Abstract
Since their initial availability in 1997, the thiazolidinediones (TZDs) have become one of the most commonly prescribed classes of medications for type 2 diabetes. In addition to glucose control, the TZDs have a number of pleiotropic effects on myriad traditional and non-traditional risk factors for diabetes. TZDs may benefit cardiovascular parameters, such as lipids, blood pressure, inflammatory biomarkers, endothelial function and fibrinolytic state. In this review, we summarise the experimental, preclinical and clinical data regarding the effects of the TZDs in conditions for which they are indicated and discuss their potential in the treatment of other conditions.
Introduction
Thiazolidinediones (TZDs) agonists for peroxisome proliferators-activated receptor (PPAR) γ are currently used therapeutically. Troglitazone, the first agent of this class to be approved, was effective in controlling glycemia but was removed from the market because of serious liver toxicity. Pioglitazone and Rosiglitazone are PPARγ agonists currently licensed for the management of hyperglycemia in Type 2 diabetes mellitus (T2DM). TZDs induced glycemic improvement is accompanied by a significant reduction in fasting insulin. The magnitude of the improvement depends on many factors such as body mass index (BMI), basal glucose levels, the degree of insulin resistance and the extent of β-cell failure. Interestingly, TZDs may benefit cardiovascular parameters, such as lipids, blood pressure, inflammatory biomarkers, endothelial function and fibrinolytic state (CitationParulkar et al 2001; CitationHaffner et al 2002). Moreover, they have been successfully used in non-diabetic insulin resistant conditions such as polycystic ovary syndrome (CitationRomualdi et al 2003; CitationGlintborg et al 2005).
The purpose of this review is to give a timely examination of the efficacy of PPARγ agonists in conditions for which they are currently indicated and discuss their potential in the treatment of other conditions.
Mechanism of action and insulin resistance
The mechanism of action of thiazolidinediones involves their binding to the nuclear PPARγ receptor. PPARγ is a nuclear receptor that acts as a transcription factor upon activation, by regulating the transcription and expression of specific genes. Together with the isoforms PPAR-α and PPAR-δ, is a member of a family of nuclear hormone receptors that includes the retinoid X receptor (RXR), the vitamin D receptor and the thyroid hormone receptor. PPARs play a critical role as lipid sensors and regulators of lipid metabolism.
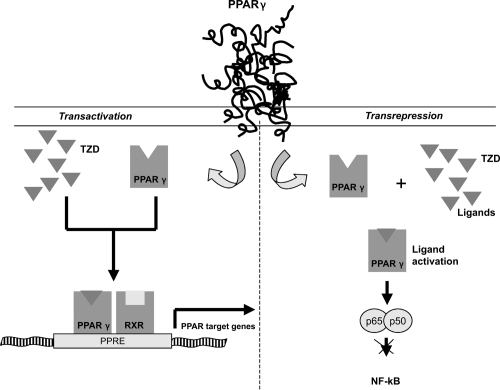
PPARs seem to regulate gene transcription by two mechanisms (). Transactivation, a DNA-dependent mechanism that involves binding to PPAR response elements of target genes (see below) and a second mechanism, transrepression, that would explain the anti-inflammatory actions of PPARs. It involves interfering with other transcription-factor pathways in a DNA-independent way (CitationJarvinen 2004).
Figure 1 Molecular mechanisms of Thiazolidinediones. In transactivations, perozisome-proliferator-activated receptor γ (PPARγ) is a nuclear receptor that acts as a transcription factor upon activation. Thiazolidinediones can active PPARγ. On ligand binding, the PPAR forms a heterodimer with the retinoid X receptor (RXR) and they bind to specific peroxisome proliferators response elements (PPRE) on a number of key target genes involved in the carbohydrate and lipid metabolism. In transespression PPARs can repress gene trascription of other pathways, such as nuclear factor −kB (NF-kB).
PPAR-α is principally expressed in tissues that exhibit a high rate of fatty acid metabolism (eg, brown adipose tissue, liver, kidney and heart) and is the molecular target for the fibrate class of drugs (CitationWillson et al 2000). Less is known about the physiological roles of PPAR-δ, which is expressed more ubiquitously, although recent studies have implicated it in lipid trafficking in macrophages and trophoblast (CitationOliver et al 2001; CitationChawla et al 2003), blastocyst implantation (CitationLim and Dey 2000), wound healing (CitationTan et al 2001), and the regulation of fatty acid catabolism and energy homeostasis (CitationPeters et al 2000; CitationWang et al 2003). PPAR-γ is mainly found in adipose tissue, intestinal cells and macrophages, although it is also expressed in other tissues including skeletal muscle and endothelium at lower concentrations (CitationPerry and Petrie 2002).
On ligand binding, the PPAR forms a heterodimer with the RXR and they bind to specific peroxisome proliferators response elements (PPRE) on a number of key target genes involved in the carbohydrate and lipid metabolism. Endogenous ligands of PPAR-γ include long chain unsaturated fatty acids and prostanoids (CitationShiraki et al 2005). Insulin resistance is caused by multiple factors, most likely involving many different aspects of the metabolic regulation of insulin in muscle, liver and adipose tissue.
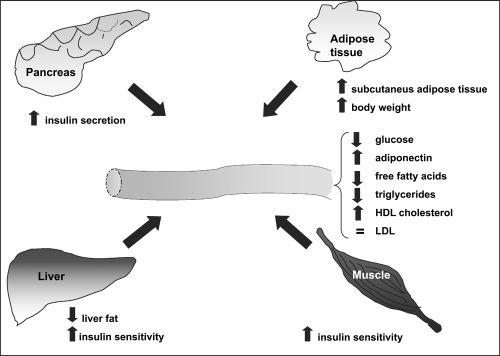
Current evidence supports the notion that the primary target tissue of TZDs is the adipose tissue. Their effect on insulin-stimulated glucose disposal may in part be secondary to changes in adipose tissue, where PPAR-γ is predominantly expressed, and involve factors that alter peripheral insulin sensitivity. TZDs have been shown to selectively stimulate lipogenic activities in fat cells resulting in greater insulin suppression of lipolysis (CitationOakes et al 2001). They decrease FFAs available for infiltration into other tissues; thus TZDs treatment target the insulin-desensitizing effects of FFAs in muscle and liver (CitationVasudevan and Balasubramanyam 2004). Finally, TZDs have been shown to alter expression and release of adipokines. Resistin and TNF-α, which have the potential to decrease insulin sensitivity, are reduced following incubation with TZDs (CitationSteppan et al 2001: CitationHofmann et al 1994; CitationDe Vos et al 1996). Conversely, the level of adiponectin is increased after TZD treatment in vitro (CitationMaeda et al 2001). Additionally, TZDs enhance the lipid storage capacity of adipose tissue by increasing the number of small adipocytes. This lipogenic effect may lead to a reduction of deleterious lipid accumulation in other insulin-sensitive tissues such as the muscle and liver, thus reducing insulin resistance (CitationOkuno et al 1998).
The TZDs therefore hold promise as agents that can ameliorate insulin resistance due to lipotoxicity in obesity- and lipodystrophy-associated T2DM (CitationUnger and Orci 2001).
Clinical effects
The TZDs have been shown to mediate a variety of effects on the complex pathophysiology associated with insulin resistance (CitationBhatia and Viswanathan 2006) ().
They are now approved for the treatment of T2DM and offer a new angle of attach in the pharmacologic management of T2DM.
Unlike other oral antidiabetic agents, TZDs are unique because they improve insulin sensitivity by increasing insulin-mediated glucose uptake in skeletal muscle, suppressing hepatic glucose output and improving the secretory response of insulin in pancreatic β-cells (CitationCamp 2003).
The TZDs are indicated in the United States for monotherapy and as combination therapy with sulfonylureas, metformin and insulin for treatment of T2DM (CitationDiamant and Heine 2003).
Rosiglitazone and pioglitazone are the only TZDs available for clinical uses.
As monotherapies, both agents significantly improve insulin sensitivity and glycemic control in T2DM patients by decreasing plasma glucose and plasma insulin levels, thus reducing HbA1c levels from 0.5% to 1.9% (CitationLebovitz et al 2001; CitationMiyazaki et al 2001). In combination therapy with other oral diabetes medications, both rosiglitazone and pioglitazone demonstrate additive effects on glycemic control compared with monotherapy (CitationCamp 2003).
Effect of adipocytes and serum lipid profile
The typical lipid profile of T2DM is characterized by low HDL, hypertriglyceridemia, and degeneration of atherogenic, small, dense LDL-cholesterol particles which are prone to oxidation and glication (CitationGinsberg et al 2005). Pioglitazone have beneficial effects in attenuating this complex dyslipidemic states. Typical beneficial effects in attenuating this complex dyslipidemic state have been described in studies using pioglitazone (CitationEinhorn et al 2000; CitationRosenstck et al 2002) and rosiglitazone (CitationPatel et al 1999; CitationRaskin et al 2000; CitationFhillips et al 2001). Data for rosiglitazone, in combination with metformin for 26 weeks showed an HDL increase of 13.3%, LDL increase of 18.6% and a neutral effect on total cholesterol (CitationFonseca et al 2000). A study reported by Khan et al found that pioglitazone was superior to rosiglitazone in terms of lowering triglyceride (TG) and total cholesterol (TC) in T2DM patients (CitationKhan et al 2002). By modulating adipogenic differentiation, the in vivo effects of TZDs on lipid profile may be do to the repartitioning of lipids. Studies in human tissue point to a critical role for PPARγ in the regulation of adipogenesis. Exposure of human pre-adipocytes to TZDs induces differentiation (CitationAdams et al 1997). Intriguingly, pre-adipocytes isolated from subcutaneous abdominal adipose tissue have been shown to differentiate more readily in response to TZDs than from visceral depots taken from the same subjects (CitationAdams et al 1997).
Evidence emerging from studies of humans and mice has indicated PPARγ to be not only a key factor for adipogenesis but also a critical determinant of body fat distribution (CitationTsai and Maeda 2005). In patients with T2DM studied by computerized tomography, visceral adipose tissue decreased following TZDs treatment while subcutaneous adipose tissue increased (CitationMori et al 1999).
These findings correlate with the adipogenic effects of TZDs used in clinical practice; treated type 2 diabetic patients accumulate subcutaneous fat, whereas visceral adipose tissue volume is reduced or unchanged (CitationBays et al 2004). It is well-established that subcutaneous adipose tissue is metabolically less harmful than visceral adipose tissue (CitationMontague et al 2000). This may, in part, explain the beneficial effects of TZDs despite their tendency to induce weight gain (CitationBays et al 2004).
Effect on inflammation and atherosclerosis
Atherogenesis is believed to be initiated by passage of lipid through the vascular endothelium, and deposition in the arterial intima, where it stimulates an inflammatory response. The anti-inflammatory properties of TZDs, in addition to their metabolic effects, may confer further cardiovascular benefits (CitationBlaschke et al 2006).
PPARγ agonists have been shown to reduce endothelial expression of VCAM-1 (CitationPasceri et al 2001) and intercellular adhesion molecule-1 (CitationWang et al 2002) on activated endothelium. Thus TZDs, through modulating inflammation, may influence the early stages of atherosclerosis.
The subsequent immune response, monocyte chemotaxis, T-cell activation and vascular smooth cell migration, forms the atherosclerotic plaque. It was recently demonstrated that pioglitazone and rosiglitazone have direct anti-inflammatory effects in a rat model of atherosclerosis via interference with monocyte chemoattractant protein 1 and its monocyte receptor (CCR2) (CitationIshibashi et al 2002; CitationHan et al 2000).
As monocytes enter in the intima they undergo transformation into macrophages. They take up lipid deposited in the intima forming the “foam cells”. Foam cells may play a pivotal role in atherogenesis, secreting reactive oxygen species and inflammatory cytokines. PPARγ is upregulated in activated macrophages while PPARγ agonists have been shown to attenuate the inflammatory response in activated monocytes and macrophages. Thus, activation of PPARγ receptors in macrophages within the arterial intima may reduce cytokine production, limiting the local inflammatory response, hence arresting atherosclerosis (see below) (CitationRoberts et al 2003).
Effect on endothelial dysfunction and blood pressure
The TZDs have salutary effects on endothelial dysfunction, a key factor in the pathogenesis of cardiovascular disease (CitationWidlansky et al 2003). Endothelium-derived nitric oxide (NO) is responsible for vasodilatation, and No- synthase (eNOS) expression, is diminished in diabetic patients. It has been shown that TZDs up-regulates eNOS expression (CitationGoya et al 2006). Expression of endothelin-1 (ET-1), a potent endothelial-derived vasoconstrictor compound, thought to be involved in the pathogenesis of numerous complications of diabetes, is regulated by TZDs action (Hopfner and Gopalakrishnan 1999). In a randomized study rosiglitazone was shown to improve endothelial dysfunction (CitationNatali et al 2002).
TZDs are also able to exert direct beneficial vascular effects beyond their effect on glycemia, thus reducing cardiovascular risk in type 2 diabetics. For example, rosiglitazone has been reported to increase differentiation towards the endothelial lineage when treating a heterogeneous population of angiogenic precursors, an effect mediated by PPAR-γ (CitationWang et al 2004)
Twelve-week treatment with rosiglitazone improved endothelial progenitor cells (EPC) number and migratory activity of T2DM patients (CitationPistrosch et al 2005).
Also pioglitazone was demonstrated to have a PPAR-γ dependent stimulation effect on EPCs (CitationRedondo et al 2007).
Some observations strongly suggest that insulin resistance per se is related to endothelial dysfunction, independent of glycemic control, and that rosiglitazone has therapeutic effects on this endothelial dysfunction (CitationPistrosch et al 2004). Interestingly, these endothelium-related effects are observed even in non-diabetic subjects (CitationHorio et al 2005).
Studies conducted on mammalian models of diabetes suggest that TZDs may be useful in the treatment of hypertension, particularly when associated with insulin resistance (CitationGrinsell et al 2000; CitationDiep et al 2002; CitationSchiffrin et al 2003). Bakris et al demonstrated significant reduction in systolic, diastolic and mean arterial blood pressure after 52 week of rosiglitazone treatment (CitationBakris et al 2000). In another study, decreases in mean arterial blood pressure with the use of troglitazone correlated significantly with reductions in plasma insulin, suggesting a link between improvement of insulin sensitivity and blood pressure reduction (CitationOgihara et al 1995). ET-1 is a potent vasoconstrictor, as well as a mediator of smooth muscle mitogenesis, and thus a determinant of systemic blood pressure (CitationMiller et al 1993). TZDs treatment has been shown to attenuate the release of ET-1 from vascular cells, which may contribute to the observed blood pressure lowering effect (CitationSatoh et al 1999; CitationIglarz M et al 2003).
Use in diabetes prevention
Insulin resistance is the key mechanism underlying impaired glucose tolerance (IGT) (CitationTurner and Clapham 1998). Three large-scale intervention trials have demonstrated the beneficial effect of weight reduction, dietary modifications and regular exercise on IGT (CitationPan et al 1997; CitationKnowler et al 2002; CitationTuomilehto et al 2001). The overall risk that patients with IGT will develop diabetes is 3–9% per year in the United States (CitationIpp 2000). TZDs improve insulin sensitivity in a range of insulin-resistant states without diabetes, including obesity and IGT (CitationGhanim et al 2001; CitationSekino et al 2003; CitationBennett et al 2004).
Durbin et al studied 172 IGT patients and demonstrated that the incidence of diabetes after three years was 88.9% lower in the TZD’s treated group compared with the control group (CitationDurbin 2004).
The pivotal problem of progression to overt diabetes from a prediabetic state is impaired β-cell function to begin with decline due to glucolipotoxicity; β-cell is unable to meet the insulin requirements imposed by insulin resistance The TRIPOD Study (Troglitazone in the Prevention of Diabetes Mellitus) (CitationBuchanan et al 2002)was a double blind prospective, randomized, placebo controlled, study examining Hispanic women without diabetes but with previous gestational diabetes. In this study troglitazone had a sustained beneficial effect on β-cell function in addition to the insulin-sensitizing and glucose lowering properties still present 8 months after discontinuation of the medication. The administration of troglitazone reduced the incidence of diabetes by 50% in high-risk women. All these data strongly advocate a role of this class of drugs in the prevention of T2DM.
Safety
The TZDs are generally safe and well tolerated. The liver toxicity that resulted in withdrawal of troglitazone from market does not seem to be a problem with rosiglitazone and pioglitazone (CitationScheen 2001). The incidence of liver toxicity occurred in approximately one in 100000 patients (CitationTolman 2000; CitationParulkar et al 2001). There is no clear molecular explanation for this adverse effect, however, it was reported that troglitazone, unique among the TZDs, activates the pregnane X receptor, a transcription factor that induces the expression of the hepatic cytochrome P450 3A4 gene (CitationKliewer et al 1999; CitationLehmann et al 1998). Following the withdrawal of troglitazone, the liver enzyme levels of patients prescribed rosiglitazone or pioglitazone have been carefully monitored, and no increase in TZD-induced liver toxicity has been reported.
The TZDs have been associated with weight gain of 3–5 Kg in a dose-dependent manner, most likely due to an increase in fat mass, peripheral edema or both. In particular the weight gain noted with TZD therapy may actually be associated with a beneficial redistribution of fat between the visceral versus subcutaneous body compartments (CitationNakamura et al 2001; CitationNesto et al 2003). Visceral adipose tissue is associated with the metabolic syndrome, being morphologically and functionally different from subcutaneous adipose tissue. Insulin effects trend to be blunted, and glucocorticoid and catecholamine effects trend to be enhanced in visceral adipose tissue compared with subcutaneous adipose tissue.
Fluid retention, expanded plasma volume and dependent edema have been noted with TZD therapy (CitationNesto et al 2003). In most of these cases, patients showed peripheral edema (swelling of legs, ankles, hands and face). In clinical practice, 3–5% of patients on either pioglitazone or rosiglitazone develop peripheral edema (CitationNesto et al 2003). The incidence of edema is much higher (up to 15%) in combination therapies that include insulin or sulfonylureas (CitationMalinowski and Bolesta 2000). Although the precise mechanism underlying the edema are unclear, some studies have suggested a vascular leak syndrome, possibly due to increased concentrations of vascular permeability factors and enhancement of endothelial-mediated vasodilation (CitationEmoto et al 2001; CitationNiemeyer and Janney 2002). Exacerbation of congestive heart failure (CHF) has rarely been reported with the use of TZDs (CitationNesto et al 2003). Because patients with advanced heart failure were not evaluated in clinical studies, TZDs are currently not recommended for use in individuals with New York Heart Association Class III or IV heart failure symptoms.
In their meta-analysis, CitationNissen and Wolski (2007) still suggest that rosiglitazone is associated with an increased risk of myocardial infarction. The main limitations of this study are the quality and quantity of the available data (CitationPsaty and Furberg 2007). The mechanism for the apparent increase death from cardiovascular causes associated with rosiglitazone remains uncertain. One potential contributing factor may be a modest reduction in the hemoglobin level. In susceptible patients, a reducted hemoglobin level may result in increased physiological stress, thereby provoking myocardial ischemia. These data point to the urgent need for comprehensive evaluations to clarify the cardiovascular risk of TZDs.
Future applications of PPAR agonists
TZD therapy appears to result in a variety of effects independent of blood glucose lowering which may have the potential to revolutionize the management of T2DM (CitationBoden and Zhang 2006). Evidently, TZDs improve insulin sensitivity not only of T2DM but also of primarily non-diabetic conditions. Theoretically, this opens the possibility of pharmacological prevention of T2DM and associated disease as strongly suggested by the sustained beneficial effect in women with previous gestational diabetes (CitationStumvoll 2003).
The anti-inflammatory properties of the TZDs are particularly interesting in the context of atherosclerosis, now recognised to have a central inflammatory component. Their immunomodulatory and anti-inflammatory effects may have a powerful impact on the cardiovascular disease burden associated with T2DM. There are some very recent results from the Prospective Pioglitazone Clinical Trial In Macrovascular Events (PROactive study). They show that pioglitazone reduces the occurrence of fatal and nonfatal myocardial infarction (MI) and acute coronary syndrome (ACS) (CitationErdmann et al 2007)and recurrent stroke (CitationWilcox et al 2007) in high-risk patients with T2DM. On the other hand, metabolic control as a surrogate endpoint did not demonstrate clinically relevant differences to the other oral antidiabetic drugs and the occurrence of edema was significantly raised. Therefore, until new evidence becomes available, the benefit-risk ratio of pioglitazone remains unclear (CitationRichter et al 2006).
In ADOPT study is compared rosiglitazone with metformin and glyburide in terms of the duration of glycemic control. Recent data show a cumulative incidence of monotherapy failure at 5 years of 15% with rosiglitazone, 21% with metformin and 34% with glyburide (CitationKahn et al 2006).
The DREAM trial and related studies are determining if rapamil or rosiglitazone reduces the number of cases of diabetes and atherosclerosis, and are trying to identify novel risk factors for diabetes (DREAM Study 2004).
These and other large randomized studies are under way to evaluate this theme including, RECORD (Rosiglitazone evaluated for cardiac outcomes and regulation of glycemia in diabetes), ACCORD (Action to control cardiovascular risk in diabetes), PPAR (PPARg agonists for the prevention of late adverse events following percutaneous revascularisation).
Final results of these studies will shed light on effects of TZDs in treatment and prevention of insulin resistance, IGT and T2DM.
Abbreviations
| T1DM | = | type 1 diabetes mellitus |
| T2DM | = | type 2 diabetes |
References
- AdamsMMontagueCTPrinsJBActivators of PPAR-gamma have depot-specific effects on human preadipocyte diffentiationJ Clin Invest19971003149539399962
- BakrisFLDoleJFPorterLERosiglitazone improves blood pressure in patients with type 2 diabetes mellitusDiabetes200049A96
- BaysHMandarinoLDeFronzoRARole of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferators-activated receptor agonists provide a rational therapeutic approachJ Clin Endocrinol Metab2004894637814764748
- BennettSMAgrawalAElashaHRosiglitazone improves insulin sensitivity, glucose tolerance and ambulatory blood pressure in subjects with impaired glucose toleranceDiabet Med2004214152215089784
- BhatiaVViswanathanPInsulin resistance and PPAR insulin sensitizersCurr Opin Investig Drugs200678917
- BlaschkeFCaglayanEHsuehWAPeroxisome proliferator-activated receptor gamma agonists: their role as vasoprotective agents in diabetesEndocrinol Metab Clin North Am2006355617416959586
- BodenGZhangMRecent findings concerning thiazolidinediones in the treatment of diabetesExpert Opin Invest Drugs20061524350
- BuchananTAXiangAHPetersRKPreservation of pancreatic beta-cell function and prevention of Type 2 diabetes by pharmacological treatment of insulin resistance in high risk hispanic womenDiabetes200251279680312196473
- CampHSThiazolidinediones in diabetes: current status and future outlookCurr Opin in Investig Drugs2003440611
- ChawlaALeeCHBarakYPPARdelta is a very low-density lipoprotein sensor in macrophagesProc Natl Acad Sci USA200310012687312540828
- De VosPLefebvreAMMillerSGThiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor-gammaJ Clin Invest199698100498770873
- DiamantMHeineRJThiazolidinediones in type 2 diabetes mellitus: current clinical evidenceDrugs200363137340512825962
- DiepQNEl MabroukMCohnJSStructure, endothelial function, cell growth, and inflammation in blood vassels of angiotensina II-infused rats: role of peroxisome proliferator-acvtivated receptor-gammaCirculation2002105229630212010913
- DurbinRJThiazolidinedione therapy in the prevention/delay of type 2 diabetes in patients with impaired glucose tolerance and insulin rsistanceDiabetes Obes Metab20046280515171752
- EinhornDRendellMRosenzweigJFor the pioglitazone 027 study group: pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo-controlled studyClin Ther200022139540911192132
- EmotoMAnnoTSatoYTroglitazone treatment increases plasma vascular endothelial growth factor in diabetic patients and its mRNA in 3T3-L1 adipocytesDiabetes20015011667011334422
- ErdmannEDormandyJACharbonnelBThe effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) StudyJAm Coll Cardiol20074917728017466227
- FhillipsLSGrunbergerGMillerEfor the Rosiglitazone Clinical Trials Study GroupOnce- and twice-daily dosing with rosiglitazone improves glycemic control in patients with type 2 diabetesDiabetes Care2001243081511213884
- FonsecaVRosenstockJPatwardhanREffect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trialJAMA2000283169570210755495
- GersteinHCYusufSHolmanRThe DREAM Trial Investigators. Rationale, design and recruitment characteristics of a large, simple international trial of diabetes prevention: the DREAM trialDiabetologia20044715192715322749
- GhanimHGargRAljadaASuppression of nuclear factor-kappaB and stimulation of inhibitor kappaB by troglitazone: evidence for an anti-inflammatory effect and a potential antiatherosclerotic effect in the obeseJ Clin Endocrinol Metab20018613061211238525
- GinsbergHNZhangYLHernandez-OnoARegulation of plasma triglycerides in insulin resistance and diabetesArch Med Res2005362324015925013
- GlintborgDStovingRKHagenCPioglitazone treatment increases spontaneous growth hormone (GH) secretion and stimulated GH levels in polycystic ovary syndromeJ Clin Endocrinol Metab20059056051216076946
- GoyaKSumitaniSOtsukiMThe thiazolidinedione drug troglitazone up-regulates nitric oxide synthase expression in vascular endothelial cellsJ Diabetes Complications2006203364216949522
- GrinsellJWLardinoisCKSwislockiAPioglitazone attenuates basal and postprandial insulin concentrations and blood pressure in the spontaneously hypertensive ratA J Hypertens2000133705
- HaffnerSMGreenbegASWestonWMEffects of rosiglitazone treatment on non-traditional markers of cardiovascular disease in patients with type 2 diabetes mellitusCirculation20021066798412163427
- HanK-HChangMKBoullierAOxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptorJ Clin Invest200010679380210995790
- HofmannCLorenzKBraithwaiteSSAltered gene expression for tumor necrosis factor-alfa and its receptor during drug and dietary modulation of insulin resistanceEndocrinology1994134264708275942
- HorioTSuzukiMTakamisawaIPioglitazone-induced insulin sensitization improves vascular endothelial function in nondiabetic patients with essential hypertensionAm J Hypertens20051816263016364837
- IglarzMTouyzRMAmiriFEffect of peroxisome proliferator-activated receptor-delta and-gamma activators on vascular remodelling in endothelin-dependent hypertensionArterioscler Thromb Vasc Biol200323455112524223
- IppEImpaired glucose tolerance: the irrepressible alpha-cell?Diabetes Care2000235697010834408
- IshibashiMEgashiraKHiasaKAntiinflammatory and antiarteriosclerotic effects of pioglitazoneHypertension2002406879312411463
- JarvinenHThiazolidinedionesN Engl J Med200435111061815356308
- KhanMASt PeterJVXueJLA prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazoneDiabetes Care2002257081111919129
- KahnSEHaffnerSMHeiseMAADOPT Study GroupGlycemic durability of rosiglitazone, metformin, or glyburide monotherapyNEngl J Med2006355242743
- KliewerSALehmannJMMilburnMVThe PPARs and PXRs: nuclear xenobiotic receptors that define novel hormone signalling pathwaysRecent Prog Horm Res1999543456710548883
- KnowlerWCBarrett-ConnorEFowlerSEReduction in the incidence of Type 2 diabetes with lifestyle intervention or metforminN Engl J Med200234639340311832527
- LebovitzHEDoleJFPatwardhanRRosiglitazone monotherapy is effective in patients with type 2 diabetesJ Clin Endocrinol Metab200186280811232013
- LehmannJMMcKeeDDWatsonMAThe human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactionsJ Clin Invest199810210161239727070
- LimHDeySKPPAR delta functions as a prostacyclin receptor in blastocyst implantationTrends Endocrinol Metab2000111374210754535
- MaedaNTakahashiMFunahashiTPPAR-gamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived proteinDiabetes2001502094911522676
- MalinowskiJMBolestaSRosiglitazone in the treatment of type 2 diabetes mellitus: a critical reviewClin Ther20002211516811110228
- MillerRCPeltonJTHugginsJPEndothelins: from receptors to medicineTrends Pharmacol Sci1993254608480375
- MiyazakiYMahankaliAMatsudaMImproved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazoneDiabetes Care2001247101911315836
- MontagueCTO’RahillySThe perils of portiliness: causes and consequences of visceral adiposityDiabetes200049883810866038
- MoriYMurakawaYOkadaKEffect of troglitazone on body fat distribution in type 2 diabetic patientsDiabetes Care1999229081210372240
- NakamuraTFunahashiTYamashitaSThiazolidinedione derivative improves fat distribution and multiple risk factors in subjects with visceral fat accumulation-double-blind placebo-controlled trialDiabetes Res Clin Pract2001541819011689273
- NataliABaldewegSToschiERosiglitazone directly improves endothelial function in type 2 diabetic patientsDiabetes200251Suppl 2P-573A142
- NestoRWBellDBonowROFonsecaVThiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes AssociationCirculation20031082941814662691
- NiemeyerNVJanneyLMThiazolidinedione-induced edemaPharmacotherapy200222924912126225
- NissenSEWolskiKEffect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causesN Engl J Med200735624577117517853
- OakesNDThalenPGJacintoSMThiazolidinediones increase plasma adipose tissue FFA exchange capacity and enhance insulin-mediated control of systemic FFA availabilityDiabetes20015011586511334421
- OgiharaTRakugiHIkegamiHEnhancement of insulin sensitivity by troglitazone lowers blood pressure in diabetic hypertensivesAm J Hypertens19958316207794582
- OkunoATamemotoHTobeKTroglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker ratsJ Clin Invest19981011354619502777
- OliverWRJrShenkJLSnaithMRSelective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transportProc Natl Acad Sci USA20019853061111309497
- ParulkarAAPendergrassMLGranda-AyalaRNonhypoglycemic effects of thiazolidinedionesAnn Intern Med200113530711511161
- PetersJMLeeSLiWGrowth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferators activated receptor beta (delta)Mol Cell Biol20002051192810866668
- PanXRLiGWHuYHEffects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes StudyDiabetes Care199720537449096977
- ParulkarAAPendergrassMLGranda-AyalaRNonhypoglycemic effects of thiazolidinedionesAnn Intern Med2001134617111187421
- PasceriVWuHWillersenJYehEModular of vascular inflammation in vitro and in vivo by peroxisome proliferator activated receptor- activatorsCirculation2001101235810645917
- PatelJAndersonRJRappaportEBRosiglitazone monotherapy improves glycaemic control in patients with type 2 diabetes: a twelve-week, randomized, placebo controlled studyDiabetes Obes Metab199911657211220295
- PerryCGPetrieJRInsulin-sensitising agents:beyond thiazolidinedionesExpert Opin Emerg Drugs200271657415989542
- PistroschFPassauerJFischerSIn type 2 diabetes, rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose controlDiabetes Care2004274849014747233
- PistroschFHerbringKOelschlaegelUPPARgamma-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cellsAtherosclerosis2005183163715907852
- PsatyBMFurbergCDThe record on rosiglitazone and risk of myocardial infarctionN Engl J Med200735767917551162
- RaskinPRappaportEBColeSTRosiglitazone short term monotheraphy lowers fasting and postprandial glucosze in patients with type 2 diabetesDiabetologia2000432788410768088
- RedondoSHristovMGumbelDBiphasic effect of pioglitazone on isolated human endothelial progenitor cells: involvement of peroxisome proliferator-activated receptor-gamma and transforming growth factor-beta 1Thromb Haemost2007979798717549301
- RichterBBandeira-EchtlerEBergerhoffKPioglitazone for type 2 diabetes mellitusCochrane Database Syst Rev200618CD00606017054272
- RobertsAWThomasAReesAPeroxisome proliferator-activated receptor-gamma agonists in atherosclerosis: current evidence and future directionsCurr Opin Lipidol2003145677314624133
- RomualdiDGuidoMCiampelliMSelective effects of pioglitazone on insulin androgen abnormalities in normo- and hyperinsulineamic obese patients with polycystic ovary sindromeHum Reprod20031812101812773448
- RosenstckJEinhornDHershonKthe pioglitazone 014 study groupEfficay and safety of pioglitazone in type 2 diabetes: a randomised, placebo-controlled study in patients receiving stable insulin theraphyInt J Clin Pract200256251712074206
- SatohHTsukamotoKHashimotoYThiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPAR on vascular endothelial functionBiochem Biophys Res Commun1999254757639920814
- ScheenAJThiazolidinediones and liver toxicityDiabetes Metab2001273051311431595
- SchiffrinELAmiriFBenkiraneKPeroxisome proliferator-activated receptors: vascular and cardic effects in hypertensionHypertension200342664812874098
- SekinoNKashiwabaraAInoueTUsefulness of troglitazone administration to obese hyperglycaemic patients with near-normoglycaemiaDiabetes Obes Metab20035145912681020
- ShirakiTKamiyaNShikiSAlpha, beta-unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor gammaJ Biol Chem2005280141455315695504
- SteppanCMBaileySTBhatSThe hormone resistin links obesity to diabetesNature20014093071211201732
- StumvollMThiazolidinediones- some recent developmentsExpert Opin Investig Drugs200312117987
- TanNSMichalinLNoyNCritical roles of PPAR beta/delta in keratinocyte response to inflammationGenes Dev20011532637711751632
- TolmanKGThiazolidinedione hepatotoxicity: a class effect?Int J Clin Pract2000113Suppl2934
- TsaiYSMaedaNPPAR-gamma: a critical determinant of body fat distribution in humans and miceTrends Cardiovasc Med20051581516039966
- TuomilehtoJLindstromJErikssonJCPrevention of Type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose toleranceN Engl J Med200134413435011333990
- TurnerNCClaphamJCInsulin resistance, impaired glucose tolerance and non-insulin dependent diabetes, pathologic mechanisms and treatment: current status and therapeutic possibilitiesProg Drug Res19985133949949859
- UngerRHOrciLDiseases of liporegulation: new perspective on obesity and related disordersFASEB J2001153122111156947
- VasudevanARBalasubramanyamAThiazolidinediones: a review of their mechanism of insulin sensitiziation, therapeutic potential, clinical efficacy, and tolerabilityDiabetes Technol Ther200468506315684639
- WangNVernaLChenN-GConstitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cellsJ Biol Chem2002277341768112107164
- WangYXLeeCHTiepSPeroxisome-prolifator-activated receptor delta activates fat metabolism to prevent obesityCell20031131597012705865
- WangCHCilibertiNLiSHRosiglitazone facilitates angiogenic progenitor cell differentiation toward endothelial lineage: a new paradigm in glitazone pleiotropyCirculation2004109139240014993120
- WidlanskyMEGokceNKeaneyJFJrThe clinical implications of endothelial dysfunctionJ Am Coll Cardiol20034211496014522472
- WilcoxRBousserMGBetteridgeDJEffects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04)Stroke2007388657317290029
- WillsonTMBrownPJSternbachDDThe PPARs: fro orphan receptors to drug discoveryJ Med Chem2000435275010691680