of CCBs compared with placebo for reducing the risk of stroke.Citation21–Citation23 A meta-analysis of these two trials provided clear evidence of a reduction in stroke risk with CCBs vs placebo of 39%.Citation18 The Systolic Hypertension in China (Syst-China) study has also confirmed the benefits of the dihydropyridine CCB, nitrendipine, for improving prognosis in Chinese patients. Indeed, nitrendipine-based treatment reduced the incidence of fatal and nonfatal stroke by 38% (hazard ratio [HR] 0.62 [95% confidence interval (CI) 0.42–0.91]; P < 0.05).Citation24 In addition, in the ACTION (A Coronary disease Trial Investigating Outcome with Nifedipine GITS) trial, a CCB reduced the risk of any stroke or TIA by 30% compared with placebo in patients with hypertension and stable angina.Citation25 Following ischemic stroke, CCB treatment has been associated with a reduction in mortality (odds ratio [OR] 0.38 [0.17–0.88] vs no CCB treatment) and improvements in the stroke impact scale-16.Citation26
Table 2 Stroke outcomes in various trials with CCBs, ACeis and ARBs
In addition to their benefits compared with placebo, CCBs have also been shown to provide better protection against fatal and nonfatal stroke than older drugs, such as β-blockers and diuretics.Citation27,Citation28 In addition, CCBs have been shown to provide benefit over angiotensin-converting enzyme inhibitors (ACEIs) (11% relative risk [RR] reduction) in a meta-analysis of 4 trials (the Appropriate Blood Pressure Control in Diabetes [ABCD], the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial [ALLHAT], the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial [FACET] and the Swedish Trial in Old Patients with Hypertension [STOP-2].Citation18 A meta-regression analysis has confirmed that CCBs are superior to ACEIs for the prevention of stroke (P = 0.042).Citation29
Amlodipine
In the BP-lowering arm of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), amlodipine-based treatment reduced fatal and nonfatal stroke by 23% (HR 0.77 [0.66–0.89]; P < 0.0003) compared with atenolol-based treatment in a range of high cardiovascular (CV) risk patients (11% with a previous stroke or TIA) with uncontrolled BP (SBP ≥ 160 mmHg and/or diastolic blood pressure [DBP] ≥ 100 mmHg BP not on antihypertensive treatment or SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg; n = 19342).Citation30 On average, BP levels were lower throughout the trial in patients allocated to amlodipine-based treatment compared with atenolol-based treatment (average difference 2.7/1.9 mmHg). Although BP was the largest contributor to stroke events, peripheral BP measurements could not fully account for the treatment differences in stroke.Citation31
The Comparison of AMlodipine vs Enalapril to Limit Occurrences of Thrombosis (CAMELOT) study compared amlodipine with enalapril or placebo in 1991 patients with angiographically documented coronary artery disease and DBP < 100 mmHg. Amlodipine reduced the risk of stroke or TIA by 50% compared with placebo (HR 0.50 [0.19–1.32]) and 24% compared with enalapril (HR 0.76 [0.26–2.20]), although these reductions did not achieve statistical significance (P = 0.15 and P = 0.61, respectively), possibly due to the small numbers of events.Citation32
ALLHAT compared three different antihypertensive regimens (amlodipine, chlorthalidone, and lisinopril) in 33357 patients with stage 1 or 2 hypertension and at least one other risk factor for coronary heart disease.Citation33 Almost one quarter (23%) of patients had a previous history of stroke or myocardial infarction (MI) at baseline. Stroke was assessed as a secondary endpoint and there were significantly more strokes for lisinopril compared with amlodipine (RR 1.23 [1.08–1.41]; P < 0.003).Citation34 On average, follow-up BP was 1.5/1.1 mmHg higher in patients treated with lisinopril compared with amlodipine.Citation34 However, there was no significant difference in stroke incidence between amlodipine and chlorthalidone (RR 0.93 [0.82–1.06]; P = 0.28) in this study.Citation33
An analysis of six actively controlled trials involving an amlodipine treatment group (including the three trials described above plus the Candesartan Antihypertensive Survival Evaluation in Japan [CASE-J] trial, the Valsartan Antihypertensive Long-term Use Evaluation [VALUE] and the Irbesartan Diabetic Nephropathy Trial [IDNT]) showed that amlodipine provided more protection against stroke than other antihypertensive agents (OR 81 [95% CI 0.75–0.87]; P < 0.0001).Citation35 Moreover, the risk of stroke with amlodipine was statistically less when compared with non-ARB antihypertensive drugs (OR 0.79 [95% CI 0.72–0.87]; P < 0.0001) and ARB therapies separately (OR 0.84 [95% CI 0.73–0.97]; P = 0.02).
What evidence is available with ARBs?
The RAS
The RAS has been linked to the development and progression of cerebrovascular disease in patients with hypertension.Citation36,Citation37 Indeed, angiotensin II is thought to induce cerebrovascular hypertrophy and remodeling, inhibit endothelium-dependent relaxation and disrupt the blood-brain barrier.Citation36 Therefore, it might be assumed that RAS blockade would provide cerebroprotection. However, studies with ACEIs have produced mixed results ().
In the Heart Outcomes Prevention Evaluation (HOPE) study ramipril reduced all stroke by 32% (RR 0.68 [0.56–0.84]) and fatal stroke by 61% (RR 0.39 [0.22–0.67]) compared with placebo in a study of 9297 patients with high CV risk (~11% had a prior history of stroke).Citation38,Citation39 In the Perindopril Protection Against Recurrent Stroke Study (PROGRESS), active treatment with perindopril monotherapy or perindopril plus a diuretic (indapamide) reduced stroke by 28% in 6105 patients with a history of stroke or TIA.Citation40 However, in PROGRESS, monotherapy with perindopril had little beneficial effect on stroke when compared with placebo, despite a reduction in BP of 5/3 mmHg.Citation40 This observation is consistent with a meta-analysis of three smaller trials (Survival And Ventricular Enlargement [SAVE], Acute Infarction Ramipril Efficacy [AIRE] and TRAndolapril Cardiac Evaluation [TRACE]) which did not observe a beneficial effect of ACEIs on stroke compared with placebo (OR 1.10 [0.84–1.43]; P = 0.48) in patients with heart failure (HF) or left ventricular dysfunction.Citation41
In studies with an active comparator, data supporting the use of ACEIs are even less convincing. In the Captopril Prevention Project (CAPPP), fatal/nonfatal stroke was found to be 1.25 times more frequent in patients randomized to captopril vs conventional therapy with diuretics, β-blockers or both,Citation42 although a subanalysis found no difference in stroke between study groups in patients with diabetes.Citation43 In ALLHAT, lisinopril was less effective in preventing stroke vs chlorthalidone (RR 1.15 [1.02–1.30]; P = 0.02),Citation33 although interpreting these findings is confounded by the different BPs achieved.
It has subsequently been suggested that angiotensin II might have a protective effect on stroke.Citation44–Citation46 In an analysis of 26 prospective randomized trials during which 7108 strokes occurred in 206,632 patients without HF, Boutitie et al noted that differences in BP do not totally account for differences in stroke risk and that the relative risk of stroke was 17% greater with agents that potentially decrease angiotensin II levels (β-blockers and ACEIs) compared with those that increase angiotensin II levels (thiazide diuretics, dihydropyridine CCBs and ARBs).Citation44 It was hypothesized that increased angiotensin II may act on angiotensin type 2 (AT2) receptors and mediate protective effects such as improving collateral circulation and neuronal resistance to anoxia.Citation44 However, mechanistic data to support such an effect in the cerebral circulation in humans are lacking and data from animal models should be interpreted with caution as the presence and role of receptors can differ from that in humans.
Stroke protection with ARBs
According to the hypothesis proposed by Boutitie et al ARBs should help protect against stroke as, in addition to lowering BP, they inhibit the negative effects of angiotensin type I (AT1) receptors in the cerebral circulation, but allow angiotensin to mediate potentially stroke-protective effects through the AT2 receptor. Observations from large clinical trials would support this suggestion.
In the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study, losartan substantially reduced the rate of fatal and nonfatal stroke by 25% vs atenolol (HR 0.75 [0.63–0.89]; P = 0.001) in 9193 patients with hypertension and LVH.Citation47 A small (1.1 mmHg) but significant difference in the reduction in systolic BP (P = 0.017) was observed between treatments in favor of losartan. A substudy of patients with LVH and isolated systolic hypertension in the LIFE trial demonstrated an even more impressive 40% stroke reduction.Citation48 AF is a known risk factor for stroke and losartan reduced the incidence of stroke by 51% (HR 0.49 [0.29–0.86]; P = 0.01) in patients with new-onset AF in the LIFE study.Citation49 In the Study on Cognition and Prognosis in the Elderly (SCOPE), candesartan-based treatment reduced nonfatal stroke by 27.8% and all stroke by 23.6% compared with placebo in 4964 elderly patients.Citation50 The Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) study reported a nonsignificant 17% reduction in stroke with telmisartan compared with placebo in high-risk patients who were intolerant to ACEIs.Citation51 The TRANSCEND trial included a large proportion of patients without hypertension, in whom the benefits of BP lowering remains highly uncertain.
In addition to the strong data with ARBs for the primary prevention of stroke in placebo-controlled trials, several studies have indicated that ARBs are at least as effective as other antihypertensive agents for preventing stroke (). For example, in the CASE-J study there was no significant difference in cerebrovascular events between amlodipine- and candesartan-based regimens in Japanese high-risk patients (n = 4728) with hypertension, including approximately 10% of patients with a history of cerebrovascular events.Citation52 Recently, the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) programme compared the effects of an ARB, telmisartan, with an ACEI, ramipril, and both agents in combination, in a range of patients at high risk of CV disease (n = 25620). ONTARGET reported no significant difference between ramipril and telmisartan for reducing stroke.Citation53 In addition, a combination of ramipril and telmisartan provided no additional benefit to either monotherapy. These findings in ONTARGET may seem to contradict the hypothesis suggested by Boutitie et al ONTARGET enrolled individuals mostly at high risk of cardiac events rather than cerebrovascular events, where ramipril has already been shown to improve stroke in these patients.Citation38 ACEIs are known to reduce cardiac risk and complications.Citation39 Thus, it may be that many of the strokes in HOPE and ONTARGET occurred secondary to cardiac complications and this would explain some of the benefit of these agents on stroke. A recent meta-analysis covering 49924 patients in 6 trials (ONTARGET, Valsartan In Acute Myocardial Infarction Trial [VALIANT], Evaluation of Losartan In The Elderly study [ELITE] I and II, OPtimal Trial In Myocardial infarction with the Angiotensin II Antagonist Losartan [OPTIMAAL] and Diabetics Exposed to Telmisartan and enalaprIL [DETAIL]) comparing ACEIs and ARBs head-to-head noted that, despite similar effects on MI, ARBs were associated with an 8% lower risk of stroke compared with ACEIs (OR 0.92 [95% CI 0.85–0.99]; P = 0.036).Citation54
The benefits of ARBs for the prevention of secondary stroke are less well known and are undergoing intense scrutiny. Indeed, it has long been debated whether elevated BP should be lowered in the acute phase of stroke as it is feared that lowering BP would reduce cerebral blood perfusion. The Acute Candesartan Cilexetil Therapy in Stroke Survivors (ACCESS) study assessed the safety of a modest BP reduction by candesartan in the early treatment of stroke (n = 342) and showed significant reductions in 12-month mortality and vascular events with candesartan compared with placebo (OR 0.475 [95% CI 0.252–0.895]).Citation55 The Scandinavian Candesartan Acute Stroke Trial (SCAST) is designed to compare the effects of an ARB (candesartan) or placebo on CV morbidity and mortality in approximately 2500 patients with acute stroke (<30 hours) and elevated SBP (≥ 140 mmHg).Citation56
The Morbidity and Mortality After Stroke, Eprosartan compared with nitrendipine for Secondary Prevention (MOSES) study was the first to compare an ARB with a short-acting CCB in a population of patients with hypertension and a history of cerebrovascular events. The trial reported a significant (P = 0.026) 25% reduction in cerebrovascular events with eprosartan compared with nitrendipine, despite similar reductions in BP.Citation57 Thus, the MOSES and ACCESS studies demonstrate that ARBs are effective for the secondary prevention of stroke. In contrast, the PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes) study, the largest randomized double-blind secondary stroke prevention trial to date,Citation58 did not find any significant benefit of telmisartan treatment compared with placebo on recurrent stroke in 20332 patients with an ischemic stroke within the last <120 days and who were stable (HR 0.95 [95% CI 0.86–1.04]; P = 0.23).Citation59 The lack of a significant benefit between telmisartan and placebo in these patients could be due to methodological considerations, such as the inclusion of patients with low BP (baseline SBP was 144 ± 17 mmHg) and carotid plaques. However, a prespecified subgroup analyses indicated no heterogeneity of effects on stroke across baseline SBP categories (<135, 135 to ≤150 and >150 mmHg). The presence of a J-curve relationship between BP and stroke, similar to that reported for a composite of all-cause mortality, nonfatal MI and nonfatal stroke and BP in patients with hypertension and coronary artery disease in the INternational VErapamil SR-trandolapril Study (INVEST),Citation60 is unlikely to account for the lack of benefit with telmisartan in PRoFESS. Indeed, several studies have noted that a reduction in SBP to <140 mmHg is associated with a reduced risk of stroke in patients with a prior stroke/TIACitation61 or in high-risk hypertension.Citation62 Moreover, an analysis of PROGRESS observed similar risk reduction in each of four subgroups defined by baseline BP of less than 120, 120 to 139, 140 to 159, and 160 mmHg or greater (P = 0.5 for homogeneity), indicating that achieving low BP levels should not be a concern in patients with prior cerebrovascular disease.Citation63
In the Japanese Investigation of Kinetic Evaluation in Hypertensive Event and Remodelling Treatment (JIKEI HEART) study, valsartan has been examined in a Japanese population (n = 4728) with hypertension and other CV disease (patients with a cerebrovascular event in the previous 3 months were excluded) who were receiving usual treatment. Of patients who received valsartan on top of usual treatment, 29 had stroke (or TIA), compared with 48 in patients receiving non-ARB-based treatment (HR 0.60; P = 0.0280).Citation64 The VALUE trial compared the effects of the ARB valsartan with the CCB amlodipine on cardiac morbidity and mortality in 15245 patients with hypertension and high CV risk.Citation65 Almost 20% of the patients in VALUE had a history of stroke or TIA at baseline. No significant difference in the incidence of stroke was noted between the two treatment arms.Citation62,Citation65 In the VALUE study, an ARB was shown to reduce AF significantly more than amlodipine,Citation66 although this was not associated with a significant reduction in stroke,Citation65 possibly due to the small numbers of patients with these events. Thus, valsartan and other ARBs appear to reduce the risk of stroke more than placebo and to a similar extent as CCBs in primary prevention populations.
In general, the cerebrovascular benefits of ARBs seem to be class-related rather than drug-related.Citation54 All ARBs might be expected to reduce the risk of stroke. Any differences in stroke protection between individual trials may be accounted for by difference in study design and/or patient populations.
What is the source of the benefit of ARBs and CCBs on stroke?
Reductions in BP are the most important determinant of CV outcome, and stroke in particular.Citation29 Most of the benefit of amlodipine on stroke can be explained by differences in BP control.Citation35 The relationship between BP and stroke is strong and even small changes in BP between treatments can result in differences in stroke ().Citation16,Citation67,Citation68 However, there does appear to be a BP-independent component that contributes to the benefit of CCBs on stroke.Citation31,Citation35 Similarly, reductions in the incidence of stroke with ARBs in the MOSES and ACCESS studies occurred despite reductions in BP being similar to that observed with the comparators used, suggesting that these agents also have some BP-independent benefits. Preclinical studies also support a BP-independent effect of ARBs on stroke. In normotensive rats, pretreatment of an ARB at a subantihypertensive dose was more effective than an ACEI for reducing infarct size and neurological deficits following transient focal ischemia.Citation69
Figure 1 Relationship between SBP and stroke. Reprinted from The Lancet, 362, Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials, 1527–1535.Citation67 Copyright © 2003, with permission from elsevier.
Abbreviations: ACei, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; SBP, systolic blood pressure.
There are several theoretical mechanisms whereby ARBs and CCBs might prevent stroke beyond BP reductions. For example, increased carotid intima-media thickness (CIMT) is associated with an increased risk for stroke Citation70 and it is known that CCBs can reduce carotid intima-media thickening to a greater degree than observed with ACEIs, despite similar reductions in BP.Citation71 It has been suggested that this effect on CIMT might explain the superior protection against stroke with these agents.Citation71 ARBs have also been shown to reduce CIMT in patients with hypertension,Citation72–Citation74 an effect greater than observed with atenolol despite similar reductions in BP.Citation72 This effect on CIMT observed with ARBs is thought to be mediated by improvements in nitric oxide production and decreases in oxidative stress.Citation74
Increased left ventricular mass (LVM) is a risk factor for stroke.Citation6 Increased LVM is also a risk factor for AF,Citation75 a known cause of stroke.Citation76,Citation77 Thus, a beneficial effect of ARBs and CCBs on LVH relative to other antihypertensive agents could also explain the strong supportive data for stroke prevention with these agents. Indeed, in a meta-analysis of the effects of antihypertensive treatment on LVM, CCBs and ARBs were reported to reduce LVM index by 11% and 13%, respectively, which are numerically greater reductions than those observed with other antihypertensive agents.Citation78
Changes in central aortic pressure but not peripheral BP could explain some differences between CCBs and other agents. Despite similar brachial pressures, amlodipine-based treatment reduced central SBP more than atenolol-based treatment in the ASCOT Conduit Artery Function Evaluation (CAFÉ) substudy.Citation79 It has been suggested that heart rate is a major determinant of the difference between central and brachial BP and might account for the less effective lowering of central BP with atenolol.Citation80 Thus, the effect on central BP and heart rate could account for some of the difference in stroke between atenolol and amlodipine in ASCOT. When assessing possible relationships of BP and stroke, many studies are limited by the use of sitting BP determined in the clinic. However, there are other BP parameters, such as central BP, night-time and 24-hour BP, BP variability and heart rate, which might also contribute to treatment differences in stroke, and further studies are required.
Finally, experiments in animals suggest that ARBs and CCBs might have BP-independent effects that might influence stroke outcomes. For example, studies in spontaneously hypertensive rats suggest that ARB treatment can reduce inflammation in cerebral microvesselsCitation81 and normalize the cerebral blood flow following ischemia.Citation82 Moreover, in a rat model of cerebral ischemia, ARB treatment reduced middle cerebral artery (MCA) media thickness and infarct area following occlusion of MCA.Citation83 Studies in rats also showed that the protection in cerebral circulation by improving cerebral blood flow autoregulation and reducing superoxide production, occurred with doses that do not reduce BP.Citation84 A similar effect has also been observed with amlodipine in ApoE knockout mice model of stroke.Citation85 Although these effects have been observed in animal models, these data should be cautiously translated to humans where these mechanisms have not been readily observed.
Although it is possible to speculate about the various possible cerebroprotective mechanisms of CCBs and ARBs, reductions in BP are key in preventing stroke. Moreover, caution should be used when comparing and interpreting differences in stroke reductions between clinical trials, as differences in trial design and selection criteria may influence the data. A meta-analysis of head-to head ACEI and ARB trials noting a slight benefit in stroke prevention with ARBs could not attribute any mechanistic basis to the cerebrovascular protection with ARBs, and it cannot be excluded that differences in blood pressure accounted for this observation.Citation54
Potential of combination therapy
As indicated previously, the relationship between BP reductions and the risk of stroke is well established ().Citation67 It has been suggested, therefore, that rapid, sustained reductions in BP are necessary for the optimal prevention of stroke in patients with hypertension.Citation45 Indeed, in VALUE the BP response after 1 month predicted CV events and survival.Citation62 Combination therapy has been suggested as an approach to achieve large, rapid reductions in BP and help optimize the reduction in stroke risk.Citation45
Few studies have assessed the benefits of combination therapy compared with monotherapy. The Felodipine Event Reduction (FEVER) study has compared a combination therapy (hydrochlorothiazide [HCTZ]/felodipine extended release) with monotherapy (HCTZ/placebo) in 9800 Chinese patients with hypertension and other CV risk factors. It was noted that addition of felodipine extended release to HCTZ treatment reduced BP by an additional 4.2/2.1 mmHg and reduced the incidence of fatal/nonfatal stroke by 27% vs HCTZ/placebo.Citation86 Thus, these studies would support the use of greater BP reductions with combination therapy to provide greater reductions in the risk of stroke. In contrast, combining an ARB and an ACEI in ONTARGET provided no additional benefit over monotherapy for reducing stroke despite an incremental reduction in BP of 2.4/1.4 mmHg over ramipril monotherapy.Citation53 Therefore, the choice of agents for combination may be an important consideration.
The Avoiding Cardiovascular events through COMbination therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial compared the clinical benefits of two single-pill combinations of antihypertensive agents (benazepril/HCTZ and amlodipine/benazepril) on CV mortality and morbidity in high-risk patients with hypertension.Citation87 It was noted that the CCB/ACEI combination decreased CV morbidity and mortality significantly more than the ACEI/diuretic (20% relative risk reduction; P < 0.001) despite similar reductions in BP.Citation88 There were numerically fewer strokes (fatal and nonfatal) with the CCB/ACEI compared with the CCB/diuretic (16% risk reduction) in ACCOMPLISH although this did not achieve statistical significance (P = 0.16), and it may be that there were insufficient events to establish a difference between treatments in this outcome.
In the JIKEI HEART study, addition of valsartan to conventional therapy was more effective at reducing stroke compared with non-ARB-based therapy.Citation64 Given that the majority of patients were receiving antihypertensive agents at baseline, this may suggest that that ARB-based combinations might have some utility in preventing stroke compared with non-ARB-based combinations.
In PROGRESS, combination therapy with perindopril and indapamide reduced BP by 12/5 mmHg and lowered the risk of recurrent stroke by 43% compared with placebo. However, single drug therapy with perindopril reduced BP by only 5/3 mmHg and resulted in no significant reduction in recurrent stroke risk.Citation40,Citation89 On the basis of these data, the US JNC VII guidelines recommend either treatment with a diuretic, an ACE inhibitor or both agents in combination for the prevention of recurrent stroke.Citation90 However, these recommendations were made before the results of studies investigating the use of ARBs for the prevention of secondary stroke (MOSES, ACCESS and PROFeSS) were published. The ESH-ESC guidelines recognize that antihypertensive treatment markedly reduces the incidence of stroke recurrence in patients with a history of stroke or TIA, and a BP goal of 130/80 mmHg is recommended.Citation91 Since evidence from trials suggests that the benefit predominantly depends on BP lowering, the ESH-ESC guidelines indicate that all available drugs and ‘rational’ combinations can be used.Citation91 The benefits of BP lowering in the setting of acute stroke requires more research and current recommendations are that antihypertensive treatment should start when poststroke clinical conditions are stable, usually several days after the event.Citation91 Both JNC VII and ESH-ESC guidelines recognize that combination therapy is required to reduce BP to recommended levels in a large proportion of patients.Citation90,Citation91 In addition, more evidence is needed before the specific cerebrovascular protective properties of individual agents or particular combinations are established.
Rationale for a CCB/ARB single-pill combination for stroke prevention
Multiple regulatory pathways are involved in the regulation of BP, and therefore combinations of agents that act by different mechanisms can have complementary actions and be more effective at reducing BP than monotherapy.Citation92 To optimize the benefits on stroke prevention it seems logical, when combining agents, to employ agents that (1) have complementary effects, (2) are effective at reducing BP, (3) might possess BP-independent effects, such as those discussed earlier, and (4) are associated with strong supportive evidence for the prevention of stoke. As indicated earlier, protection against stroke was greater with ARBs than with ACEIs.Citation54
Individually, amlodipine and ARBs seem to possess strong clinical trial data for antihypertensive agents in the protection against stroke.Citation93 Clinical studies have demonstrated that a combination of valsartan and amlodipine is an effective antihypertensive strategy capable of reducing BP more effectively than either treatment as monotherapy.Citation94–Citation96 Indeed, amlodipine/valsartan 5 to 10/160 mg reduces BP across all stages of hypertension, with reductions from baseline in mean sitting systolic BP of 20, 30 and 36 to 43 mmHg, respectively, in patients with mild, moderate and severe hypertension.Citation94,Citation96,Citation97 The large BP reductions with this combination coupled with the data supporting the protective effect of these agents as monotherapy would suggest that this combination might be an effective approach for stroke prevention. Indeed, in the JIKEI HEART study, a large proportion (67%) of patients in this study were also receiving a CCB and valsartan therapy reduced the risk of stroke by 40% compared with non-ARB-based therapy.Citation64 These data may suggest that combining valsartan with a CCB, such as amlodipine, has potential for protecting against stroke. However, studies on this combination in the context of stroke prevention have not been conducted to date.
Finally, the presence of CCB/ARB combinations in single-pill formulation may have indirect benefits. It is known that the use of single-pill antihypertensive combinations can improve persistence with therapy beyond that provided by free combinations.Citation98 Patients who persist on antihypertensive therapy have been reported to have a 28% reduction in the relative risk of stroke compared with patients who do not persist with therapy.Citation20 Thus, the use of single-pill antihypertensive combinations may help to reduce stroke through improvements in adherence.
Concluding remarks
In conclusion, antihypertensive agents can reduce the risk of stroke, predominantly by reductions in BP. However, there may be some differences in stroke protection between antihypertensive treatments, which may not be explained solely by differences in BP. Possible mechanisms for this additional benefit might include reductions in CIMT, LVH or central BP, or improvements in cerebral blood flow autoregulation. ARBs and CCBs have particularly strong supportive data for a protective effect against stroke. The choice of these agents or combinations of these agents could help to optimize the cerebrovascular benefits of antihypertensive treatment. However, further studies are needed to confirm the benefits of different combination strategies on stroke.
Acknowledgments and disclosures
Writing assistance in drafting this article was provided by Graham Allcock, a professional medical writer for ACUMED®. Financial support for this assistance was provided by Novartis Pharma AG.
Professor Wang has received consulting and lecture fees from Astra-Zeneca, GSK, Novartis, Pfizer, Sanofi-Aventis, Servier, and Takeda, and grants from Omron and Pfizer via the Shanghai Institute of Hypertension.
References
- MeairsSWahlgrenNDirnaglULindvallORothwellPBaronJCStroke research priorities for the next decade – a representative view of the European scientific communityCerebrovasc Dis200622237582
- WHO atlas of heart disease and stroke http://www.who.int/cardiovascular_diseases/en/cvd_atlas_15_burden_stroke.pdfAccessed July 8, 2009
- TruelsenTPiechowski-JóŸwiakBBonitaRMathersCBogousslavskyJBoysenGStroke incidence and prevalence in Europe: a review of available dataEur J Neurol200613658159816796582
- PaulSLSrikanthVKThriftAGThe large and growing burden of strokeCurr Drug Targets20078778679317630931
- GoldsteinLBAdamsRAlbertsMJAppelLJBrassLMBushnellCDAmerican Heart Association; American Stroke Association Stroke CouncilPrimary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working GroupCirculation200611324e873e92316785347
- Di TullioMRZwasDRSaccoRLSciaccaRRHommaSLeft ventricular mass and geometry and the risk of ischemic strokeStroke200334102380238412958319
- LüdersSDrug therapy for the secondary prevention of stroke in hypertensive patients: current issues and optionsDrugs200767795596317488141
- CoullAJLovettJKRothwellPMOxford Vascular StudyPopulation based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of servicesBMJ2004328743532614744823
- XuGLiuXWuWZhangRYinQRecurrence after ischemic stroke in Chinese patients: impact of uncontrolled modifiable risk factorsCerebrovasc Dis20072323117120
- SinghRBSuhILSinghVPChaithiraphanSLaothavornPSyRGHypertension and stroke in Asia: prevalence, control and strategies in developing countries for preventionJ Hum Hypertens2000141011749763
- LewingtonSClarkeRQizilbashNPetoRCollinsRProspective Studies CollaborationAge-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studiesLancet200236093491903191312493255
- LawesCMVander HoornSRodgersAInternational Society of HypertensionGlobal burden of blood-pressure-related disease, 2001Lancet200837196231513151818456100
- GorelickPBNew horizons for stroke prevention: PROGRESS and HOPELancet Neurol20021314915612849483
- ManciaGGrassiGSecondary prevention of stroke: old and new evidenceAging Clin Exp Res200214321622012387531
- GrassiGArenareFTrevanoFQDell’OroRManciaAGPrimary and secondary prevention of stroke by antihypertensive treatment in clinical trialsCurr Hypertens Rep20079429930417686381
- StaessenJAWangJGThijsLCardiovascular protection and blood pressure reduction: a meta-analysisLancet200135892901305131511684211
- LawesCMRodgersABennettDAParagVSuhIUeshimaHAsia Pacific Cohort Studies Collaboration. Blood pressure and cardiovascular disease in the Asia Pacific regionJ Hypertens200321470771612658016
- LawesCMBennettDAFeiginVLRodgersABlood pressure and stroke: an overview of published reviewsStroke20043541024103315053002
- GueyffierFBoisselJPBoutitieFPocockSCoopeJCutlerEffect of antihypertensive treatment in patients having already suffered from stroke. Gathering the evidence. The INDANA (INdividual Data Analysis of ANtihypertensive intervention trials) project collaboratorsStroke19972812255725629412649
- Breekveldt-PostmaNSPenning-vanBeest FJSiiskonenSJFalveyHVinczeGKlungelOHThe effect of discontinuation of antihypertensives on the risk of acute myocardial infarction and strokeCurr Med Res Opin200824112112718031596
- PittBByingtonRPFurbergCDHunninghakeDBManciniGBMillerMEEffect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT InvestigatorsCirculation2000102131503151011004140
- StaessenJAFagardRThijsLCelisHArabidzeGGBirkenhägerWHRandomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial InvestigatorsLancet199735090807577649297994
- StaessenJAThijsLFagardRHBirkenhägerWHArabidzeGGBabeanuSCalcium channel blockade and cardiovascular prognosis in the European trial on isolated systolic hypertensionHypertension19983234104169740604
- WangJGStaessenJAGongLLiuLChinese trial on isolated systolic hypertension in the elderly. Systolic Hypertension in China (Syst-China) Collaborative GroupArch Intern Med2000160221122010647760
- LubsenJWagenerGKirwanBAdeBrouwer SPoole-WilsonPAACTION (A Coronary disease Trial Investigating Outcome with Nifedipine GITS) investigatorsEffect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with symptomatic stable angina and hypertension: the ACTION trialJ Hypertens200523364164815716708
- DowlatshahiDFangJKawajaMHakimAUse of calcium channel blockers after stroke is not associated with poor outcome: a cohort from the registry of the Canadian stroke networkJ Neurol2006253111478148316786208
- BangaloreSMesserliFHA review of stroke in patients with hypertension and coronary artery disease: Focus on calcium channel blockersInt J Clin Pract200660101281128616942591
- StaessenJALiYThijsLWangJGBlood pressure reduction and cardiovascular prevention: an update including the 2003–2004 secondary prevention trialsHypertens Res200528538540716156503
- VerdecchiaPReboldiGAngeliFGattobigioRBentivoglioMThijsLAngiotensin-converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke preventionHypertension200546238639216009786
- DahlöfBSeverPSPoulterNRWedelHBeeversDGCaulfieldMASCOT InvestigatorsPrevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required vs atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trialLancet2005366948989590616154016
- PoulterNRWedelHDahlöfBSeverPSBeeversDGCaulfieldMASCOT InvestigatorsRole of blood pressure and other variables in the differential cardiovascular event rates noted in the Anglo- Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA)Lancet2005366948990791316154017
- NissenSETuzcuEMLibbyPThompsonPDGhaliMGarzaDCAMELOT InvestigatorsEffect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trialJAMA2004292182217222515536108
- ALLHAT Collaborative Research GroupMajor outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)JAMA2002288232981299712479763
- LeenenFHNwachukuCEBlackHRCushmanWCDavisBRSimpsonLMALLHAT Collaborative Research GroupClinical events in high-risk hypertensive patients randomily assigned to calcium channel blocker vs angiotensin-converting enzyme inhibitor in the antihypertensive and lipid-lowering treatment to prevent heart attack trialHypertension200648337438416864749
- WangJGLiYFranklinSSSafarMPrevention of stroke and myocardial infarction by amlodipine and angiotensin receptor blockers. A quantitative overviewHypertension200750118118817502490
- IadecolaCGorelickPBHypertension, angiotensin, and stroke: beyond blood pressureStroke200435234835014757875
- SchraderJKulschewskiADendorferAInhibition of the renin-angiotensin system and the prevention of strokeAm J Cardiovasc Drugs200771253717355164
- BoschJYusufSPogueJSleightPLonnERangoonwalaBHOPE InvestigatorsHeart outcomes prevention evaluation Use of ramipril in preventing stroke: double blind randomised trialBMJ20023247339699702
- YusufSSleightPPogueJBoschJDaviesRDagenaisGEffects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study InvestigatorsN Engl J Med2000342314515310639539
- PROGRESS Collaborative GroupRandomised trial of a perindopril-based blood pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attackLancet200135892871033104111589932
- FlatherMDYusufSKøberLPfefferMHallAMurrayGLong-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative GroupLancet200035592151575158110821360
- HanssonLLindholmLHNiskanenLLankeJHednerTNiklasonAEffect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trialLancet1999353915361161610030325
- NiskanenLHednerTHanssonLLankeJNiklasonACAPPP Study GroupReduced cardiovascular morbidity and mortality in hypertensive diabetic patients on first-line therapy with an ACE inhibitor compared with a diuretic/beta blocker-based treatment regimen: a subanalysis of the Captopril Prevention ProjectDiabetes Care200124122091209611723089
- BoutitieFOprisiuRAchardJMMazouzHWangJMesserliFHDoes a change in angiotensin II formation caused by antihypertensive drugs affect the risk of stroke? A meta-analysis of trials according to treatment with potentially different effects on angiotensin IIJ Hypertens20072581543155317620946
- EpsteinBJGumsJGCan the renin-angiotensin system protect against stroke? A focus on angiotensin II receptor blockersPharmacotherapy200525453153915977915
- FournierAMesserliFHAchardJMFernandezLCerebroprotection mediated by angiotensin II: a hypothesis supported by recent randomized clinical trialsJ Am Coll Cardiol20044381343134715093864
- DahlöfBDevereuxRBKjeldsenSEJuliusSBeeversGde FaireULIFE Study GroupCardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenololLancet20023599311995100311937178
- KjeldsenSEDahlöfBDevereuxRBJuliusSAurupPEdelmanJLIFE (Losartan Intervention for Endpoint Reduction) Study GroupEffects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a Losartan Intervention for Endpoint Reduction (LIFE) substudyJAMA2002288121491149812243636
- WachtellKLehtoMGerdtsEOlsenMHHornestamBDahlöfBAngiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) studyJ Am Coll Cardiol200545571271915734615
- LithellHHanssonLSkoogIElmfeldtDHofmanAOlofssonBSCOPE Study GroupThe Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trialJ Hypertens200321587588612714861
- The Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) InvestigatorsEffects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensinconverting enzyme inhibitors: a randomised controlled trialLancet200837296441174118318757085
- OgiharaTNakaoKFukuiTFukiyamaKUeshimaKObaKCandesartan Antihypertensive Survival Evaluation in Japan Trial GroupEffects of candesartan compared with amlodipine in hypertensive patients with high cardiovascular risks: candesartan antihypertensive survival evaluation in Japan trialHypertension200851239339818172059
- ONTARGET InvestigatorsYusufSTeoKKPogueJDyalLCoplandITelmisartan, ramipril, or both in patients at high risk for vascular eventsN Engl J Med2008358151547155918378520
- ReboldiGAngeliFCavalliniCGentileGManciaGVerdecchiaPComparison between angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the risk of myocardial infarction, stroke and death: a meta-analysisJ Hypertens20082671282128918550998
- SchraderJLüdersSKulschewskiABergerJZidekWTreibJAcute Candesartan Cilexetil Therapy in Stroke Survivors Study GroupThe ACCESS Study: evaluation of Acute Candesartan Cilexetil Therapy in Stroke SurvivorsStroke20033471699170312817109
- ClinicalTrials.gov [webpage]Scandinavian Candesartan Acute Stroke Tirial (SCAST) [updated 2009 May 13; cited 2008 July 15]. Available from: http://clinicaltrials.gov/ct2/show/NCT00120003
- SchraderJLüders SKulschewskiAHammersenFPlateKBergerJMOSES Study GroupMorbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention. Principal results of a prospective randomized controlled study (MOSES)Stroke20053661218122615879332
- DienerHCSaccoRYusufSPRoFESS Study GroupRationale, design and baseline data of a randomized, double-blind, controlled trial comparing two antithrombotic regimens (a fixed-dose combination of extended-release dipyridamole plus ASA with clopidogrel) and telmisartan vs placebo in patients with strokes: the Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS)Cerebrovasc Dis20072356368380
- YusufSDienerHCSaccoRLCottonDOunpuuSLawtonWAPRoFESS Study GroupTelmisartan to prevent recurrent stroke and cardiovascular eventsN Engl J Med2008359121225123718753639
- MesserliFHManciaGContiCRHewkinACKupferSChampionADogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous?Ann Intern Med20061441288489316785477
- CocaAMesserliFHBenetosAZhouQChampionACooper-DeHoffRMPredicting stroke risk in hypertensive patients with coronary artery disease: a report from the INVESTStroke200839234334818162623
- WeberMAJuliusSKjeldsenSEBrunnerHREkmanSHanssonLBlood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE trialLancet200436394262049205115207957
- ArimaHChalmersJWoodwardMAndersonCRodgersADavisSPROGRESS Collaborative GroupLower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trialJ Hypertens20062461201120816685221
- MochizukiSDahlöfBShimizuMIkewakiKYoshikawaMTaniguchiIJikei Heart Study groupValsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality studyLancet200736995711431143917467513
- JuliusSKjeldsenSEWeberMBrunnerHREkmanSHanssonLVALUE trial groupOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet200436394262022203115207952
- SchmiederREKjeldsenSEJuliusSMcInnesGTZanchettiAHuaTAVALUE Trial GroupReduced incidence of new-onset atrial fibrillation with angiotensin II receptor blockade: the VALUE trialJ Hypertens200826340341118300848
- TurnbullFBlood Pressure Lowering Treatment Trialists’ CollaborationEffects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trialsLancet200336293951527153514615107
- MesserliFHStaessenJAAmlodipine better than lisinopril? How one randomized clinical trial ended fallacies from observational studiesHypertension200648335936116894055
- Thone-ReinekeCKrikovMSchmerbachKMullerSVillringerASteckelingsUComparison of the effect of systematic pretreatment with telmisartan, ramipril and their combination on neurological status and infarct volume in rats after strokeJ Hypertens200826Suppl1S118815510
- LorenzMWMarkusHSBotsMLRosvallMSitzerMPrediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysisCirculation2007115445946717242284
- WangJGStaessenJALiYVan BortelLMNawrotTFagardRCarotid intima-media thickness and antihypertensive treatment: a meta-analysis of randomized controlled trialsStroke20063771933194016763185
- MörtsellDMalmqvistKHeldCKahanTIrbesartan reduces common carotid artery intima-media thickness in hypertensive patients when compared with atenolol: the Swedish Irbesartan Left Ventricular Hypertrophy Investigation vs Atenolol (SILVHIA) studyJ Intern Med2007261547247917444886
- OlsenMHWachtellKNelandKBellaJNRokkedalJDige-PetersenHLosartan but not atenolol reduce carotid artery hypertrophy in essential hypertension. A LIFE substudyBlood Press200514317718316036498
- OnoHMinatoguchiSWatanabeKYamadaYMizukusaTKawasakiHCandesartan decreases carotid intima-media thickness by enhancing nitric oxide and decreasing oxidative stress in patients with hypertensionHypertens Res200831227127918360047
- VerdecchiaPReboldiGGattobigioRBentivoglioMBorgioniCAngeliFAtrial fibrillation in hypertension: predictors and outcomeHypertension200341221822312574085
- SampsonUKPfefferMAMcMurrayJJLokhnyginaYWhiteHDSolomonSDVALIANT Trial InvestigatorsPredictors of stroke in high-risk patients after acute myocardial infarction: insights from the VALIANT TrialEur Heart J200728668569116984929
- WattigneyWAMensahGACroftJBIncreasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary preventionCirculation2003108671171612885749
- KlingbeilAUSchneiderMMartusPMesserliFHSchmiederREA meta-analysis of the effects of treatment on left ventricular mass in essential hypertensionAm J Med20031151414612867233
- WilliamsBLacyPSThomSMCruickshankKStantonACollierDCAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing CommitteeDifferential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) studyCirculation200611391213122516476843
- LacyPSWilliamsBImpact of heart rate on the differential impact of blood pressure lowering drugs on central and peripheral pressures: Data from the Conduit Artery Function Evaluation (CAFÉ) studyJ Hypertens200826Suppl1S459
- AndoHZhouJMacovaMImbodenHSaavedraJMAngiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive ratsStroke20043571726173115143297
- NishimuraYItoTSaavedraJMAngiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive ratsStroke200031102478248611022082
- ItoTYamakawaHBregonzioCTerrónJAFalcón-NeriASaavedraJMProtection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonistStroke20023392297230312215602
- KumaiYOoboshiHAgoTIshikawaETakadaJKamouchiMProtective effects of angiotensin II type 1 receptor blocker on cerebral circulation independent of blood pressureExp Neurol2008210244144818177860
- MogiMIwaiMChenRIwanamiJIdeATsukudaKAmlodipine treatment reduces stroke size in apolipoprotein E-deficient miceAm J Hypertens200619111144114917070425
- LiuLZhangYLiuGLiWZhangXZanchettiAFEVER Study GroupThe felodipine event reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patientsJ Hypertens200523122157217216269957
- JamersonKABakrisGLWunCCDahlöfBLefkowitzMManfredaSRationale and design of the avoiding cardiovascular events through combination therapy in patients living with systolic hypertension (ACCOMPLISH) trial: the first randomized controlled trial to compare the clinical outcome effects of first-line combination therapies in hypertensionAm J Hypertens200417979380115363822
- JamersonKWeberMABakrisGLDahlöfBPittBShiVACCOMPLISH Trial InvestigatorsBenazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patientsN Engl J Med20083592324172819052124
- ChalmersJMacMahonSPerindopril protection against recurrent stroke study (PROGRESS): interpretation and implementationJ Hypertens200321SupplS914
- ChobanianAVBakrisGLBlackHRCushmanWCGreenLAIzzoJLJrNational Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating CommitteeThe Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 reportJAMA2003289192560257212748199
- ManciaGDe BackerGDominiczakACifkovaRFagardRGermanoG2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens20072561105118717563527
- SicaDARationale for fixed-dose combination in the treatment of hypertension: the cycle repeatsDrugs200262344346211827559
- StaessenJAWangJGThijsLCardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003J Hypertens20032161055107612777939
- DestroMLuckowASamsonMKandraABrunelPEfficacy and safety of amlodipine/valsartan compared with amlodipine monotherapy in patients with stage 2 hypertension: a randomized, double-blind, multicenter study: the EX-EFFeCTS studyJ Am Soc Hypertens2008229430220409909
- PhilippTSmithTRGlazerRWernsingMYenJJinJTwo multicenter, 8-week, randomized, double-blind, placebo-controlled, parallel-group studies evaluating the efficacy and tolerability of amlodipine and valsartan in combination and as monotherapy in adult patients with mild to moderate essential hypertensionClin Ther200729456358017617280
- SmithTRPhilippTVaisseBBakrisGLWernsingMYenJAmlodipine and valsartan combined and as monotherapy in stage 2, elderly, and black hypertensive patients: subgroup analyses of 2 randomized, placebo-controlled studiesJ Clin Hypertens200795355364
- PoldermansDGlazesRKargiannisSWernsingMKaczorJChiangYTTolerability and blood pressure-lowering efficacy of the combination of amlodipine plus valsartan compared with lisinopril plus hydrochlorothiazide in adult patients with stage 2 hypertensionClin Ther200729227928917472820
- BrixnerDIJacksonKC2ndShengXNelsonREKeskinaslanAAssessment of adherence, persistence, and costs among valsartan and hydrochlorothiazide retrospective cohorts in free-and fixed-dose combinationsCurr Med Res Opin20082492597260718812017