Abstract
This review highlights a number of nitric oxide (NO)-related mechanisms that contribute to coronary vascular function and that are likely affected by hypertension and thus become important clinically as potential considerations in prevention, diagnosis, and treatment of coronary complications of hypertension. Coronary vascular resistance is elevated in hypertension in part due to impaired endothelium-dependent function of coronary arteries. Several lines of evidence suggest that other NO synthase isoforms and dilators other than NO may compensate for impairments in endothelial NO synthase (eNOS) to protect coronary artery function, and that NO-dependent function of coronary blood vessels depends on the position of the vessel in the vascular tree. Adaptations in NOS isoforms in the coronary circulation to hypertension are not well described so the compensatory relationship between these and eNOS in hypertensive vessels is not clear. It is important to understand potential functional consequences of these adaptations as they will impact the efficacy of treatments designed to control hypertension and coronary vascular disease. Polymorphisms of the eNOS gene result in significant associations with incidence of hypertension, although mechanistic details linking the polymorphisms with alterations in coronary vasomotor responses and adaptations to hypertension are not established. This understanding should be developed in order to better predict those individuals at the highest risk for coronary vascular complications of hypertension. Greater endothelium-dependent dilation observed in female coronary arteries is likely related to endothelial Ca2+ control and eNOS expression and activity. In hypertension models, the coronary vasculature has not been studied extensively to establish mechanisms for sex differences in NO-dependent function. Genomic and nongenomic effects of estrogen on eNOS and direct and indirect antioxidant activities of estrogen are discussed as potential mechanisms of interest in coronary circulation that could have implications for sex- and estrogen status-dependent therapy for hypertension and coronary dysfunction. The current review identifies some important basic knowledge gaps and speculates on the potential clinical relevance of hypertension adaptations in factors regulating coronary NO function.
Introduction to coronary hemodynamics in hypertension
Coronary blood flow is highly regulated to ensure an adequate matching of coronary perfusion to meet the metabolic demands imposed by a constantly beating heart.Citation1–Citation4 The main mechanisms controlling coronary artery tone are: metabolic, myogenic, neurohormonal, and endothelial.Citation1,Citation3,Citation4 These factors all interact to determine myocardial perfusion, and the relative importance of each mechanism varies as a function of the anatomical location of the vessel type of interest in the vascular tree. For instance, large coronary arteries have a greater dependency on endothelium-dependent mechanisms for maintenance of proper tone, while smaller arterioles depend more on metabolic and myogenic mechanisms.Citation2,Citation5,Citation6 Vascular resistance is dependent on position in the vascular network with approximately 75% of the resistance lying in the arteries between 75–200 μm in diameter,Citation2,Citation5,Citation7 and resistance also varies depending on the location of the vasculature within the depth of the myocardium.Citation2,Citation5,Citation8
Several humanCitation9–Citation12 and experimental animalCitation13–Citation22 studies indicate that coronary vascular resistance is increased, and coronary flow reserve is decreased with hypertension. The pathophysiology of hypertension is undoubtedly heterogeneous and a variety of animal models have been developed to investigate essential hypertension. The spontaneously hypertensive rat (SHR) model has been particularly useful since several defining characteristics of the SHR are similar to those observed in human essential hypertension including hemodynamic abnormalities, humoral and sympathetic nervous system involvement, renal abnormalities and vascular cellular adaptations.Citation23–Citation28 For example, even though SHR can have similar coronary blood flow compared to their normotensive counterpart Wistar–Kyoto rats (WKY) on a ventricular mass-corrected basis,Citation13 SHRs have higher coronary vascular resistance (CVR) over a wide pressure range,Citation14,Citation19 and higher minimal CVR (lower maximal conductance) during maximal coronary vasodilation.Citation13,Citation16
Changes in coronary hemodynamics accompanying hypertension occur as a result of both structural and functional adaptations in the coronary vasculature. Structural remodeling to hypertension includes hypertrophic and eutrophic inward remodeling and frank rarefaction, which can result in loss of up to half of the normal number of microvessels.Citation29,Citation30 A number of genetic, neurohumoral, and local factors contribute.Citation9,Citation11,Citation19,Citation29–Citation36 to these adaptations which result in increases in resistance, as well as reduced flow and increased diffusion distances, all of which impair oxygen delivery and organ function. Although nitric oxide (NO) likely contributes to the structural remodeling accompanying hypertension, the focus of this review does not include description of structural adaptations, and the reader is referred to other works dealing with these topics.Citation29,Citation30,Citation34,Citation35 Rather, the current focus is on the contribution of vascular NO to the functional adaptations in the coronary circulation in hypertension.
Multiple endothelium-derived products may contribute to the control of the coronary vasculature.Citation37,Citation38 For instance, it is quite apparent that one or more endothelium-derived hyperpolarizing factors (EDHFs) contribute greatly to the control of the coronary microcirculation.Citation39 Potentially important compensatory roles for EDHF may make these factors even more dominant in coronary vascular regulation under conditions of impairment of NO bioavailability.Citation40–Citation46 The importance and emerging knowledge concerning EDHF notwithstanding, the focus of the current review is on NO and coronary adaptations to hypertension, and the reader is referred to the cited works for further information on EDHF in the coronary vasculature.
Several reports have suggested that altered NO bioavailability contributes to altered vasomotion seen in hypertensionCitation38,Citation47,Citation48 and NO is the primary dilator of large epicardial coronary arteriesCitation3,Citation6,Citation49 as well as a mediator of flow-induced dilation in the coronary microcirculation.Citation2,Citation5,Citation6 Given the potential importance of the NO system in the etiology of hypertensive large artery disease, and given the fact that studies in other vascular beds have revealed a number of patterns of regulation of the NO system, the importance of which is not known in the coronary bed and in hypertension, it is important to bring attention to these factors as they may be important clinically and therapeutically. Thus, this review attempts to highlight a number of factors that influence NO function and which may be relevant from both basic science and clinical perspectives of understanding the function of the coronary circulation in hypertension.
Nitric oxide bioavailability in the control of coronary hemodynamics in hypertension
The NO synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (L-NAME) has been a useful tool in studies determining the NO component of flow and CVR alterations in the intact coronary circulation. Reductions in baseline coronary flow in the presence of L-NAME were smaller in SHR than in WKY hearts,Citation19,Citation22 suggesting a reduced basal NO bioavailability in the coronary circulation of hypertensive animals. NO bioavailability is a function of the production and destruction of NO, and of the sensitivity of the target tissue to NO.Citation48 Further investigation revealed that the smaller L-NAME-dependent reduction in baseline coronary flow was not correlated to decreases in NO production, suggesting that increased destruction of NO and/or decreased sensitivity to NO contributed to the reduced basal coronary NO bioavailability in hypertension.Citation22
In contrast to baseline effects, acetylcholine (ACh)-stimulated dilation of the coronary circulation was similar in normotensive and hypertensive animals, and was abolished in both groups by L-NAME, suggesting stimulated NO release and/or bioavailability may be unaltered by hypertension in this vascular bed.Citation50 Further analysis revealed, however, that the relationship between CVR and NO production is altered in SHR hearts so a greater amount of NO production occurs despite a persistently much higher CVR than in WKY controls.Citation18 Furthermore, inhibition of eNOS with l-arginine analogues also blunts constrictory responses in the majority of the coronary perfusion studies in hypertensive hearts,Citation19,Citation21 suggesting that NO is contributing to constrictory responses in the hypertensive coronary vascular bed likely via its destruction by superoxide and the consequent effects of reactive species formed.Citation51 This is supported by observations that supplementation of the coronary perfusate with the superoxide scavenging enzyme superoxide dismutase restored the maximal endothelium-dependent dilation in hypertensive animals.Citation21 Sensitivity to NO is likely not altered in hypertensive perfused heart studies,Citation18,Citation21,Citation22 acknowledging some dissenting reports.Citation52 Together this evidence suggests that increased NO destruction is a dominant contributor to the reduced NO bioavailability and consequent elevated CVR in the intact coronary circulation of hypertensive animals. Complementary work using isolated blood vessels reveals additional details regarding potential mechanisms accounting for hypertensive adaptations in the coronary circulation.
Nitric oxide-mediated vasomotor function of isolated coronary arteries in hypertension
Studies using isolated coronary arteries and arterioles support the findings from the intact coronary circulation that endothelium-dependent dilatory function is impaired in hypertensive animals, and that reduced NO bioavailability, and increased oxidative stress contribute to the mechanism of this impairment (). Indeed, in general, endothelium-mediated (drug- and shear stress-stimulated) dilation of isolated coronary arteries is reduced in hypertensive humansCitation11,Citation53–Citation57 and animals,Citation19,Citation21,Citation32,Citation36,Citation45,Citation51,Citation52,Citation58–Citation62 while the endothelium-independent vasodilatory responses to sodium nitroprusside and adenosine are often unaltered.Citation21,Citation32,Citation45,Citation55,Citation63 There are exceptions to these general observationsCitation18,Citation33,Citation50,Citation63–Citation66 and the vessel type (conduit vs resistance artery),Citation45,Citation57 the mode of precontraction,Citation33 and the vasodilator protocolCitation45,Citation64 must be scrutinized to compare and analyze mechanisms of coronary vasomotor control.
Figure 1 Factors affecting NO-mediated endothelium-dependent relaxation of coronary arteries in hypertension. Chemical and hemodynamic forces on the luminal side of the endothelium stimulate eNOS production of NO which can be scavenged by superoxide or diffuse to the vascular smooth muscle cells. At the smooth muscle, available NO activates sGC ultimately affecting Ca2+ regulatory proteins, cytosolic [Ca2+], and contractile elements, thereby causing arterial relaxation. In hypertension, NO-bioavailability and relaxation of the coronary vascular smooth muscle can be altered due to many factors as discussed in the text and indicated in the Figure by the small arrows.
![Figure 1 Factors affecting NO-mediated endothelium-dependent relaxation of coronary arteries in hypertension. Chemical and hemodynamic forces on the luminal side of the endothelium stimulate eNOS production of NO which can be scavenged by superoxide or diffuse to the vascular smooth muscle cells. At the smooth muscle, available NO activates sGC ultimately affecting Ca2+ regulatory proteins, cytosolic [Ca2+], and contractile elements, thereby causing arterial relaxation. In hypertension, NO-bioavailability and relaxation of the coronary vascular smooth muscle can be altered due to many factors as discussed in the text and indicated in the Figure by the small arrows.](/cms/asset/0dd2e1ef-f332-4263-be9c-e6f38d31ed08/dvhr_a_7464_f0001_b.jpg)
Agonist-induced dilation from the pre-contracted state is reducedCitation45,Citation56,Citation61,Citation67 or eliminatedCitation58,Citation68,Citation69 by inhibitors of eNOS in isolated coronary vessels from hypertensive animals, suggesting that NO remains an important component of vascular function even when its bioavailability is reduced in hypertension. In isolated pressurized coronary microvessels it was observed that removal of the endothelium increased the amount of myogenic constriction to a greater extent in SHR than in WKY over a wide pressure range.Citation31 Similarly, inhibition of the endothelial NOS isoform eNOS resulted in greater constriction in the SHR at moderate-to-high pressures,Citation31 while inhibition of the cyclooxygenase (COX) pathway did not affect the myogenic response in either WKY or SHR.Citation70 These studies suggest that NO is an important mediator of basal tone, especially at higher pressures, which are more likely to be physiologically relevant, especially in hypertension.
Regarding NO bioavailability in isolated arteries, while several reports suggest that NO production per se is not altered in isolated arteries from hypertensive animals,Citation18,Citation22,Citation66 a recent report indicates that hypertension is associated with an increase in arginase activity, which results in reduced basal and stimulated NO production.Citation71 In general, however, increased local vascular superoxide production-induced destruction of NO is thought to be the major mechanism limiting NO bioavailability in isolated coronary vessels from hypertensive animals,Citation48,Citation72 as also seemed to be the case when evaluating the intact coronary vascular bed.
The reaction rate of NO and superoxide is much faster than that of superoxide with superoxide dismutase.Citation47 Thus it is not surprising that in hypertension the increased production of reactive oxygen species (ROS) is associated with an increased production of peroxynitrite in the heart and coronary blood vessels.Citation47,Citation48,Citation73–Citation75 The production of peroxynitrite itself has an impact on several pathways of vasodilation including reduced prostaglandin synthesis and inhibition of K+ channels.Citation8,Citation76 Additionally peroxynitrite impairs NO production through oxidation of BH4, a NOS co-factorCitation8,Citation76–Citation78 and also impairs the sGC-mediated response to NO.Citation8,Citation76
Coronary hemodynamics and vasomotor activity in the eNOS knockout mouse
Given that hypertension is associated with impairments in NO bioavailability in the coronary vasculature as illustrated above, it is instructive to consider whether experimental models that specifically disrupt NO availability can provide insight to help understand and interpret hypertensive adaptations. One such model is the eNOS knockout mouse. Early work by Huang and colleagues demonstrated an increase in blood pressure (~20 mm Hg) in eNOS knockout (eNOS−/−) compared to wild-type (WT) mice,Citation79 and subsequent studies have demonstrated that the blood pressure effect is age-dependent; absent at eight weeks, but elevated by up to ~50 mm Hg at 12 weeks.Citation80,Citation81 These observations are consistent with the general view that NO is of critical importance in controlling vascular resistance and blood pressure.
The coronary vasculature of WT mice depends on NO for the majority of its overall total endothelium-dependent dilation since acute NOS inhibition eliminates most of the ACh-induced dilation in isolated preparations of the left anterior descending and left circumflex coronary arteriesCitation82,Citation83 and about half of the bradykinin (BK)-induced dilation in isolated heart preparations.Citation44 However, in the eNOS−/−, overall total endothelium-dependent dilation of the coronary vasculature can be either unaltered,Citation83 reduced,Citation44 or eliminatedCitation82 when compared to WT littermates. Since dilatory responses to endothelium-independent dilators in eNOS−/− are identical to those in WT in both isolated vesselsCitation82,Citation83 and perfused heart preparations,Citation40,Citation44 these results suggest that alterations occur in the eNOS−/− as a result of changes in the activity of other endothelium-derived vasodilators to compensate for the loss of eNOS-derived NO.
Little consensus has been reached as to the chemical identity of the dilator(s) released from the endothelium in eNOS−/−. Possibilities include NO (derived from other NOS isoforms; iNOS, nNOS), prostacylin, and non-NO, nonprostanoid endothelium-derived dilators.Citation42 For instance, in isolated coronary arteries, the specific nNOS inhibitor trifluoromethylphenylimidazole (TRIM) significantly reduced ACh-induced dilation in eNOS−/− by approximately half, and additional COX inhibition with indomethacin almost completely eliminated this remaining ACh-induced dilation. In contrast, neither inhibitor affected the responses in WT vessels.Citation83 This suggests that nNOS-derived NO and prostacyclin may compensate for the loss of eNOS in eNOS−/− to preserve coronary artery endothelium-dependent dilation. It is also possible that upregulation of cytochrome-P450 metabolites may be responsible for some of the compensatory endothelium-dependent dilation observed in the eNOS−/− coronary vasculature.Citation44
Thus, although it is clear that overall endothelium-dependent dilation may be maintained in eNOS−/− via compensatory changes in alternate dilatory pathways, the precise mechanisms signaling this compensation by alternate pathways is not resolved and seems to involve multiple factors.Citation42 The hypertension itself, and the compensatory changes in endothelium-derived vasoactive pathways that occur in the eNOS−/− model must be accounted for in studies utilizing this model to study the importance of eNOS and adaptations of the coronary circulation to hypertension. Known responses of NOS isoforms in hypertension may help to determine the mechanisms controlling compensatory responses in the regulation of coronary endothelial function. It could be important to understand this issue for the effective clinical/therapeutic management of vascular dysfunction in hypertension and other cardiovascular disease.
Adaptations in coronary NOS isoforms to hypertension
The NOS family of enzymes is composed of three isoforms; neuronal nNOS, inducible iNOS, and endothelial eNOS.Citation84 For all isoforms, NO production is controlled through protein expression level, and a number of post-translational mechanisms; however, many regulatory mechanisms are isoform-specific.Citation84 In terms of coronary vascular control in hypertension and heart failure, eNOS is the major isoform of interest, and is expressed in the coronary endothelium, the endocardium, and in cardiomyocytes;Citation85,Citation86 however, nNOS and iNOS may also play a role in vascular control under certain conditions (),Citation87–Citation90 as alluded to above.Citation83
eNOS
Observations that eNOS expression level of coronary artery endothelium,Citation52 and intramyocardial arteriolesCitation19 are reduced in SHR vs WKY animals have led to the suggestion that reduced eNOS expression contributes to the coronary endothelial dysfunction accompanying hypertension. However, other studies report that eNOS expression is actually increased in the coronary vessels in hypertension, suggesting that compensatory upregulation of this enzyme may be a strategy to help preserve vascular function.Citation85
In contrast to the disparate findings regarding coronary eNOS expression in hypertension,Citation19,Citation52,Citation85,Citation88 eNOS levels in large systemic arteries such as the aorta are generally increased in hypertensive rats.Citation48 For example, eNOS protein expression was elevated by ~60% in male SHR compared to WKY thoracic aorta.Citation91 Although eNOS expression is elevated in the SHR aorta, the elevated ROS environment because of increased NAD(P)H oxidase expressionCitation48 may scavenge the available NO, and/or uncoupled eNOS could be producing ROS rather than NO,Citation92 both of which would lead to the impaired NO-mediated dilation. The importance of these mechanisms in the coronary circulation in hypertension has not been resolved. In this regard, it will be important to assess the susceptibility of the coronary vascular bed to ROS-mediated reductions in NO bioavailability in hypertension via the actions of ROS sources such as NADPH oxidase and possibly uncoupled NOS activity.
Uncoupled eNOS has been the subject of several extensive reviews.Citation76–Citation78,Citation93 Briefly, this process is a result of reduced substrate l-arginine or cofactor BH4. Under either of these conditions the flow of electrons is delivered to molecular oxygen and superoxide is formed.Citation76–Citation78,Citation93 In hypertensive animals chronic treatment with BH4 has been shown to improve endothelium-dependent dilation though this treatment both increased NO production and reduced superoxide production.Citation74,Citation94 These results suggest that at least part of the increased production of ROS and altered vasodilation may be explained by increased uncoupled eNOS-mediated production of superoxide, and subsequent reduced NO bioavailability. The reduction in BH4 and/or increase in arginaseCitation71 may propagate the uncoupling of eNOS and enhance superoxide production under these conditions. It needs to be determined if uncoupled eNOS contributes to suppression of NO bioavailability in the coronary vascular bed in hypertension in order to determine if this should be a potentially important treatment target to correct the vascular dysfunction.
iNOS
The potential role for iNOS as a vasoactive NO source in the coronary vasculature of hypertensive animals is supported by observations of increased iNOS expression in many SHR tissues (including heart) which is attenuated by antioxidant treatment.Citation88 However, other studies show that iNOS activity is either no different between SHR and WKY,Citation85 or completely undetectable in either strain.Citation95 Similarly, in dogs with acute perinephritic hypertension, iNOS protein is undetectable in the heart and no difference in Ca2+-independent NOS activity is apparent between normotensive and hypertensive conditions.Citation86 Thus, the contribution of iNOS to altered NO production in hypertension remains unknown at this time. The involvement of iNOS may be complex, as this isoform is known to act in an uncoupled manner, producing superoxide in some diseased arteries ().Citation90 It is also possible that the reduction in both BH4 and l-arginine could account for increased superoxide production from uncoupled iNOS, as described above for eNOS.
nNOS
Isolated left anterior descending coronary arteries from eNOS−/− exhibit similar overall total endothelium-dependent dilation in response to increased flow as do those from WT.Citation89 Flow-induced dilation of eNOS−/− coronary arteries was inhibited by the nNOS specific inhibitor 7-nitroindazole (7-Ni), and by the soluble guanylate cyclase (sGC) inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) to a similar extent as by L-NAME.Citation89 However, while L-NAME inhibited dilation in WT, 7-Ni had no effect in WT, suggesting that the vasodilatory role for nNOS-derived NO was limited to conditions under which NO availability from eNOS is impaired (ie, the eNOS−/−), similar to the findings of a previous report that used the nNOS inhibitor TRIM to demonstrate a vasodilatory contribution of nNOS in isolated coronary arteries of eNOS−/− but not WT.Citation83 The presence of nNOS protein in the coronary endothelium of eNOS−/−, but not in WT animalsCitation89 supports the functional findings. Thus, in the absence of eNOS, nNOS may be upregulated, and act through sGC to help maintain coronary flow-mediated dilation. This reveals a possible compensatory role for nNOS in the control of coronary vascular function in hypertension, especially if the elevated blood pressure associated with eNOS−/− is involved in signaling the increase in nNOS expression.Citation83
The work with knockout animals and with isoform-specific pharmacological blockade reveals that shifts can occur in the origin of NO in the coronary vasculature under conditions of impairment of eNOS function and of oxidative stress, such as occurs in a variety of cardiovascular diseases including hypertension. Currently, these adaptations are predominantly interpreted as compensatory changes to protect NO bioavailability under the indicated conditions. The findings highlight an important response that must be taken into account to understand the role of NO in coronary vascular adaptations to hypertension and in therapeutic interventions designed to protect coronary vascular function. Factors affecting individual variability in eNOS regulation could impact these considerations, including emerging evidence that gene polymorphisms in eNOS are associated with cardiovascular disease risk.
eNOS polymorphisms associated with hypertension and coronary vascular function
Gene polymorphisms in eNOS and resultant impacts on eNOS expression levels have been associated with increased risk of hypertension,Citation96 as well as a variety of conditions affecting the coronary circulation including coronary artery disease and coronary spasm.Citation97 However, little evidence is available regarding the precise mechanisms by which eNOS polymorphisms may lead to altered levels of cardiovascular disease. Although a variety of locus- and ethnicity-dependent polymorphism effects exist,Citation97 the current review will briefly focus specifically on the single nucleotide polymorphisms and grouped haplotypes that are associated with hypertension, and the limited known effects on coronary vascular function ().
Figure 2 Effects of genetic factors and estrogen on eNOS function and NO bioavailability in coronary arteries with hypertension. Activation of the membrane-bound estrogen receptor can increase eNOS expression and activation as well as reduce the destruction of NO by superoxide, thereby increasing NO available for relaxation. Local hemodynamics, location in the coronary vascular bed, and genetic polymorphisms can also affect eNOS expression and may impact coronary relaxation in hypertension.
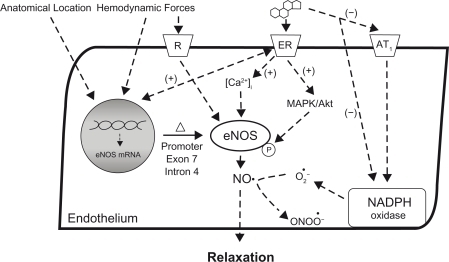
An exon 7 polymorphism results in eNOS Glu298Asp, with the Glu298Asp variant associated with 2.3X greater odds of developing hypertension.Citation96,Citation97 Several recent studies also associate eNOS combined haplotypes of the T-786C, Glu298Asp, and intron 4 polymorphisms with incidence of hypertension and plasma NO metabolites,Citation98,Citation99 implying a functional change at the eNOS enzyme. Available information suggests that eNOS polymorphisms attenuate the eNOS promoter efficiency.Citation100,Citation101 However, combinations of single polymorphisms can interact in a complex manner, and this supports the need to investigate haplotypes in order to assess and understand the role of eNOS polymorphisms in hypertension and vascular function.Citation101
Although eNOS polymorphisms and altered coronary eNOS expression are associated with hypertension,Citation96,Citation98,Citation99 little direct evidence links mutations in the eNOS gene to specific mechanisms of coronary vascular dysfunction in hypertension. Certain polymorphisms for the 27 bp repeat at intron 4 and for the G894T have been associated with alterations in coronary vasomotor responses,Citation102,Citation103 but this has not been assessed in a manner that allows for conclusions regarding the endothelium, or NO-dependency of the response. Thus, the precise mechanisms by which eNOS polymorphisms might affect coronary vascular function in hypertension have not yet been well established, and this important issue remains to be elucidated experimentally. In light of the involvement of NO in coronary hemodynamic and vasomotor adaptations to hypertension established in the previous sections, it is likely that human eNOS polymorphisms will be a factor contributing to the overall coronary vascular endothelial dysfunction accompanying hypertension in humans. Knowledge of the eNOS polymorphism status could be of possible diagnostic and therapeutic utility in the management of individual hypertensive patients as it may provide useful information concerning the potential severity of coronary vascular dysfunction.
Heterogeneity of eNOS expression in the coronary vascular bed
As might be expected from the known dependency of vasomotor control mechanisms on the position of a given vessel type in the vascular tree,Citation2,Citation5,Citation6 the distribution of the eNOS protein is likewise not uniform throughout the coronary vasculature of normal healthy hearts. Laughlin and colleagues demonstrated a reduced eNOS protein content per mg total vessel protein in the smallest porcine coronary resistance arteries (≤50 μM) compared to larger coronary arteries, despite a large reduction in the smooth muscle cell-to-endothelial cell ratio as coronary artery diameter decreases.Citation104 These data suggest that the largest coronary conduit vessels have a very large expression of eNOS protein in each endothelial cell. The authors postulate that the greater eNOS content in conduit arteries may be necessary to provide adequate NO for dilation of multiple layers of vascular smooth muscle, or may provide NO for dilation of downstream vessels.Citation104 The reported expression pattern for eNOS is consistent with a greater dependence on NO-mediated, endothelium-dependent dilation in larger arteries compared to the smallest arterioles.Citation2,Citation5,Citation6
The coronary artery is characterized by asynchronous hemodynamics, wherein the wall shear stress from blood flow is out of phase with the circumferential strain from blood pressureCitation105 and this may play a role in determining the heterogeneous eNOS expression in the coronary arterial tree.Citation106,Citation107 Further support for the role of hemodynamics in eNOS protein expression comes from studies in miniature swine following prolonged aerobic exercise training.Citation108 Following weeks of training, eNOS protein content was increased by over 50% in coronary arteries and small and large arterioles, but unaltered in coronary conduit and intermediate arterioles,Citation108 suggesting that steady state adaptations to exercise hemodynamic stimuli include nonuniform changes in eNOS expression that lead to an NO-mediated improvement in coronary resistance artery endothelium-dependent dilation.Citation109
Thus, eNOS protein expression is nonuniformly distributed throughout the coronary vasculature, possibly as the result of local hemodynamic influences, and eNOS expression may be altered in response to changes in coronary hemodynamics caused by exercise (). The hemodynamics of the coronary circulation are altered in hypertensionCitation9,Citation59 and likely affect the distribution of eNOS expression throughout the coronary vasculature. Whether the heterogeneous distribution of eNOS and NO-mediated vasomotor activity in the coronary vasculature may be altered by hypertension, and the mechanisms responsible have not been directly tested. If so, there may be implications for the loci of NO dependent events such as blood flow control, thrombosis, adhesion and cellular infiltration, and VSM hypertrophy and proliferation.
Sex-dependent function of the coronary vasculature and the role of estrogen
It is widely recognized that young adult females compared to males or postmenopausal females have a lower incidence of morbidity and mortality from coronary artery disease.Citation110 It is likely that sex-dependent differences in vascular endothelial function contribute to this phenomenon. Direct studies of coronary vascular endothelium function have demonstrated greater maximal relaxations and sensitivity to endotheliumdependent agonists in isolated coronary arteries from healthy female compared to male pigs.Citation111 Furthermore, increases in intravascular pressure elicited smaller myogenic constrictions in isolated rat coronary arteries from females compared to males, and the larger diameters of the female coronary arteries were associated with higher endothelial Ca2+ concentrations and eNOS activity.Citation112 Elevated NO release from female compared to male coronary arteries has been observed in a number of studies,Citation113–Citation115 and is consistent with findings in a variety of other artery types.
There is also a consistent sex-dependent effect in the endothelium-dependent NO-mediated vasorelaxation in hypertensive rats. Thus, although both male and female SHR have lower endothelium-dependent relaxation responses in isolated aortas compared to their respective normotensive WKY counterparts, ACh elicited greater relaxations in female than in male SHR aorta.Citation116,Citation117 Furthermore, whereas high ACh concentrations result in re-contractions of isolated aortic segments from male SHR, this response did not occur in aortas of female SHR.Citation116,Citation117 This general response was also observed in isolated aortas of stroke-prone SHR.Citation118 These sex-dependent functional effects have not been studied in the coronary circulation of hypertensive animals. It would be valuable to systematically assess this and to determine the mechanisms accounting for sex-differences in the coronary vascular function in order to better understand the molecular and functional basis for sex differences in vascular disease, and to provide foundation for possible sex-dependent diagnostic and treatment strategies.
It is possible that estrogen-independent mechanisms contribute to sex differences in endothelium-dependent vasomotor function and eNOS expression and activity, but there is general consensus that estrogen is a major signal coordinating the sex-dependent vascular function and phenotype ().Citation119–Citation121 Estrogen treatment has been demonstrated to enhance coronary blood flow,Citation122–Citation125 endothelial eNOS expression and activity levels,Citation126–Citation129 and NO release.Citation114,Citation125,Citation130,Citation131 Estrogen’s effect on endothelium NO-mediated action may occur by endothelium dependent genomic, nongenomic, and antioxidant mechanisms in the coronary vasculature. Although these specific mechanisms have not been examined extensively in hypertension models, the following sections briefly outline evidence for these mechanisms affecting the coronary vascular bed, and are intended to provide provocation for further study in the context of sex-dependent coronary vascular phenotypes in hypertension.
Endothelium-dependent genomic action of estrogen in the coronary vasculature
Activation of estrogen receptors (ER) mediates the upregulation of eNOSCitation128,Citation132 via a specific estrogen response element in the eNOS gene promoter region ().Citation133 Muller-Delp and colleagues demonstrated that estrogen treatment increased eNOS protein in coronary arteries of ovariectomized ERα-deficient mice.Citation134 However, eNOS levels were not restored to those seen in estrogen-treated ovariectomized wild-type mice, suggesting partial control through ERα and ERβ. In human coronary artery endothelial cells, 17β-estradiol treatment resulted in significantly increased eNOS protein levels and attendant elevations in basal and A23187-induced NO release,Citation135 effects which were completely inhibited in the presence of ICI182,780, a specific estrogen-receptor antagonist. Collectively, these and other studies indicate that genomic effects of estrogen on eNOS expression could influence coronary vascular function and account for sex-differences. Application of this knowledge to studies of the coronary vascular bed of hypertensive individuals should be undertaken to assess whether these effects occur or are disrupted in hypertension and when estrogen status changes in hypertensive individuals.
Endothelium-dependent nongenomic action of estrogen in the coronary vasculature
At physiological concentrations, estrogen can modulate vascular tone by inducing rapid release of NO from the endothelium that is not dependent on eNOS transcription (). For instance, 15 min of intracoronary 17β-estradiol infusion potentiated coronary microvascular vasodilator responses to ACh in postmenopausal women in an endothelium-dependent manner.Citation136 Animal models support this nongenomic activation of eNOS potentiating endothelium-dependent vasodilation.Citation126,Citation129 Although enhanced basal NO levels in the female coronary vasculature have been attributed to sex differences in Ca2+-handling mechanisms of the vascular endothelium,Citation112 the stimulation of NO production during estrogen administration has also been reported to occur independently of Ca2+ mobilization.Citation137 This mechanism likely involves a functional signaling unit localized in the endothelial plasmalemmal caveolae where ERs and eNOS are found.Citation138 Estrogen binding thus leads to eNOS phosphorylation via ERK1/2 and PI3-kinase/Akt-dependent pathways. Rapid release of NO occurs once eNOS has dissociated from caveolin-1 and united with the scaffolding protein Hsp90.Citation137 Regardless of the particular cell signaling mechanisms involved, these preliminary findings suggest an acute sensitivity to changes in estrogen that may have functional impact in the coronary circulation. It could be important to know whether this plays a role in heterogeneity between sexes and within females with different estrogen status, with respect to the overall control of the coronary vascular bed in hypertension as this could affect prevention, diagnosis and treatment decisions.
Antioxidant effects of estrogen on the coronary vasculature
The specific mechanisms of estrogen’s antioxidant effect are likely manifold and likely involve both genomic and nongenomic motifs.Citation133 One intriguing possibility related to vascular adaptations in hypertension involves potential effects of estrogen on AII-induced NAD(P)H oxidase activity.Citation139 Pretreatment of bovine coronary microvascular endothelial cells with 17β-estradiol prevented increases in NAD(P)H oxidase expression observed after 24 hours of AII stimulation alone. Inhibition of ERs by ICI182,780 did not alter the estradiol-induced decrease in AII-stimulated NAD(P)H expression, suggesting that the effect of estrogen was not ER-mediated.Citation139 However, estrogen administration did prevent AII-induced increases in type 1 angiotensin II receptors (AT1).Citation139 It has been proposed that estrogen’s antioxidant effects may be mediated via this down-regulation of AT1 receptors, causing decreased superoxide anion production and improved NO bioavailability. This estrogen signaling may occur via non-ER-dependent modulation of either the endothelial membrane properties, or via chemical antioxidant properties of estrogen itself.Citation140 Regardless of the specific mechanism linking estrogen to NAD(P)H oxidase expression in coronary vascular endothelium, these observations seem to be of great importance in the context of hypertension, as upregulation of vascular cell NAD(P)H oxidase is the major source of elevated vascular ROS which are thought to make a large contribution to the endothelial dysfunction accompanying hypertension.Citation48,Citation72 Thus, this mechanism should be of interest in examining potential sex-dependent coronary vascular adaptations in hypertension.
Summary
Many specific details regarding the regulation of NO bioavailability in the coronary vascular bed in hypertension are still unclear. Compensations, both from non-NO endothelium-derived vasodilators, and possibly by other isoforms of NOS occur when eNOS functionality is impaired. There is some evidence that similar compensations may occur in hypertension, but much more research is required to define mechanisms of the compensatory changes and to describe the signals associated with hypertension that trigger these changes. Polymorphisms of eNOS have been associated with hypertension incidence, and some emerging data suggest that coronary vascular function is also associated with certain eNOS polymorhisms; although these are promising findings, again the mechanistic details linking eNOS polymorphisms with coronary vascular function in hypertension remain to be rigorously established. Changes in endothelium-dependent function contribute to sex differences in cardiovascular disease. It has been demonstrated that estrogen has several mechanisms of action that improve NO bioavailability, including some known to be altered in hypertension.
Based on known functional effects of the identified factors that regulate coronary vascular NO mechanisms, it is intriguing to speculate that these will also be important mechanisms in the coronary vascular adaptations to hypertension. Basic information concerning compensatory vasodilator pathways, NOS isoform shifts, eNOS polymorphisms and sex- and estrogen status-dependent effects on NO bioavailability may be very helpful in designing more effective prevention, diagnostic and therapeutic strategies to deal with coronary vascular dysfunction in hypertension and cardiovascular disease.
Acknowledgements
Related work in the authors’ laboratory is funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Heart and Stroke Foundation of Ontario. JT Kroetsch, AS Levy, and JCS Chung were supported by Canada Graduate Scholarships from NSERC. JWE Rush is Canada Research Chair in Integrative Vascular Biology. The authors report no conflicts of interest in this work.
References
- FeiglEOCoronary physiologyPhysiol Rev19836312056296890
- MullerJMDavisMJChilianWMIntegrated regulation of pressure and flow in the coronary microcirculationCardiovasc Res1996326686788915185
- TuneJDGormanMWFeiglEOMatching coronary blood flow to myocardial oxygen consumptionJ Appl Physiol20049740441515220323
- WesterhofNBoerCLambertsRRSipkemaPCross-talk between cardiac muscle and coronary vasculaturePhysiol Rev2006861263130817015490
- JonesCJKuoLDavisMJChilianWMRegulation of coronary blood flow: coordination of heterogeneous control mechanisms in vascular microdomainsCardiovasc Res1995295855967606744
- FelicianoLHenningRJCoronary artery blood flow: physiologic and pathophysiologic regulationClin Cardiol19992277578610626079
- ChilianWMEasthamCLMarcusMLMicrovascular distribution of coronary vascular resistance in beating left ventricleAm J Physiol1986251H779H7883766755
- ChilianWMMicrovascular pressures and resistances in the left ventricular subepicardium and subendocardiumCirc Res1991695615701873859
- NitenbergAAntonyIEpicardial coronary arteries are not adequately sized in hypertensive patientsJ Am Coll Cardiol1996271151238522684
- AntonyINitenbergACoronary vascular reserve is similarly reduced in hypertensive patients without any other coronary risk factors and in normotensive smokers and hypercholesterolemic patients with angiographically normal coronary arteriesAm J Hypertens1997101811889037326
- KozakovaMGalettaFGregoriniLCoronary vasodilator capacity and epicardial vessel remodeling in physiological and hypertensive hypertrophyHypertension20003634334910988262
- PalomboCKozakovaMMagagnaAEarly impairment of coronary flow reserve and increase in minimum coronary resistance in borderline hypertensive patientsJ Hypertens20001845345910779097
- WanglerRDPetersKGMarcusMLTomanekRJEffects of duration and severity of arterial hypertension and cardiac hypertrophy on coronary vasodilator reserveCirc Res19825110186211294
- EdouteYLuscherTFRubanyiGMAutoregulation and vascular reserve in the coronary circulation of the spontaneously hypertensive ratJ Hypertens Suppl19864S290S2923471908
- FribergPWahlanderHNordlanderMStructural and functional adaptations within the myocardium and coronary vessels after antihypertensive therapy in spontaneously hypertensive ratsJ Hypertens Suppl19864S519S5213465913
- IsoyamaSSatoFTakishimaTEffect of age on coronary circulation after imposition of pressure-overload in ratsHypertension1991173693771825648
- FujitaHTakedaKNakamuraKRole of nitric oxide in impaired coronary circulation and improvement by angiotensin II receptor antagonist in spontaneously hypertensive ratsClin Exp Pharmacol Physiol Suppl199522S148S1509072332
- KelmMFeelischMKrebberTDeussenAMotzWStrauerBERole of nitric oxide in the regulation of coronary vascular tone in hearts from hypertensive rats. Maintenance of nitric oxide-forming capacity and increased basal production of nitric oxideHypertension1995251861937843768
- CrabosMCostePPaccalinMReduced basal NO-mediated dilation and decreased endothelial NO-synthase expression in coronary vessels of spontaneously hypertensive ratsJ Mol Cell Cardiol19972955659040021
- SusicDNunezEHosoyaKFrohlichEDCoronary hemodynamics in aging spontaneously hypertensive and normotensive Wistar-Kyoto ratsJ Hypertens1998162312379535151
- MilletteEdeCJLamontagneDAltered coronary dilation in deoxycorticosterone acetate-salt hypertensionJ Hypertens2000181783179311132602
- MokunoSItoTNumaguchiYImpaired nitric oxide production and enhanced autoregulation of coronary circulation in young spontaneously hypertensive rats at prehypertensive stageHypertens Res20012439540111510752
- TrippodoNCFrohlichEDSimilarities of genetic (spontaneous) hypertension. Man and ratCirc Res1981483093197460205
- BohrDFDominiczakAFExperimental hypertensionHypertension199117I39I441846123
- YamoriYOverview: studies on spontaneous hypertension-development from animal models toward manClin Exp Hypertens A1991136316441773499
- PintoYMPaulMGantenDLessons from rat models of hypertension: from Goldblatt to genetic engineeringCardiovasc Res19983977889764191
- YagilYYagilCGenetic models of hypertension in experimental animalsExp Nephrol200191911053974
- LermanLOChadeARSicaVNapoliCAnimal models of hypertension: an overviewJ Lab Clin Med200514616017316131455
- HutchinsPMLynchCDCooneyPTCurseenKAThe microcirculation in experimental hypertension and agingCardiovasc Res1996327727808915195
- GreeneASMicrovascular regulation and dysregulationIzzoJLJrBlackHRHypertension Primer: The essentials of high blood pressure3rd edPhilidelphia, PALippincott Williams & Wilkins2003183185
- GarciaSRIzzardASHeagertyAMBundSJMyogenic tone in coronary arteries from spontaneously hypertensive ratsJ Vasc Res1997341091169167643
- GhalehBHittingerLKimSJSelective large coronary endothelial dysfunction in conscious dogs with chronic coronary pressure overloadAm J Physiol1998274H539H5519486258
- BundSJInfluence of mode of contraction on the mechanism of acetylcholine-mediated relaxation of coronary arteries from normotensive and spontaneously hypertensive ratsClin Sci (Lond)1998942312389616256
- BaumbachGLHeistadDDRemodeling of cerebral arterioles in chronic hypertensionHypertension1989139689722737731
- BaumbachGLMechanisms of vascular remodelingIzzoJLJrBlackHRHypertension Primer: The essentials of high blood pressure3rd edPhiladelphia, PALippincott Williams & Wilkins2003180183
- MilletteEDemeilliersBWuRComparison of the cardiovascular protection by omapatrilat and lisinopril treatments in DOCA-salt hypertensionJ Hypertens20032112513512544444
- VanhouttePMFeletouMTaddeiSEndothelium-dependent contractions in hypertensionBr J Pharmacol200514444945815655530
- FeletouMVanhouttePMEndothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture)Am J Physiol Heart Circ Physiol2006291H985H100216632549
- LiuYGuttermanDDVascular control in humans: focus on the coronary microcirculationBasic Res Cardiol200910421122719190954
- GodeckeADeckingUKDingZCoronary hemodynamics in endothelial NO synthase knockout miceCirc Res1998821861949468189
- MiuraHLiuYGuttermanDDHuman coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization: contribution of nitric oxide and Ca2+-activated K+ channelsCirculation1999993132313810377076
- GodeckeASchraderJAdaptive mechanisms of the cardiovascular system in transgenic mice--lessons from eNOS and myoglobin knockout miceBasic Res Cardiol20009549249811192371
- MiuraHWachtelRELiuYFlow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channelsCirculation20011031992199811306529
- DingZGodeckeASchraderJContribution of cytochrome P450 metabolites to bradykinin-induced vasodilation in endothelial NO synthase deficient mouse heartsBr J Pharmacol200213563163811834610
- AubinMCGendronMELebelVAlterations in the endothelial G-protein coupled receptor pathway in epicardial arteries and subendocardial arterioles in compensated left ventricular hypertrophyBasic Res Cardiol200710214415317006634
- HeintzADammMBrandMKochTDeussenACoronary flow regulation in mouse heart during hypercapnic acidosis: role of NO and its compensation during eNOS impairmentCardiovasc Res20087718819618006478
- CaiHHarrisonDGEndothelial dysfunction in cardiovascular diseases: the role of oxidant stressCirc Res20008784084411073878
- RushJWDennissSGGrahamDAVascular nitric oxide and oxidative stress: determinants of endothelial adaptations to cardiovascular disease and to physical activityCan J Appl Physiol20053044247416258183
- KelmMSchraderJControl of coronary vascular tone by nitric oxideCirc Res199066156115752160870
- TschudiMRNollGArnetUNovoselDGantenDLuscherTFAlterations in coronary artery vascular reactivity of hypertensive Ren-2 transgenic ratsCirculation199489278027868205692
- BouloumieABauersachsJLinzWEndothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion productionHypertension1997309349419336396
- BauersachsJBouloumieAMulschAWiemerGFlemingIBusseRVasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression, and in superoxide anion productionCardiovasc Res1998377727799659462
- BrushJEJrFaxonDPSalmonSJacobsAKRyanTJAbnormal endothelium-dependent coronary vasomotion in hypertensive patientsJ Am Coll Cardiol1992198098151545076
- TreasureCBManoukianSVKleinJLEpicardial coronary artery responses to acetylcholine are impaired in hypertensive patientsCirc Res1992717767811516154
- TreasureCBKleinJLVitaJAHypertension and left ventricular hypertrophy are associated with impaired endothelium-mediated relaxation in human coronary resistance vesselsCirculation19938786938419028
- QuyyumiAADakakNAndrewsNPNitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosisJ Clin Invest199595174717557706483
- HoughtonJLDavisonCAKuhnerPATorossovMTStrogatzDSCarrAAHeterogeneous vasomotor responses of coronary conduit and resistance vessels in hypertensionJ Am Coll Cardiol1998313743829462582
- PourageaudFFreslonJLEndothelial and smooth muscle properties of coronary and mesenteric resistance arteries in spontaneously hypertensive rats compared to WKY ratsFundam Clin Pharmacol1995937457768486
- PourageaudFFreslonJLImpaired endothelial relaxations induced by agonists and flow in spontaneously hypertensive rat compared to Wistar-Kyoto rat perfused coronary arteriesJ Vasc Res1995321901997772679
- MacCarthyPAShahAMImpaired endothelium-dependent regulation of ventricular relaxation in pressure-overload cardiac hypertrophyCirculation20001011854186010769288
- MaloOCarrierMShiYFTardifJCTanguayJFPerraultLPSpecific alterations of endothelial signal transduction pathways of porcine epicardial coronary arteries in left ventricular hypertrophyJ Cardiovasc Pharmacol20034227528612883333
- DemirciBMcKeownPPBayraktutanUBlockade of angiotensin II provides additional benefits in hypertension- and ageing-related cardiac and vascular dysfunctions beyond its blood pressure-lowering effectsJ Hypertens2005232219222716269964
- TschudiMRCriscioneLLuscherTFEffect of aging and hypertension on endothelial function of rat coronary arteriesJ Hypertens Suppl19919S164S1651818925
- Gauthier-ReinKMRuschNJDistinct endothelial impairment in coronary microvessels from hypertensive Dahl ratsHypertension1998313283349453324
- FuchsLCNunoDLampingKGJohnsonAKCharacterization of endothelium-dependent vasodilation and vasoconstriction in coronary arteries from spontaneously hypertensive ratsAm J Hypertens199694754838735179
- KelmMFeelischMKrebberTMotzWStrauerBEThe role of nitric oxide in the regulation of coronary vascular resistance in arterial hypertension: comparison of normotensive and spontaneously hypertensive ratsJ Cardiovasc Pharmacol199220Suppl 12S183S1861282963
- DuffySJCastleSFHarperRWMeredithITContribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulationCirculation19991001951195710556220
- TschudiMRCriscioneLNovoselDPfeifferKLuscherTFAntihypertensive therapy augments endothelium-dependent relaxations in coronary arteries of spontaneously hypertensive ratsCirculation199489221222188181147
- Vazquez-PerezSNavarro-CidJde lasHNRelevance of endothelium-derived hyperpolarizing factor in the effects of hypertension on rat coronary relaxationsJ Hypertens20011953954511327627
- GarciaSRBundSJNitric oxide modulation of coronary artery myogenic tone in spontaneously hypertensive and Wistar-Kyoto ratsClin Sci (Lond)1998942252299616255
- ZhangCHeinTWWangWUpregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arteriolesHypertension20044493594315492130
- RushJWFordRJNitric oxide, oxidative stress and vascular endothelium in health and hypertensionClin Hemorheol Microcirc20073718519217641408
- Rodriguez-PorcelMLermanLOHerrmannJSawamuraTNapoliCLermanAHypercholesterolemia and hypertension have synergistic deleterious effects on coronary endothelial functionArterioscler Thromb Vasc Biol20032388589112663373
- ZhuXYDaghiniEChadeARRole of oxidative stress in remodeling of the myocardial microcirculation in hypertensionArterioscler Thromb Vasc Biol2006261746175216709946
- LuZXuXHuXExtracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunctionHypertension200851192517998475
- LeeMYGriendlingKKRedox signaling, vascular function, and hypertensionAntioxid Redox Signal2008101045105918321201
- MunzelTDaiberAUllrichVMulschAVascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinaseArterioscler Thromb Vasc Biol2005251551155715879305
- ThomasSRWittingPKDrummondGRRedox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunitiesAntioxid Redox Signal2008101713176518707220
- HuangPLHuangZMashimoHHypertension in mice lacking the gene for endothelial nitric oxide synthaseNature19953772392427545787
- KubisNBesnardSSilvestreJSDecreased arteriolar density in endothelial nitric oxide synthase knockout mice is due to hypertension, not to the constitutive defect in endothelial nitric oxide synthase enzymeJ Hypertens20022027328011821712
- KubisNRicherCDomergueVGiudicelliJFLevyBIRole of microvascular rarefaction in the increased arterial pressure in mice lacking for the endothelial nitric oxide synthase gene (eNOS3pt−/−)J Hypertens2002201581158712172320
- ChataigneauTFeletouMHuangPLFishmanMCDuhaultJVanhouttePMAcetylcholine-induced relaxation in blood vessels from endothelial nitric oxide synthase knockout miceBr J Pharmacol199912621922610051139
- LampingKGNunoDWSheselyEGMaedaNFaraciFMVasodilator mechanisms in the coronary circulation of endothelial nitric oxide synthase-deficient miceAm J Physiol Heart Circ Physiol2000279H1906H191211009479
- MichelTFeronONitric oxide synthases: which, where, how, and whyJ Clin Invest1997100214621529410890
- NavaENollGLuscherTFIncreased activity of constitutive nitric oxide synthase in cardiac endothelium in spontaneous hypertensionCirculation199591231023137537185
- PiechAMassartPEDessyCDecreased expression of myocardial eNOS and caveolin in dogs with hypertrophic cardiomyopathyAm J Physiol Heart Circ Physiol2002282H219H23111748066
- RavalliSAlbalaAMingMInducible nitric oxide synthase expression in smooth muscle cells and macrophages of human transplant coronary artery diseaseCirculation199897233823459639378
- VaziriNDNiZOveisiFTrnavsky-HobbsDLEffect of antioxidant therapy on blood pressure and NO synthase expression in hypertensive ratsHypertension20003695796411116107
- HuangASunDSheselyEGLeveeEMKollerAKaleyGNeuronal NOS-dependent dilation to flow in coronary arteries of male eNOS-KO miceAm J Physiol Heart Circ Physiol2002282H429H43611788389
- UngvariZCsiszarAEdwardsJGIncreased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthaseArterioscler Thromb Vasc Biol20032341842412615666
- GrahamDARushJWExercise training improves aortic endotheliumdependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive ratsJ Appl Physiol2004962088209614752124
- LiHWitteKAugustMReversal of endothelial nitric oxide synthase uncoupling and up-regulation of endothelial nitric oxide synthase expression lowers blood pressure in hypertensive ratsJ Am Coll Cardiol2006472536254416781385
- SchulzEJansenTWenzelPDaiberAMunzelTNitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertensionAntioxid Redox Signal2008101115112618321209
- MaloODesjardinsFTanguayJFTardifJCCarrierMPerraultLPTetrahydrobiopterin and antioxidants reverse the coronary endothelial dysfunction associated with left ventricular hypertrophy in a porcine modelCardiovasc Res20035950151112909333
- KhadourFHKaoRHParkSArmstrongPWHolycrossBJSchulzRAge-dependent augmentation of cardiac endothelial NOS in a genetic rat model of heart failureAm J Physiol1997273H1223H12309321810
- MiyamotoYSaitoYKajiyamaNEndothelial nitric oxide synthase gene is positively associated with essential hypertensionHypertension199832389674630
- WangXLWangJEndothelial nitric oxide synthase gene sequence variations and vascular diseaseMol Genet Metab20007024125110993711
- SandrimVCde SyllosRWLisboaHRTresGSTanus-SantosJEEndothelial nitric oxide synthase haplotypes affect the susceptibility to hypertension in patients with type 2 diabetes mellitusAtherosclerosis200618924124616427644
- SandrimVCde SyllosRWLisboaHRTresGSTanus-SantosJEInfluence of eNOS haplotypes on the plasma nitric oxide products concentrations in hypertensive and type 2 diabetes mellitus patientsNitric Oxide20071634835517306574
- NakayamaMYasueHYoshimuraMT-786 – >C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasmCirculation1999992864287010359729
- WangJDudleyDWangXLHaplotype-specific effects on endothelial NO synthase promoter efficiency: modifiable by cigarette smokingArterioscler Thromb Vasc Biol200222e1e412006409
- NaberCKBaumgartDAltmannCSiffertWErbelRHeuschGeNOS 894T allele and coronary blood flow at rest and during adenosine-induced hyperemiaAm J Physiol Heart Circ Physiol2001281H1908H191211668050
- KunnasTALehtimakiTLaaksonenREndothelial nitric oxide synthase genotype modulates the improvement of coronary blood flow by pravastatin: a placebo-controlled PET studyJ Mol Med20028080280712483466
- LaughlinMHTurkJRSchrageWGWoodmanCRPriceEMInfluence of coronary artery diameter on eNOS protein contentAm J Physiol Heart Circ Physiol2003284H1307H131212595288
- QiuYTarbellJMNumerical simulation of pulsatile flow in a compliant curved tube model of a coronary arteryJ Biomech Eng2000122778510790833
- DancuMBBerardiDEVanden HeuvelJPTarbellJMAsynchronous shear stress and circumferential strain reduces endothelial NO synthase and cyclooxygenase-2 but induces endothelin-1 gene expression in endothelial cellsArterioscler Thromb Vasc Biol2004242088209415345505
- DancuMBTarbellJMCoronary endothelium expresses a pathologic gene pattern compared to aortic endothelium: correlation of asynchronous hemodynamics and pathology in vivoAtherosclerosis200719291416806232
- LaughlinMHPollockJSAmannJFHollisMLWoodmanCRPriceEMTraining induces nonuniform increases in eNOS content along the coronary arterial treeJ Appl Physiol20019050151011160048
- MullerJMMyersPRLaughlinMHVasodilator responses of coronary resistance arteries of exercise-trained pigsCirculation199489230823148181157
- NathanLChaudhuriGEstrogens and atherosclerosisAnnu Rev Pharmacol Toxicol1997374775159131262
- BarberDAMillerVMGender differences in endothelium-dependent relaxations do not involve NO in porcine coronary arteriesAm J Physiol1997273H2325H23329374769
- KnotHJLounsburyKMBraydenJENelsonMTGender differences in coronary artery diameter reflect changes in both endothelial Ca2+ and ecNOS activityAm J Physiol1999276H961H96910070080
- WellmanGCBonevADNelsonMTBraydenJEGender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca2+-dependent K+ channelsCirc Res199679102410308888695
- DarkowDJLuLWhiteREEstrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMPAm J Physiol1997272H2765H27739227556
- MaLRobinsonCPThadaniUPattersonEEffect of 17-beta estradiol in the rabbit: endothelium-dependent and -independent mechanisms of vascular relaxationJ Cardiovasc Pharmacol1997301301359268232
- KauserKRubanyiGMGender difference in endothelial dysfunction in the aorta of spontaneously hypertensive ratsHypertension1995255175237721392
- GrahamDARushJWECyclooxygenase and thromboxane/prostaglandin receptor contribute to aortic endothelium-dependent dysfunction in aging female spontaneously hypertensive ratsJ Appl Physiol20091071059106719696359
- McIntyreMHamiltonCAReesDDReidJLDominiczakAFSex differences in the abundance of endothelial nitric oxide in a model of genetic hypertensionHypertension199730151715249403576
- PintoSVirdisAGhiadoniLEndogenous estrogen and acetylcholine-induced vasodilation in normotensive womenHypertension1997292682739039113
- LimaSMAldrighiJMConsolim-ColomboFMAcute administration of 17beta-estradiol improves endothelium-dependent vasodilation in postmenopausal womenMaturitas20055026627415780525
- NewGDuffySJHarperRWMeredithITEstrogen improves acetylcholine-induced but not metabolic vasodilation in biological malesAm J Physiol1999277H2341H234710600854
- CollinsPShayJJiangCMossJNitric oxide accounts for dose-dependent estrogen-mediated coronary relaxation after acute estrogen withdrawalCirculation199490196419687923686
- GorodeskiGIYangTLevyMNGoldfarbJUtianWHEffects of estrogen in vivo on coronary vascular resistance in perfused rabbit heartsAm J Physiol1995269R1333R13388594934
- LangUBakerRSClarkKEEstrogen-induced increases in coronary blood flow are antagonized by inhibitors of nitric oxide synthesisEur J Obstet Gynecol Reprod Biol1997742292359306125
- NodeKKitakazeMKosakaHRoles of NO and Ca2+-activated K+ channels in coronary vasodilation induced by 17beta-estradiol in ischemic heart failureFASEB J1997117937999271364
- BellDRRensbergerHJKoritnikDRKoshyAEstrogen pretreatment directly potentiates endothelium-dependent vasorelaxation of porcine coronary arteriesAm J Physiol1995268H377H3837840287
- HayashiTYamadaKEsakiTEstrogen increases endothelial nitric oxide by a receptor-mediated systemBiochem Biophys Res Commun19952148478557575554
- HishikawaKNakakiTMarumoTSuzukiHKatoRSarutaTUp-regulation of nitric oxide synthase by estradiol in human aortic endothelial cellsFEBS Lett19953602912937533729
- ChenZYuhannaISGalcheva-GargovaZKarasRHMendelsohnMEShaulPWEstrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogenJ Clin Invest19991034014069927501
- HuangASunDKollerAKaleyGGender difference in myogenic tone of rat arterioles is due to estrogen-induced, enhanced release of NOAm J Physiol1997272H1804H18099139966
- ThompsonLPWeinerCPLong-term estradiol replacement decreases contractility of guinea pig coronary arteries to the thromboxane mimetic U46619Circulation1997957097149024161
- KleinertHWallerathTEuchenhoferCIhrig-BiedertILiHForstermannUEstrogens increase transcription of the human endothelial NO synthase gene: analysis of the transcription factors involvedHypertension1998315825889461225
- SiowRCLiFYRowlandsDJdeWPMannGECardiovascular targets for estrogens and phytoestrogens: transcriptional regulation of nitric oxide synthase and antioxidant defense genesFree Radic Biol Med20074290992517349919
- Muller-DelpJMLubahnDBNicholKERegulation of nitric oxide-dependent vasodilation in coronary arteries of estrogen receptor-alpha-deficient miceAm J Physiol Heart Circ Physiol2003285H2150H215712881205
- YangSBaeLZhangLEstrogen increases eNOS and NOx release in human coronary artery endotheliumJ Cardiovasc Pharmacol20003624224710942167
- GilliganDMQuyyumiAACannonROIIIEffects of physiological levels of estrogen on coronary vasomotor function in postmenopausal womenCirculation199489254525518205663
- RussellKSHaynesMPCaulin-GlaserTRosneckJSessaWCBenderJREstrogen stimulates heat shock protein 90 binding to endothelial nitric oxide synthase in human vascular endothelial cells. Effects on calcium sensitivity and NO releaseJ Biol Chem20002755026503010671543
- MichelJBFeronOSacksDMichelTReciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolinJ Biol Chem199727215583155869188442
- GragasinFSXuYArenasIAKainthNDavidgeSTEstrogen reduces angiotensin II-induced nitric oxide synthase and NAD(P)H oxidase expression in endothelial cellsArterioscler Thromb Vasc Biol200323384412524222
- RomerWOettelMDroescherPSchwarzSNovel “scavestrogens” and their radical scavenging effects, iron-chelating, and total antioxidative activities: delta 8,9-dehydro derivatives of 17 alpha-estradiol and 17 beta-estradiolSteroids1997623043109071739