Abstract
Angiotensin receptor blockers have emerged as a first-line therapy in the management of hypertension and hypertension-related comorbidities. Since national and international guidelines have stressed the need to control blood pressure to <140/90 mmHg in uncomplicated hypertension and <130/80 mmHg in those with associated comorbidities such as diabetes or chronic kidney disease, these goal blood pressures can only be achieved through combination therapy. Of several drugs that can be effectively combined to attain the recommended blood pressure goals, fixed-dose combinations of angiotensin receptor blockers and the calcium channel blocker amlodipine provide additive antihypertensive effects associated with a safe profile and increased adherence to therapy. In this article, we review the evidence regarding the beneficial effects of renin–angiotensin system blockade with olmesartan medoxomil and amlodipine in terms of blood pressure control and improvement of vascular function and target organ damage.
Introduction
The 33rd report on the Health Status in the United States estimates that essential hypertension affects 17.9% of the age-adjusted, 20-year-old or older white subjects and up to 26% of male African Americans (http://www.cdc.gov/nchs/data/hus/hus09.pdf). Numerous large-scale clinical trials have documented the benefits of strict blood pressure control in preventing hypertension-related cardiovascular events. It is clear that the benefit of blood pressure reduction to 120/80 mmHg in non-diabetic patients will be associated with a large reduction in the occurrence of strokes and fatal and nonfatal ischemic heart diseases. Since single-drug therapy is often not able to lower blood pressure to these ideal values, recent recommendations now emphasize the need for combination therapy to achieve these blood pressure goals.Citation1,Citation2 Although no definitive evidence is available as to which combination therapy will most effectively achieve strict blood pressure control and reduction in target organ damage, the increased use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and the calcium channel blocker (CCB) amlodipine, widely prescribed alone or in combination in most countries in the world, favors their use.
Evidence-based medicine from large controlled clinical trials supports the use of these drugs, although the long-term benefit of any one combination over the others remains to be established.Citation3 In this context, the Avoiding Cardiovascular Events through COMbination Therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) study showed that the combination of benazepril and amlodipine resulted in outcome benefits greater than those observed in subjects medicated with benazepril and hydrochlorothiazide (HCTZ) despite similar blood pressure reductions.Citation4,Citation5 Although both ACE inhibitors and ARBs have a definitive role in preventing the consequences of increased renin–angiotensin system activity in cardiac, vascular, and renal functions, enhanced tolerability and the more specific effects of ARBs on suppressing the binding of angiotensin II (Ang II) to the subtype 1 (AT1) receptor should favor the use of ARBs over blockers of ACE. This article summarizes the evidence for the combined use of the AT1 selective receptor antagonist, olmesartan medoxomil, with amlodipine on blood pressure control and target organ damage. Additional information regarding the pharmacological and clinical response to olmesartan administration are reviewed elsewhere.Citation6–Citation9
Pharmacodynamics of olmesartan and amlodipine
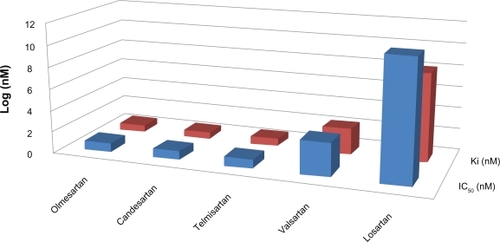
Olmesartan medoxomil is a highly selective ARB with pharmacokinetic characteristics that determine high binding to AT1 receptors and lasting effects on arterial pressure.Citation10,Citation11 The medoxomil ester of olmesartan facilitates its bioavailability as the oral bioavailability of the active product RNA-6270 is less than 4.5%.Citation12 In bovine adrenal cells, the displacement of 125I-Ang II by olmesartan has an half maximal inhibitory concentration (IC50) value of 7.7 nM, a displacement value significantly lower than that of losartan (92 nM) and its active metabolite EXP3174 (16 nM). shows that the affinity of olmesartan for AT1 receptors is high when compared with that of other ARBs. Furthermore, the displacement of olmesartan by Ang II in Hill’s plots shows that the active drug behaves as a competitive antagonist of AT1 receptors.Citation13 Pharmacokinetic properties of olmesartan support its efficacy and long duration of action when given to experimental animals and humans. Additional information on pharmacokinetics of olmesartan and its mode of action are discussed elsewhere.Citation8,Citation9,Citation14
Figure 1 Comparative pharmacodynamic characteristics of five angiotensin receptor blockers in terms of their half maximal inhibitory concentration (IC50) and dissociation constant (Ki). The active form of olmesartan shows high affinity for AT1 receptors with an IC50 equivalent to that of candesartan and much lower than the IC50 for other angiotensin receptor blockers. Similarly, the lowest IC50 for olmesartan is associated with the lowest dissociation constant from the receptor.
The antihypertensive actions of olmesartan are potentiated when used in combination with either thiazide diuretics or CCBs. Amlodipine is a potent dihydropyridine CCB having a high degree of ionization, high oral bioavailability (60%–65%), and peak plasma concentrations attainable within 6–8 hours after oral administration. Like other dihydropyridine CCB, amlodipine selectively inhibits calcium (Ca2+) influx across cell membranes in cardiac and vascular smooth muscle with a greater effect on the latter.Citation15 Rohatagi et alCitation16 reported the pharmacokinetics of olmesartan medoxomil and amlodipine besylate alone and in a fixed-dose combination in five phase I crossover studies in healthy volunteers. The similarity of the mean steady-state pharmacokinetics of olmesartan and amlodipine at doses of 40 and 10 mg, respectively, their drug concentration–time curves, and the maximum observed plasma drug concentrations within their prespecified bioequivalence (80%–125%) showed that they were well suited to coadministration in a fixed-dose combination.Citation16 Furthermore, coadministration of amlodipine besylate and olmesartan medoxomil, as commercially available separate dosage forms, for 10 days showed no evidence of any negative pharmacokinetic drug–drug interactions.Citation16
The additive mechanisms of action of the single-dose form of olmesartan/amlodipine on long-term hemodynamic and neurohormonal systems controlling blood pressure have not been studied. Results from the direct effects of olmesartan or amlodipine on cardiac and vascular structures and hypertension-induced remodeling suggest complementary actions. Olmesartan induces a reduction in peripheral vascular resistance that is associated with no changes in heart rate or cardiac output and increases in plasma renin activity (see Schindler and FerrarioCitation9 for review). In the EUropean Trial on Olmesartan and Pravastatin in Inflammation and Atherosclerosis (EUTOPIA trial),Citation17 olmesartan was found to lower the serum levels of high-sensitivity C-reactive protein, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and human monocyte chemoattractant protein-1 (MCP-1). A further reevaluation of the EUTOPIA trial showed that the vasculoprotective effects of olmesartan were associated with decreases in plasma osteopontin concentrations.Citation18 The anti-inflammatory effects of AT1 receptor blockade with olmesartan may contribute to the observation that this drug can prevent the progression of atherosclerosis in nonhuman primates.Citation19 Additional vasculotropic effects of olmesartan in the protection of vascular endothelial function have been reviewed recently.Citation8 Although the long-term effect of olmesartan on the plasma and tissue concentrations of Ang II requires further study, a report by Ichikawa et alCitation20 showed that blockade of AT1 receptors may not be associated with the typical increase in plasma Ang II concentrations, as observed with other ARBs.Citation21 This clinical study may be tentatively explained by the observation that AT1 blockade upregulates the activity and tissue expression of ACE 2, a homolog of ACE that acts as a monocarboxypeptidase degrading Ang II into the vasodilator and antitrophic peptide angiotensin-(1–7) [Ang-(1–7)].Citation22–Citation27 In keeping with these findings, an experimental study in the stroke-prone rat suggested that olmesartan may act as an inhibitor of ACE through the stimulation of Ang-(1–7) actions and release of nitric oxide.Citation28
The beneficial effects of olmesartan on the prevention of vascular remodeling and carotid artery atherosclerotic plaque progression in subjects with hypertension are now documented.Citation29,Citation30 The Multicentre Olmesartan atherosclerosis Regression Evaluation (MORE) study was a double-blind trial conducted in patients with hypertension who are at increased cardiovascular risk (presence of carotid wall thickening and a defined atherosclerotic plaque), using noninvasive two- and three-dimensional ultrasonography. The trial compared the effects of a 48-month treatment based on either olmesartan medoxomil or atenolol on common carotid intima-media thickness and plaque volume (PV).Citation30 Large PVs (>33 μL) were significantly reduced over the 102-week treatment period only in those subjects assigned to the olmesartan-based therapy. In agreement with these findings, administration of olmesartan to subjects with diabetes was associated with reduced arterial stiffness while amlodipine had no effect.Citation31
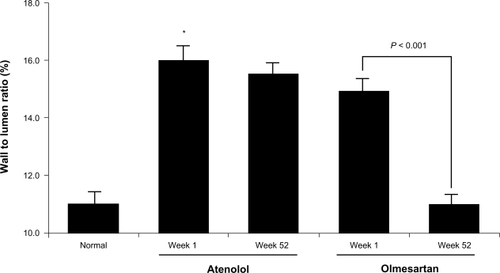
The Vascular Improvement with Olmesartan Study (VIOS) enrolled 100 subjects with stage 1 hypertension without diabetes, to evaluate whether an olmesartan-based therapeutic regimen could reverse vascular hypertrophy independent of the magnitude of blood pressure lowering.Citation29,Citation32 The trial compared the effects of olmesartan-based therapy versus atenolol-based therapy on blood pressure control and changes in wall/media lumen (W/L) ratio from small resistance arterioles obtained from these patients through the technique of gluteal biopsies.Citation32–Citation34 Biopsies were available from 22 atenolol recipients (100 mg/day), 27 olmesartan medoxomil recipients (40 mg/day), and 11 normal volunteer controls. Additional antihypertensive medications (HCTZ 12.5–25 mg/day, amlodipine 5–10 mg/day, or hydralazine 50–100 mg twice daily) were dispensed to achieve blood pressure control below 140/90 mmHg. Overall, patients in the atenolol-based regimen group required more medications versus patients randomized to the olmesartan-based group. Furthermore, a greater percentage of patients assigned to the olmesartan-based therapy achieved and maintained an ideal blood pressure of ≤120/80 mmHg at 4 weeks (24% in the olmesartan-based therapy vs 8% in those assigned to the atenolol-based therapy [P < 0.05]). At the completion of the 52-week period, comparable decreases in arterial pressure resulting in the physiological levels of blood pressure (≤120/80 mmHg) were observed in patients assigned to each of the two regimens. Normalization of blood pressure, however, was associated with the regression of vascular hypertrophy only in those subjects assigned to the olmesartan-based therapy (). In these subjects, the reduction in W/L ratio of small resistance vessels (from 14.9% to 11.1%; P < 0.01) was numerically equivalent to the W/L ratio determined in the subset of normotensive volunteers from whom subcutaneous small arteriole resistance vessels were obtained.Citation32 Since the addition of HCTZ and amlodipine were required in more than 59% of the subjects and no differences existed in the dosing and time periods in which these agents were incorporated to the treatment regimen for both arms of the study, the data demonstrated that the selective effect of AT1 receptor blockade in the reversal of vascular hypertrophy in small resistance vessels was directly responsible for the reduction of peripheral vascular resistance.Citation32,Citation33 Furthermore, noninvasive measurements of central aortic pressure and determination of the augmentation index by applanation tonometryCitation35 showed decreases in the indices of vascular compliance only on those subjects receiving the olmesartan-based therapy.Citation32 On the other hand, a study that investigated the role of cellular oxidant stress and inflammation on patients with hypertension and the cardiometabolic syndrome showed comparative effects induced by treatment with either olmesartan or amlodipine.Citation36 A small sample size and the presence of comorbidities may have contributed to the reported conclusions.Citation36
Figure 2 Bar graph denotes the average value of wall/media lumen ratio from small resistance arterioles obtained from normotensive subjects (normal) and patients with hypertension without diabetes assigned to either an atenolol-based or olmesartan-based therapy before and at week 52 after completion of the treatment regimen.
Clinical studies
A series of studies have documented the effective control of arterial pressure achieved with the daily fixed-dose administration of a single tablet of olmesartan/amlodipine. A multicenter, double-blind, randomized, placebo-controlled, parallel-group, factorial study, lasting 8 weeks and enrolling 1,940 subjects with stage 1 and stage 2 hypertension, evaluated the blood pressure response to placebo, amlodipine (5–10 mg/day), olmesartan (10, 20, and 40 mg/day), and the fixed combination of olmesartan and amlodipine at doses of 5/10, 5/20, 5/40, 10/10, 10/20, and 10/40 mg/day.Citation37 At the highest dose combination of olmesartan/amlodipine (40/10 mg/day), the reduction in systolic and diastolic blood pressures amounted to 28.5 and 19.4 mmHg, respectively.Citation37 The decreases in arterial pressure were significantly greater than those obtained with either olmesartan or amlodipine when given alone.Citation37 The beneficial effects of the single-tablet combination were associated with increased target blood pressure of <140/90 mmHg.Citation37 Although all treatment regimens in the Combination of Olmesartan medoxomil and Amlodipine besylate in Controlling High blood pressure (COACH) study were well tolerated and were free of major side effects, the occurrence of pedal edema was less in those subjects medicated with the fixed-dose combination of 40/10 mg of olmesartan/amlodipine (23.5%) than in those subjects medicated with amlodipine alone (36.8%). The reduction in peripheral edema in response to the addition of an ARB to a CCB is a product of the concurrent vasodilator effect of Ang II blockade inducing venular capillary dilatation, thus diminishing the pressure gradient across the peripheral microcirculation.Citation38
A trial performed in Europe compared the effectiveness of a single-pill combination of olmesartan and amlodipine in a randomized, double-blind, parallel-group, multicenter trial in patients with moderate to severe hypertension (systolic blood pressure [SBP] ≥160 mmHg and diastolic blood pressure [DBP] ≥100 mmHg).Citation39 Nonresponders to an open-label monotherapy phase with olmesartan (8 weeks at 20 mg/day) were randomized to 20 mg/day olmesartan plus placebo, the fixed-dose combination of 20 mg/day olmesartan plus 5 mg/day amlodipine, or 20 mg/day olmesartan plus 10 mg/day amlodipine for an additional 8 weeks.Citation39 The primary end point evaluated the intention-to-treat population of all subjects with hypertension who received at least one dose of the double-blind study medication, had baseline measures of sitting DBP, and received at least one postrandomization measure of DBP > 140/90 mmHg. Potential confounders due to the use of the less rigorous statistical approach of last observation carried forward for missing data during the double-blind period were compensated by the inclusion of an observed case approach in which the last observation was not carried forward. Of the 1,519 screened subjects, 722 patients entered the open-label phase of the study with 20 mg/day olmesartan. The 538 subjects who completed this phase of the study were randomized to the 8-week double-blind period of one of the three interventions. The blood pressure of <140/90 mmHg after the 8-week double-blind period was achieved in 28.5%, 44.5%, and 45.8% of subjects randomized to olmesartan/placebo, 20/5 mg/day olmesartan/amlodipine, and 20/10 mg/day olmesartan/amlodipine, respectively.Citation39 A post hoc analysis showed that the number of subjects reaching a DBP of <90 mmHg were greater in those using the fixed-dose combinations of olmesartan/amlodipine.Citation39 In addition, the study showed that the combination therapy was associated with an earlier reaching of their goal blood pressure when compared with the monotherapy phase. These data are in agreement with another study in which the combination of 10–40 mg/day olmesartan with 5 mg/day amlodipine for 8 weeks reduced the mean SBP and DBP by 16.8 and 9.6 mmHg, respectively.Citation40
SBP is a predictor of increased cardiovascular risk.Citation41 In a post hoc analysis of changes in sitting SBP in patients treated with 40 mg of olmesartan plus 5–10 mg/day amlodipine,Citation40 the combination therapy was shown to be most effective in reducing SBP in the subjects with highest levels of SBP.Citation42 As reviewed elsewhere,Citation43 this combination therapy is superior to the single-agent administration in other high-risk populations, such as African Americans,Citation44 obese, and patients with diabetes. The predominant effect of improvement in insulin resistance and reduced oxidative stress seems to be related to blockade of Ang II receptors.Citation45–Citation49
Presence of chronic kidney disease aggravates the odds of cardiovascular events in patients with hypertension.Citation50 The potential for renoprotective effects of combining a CCB with olmesartan in elderly patients (age 65–85 years) with chronic kidney disease was investigated in a crossover study using an open-label, randomized design with albuminuria (creatinine > 5 mg/g).Citation51 Following a 2-week run in observation period, the subjects were randomized to receive a starting dose of benidipine (4 mg/day) or amlodipine combined with olmesartan (5/10 mg/day). Three months later, the patients were switched from benidipine to amlodipine and followed-up for an additional 3 months. Benidipine is a dihydropyridine CCB that induces efferent arteriolar dilation through blockade of both L- and T-type calcium channels.Citation52,Citation53 Combination of olmesartan with either CCBs produced comparable decreases in arterial blood pressure, whereas the combination of benidipine and olmesartan achieved slightly greater statistically significant decreases in albumin excretion.Citation51 The Randomized Olmesartan and Diabetes Prevention (ROADMAP) study determined the factors correlating with albumin excretion rates across the range of normoalbuminuric values in patients with type 2 diabetes using olmesartan medoxomil.Citation54,Citation55
There is evidence that the administration of olmesartan is the key driver in achieving the target blood pressure in patients with stage II hypertensionCitation56 and reducing the effects of hypertension in vascular remodeling.Citation31,Citation32 A multicenter, 12-week study compared the efficacy, safety, and tolerability of a combination of olmesartan medoxomil/HCTZ with that of benazepril plus amlodipine besylate in patients with stage II hypertension.Citation57 The data showed that the primary efficacy end point of change in mean seated SBP at week 12 was significantly greater with olmesartan medoxomil/HCTZ than with benazepril plus amlodipine besylate.Citation57 These findings are in agreement with other studies documenting the efficacy of the combination of olmesartan/HCTZ in subjects with hypertension and in those with isolated systolic hypertension.Citation44,Citation58–Citation60 As reviewed by Quan et alCitation61 a multi-factorial analysis of the published studies reported that the daily combination of 40 mg olmesartan and 25 mg HCTZ produced greater blood pressure reductions than with the administration of 300 mg irbesartan/25 mg HCTZ, 80 mg telmisartan/12.5 mg HCTZ, and 160 mg valsartan/25 mg HCTZ.Citation62 The addition of HCTZ to patients receiving a fixed-dose combination of olmesartan/amlodipine (40/5–10 mg/day) increased the overall proportion of patients reaching the goal blood pressure.Citation63 In an additional study that focused on reaching the blood pressure goals rather than the responder rates, with the combination of olmesartan medoxomil, amlodipine, and HCTZ, 90% of patients with stage 2 hypertension reached the blood pressure of <140/90 mmHg and 81% patients attained <130/85 mmHg.Citation64
Long-term outcome studies as to the benefit of the combination of olmesartan/amlodipine in the prevention of cardiovascular events are not yet available. To meet this objective, an on-going study will evaluate whether high-dose ARB monotherapy is superior to the combination therapy of ARB plus CCB in the prevention of cardiovascular morbidity and mortality in elderly Japanese high-risk patients with hypertension (OlmeSartan and Calcium Antagonists Randomized [OSCAR] Study).Citation65
Conclusions
The importance of blood pressure control in the prevention of cardiovascular events is well established. Given the relative success in achieving appropriate blood pressure control in the general population, effective drug combinations as first-line therapy can meet the need to attain blood pressure levels <140/90 mmHg in uncomplicated hypertension and ≤130/80 mmHg in subjects with diabetes or in those in whom hypertension is accompanied by chronic kidney disease. Although published guidelines advocate the combination of a thiazide diuretic with another antihypertensive agent as initial therapy, emerging evidence suggests that the association of a CCB with either an ACE inhibitor or an ARB may be a safer and more effective combination.Citation66 Evidence for this approach is buttressed by the recent publication of the ACCOMPLISH study.Citation5 In this study, the combination of the ACE inhibitor benazepril with the CCB amlodipine was more effective than the combination of benazepril with HCTZ in reducing the primary composite end point of cardiovascular events and death from cardiovascular causes over the 36-month mean follow-up period.Citation5 The combination of olmesartan/amlodipine in a fixed-dose combination has proven to be effective in controlling blood pressure in patients with stage 1 and stage 2 hypertension.Citation4,Citation37,Citation38,Citation44,Citation56,Citation66–Citation73 Their additive anti-hypertensive effect is associated with complimentary actions that in part may be related to the buffering of the reactive increase in renin–angiotensin system activity triggered by the vasodilator action of amlodipine.Citation74
Among the advantages of fixed combination therapy, several studies suggest that this approach overcomes issues related to side effects, patient and physician inertia, the proportion of subjects classified as resistant hypertension, and cost-effectiveness issues such as co-pays.Citation43 In several observational studies, fixed-dose combinations were associated with higher rates of compliance, persistence, and adherence to treatment regimens.Citation75–Citation82 A meta-analysis of 15 published studies with a total of 32,331 patients concluded that fixed-dose combinations of antihypertensive agents were associated with increased compliance and no changes in the frequency of adverse events when compared with free drug components given separately.Citation80
Interest in the effect of the circadian rhythm of blood pressure in terms of its association with the occurrence of cardiovascular events posits the question as to whether single-pill, fixed-dose combinations may provide greater benefit when administered at bedtime. Although the effectiveness of such a chronotherapeutic approach remains unexplored for single-pill, fixed-dose combinations, Minutolo et alCitation83 reported that in nondipper subjects with chronic kidney disease changing the timing of antihypertensive therapy decreases nocturnal blood pressure and proteinuria. As reviewed by Stergiou et alCitation84 morning administration of single- or fixed-dose combinations of drugs have been used in assessing the efficacy of antihypertensive therapy. In bedtime dosing of treatment as used in the Controlled Onset Verapamil Investigation of Cardiovascular Endpoints (CONVINCE) trial, chronotherapeutical dosing of verapamil failed to blunt the early morning surge in blood pressure.Citation85 In contrast, the bedtime dosing of ramipril in the Heart Outcomes Prevention Evaluation (HOPE) trial has been suggested to partially account for the vascular benefits found in this study.Citation86 The efficacy of olmesartan medoxomil in controlling blood pressure over a 24-hour period showed that the ARB was more effective than losartan and valsartan in maintaining lower levels of blood pressure for mean daytime and nighttime ambulatory blood pressure.Citation87 Although further work will be necessary to evaluate these possibilities, it is undeniable that the use of a single-pill, fixed-dose combination at bedtime should be explored in well-controlled clinical trials.
Acknowledgements
In addition to the support provided by NHLBI grant PO1 HL051952, the authors gratefully acknowledge the grant support provided by Unifi, Inc., Greensboro, NC, and Farley-Hudson Foundation, Jacksonville, NC.
Disclosure
Carlos M Ferrario receives compensation for speaking and consulting with Daiichi Sankyo Corporation, Novartis, Inc., Forest Research, Bristol-Myers Squibb, Takeda, and Merck Inc.
References
- ManciaGLaurentSAgabiti-RoseiEReappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force documentBlood Press20091830834720001654
- ManciaGZanchettiAEuropean Society of Hypertension-European Society of CardiologyChoice of antihypertensive drugs in the European Society of Hypertension-European Society of Cardiology guidelines: specific indications rather than ranking for general usageJ Hypertens20082616416818192825
- ZanchettiAManciaGBlackHRFacts and fallacies of blood pressure control in recent trials: implications in the management of patients with hypertensionJ Hypertens20092767367919516168
- IzzoJLJrPurkayasthaDHallDHilkertRJComparative efficacy and safety of amlodipine/benazepril combination therapy and amlodipine monotherapy in severe hypertensionJ Hum Hypertens20102440340919890370
- JamersonKWeberMABakrisGLBenazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patientsN Engl J Med20083592417242819052124
- BrunnerHRThe new oral angiotensin II antagonist olmesartan medoxomil: a concise overviewJ Hum Hypertens200216Suppl 2S13S1611967728
- BrunnerHRLaeisPClinical efficacy of olmesartan medoxomilJ Hypertens Suppl200321S43S4612929907
- FerrarioCEffect of angiotensin receptor blockade on endothelial function: focus on olmesartan medoxomilVasc Health Risk Manag2009530131419436655
- SchindlerCFerrarioCMOlmesartan for the treatment of arterial hypertensionFuture Cardiol2008435737219804316
- KoikeHSadaTMizunoMIn vitro and in vivo pharmacology of olmesartan medoxomil, an angiotensin II type AT1 receptor antagonistJ Hypertens Suppl200119S3S1411451212
- KoikeHNew pharmacologic aspects of CS-866, the newest angiotensin II receptor antagonistAm J Cardiol20018733C36C
- LaeisPPuchlerKKirchWThe pharmacokinetic and metabolic profile of olmesartan medoxomil limits the risk of clinically relevant drug interactionJ Hypertens Suppl200119S21S3211451211
- MizunoMSadaTIkedaMPharmacology of CS-866, a novel nonpeptide angiotensin II receptor antagonistEur J Pharmacol19952851811888566137
- YoshidaKKohzukiMClinical and experimental aspects of olmesartan medoxomil, a new angiotensin II receptor antagonistCardiovasc Drug Rev20042228530815592575
- MurdochDHeelRCAmlodipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cardiovascular diseaseDrugs1991414785051711448
- RohatagiSLeeJShenoudaMPharmacokinetics of amlodipine and olmesartan after administration of amlodipine besylate and olmesartan medoxomil in separate dosage forms and as a fixed-dose combinationJ Clin Pharmacol2008481309132218974285
- FliserDBuchholzKHallerHAntiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammationCirculation20041101103110715313950
- LorenzenJMNeunhofferHDavidSKielsteinJTHallerHFliserDAngiotensin II receptor blocker and statins lower elevated levels of osteopontin in essential hypertension – results from the EUTOPIA trialAtherosclerosis201020918418819801149
- MiyazakiMTakaiSAnti-atherosclerotic efficacy of olmesartanJ Hum Hypertens200216Suppl 2S7S1211967727
- IchikawaSTakayamaYLong-term effects of olmesartan, an Ang II receptor antagonist, on blood pressure and the renin-angiotensin-aldosterone system in hypertensive patientsHypertens Res20012464164611768722
- SchindlerCBrosnihanKBFerrarioCMComparison of inhibitory effects of irbesartan and atorvastatin treatment on the renin angiotensin system (RAS) in veins: a randomized double-blind crossover trial in healthy subjectsJ Clin Pharmacol20074711212017192509
- CrackowerMASaraoROuditGYAngiotensin-converting enzyme 2 is an essential regulator of heart functionNature200241782282812075344
- FerrarioCMJessupJChappellMCEffect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2Circulation20051112605261015897343
- FerrarioCMTraskAJJessupJAAdvances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular functionAm J Physiol Heart Circ Physiol2005289H2281H229016055515
- FerrarioCMNew physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolismHypertension20105544545220026757
- GallagherPEFerrarioCMTallantEARegulation of ACE2 in cardiac myocytes and fibroblastsAm J Physiol Heart Circ Physiol2008295H2373H237918849338
- TallantEAFerrarioCMGallagherPEAngiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptorAm J Physiol Heart Circ Physiol2005289H1560H156615951342
- AgataJUraNYoshidaHOlmesartan is an angiotensin II receptor blocker with an inhibitory effect on angiotensin-converting enzymeHypertens Res20062986587417345786
- SmithRDYokoyamaHAverillDBThe protective effects of angiotensin II blockade with olmesartan medoxomil on resistance vessel remodeling (The VIOS study): rationale and baseline characteristicsAm J Cardiovasc Drugs2006633534217083268
- StumpeKOOlmesartan compared with other angiotensin II receptor antagonists: head-to-head trialsClin Ther200426Suppl AA33A3715291378
- MiyashitaYSaikiAEndoKEffects of olmesartan, an angiotensin II receptor blocker, and amlodipine, a calcium channel blocker, on Cardio-Ankle Vascular Index (CAVI) in type 2 diabetic patients with hypertensionJ Atheroscler Thromb20091662162619907103
- SmithRDYokoyamaHAverillDBSchiffrinELFerrarioCMReversal of vascular hypertrophy in hypertensive patients through blockade of angiotensin II receptorsJ Am Soc Hypertens2008216517220409899
- SchiffrinELDengLYLarochellePMorphology of resistance arteries and comparison of effects of vasoconstrictors in mild essential hypertensive patientsClin Invest Med1993161771868365045
- SchiffrinELDengLYLarochelleP[Prospective study of the effects of an angiotensin converting enzyme inhibitor and a beta blockader on the structure and function of resistant arteries in mild essential hypertension]Arch Mal Coeur Vaiss1994879799817755476
- AsmarRRudnichiABlacherJLondonGMSafarMEPulse pressure and aortic pulse wave are markers of cardiovascular risk in hypertensive populationsAm J Hypertens200114919711243313
- RosensonRSTreatment of hypertension in metabolic syndrome subjects with amlodipine and olmesartan-effects on oxidized non-esterified free fatty acids and cytokine productionCardiovasc Drugs Ther20092328929419641984
- ChrysantSGMelinoMKarkiSLeeJHeyrmanRThe combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety studyClin Ther20083058760418498909
- ChrysantSGAmlodipine besylate/olmesartan medoximil fixed combination for the treatment of hypertensionExpert Rev Cardiovasc Ther2009788789519673666
- BarriosVBrommerPHaagUCalderonAEscobarCOlmesartan medoxomil plus amlodipine increases efficacy in patients with moderate-to-severe hypertension after monotherapy: a randomized, double-blind, parallel-group, multicentre studyClin Drug Investig200929427439
- VolpeMBrommerPHaagUMieleCEfficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: a randomized, double-blind, parallel-group, multicentre studyClin Drug Investig2009291125
- LewingtonSClarkeRQizilbashNPetoRCollinsRProspectiveSCAge-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studiesLancet20023601903191312493255
- MouradJJLeJSEffective systolic blood pressure reduction with olmesartan medoxomil/amlodipine combination therapy: post hoc analysis of data from a randomized, double-blind, parallel-group, multicentre studyClin Drug Investig200929419425
- BasileJNeutelJOvercoming clinical inertia to achieve blood pressure goals: the role of fixed-dose combination therapyTher Adv Cardiovasc Dis2010411912720042448
- OparilSChrysantSGKereiakesDResults of an olmesartan medoxomil-based treatment regimen in hypertensive patientsJ Clin Hypertens (Greenwich)20081091192119120717
- de VinuesaSGGoicoecheaMKanterJInsulin resistance, inflammatory biomarkers, and adipokines in patients with chronic kidney disease: effects of angiotensin II blockadeJ Am Soc Nephrol200617S206S21217130263
- FogariRDerosaGZoppiAEffects of manidipine/delapril versus olmesartan/hydrochlorothiazide combination therapy in elderly hypertensive patients with type 2 diabetes mellitusHypertens Res200831435018360017
- FogariRDerosaGZoppiAEffect of delapril/manidipine vs olmesartan/hydrochlorothiazide combination on insulin sensitivity and fibrinogen in obese hypertensive patientsIntern Med20084736136618310964
- KuritaSTakamuraTOtaTOlmesartan ameliorates a dietary rat model of non-alcoholic steatohepatitis through its pleiotropic effectsEur J Pharmacol200858831632418501344
- ZamanAKFujiiSGotoDSalutary effects of attenuation of angiotensin II on coronary perivascular fibrosis associated with insulin resistance and obesityJ Mol Cell Cardiol20043752553515276022
- KlagMJWheltonPKRandallBLBlood pressure and end-stage renal disease in menN Engl J Med199633413187494564
- MiyagawaKDohiYNakazawaARenoprotective effect of calcium channel blockers in combination with an Angiotensin receptor blocker in elderly patients with hypertension. A randomized crossover trial between benidipine and amlodipineClin Exp Hypertens2010321720144066
- FurukawaTNukadaTMiuraRDifferential blocking action of dihydropyridine Ca2+ antagonists on a T-type Ca2+ channel (alpha1G) expressed in Xenopus oocytesJ Cardiovasc Pharmacol20054524124615725949
- MorikawaTOkumuraMKonishiYOkadaNImanishiMEffects of benidipine on glomerular hemodynamics and proteinuria in patients with nondiabetic nephropathyHypertens Res20022557157612358143
- HallerHVibertiGCMimranAPreventing microalbuminuria in patients with diabetes: rationale and design of the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) studyJ Hypertens20062440340816508590
- RitzEVibertiGCRuilopeLMDeterminants of urinary albumin excretion within the normal range in patients with type 2 diabetes: the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) studyDiabetologia201053495719876613
- ChrysantSGMarburyTCSilfaniTNUse of 24-h ambulatory blood pressure monitoring to assess blood pressure control: a comparison of olmesartan medoxomil and amlodipine besylateBlood Press Monit20061113514116702822
- KereiakesDJNeutelJMPunziHAXuJLipkaLJDubielREfficacy and safety of olmesartan medoxomil and hydrochlorothiazide compared with benazepril and amlodipine besylateAm J Cardiovasc Drugs2007736137217953475
- ChrysantSGChrysantGSAntihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazideExpert Opin Pharmacother2004565766715013933
- ChrysantSGWeberMAWangACHinmanDJEvaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazideAm J Hypertens20041725225915001200
- IzzoJLJrNeutelJMSilfaniTDubielRWalkerFEfficacy and safety of treating stage 2 systolic hypertension with olmesartan and olmesartan/HCTZ: results of an open-label titration studyJ Clin Hypertens (Greenwich)20079364417215657
- QuanAChavanuKMerkelJA review of the efficacy of fixed-dose combinations olmesartan medoxomil/hydrochlorothiazide and amlodipine besylate/benazepril in factorial design studiesAm J Cardiovasc Drugs2006610311316555863
- RamCVAntihypertensive efficacy of angiotensin receptor blockers in combination with hydrochlorothiazide: a review of the factorial-design studiesJ Clin Hypertens (Greenwich)2004656957715470286
- VolpeMMieleCHaagUEfficacy and safety of a stepped-care regimen using olmesartan medoxomil, amlodipine and hydrochlorothiazide in patients with moderate-to-severe hypertension: an open-label, long-term studyClin Drug Investig200929381391
- NeutelJMSmithDHSilfaniTNLeeYWeberMAEffects of a structured treatment algorithm on blood pressure goal rates in both stage 1 and stage 2 hypertensionJ Hum Hypertens20062025526216397514
- OgawaHKim-MitsuyamaSJinnouchiTMatsuiKArakawaKRationale, design and patient baseline characteristics of OlmeSartan and calcium antagonists randomized (OSCAR) study: a study comparing the incidence of cardiovascular events between high-dose angiotensin II receptor blocker (ARB) monotherapy and combination therapy of ARB with calcium channel blocker in Japanese elderly high-risk hypertensive patients (Clinical Trials gov no. NCT00134160)Hypertens Res20093257558019444280
- OparilSLeeJKarkiSMelinoMSubgroup analyses of an efficacy and safety study of concomitant administration of amlodipine besylate and olmesartan medoxomil: evaluation by baseline hypertension stage and prior antihypertensive medication useJ Cardiovasc Pharmacol20095442743619730391
- ChrysantSGAmlodipine/ARB fixed-dose combinations for the treatment of hypertension: focus on amlodipine/olmesartan combinationDrugs Today (Barc)20084444345318596998
- ChrysantSGOparilSMelinoMKarkiSLeeJHeyrmanREfficacy and safety of long-term treatment with the combination of amlodipine besylate and olmesartan medoxomil in patients with hypertensionJ Clin Hypertens (Greenwich)20091147548219751459
- NeutelJMSmithDHWeberMAWangACMasonsonHNUse of an olmesartan medoxomil-based treatment algorithm for hypertension controlJ Clin Hypertens (Greenwich)2004616817415073470
- NeutelJMPrescribing patterns in hypertension: the emerging role of fixed-dose combinations for attaining BP goals in hypertensive patientsCurr Med Res Opin2008242389240118616863
- OparilSWilliamsDChrysantSGMarburyTCNeutelJComparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertensionJ Clin Hypertens (Greenwich)2001328329131811588406
- OparilSWeberMAngiotensin receptor blocker and dihydropyridine calcium channel blocker combinations: an emerging strategy in hypertension therapyPostgrad Med2009121253919332960
- OparilSPimentaEEfficacy of an olmesartan medoxomil-based treatment algorithm in patients stratified by age, race, or sexJ Clin Hypertens (Greenwich)20101231320047622
- CappuccioFPMarkanduNDSagnellaGAEffects of amlodipine on urinary sodium excretion, renin-angiotensin-aldosterone system, atrial natriuretic peptide and blood pressure in essential hypertensionJ Hum Hypertens199151151191830107
- BramlagePFixed combination of irbesartan and hydrochlorothiazide in the management of hypertensionVasc Health Risk Manag2009521322419436667
- BramlagePHasfordJBlood pressure reduction, persistence and costs in the evaluation of antihypertensive drug treatment – a reviewCardiovasc Diabetol200981819327149
- BramlagePFixed-dose combinations of renin-angiotensin blocking agents with calcium channel blockers or hydrochlorothiazide in the treatment of hypertensionExpert Opin Pharmacother2009101755176719538001
- CalhounDAUse of single-pill combination therapy in the evolving paradigm of hypertension managementExpert Opin Pharmacother2009101869187419496740
- CalhounDACrikelairNAYenJGlazerRDAmlodipine/valsartan/hydrochlorothiazide triple combination therapy in moderate/severe hypertension: secondary analyses evaluating efficacy and safetyAdv Ther2009261012102320024680
- GuptaAKArshadSPoulterNRCompliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysisHypertension20105539940720026768
- SicaDARationale for fixed-dose combinations in the treatment of hypertension: the cycle repeatsDrugs20026244346211827559
- ZarowitzBJFixed-dose combinations for improving medication adherence in assisted living environmentsGeriatr Nurs20072834134518068817
- MinutoloRGabbaiFBBorrelliSChanging the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: an 8-week uncontrolled trialAm J Kidney Dis20075090891718037091
- StergiouGSNasothimiouEGDoes dosing antihypertensive drugs at night alter renal or cardiovascular outcome: do we have the evidenceCurr Opin Nephrol Hypertens20081746446918695386
- BlackHRElliottWJGranditsGPrincipal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trialJAMA20032892073208212709465
- YusufSSleightPPogueJBoschJDaviesRDagenaisGThe Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patientsN Engl J Med200034214515310639539
- SmithDHDubielRJonesMUse of 24-hour ambulatory blood pressure monitoring to assess antihypertensive efficacy: a comparison of olmesartan medoxomil, losartan potassium, valsartan, and irbesartanAm J Cardiovasc Drugs20055415015631537