Abstract
Aims
Guidelines recommend blood pressure (BP) in hypertensive patients should be <140 systolic BP (SBP) and <90 diastolic BP (DBP) mmHg. This analysis assessed goal rate achievement in hypertensive patients receiving olmesartan-based treatment in the OLMEBEST study.
Methods
Patients with essential hypertension (DBP ≥ 90 mmHg and <110 mmHg) received open-label olmesartan medoxomil 20 mg/day (n = 2306). After 8 weeks, patients with DBP ≥ 90 mmHg (n = 627) were randomized to 4 weeks’ double-blind treatment with olmesartan 40 mg/day monotherapy or olmesartan 20 mg/day plus hydrochlorothiazide (HCTZ) 12.5 mg/day. For this analysis, the numbers and proportions of patients who achieved SBP < 140 mmHg and/or DBP < 90 mmHg at the end of the 4 weeks were calculated.
Results
In patients who achieved DBP normalization (<90 mmHg) at week 8 (n = 1546) and continued open-label olmesartan 20 mg/day, 66.7% achieved SBP/DBP < 140/90 mmHg at Week 12. In patients who did not achieve DBP normalization at Week 8, 26.8% of those randomized to olmesartan 40 mg/day and 42.5% of those randomized to olmesartan 20 mg/day plus HCTZ 12.5 mg/day achieved a SBP/DBP < 140/90 mmHg at Week 12.
Conclusion
Olmesartan 40 mg/day and olmesartan 20 mg/day plus HCTZ 12.5 mg/day allow substantial proportions of patients to achieve BP goals.
Introduction
Hypertension is the leading cause of cardiovascular (CV) disease worldwide,Citation1 and often occurs in conjunction with other risk factors such as dyslipidemia, diabetes and obesity, putting patients at substantial risk of CV events such as stroke and myocardial infarction.Citation2 Due to the link between hypertension and CV risk, current guidelines recommend that all hypertensive patients should aim to reach the goal of <140 mmHg systolic blood pressure (SBP) and <90 mmHg diastolic BP (DBP).Citation3,Citation4 For diabetic patients and those with an elevated CV risk due to associated conditions, the BP goal is 130/80 mmHg.
Current recommendations state that the initial therapeutic regimen used to achieve BP goal should be based on a low dose of either a single agent or dual combination therapy.Citation3,Citation4 However, the majority of patients with hypertension will not achieve BP goals with initial low-dose monotherapy, regardless of the antihypertensive agent that is used.Citation4 Therefore in these patients, it is recommended to either increase the dose of single-agent therapy or initiate combination treatment.Citation4
Blockade of the angiotensin II type 1 (AT1) receptor is an effective way to block the renin–angiotensin system and reduce BP.Citation5 Olmesartan medoxomil, the most recently introduced angiotensin receptor blocker (ARB), was launched in the US and Europe in 2002, and in comparative studies has been shown to provide greater BP control relative to other ARBs for the doses used.Citation6–Citation10 In patients who do not respond adequately to the standard 20 mg/day dose of olmesartan, the dose can be increased to 40 mg/day to increase efficacy without affecting tolerability.Citation11,Citation12
Combination therapy with olmesartan and hydrochlorothiazide (HCTZ) has been shown to provide BP-lowering efficacy that is greater than that achieved with either agent individually.Citation13 OLMEBEST enrolled 2306 patients in a partially-randomized study in which olmesartan 40 mg/day and the combination of olmesartan 20 mg/day plus HCTZ 12.5 mg/day provided additional well-tolerated BP reductions in patients who had not achieved DBP normalization (<90 mmHg) after open-label treatment with olmesartan 20 mg/day.Citation14 The original report described the changes in BP seen during the OLMEBEST trial, and this additional analysis describes the levels of BP goal achievement obtained during this study.
Patients and methods
The design of the OLMEBEST study has been reported previously,Citation14 so is described only briefly here.
Study design
This was a prospective, parallel-group, double-blind, double-dummy study (). The study population comprised males and females (aged 18 to 75 years) with essential hypertension (DBP ≥ 90 mmHg and <110 mmHg). After a placebo run in, the study comprised an 8-week open-label phase followed by a final 4-week phase in which patients continued with open-label therapy or underwent treatment intensification by random assignment to dose uptitration or combination therapy. Patients with a normalized DBP (<90 mmHg) at the end of open-label treatment (Week 8) continued with this treatment for a further 4 weeks. Patients whose DBP was not normalized (ie, ≥ 90 mmHg) at Week 8 went on to receive 4 weeks of randomized therapy. Thus, the study comprised three consecutive phases:
Figure 1 Study design.
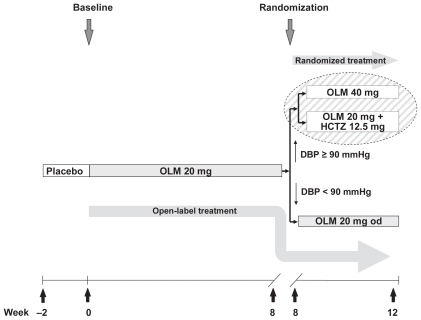
2 weeks of placebo treatment.
8 weeks of open-label treatment with olmesartan 20 mg/day.
4 weeks of either:
randomized treatment in which patients with a DBP ≥90 mmHg at Week 8 received either olmesartan 40 mg/day or olmesartan 20 mg/day plus HCTZ 12.5 mg/day.
open-label treatment in which patients with a DBP < 90 mmHg at Week 8 continued with olmesartan 20 mg/day.
Additional antihypertensive medications were not permitted during the study. The trial protocol was reviewed by an independent ethics committee or institutional review board in each country in which the trial was conducted. The trial was performed in accordance with the Declaration of Helsinki, the ethical principles of the International Conference on Harmonisation Guidelines for Good Clinical Practice, and relevant national laws of participating countries. All patients provided written informed consent.
Assessments
The assessments and timing of assessments is described in full elsewhere and is described only briefly here.Citation14
Patients attended a study center for six assessment visits between the initiation of screening and the completion of the partially randomized period; the timing of these visits was: Weeks −2, 0, 2, 4, 8, and 12, respectively. Physical investigations (including vital signs and resting electrocardiogram), blood sampling for laboratory examinations, and BP recordings were performed at Visit 1 (Week −2). At all visits, seated SBP and DBP were recorded for each patient in both arms, using an appropriately sized cuff and a well-calibrated sphygmomanometer with a maximum rate of descent of 2 mmHg. Patients were questioned about possible adverse events (AEs) at Visit 2 (baseline) and all subsequent visits.
Since analysis of BP goal achievement was not included in the original study protocol, the aim of this additional analysis was to describe the level of BP control achieved at Visit 6 (Week 12) in patients who were randomized at Visit 5 (Week 8) to receive either olmesartan 40 mg/day or olmesartan 20 mg/day in combination with HCTZ 12.5 mg/day, as well as in the non-randomized patients who were still in the study at Week 8, and continued to receive olmesartan 20 mg/day until Visit 6.
Patients
A total of 2173 patients completed the first 8 weeks of open-label treatment, of whom 71% (n = 1546) continued therapy with olmesartan 20 mg/day. A total of 627 patients did not achieve DBP normalization with olmesartan 20 mg/day at Week 8 and were randomized to either olmesartan 40 mg/day (n = 302) or a combination of olmesartan 20 mg/HCTZ 12.5 mg/day (n = 325). Demographic and clinical characteristics of these patients, approximately 10% of whom had diabetes mellitus, are shown in .
Table 1 Summary of demographic and clinical characteristics
Statistical analyses
Data were summarized by the calculation of absolute and relative frequencies of BP goal achievement. No inferential statistics were performed during this additional analysis. Missing values at Visit 6 (Week 12) were replaced on a last observation carried forwards basis.
Results
Goal rate achievement
Overall, for the total population, the proportion of patients who achieved the combined BP goal of <140/90 mmHg at Week 12 was 57.6% (1251/2173). The proportions of the total population who achieved the individual goals of SBP < 140 and DBP < 90 mmHg were 62.7 and 79.4%, respectively.
In patients who achieved DBP normalization (<90 mmHg) at Week 8 and continued to receive open-label olmesartan 20 mg/day until Week 12, approximately two thirds achieved the BP goal of <140/90 mmHg at Week 12 (). The proportions who achieved the individual goals of SBP < 140 and DBP < 90 mmHg were 69.3% and 90.1%, respectively (). For patients whose DBP was ≥90 mmHg at Week 8, treatment intensification by randomization to either olmesartan 40 mg/day or olmesartan 20 mg/day plus HCTZ 12.5 mg/day resulted in improved BP control. By Week 12, the overall proportion of patients in the two randomized groups with DBP < 90 mmHg was 53.1% (333/627), and the proportion who achieved the combined BP goal of <140/90 mmHg was 34.9% (219/627). Looking at the randomized treatment groups individually, the proportion of patients who achieved the combined BP goal of <140/90 mmHg was greater in the group that received olmesartan 20 mg/day plus HCTZ 12.5 mg/day (42.5% vs 26.8%; ). Differences between these two groups in proportions who achieved the individual goal of SBP < 140 and DBP < 90 mmHg were somewhat less marked ().
Figure 2 Blood pressure (BP) goal achievement rates at Week 12 for (a) combined systolic and diastolic BP (SBP and DBP) goals and for (b) individual SBP and DBP goals.
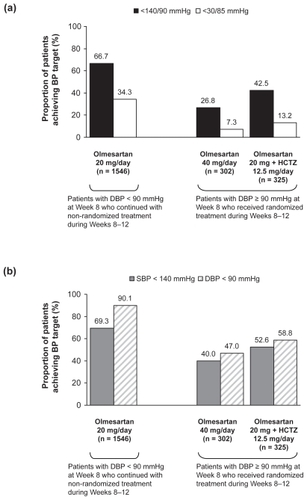
For patients who received olmesartan monotherapy only (ie, non-randomized patients who received olmesartan 20 mg/day plus the randomized patients who received olmesartan 40 mg/day), the proportion who achieved the combined BP goal of <140/90 mmHg at Week 12 was 60.3% (1113/1848). The proportions of monotherapy recipients who achieved the individual goals of SBP < 140 and DBP < 90 mmHg were 64.5% and 83.1%, respectively.
In addition to the combined BP goal of <140/90 mmHg, the proportion of patients who achieved a more rigorous combined BP goal of <130/85 mmHg was also assessed. For all patients (randomized plus non-randomized) the proportion who achieved this goal at Week 12 was 27.4% (596/2173). For the randomized groups, the overall proportion of patients who achieved the BP goal of <130/85 mmHg at Week 12 was 10.4% (65/627). In the individual randomized groups, the proportion of patients who achieved the combined BP goal of <130/85 mmHg was higher in the group that received combination therapy (). In patients who received olmesartan monotherapy only, 29.9% (553/1848) achieved the <130/85 mmHg goal.
Looking at patients with diabetes among the non-randomized patients, 16.4% achieved the guideline-recommended goal of 130/80 mmHg at Week 12. For randomized patients, the proportion who achieved the 130/80 mmHg goal was 6.7% and 9.7% in the olmesartan 40 mg/day and olmesartan 20 mg/day plus HCTZ 12.5 mg/day groups, respectively.
Safety evaluation
AEs reported in the OLMEBEST study have been reported previously,Citation14 and are described only briefly here. Generally, similar proportions of patients experienced AEs of mild, moderate and severe intensity during open-label treatment and in the two randomized treatment groups. During randomized treatment, olmesartan 40 mg/day was associated with a somewhat lower frequency of AEs than olmesartan 20 mg plus HCTZ 12.5 mg/day (21.5% vs 28.3% of patients, respectively). The majority (72.4%) of the AEs were classified as being of mild intensity and only 6.4% of events were considered probably or definitely related to study drug. Dizziness (1.4%) and headache (2.5%) were the most commonly reported AEs in each group during randomized treatment. AEs that led to withdrawal from the study were reported in 7 patients in the olmesartan 40 mg group and 9 in the olmesartan plus HCTZ group. No serious AEs were considered to be related to study medication.
At week 12, patients treated with olmesartan 40 mg/day and olmesartan 20 mg plus HCTZ 12.5 mg/day showed comparable mean serum concentrations of glucose (5.55 and 5.63 mmol/L, respectively), sodium (140.9 and 140.3 mmol/L, respectively), potassium (4.40 and 4.26 mmol/L, respectively), and creatinine (82.8 and 81.6 μmol/L, respectively). The incidence of laboratory measurements reported as being of potential clinical relevance during randomized treatment was low. Increases in serum levels were reported for alanine aminotransferase (olmesartan 20 mg plus HCTZ 12.5 mg/day group, n = 2), aspartate aminotransferase (olmesartan 20 mg plus HCTZ 12.5 mg/day, n = 2), γ-glutamyltransferase (olmesartan 40 mg/day, n = 2; and olmesartan 20 mg plus HCTZ 12.5 mg/day, n = 4), glucose (olmesartan 40 mg/day, n = 1; and olmesartan 20 mg plus HCTZ 12.5 mg/day, n = 3), and creatinine (olmesartan 40 mg/day, n = 1).
Discussion
OLMEBEST was a large study involving approximately 2300 patients that confirmed the efficacy of the standard maintenance dose of olmesartan 20 mg/day and suggested that for patients who did not show a sufficient response, antihypertensive efficacy could be increased by either dose-titration or the addition of a low dose of HCTZ. In each of the two randomized groups, additional reductions in SBP and DBP were seen relative to the end of the open-label monotherapy phase.Citation14
Monotherapy dose-titration or combination therapy is recommended in patients who do not respond adequately to initial monotherapy.Citation4 The results of this analysis show that treatment of hypertensive patients with olmesartan 20 mg/day plus HCTZ 12.5 mg/day or uptitration to olmesartan 40 mg/day for patients with a sub-optimal response, enabled more than half of all patients to achieve an SBP/DBP target of <140/90 mmHg in OLMEBEST. Looking at the whole study population, the proportion of patients who achieved the 140/90 mmHg goal approached 60% at Week 12.
Approximately 70% of patients achieved DBP normalization (<90 mmHg) after 8 weeks of open-label treatment with olmesartan 20 mg/day. However, approximately 30% of patients did not achieve DBP normalization and went on to receive randomized treatment. By the end of the study, more than half of these randomized patients who had already shown an inadequate DBP response to olmesartan 20 mg/day had achieved DBP normalisation, and more than a third had achieved the <140/90 mmHg goal.
The rate of DBP normalization achieved with olmesartan 20 mg/day during the 8-week open-label phase was higher than anticipated during the design of the study. Thus, fewer patients than planned were randomized to treatment, which meant that the study did not have sufficient power to detect non-inferiority between the two treatment groups. As such, it was not possible to determine whether there was a statistically significant difference between the two randomized groups. Despite this limitation, the olmesartan plus HCTZ arm appeared to be associated with a higher level of goal achievement compared with uptitration to olmesartan 40 mg/day. This finding is probably explained by the complementary modes of action of ARBs and HCTS that result in increased antihypertensive efficacy.Citation15 Indeed, the combination of olmesartan and HCTZ has previously been associated with larger BP reductions compared with uptitration of component monotherapy.Citation16
The partially-randomized design of the OLMEBEST study makes it difficult to compare the results with those of other studies. Adding HCTZ to an ARB increases antihypertensive efficacy, as outlined above, and the combination of olmesartan with HCTZ has been available for several years.Citation17 Rump et al looked at the effects of treatment with olmesartan 20 mg/day plus HCTZ 12.5 mg/day and found that after 12 weeks the proportion of patients with BP < 140/90 mmHg was 43%,Citation18 which is in line with the results of the present analysis for the randomized group that received combination therapy. Combining HCTZ with other ARBs has also been shown to result in comparable levels of goal rate achievement. The combination of HCTZ 12.5 mg/day with either candesartan 8 mg/day or valsartan 80 mg/day resulted in approximately 49% of hypertensive patients achieving <140/90 mmHg with each combination,Citation19 although a more recent analysis by Weir et al indicates a level closer to 40% for valsartan plus HCTZ.Citation20 For dose-titration, Neutel et al conducted a forced-titration study in which patients were treated with the aim of achieving a BP target of <130/85 mmHg.Citation21 During the initial monotherapy phase of the present study, patients received olmesartan 20 mg/day for 4 weeks, followed by uptitration to olmesartan 40 mg/day for the next 4 weeks for patients who did not achieve target BP. At the end of the 8-week monotherapy phase, the proportion of patients who achieved the BP target of <140/90 mmHg was 58.7%, a value similar to that observed for all patients who received olmesartan as monotherapy (either 20 mg/day or 40 mg/day) in OLMEBEST.
In many countries, overall levels of BP control in the general population are suboptimalCitation22–Citation24 and need to be improved in order to lower the rate of CV events such as stroke and myocardial infarction. Increasing the dose of monotherapy or using a two-drug combination is recommended in order to achieve BP control.Citation4 The results of the present analysis support this approach, and suggest that each of these approaches can be used to increase the number of patients achieving the recommended <140/90 mmHg hypertension threshold. Moreover, this analysis also looked at the more rigorous BP goal of <130/85 mmHg and found that by Week 12, 27.4% of all patients had achieved this goal and had thus achieved a level of BP control below the ESH-ESC threshold for high normalCitation4 and so could be regarded as being within the normal range.
In conclusion, treatment with olmesartan 20 mg/day enabled a substantial proportion of patients to achieve the guideline-recommended goal of <140/90 mmHg, and approximately two-thirds of patients receiving olmesartan 20 mg/day at the end of the study achieved this goal. For patients who had been unable to normalize their DBP with olmesartan 20 mg/day, addition of HCTZ 12.5 mg/day to the regimen, or dose uptitration to olmesartan 40 mg/day enabled even more patients to control their BP so that overall, nearly 60% of patients had a BP < 140/90 mmHg by study end. Such findings indicate that both uptitration and combination with HCTZ are effective options to achieve increased BP reduction and goal attainment, but clinical recommendations as to the most appropriate regimen must be decided by physicians based on individual patients’ needs.
Acknowledgments and disclosures
This study was sponsored by Daiichi Sankyo Europe GmbH, Munich, Germany. The authors thank Dr Phil Jones from Wolters Kluwer Health (Chester, UK) who provided medical writing assistance funded by Daiichi Sankyo Europe.
Professor Barrios has worked as a consultant for Daiichi Sankyo Europe.
References
- World Health OrganizationReducing risks, promoting healthy life 2002 [cited 2008 July 30] http://www.who.int/whr/2002/en/whr02_en.pdfAccessed August 6, 2009
- KannelWBBlood pressure as a cardiovascular risk factor: prevention and treatmentJAMA1996275157115768622248
- ChobanianAVBakrisGLBlackHRThe Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 reportJAMA2003 2892560257212748199
- ManciaGDe BackerGDominiczakA2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens2007251105118717563527
- BirkenhagerWHde LeeuwPWNon-peptide angiotensin type 1 receptor antagonists in the treatment of hypertensionJ Hypertens19991787388110419059
- BallKJWilliamsPAStumpeKORelative efficacy of an angiotensin II antagonist compared with other antihypertensive agents. Olmesartan medoxomil versus antihypertensivesJ Hypertens Suppl2001 19S49S5611451215
- BrunnerHRArakawaKAntihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil in achieving 24-hour blood pressure reductions and ambulatory blood pressure goalsClin Drug Investig200626185193
- BrunnerHRLaeisPClinical efficacy of olmesartan medoxomilJ Hypertens Suppl200321S43S4612929907
- GilesTDOparilSSilfaniTNComparison of increasing doses of olmesartan medoxomil, losartan potassium, and valsartan in patients with essential hypertensionJ Clin Hypertens (Greenwich)2007918719517341994
- OparilSWilliamsDChrysantSGComparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertensionJ Clin Hypertens (Greenwich)20013283291, 31811588406
- NeutelJMClinical studies of CS-866, the newest angiotensin II receptor antagonistAm J Cardiol20018737C43C
- PüchlerKLaiesPStumpeKOBlood pressure response, but not adverse event incidence, correlates with dose of angiotensin II antagonistJ Hypertens Suppl200119s41s48
- SellinLStegbauerJLaeisPAdding hydrochlorothiazide to olmesartan dose dependently improves 24-h blood pressure and response rates in mild-to-moderate hypertensionJ Hypertens2005232083209216208152
- BarriosVBoccanelliAEwaldSEfficacy and tolerability of olmesartan medoxomil in patients with mild to moderate essential hypertension: the OLMEBEST studyClin Drug Investig200727 545558
- KjeldsenSOsIH⊘ieggenAFixed-dose combinations in the management of hypertension: defining the place of angiotensin receptor antagonists and hydrochlorothiazideAm J Cardiovasc Drugs20055172215631534
- ChrysantSWeberMWangAEvaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazideAm J Hypertens20041725225915001200
- BarriosVEscobarCOlmesartan medoxomil plus hydrochlorothiazide for treating hypertensionExpert Opin Pharmacother2008912913618076344
- RumpLAmbrosioniEBurnierMInitial combination therapy with olmesartan/hydrochlorothiazide in moderate-to-severe hypertensionJ Hum Hypertens20062029930116452995
- WagstaffAJValsartan/hydrochlorothiazide: a review of its use in the management of hypertensionDrugs2006661881190117040120
- WeirMRCrikelairNLevyDEvaluation of the dose response with valsartan and valsartan/hydrochlorothiazide in patients with essential hypertensionJ Clin Hypertens (Greenwich)2007910311217268215
- NeutelJMSmithDHWeberMAUse of an olmesartan medoxomil-based treatment algorithm for hypertension controlJ Clin Hypertens (Greenwich)2004616817415073470
- BarriosVBanegasJRRuilopeLMEvolution of blood pressure control in SpainJ Hypertens2007251975197717762665
- BarriosVEscobarCCalderonABlood pressure and lipid goal attainment in the hypertensive population in the primary care setting in SpainJ Clin Hypertens (Greenwich)2007932432917485967
- Wolf-MaierKCooperRSKramerHHypertension treatment and control in five European countries, Canada, and the United StatesHypertension200443101714638619