Abstract
“3D printing has the potential to be used instead of soft lithography and injection molding for the fabrication of microfluidic devices.”
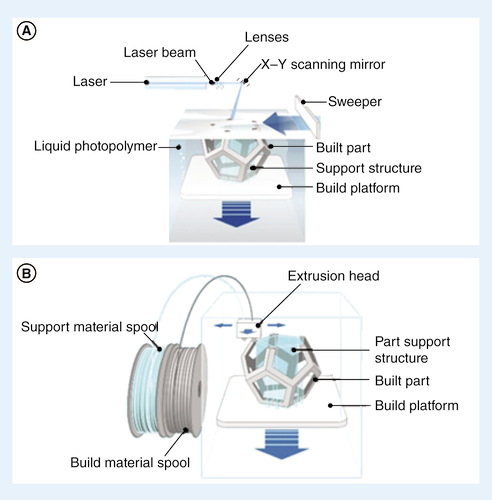
(A) Stereolithography. (B) Fused deposition modeling.
FDM: Fused deposition modeling; SLA: Stereolithography.
Illustration courtesy of Additively.com
First draft submitted: 3 November 2016; Accepted for publication: 12 December 2016; Published online: 3 March 2017
Advantages of utilizing 3D printing for microfluidics
Microfluidic devices are conventionally fabricated using soft-lithography proceeded by polydimethylsiloxane (PDMS) molding. However, automating the manual PDMS molding process is challenging, and therefore PDMS molding is usually inconvenient for high-throughput mass fabrication. Consequently, most commercial microfluidic devices are fabricated with low-cost transparent plastics using high-throughput injection molding. This process often requires large expenses for metal mold fabrication. A company that is interested in commercializing a PDMS prototype must design a plastic-molding process that is totally different from the soft lithographic method. Furthermore, both PDMS and plastic molding are multistep processes where the final device requires assembly by bonding. While this is not a critical issue for simple one-layer devices, it can be a key challenge for multilayer microfluidic devices containing interconnects and vias, particularly considering that many devices undergo multiple iterations for design improvement [Citation1].
By contrast, 3D printing is a fabrication technology that can print in clear biocompatible polymers at satisfactory resolution for various microfluidics applications [Citation2–6]. Compared with soft lithography and injection molding, 3D printing is more automated, flexible, cost–efficient and faster for iterative and incremental product development. This method is capable of producing quasiarbitrary 3D architectures that are not otherwise accommodated by plastic/PDMS molding, and it can fabricate microfluidic devices at throughputs ranging from single-device to thousands that are appealing to various commercialization routes. 3D printing provides both a more user-friendly fabrication technique and a straightforward commercialization path compared with plastic/PDMS molding, which is particularly appealing to startup companies. In addition, 3D printers are directed by CAD models, which are easily electronically transmitted through several groups of designers if necessary, further facilitating the design process.
Not surprisingly, despite the vast potential that research communities and the public recognize in microfluidics for biotechnology, only few companies are offering microfluidic devices [Citation1]. 3D printing for microfluidic device fabrication could empower small to midsized startup companies to enter the growing market of microfluidics and enable them to continuously improve and develop their designs and products.
Market opportunity
In 2015, the microfluidics market was valued at US$3.1 billion [Citation7]. With an expected compound annual growth rate of 19.3%, the market is estimated to reach $5.95–7.5 billion by 2020 [Citation8]. This excessive growth rate is mainly due to use of microfluidics in growing biotechnology fields, including gene sequencing and point-of-care (POC) diagnostics.
POC diagnostics
Many pharmaceutical companies have broadened their diagnostic platforms to include POC microfluidic devices [Citation9]. To commercialize the high-performance microfluidic diagnostic solutions, functional and marketable devices are required to address key clinical problems, particularly in resource-limited settings [Citation10–13]. Many microfluidic-based POC diagnostic solutions have been marketed (e.g., blood chemistry analyzer by Abbott Laboratories (IL, USA), complete immunoassay solution by OPKO Inc. (FL, USA), and HIV diagnostic device by Daktari Diagnostics Inc. (MA, USA), but more efforts are required to commercialize the microfluidic assays that have been developed by research groups [Citation14].
DNA sequencing
In genomics, microfluidic technology has been utilized to decrease turnaround times by increasing sample preparation automation. To enhance sample preparation, RainDance Technologies, Inc. (MA, USA) developed a microfluidic system that generates millions of droplets that each encapsulate a single molecule of genomic DNA, enabling digital PCR. Another company with a high-throughput microfluidic genetic analysis solution is Sphere Fluidics Ltd. (Cambridge, UK), whose microfluidic droplet generator system has been used for single cell genetic, proteomic and transcriptomic analysis [Citation14].
Emerging applications of 3D-printed microfluidics
3D printing techniques
Prominent 3D printing techniques include stereolithography (SLA) [Citation2,Citation15–17], selective laser sintering [Citation15], photopolymer inkjet printing [Citation2,Citation15–17], binder jetting [Citation2,Citation15–16], fused deposition modeling (FDM) [Citation2,Citation15–17], laminated objected manufacturing [Citation15], two-photon polymerization (2PP) [Citation16,Citation17] and digital micromirror device-based projection printing [Citation17]. In SLA, depicted in A, liquid polymers are cured in a layer-by-layer fashion by photopolymerization, resulting in a 3D construct [Citation2,Citation16–17]. Curing of the liquid resin is carried out either by laser or digital light projection, and resolution depends on the resin type as well as the laser spot size [Citation15,Citation16]. Selective laser sintering also builds 3D constructs using a layer-by-layer method. However, in SLS, the precursor polymers are powders that are thermally bound to previous layers using a laser [Citation2,Citation15]. In photopolymer inkjet printing, the concept of an inkjet printer is expanded to 3D printing [Citation2,Citation15–17]. Liquid photopolymers are deposited onto a build platform by a print head and are subsequently cured by UV light [Citation2,Citation15–17]. Similarly, in binder jetting, a print head deposits a liquid adhesive, which binds previously powdered layers onto the build platform [Citation2,Citation15–16]. FDM features a nozzle, which extrudes layers of melted biocompatible thermoplastics filaments that cool down and solidify postextrusion (B) [Citation2,Citation15–17]. Of the techniques discussed, SLA, photopolymer inkjet printing and FDM show the most promise for use in microfluidics [Citation2,Citation16].
Passive components
Passive microfluidic components rely on external actuation or capillary forces to move liquids within the device. Some examples of passive components include microcapillary assemblies, droplet generators, gradient generators, micromixers, check valves and reactionware [Citation2]. In one study, Lee et al. designed and characterized a modular 3D-printed microfluidic device with separate modules for inlets, outlets, channels, gradient generators, reactors, chambers and mixers. The photopolymer inkjet printing method used in this study was able to print channels of a width as small as 100 μm and a height as small as 50 μm. Rapid iterations through 3D printing can replace modules as necessary. The authors conclude that 3D printing is, therefore, a suitable method for fabrication of microfluidic devices, and the modular microfluidic chip can be integrated with applications such as biosensing, including detection of the alpha-fetoprotein (AFP) antigen prominent in the diagnosis of liver cancer [Citation18]. Other passive microfluidic devices allow for size-based ‘hydrodynamic-vortex’ separation of microparticles [Citation19], passive pumping controlled by membranes and hydrostatic pressure [Citation20] and creation of bioactive fibers through laminar flow in multi- and single-barrel capillaries [Citation21].
Active components
Active microfluidic components manipulate fluid flow by applying energy to serve many functions, including automation of the device [Citation2,Citation22]. Au et al. have successfully 3D-printed valves and pumps using SLA [Citation22]. Due to the modular design inherent to 3D-printed microfluidics, the pump consisted of three valves digitally connected in series, and the pump's function was therefore most limited by the properties of the component valves themselves [Citation22]. Another group, Gong et al., reduced the volume of their 3D-printed microfluidic valves by 90% by manipulating the resin formulation in a digital light projection SLA 3D printer. Gong et al. also designed a pump by combining two valves with a displacement chamber between them [Citation23]. Recently, Keating et al. have designed a push-up valve with variable material stiffness by printing multiple materials in one valve. This valve features a relatively flexible membrane that separates the rectangular control channel and semicircular flow channel that are both made of a stiffer material [Citation24]. It is important to note that the presence of a support material, which is a result of the photopolymer inkjet printing method used, introduces variability into the function of microfluidic devices [Citation24]. Still, active microfluidic components are useful in numerous applications including cell culture [Citation22], cell perfusion [Citation22], muscle and peristalsis simulations [Citation24] and biologically inspired robotics [Citation24].
Complete microfluidic devices
Complete microfluidics devices present a variety of applications. Here, we discuss biological applications relevant to the field of medicine. In one recent study, Morgan et al. utilized an extrusion-based 3D printing method known as fused filament fabrication to create a modular microfluidic device capable of encapsulating human dental pulp stem cells [Citation25]. Similarly, another group, Alessandri et al., used the DLP SLA 3D printing method to fabricate a microfluidic device capable of encapsulating human neural stem cells in alginate shells, thereby developing a 3D human neuronal network [Citation26]. Because the ability to encapsulate cells while maintaining their viability is extremely relevant to 3D cell culture, high-throughput biological testing, tissue engineering and drug delivery [Citation25,Citation26], this capability is crucial for the usefulness of 3D-printed microfluidic devices. The rapid prototype advantage of 3D printing, coupled with the high viability of encapsulating cells [Citation26], allows research to progress efficiently. Rapid and low-cost prototyping was especially relevant in a recent study by Patrick et al., in which 3D-printed microfluidic devices were created to assemble DNA and advance the field of synthetic biology [Citation27]. Other biological applications of complete, 3D-printed microfluidic devices include cell behavior studies, organ-on-a-chip studies and immunomagnetic assays [Citation17].
Challenges & opportunities in commercialization of 3D-printed microfluidics
3D printing resolution
Resolution, processing time and printing dimensions are important considerations that need to be taken into account while shifting from traditional fabrication techniques to 3D printing and choosing an appropriate commercial 3D printer for fabricating microfluidic devices. High resolution 3D printers could create channels with smaller dimension, high precision and different surface finishes. However, using high resolution 3D printers fabricate features with smaller layer heights, and consequently have lower throughput. In addition to printer resolution, the printing mechanism and materials are the other important factors in achieving micron-scale channels. In FDM and photopolymer inkjet printers, the channel size is limited by the channels cleaning technique from the support materials. However, in SLA printers, the use of liquid resin made cleaning of the channels easier, but as light is used for curing the resin, it can cure the liquid resin within a microchannel and block the channel when the transparent resin is used as material [Citation2]. A single device may also have slightly different dimensions when printed on different 3D printers and with different materials.
Material standardization
Commercially available 3D printing materials with a range of mechanical, chemical, biocompatibility and optical properties are mostly proprietary. Moreover, using nonproprietary materials in most commercial 3D printers needs optimization and proficiency. Also, for some particular applications, there is a lack of appropriate material [Citation15]. For example, light transmittance offered by some nonopaque materials is not adequate for high resolution optical imaging [Citation2]. Furthermore, the biocompatibility and gas permeability of the currently available materials have limitations on long-term on-chip cell culture. However, with the advances of 3D printing in different industries, these challenges can be addressed. Recently, PDMS has been proposed to be printed via SLA technique [Citation28] due to already established and widely studied advantages of PDMS. In 2015, Autodesk under a creative commons license made the formulation of their Standard Clear Prototyping material open source. Such initiatives tend to urge innovation in 3D-printable materials and advancements in availability and variability of materials.
Integration
So far microfluidic research has concentrated on the design of individual lab-on-a-chip devices, but more research on the integration of these devices is needed to make integration of these devices possible and to form fully functional integrated lab-on-a-chip devices [Citation15]. With 3D printing as a fabrication technique, we foresee that the 3D CAD designs of modular microfluidic components will be available online in the near future. These components can be integrated on a cloud-based software by teams in the companies or home-based, and then proceeded for printing to form a functional complete microfluidic device. Therefore, 3D printing will eventually eliminate the required trainings for chip fabrication, make the process more convenient for researchers and encourage them to focus on more complex system integration of microfluidic chip components at numbers and complexities that have not been studied and achieved yet. This mind-setting change will basically help the field of microfluidics to have a transformation close to the one that electronics and semiconductor industry has achieved in 1960s. For instance, the very first commercially available integrated circuit has integrated less than ten components and today's integrated circuits have billions of components integrated on a single chip.
Conclusion & future perspective
Despite the great expectations that researchers put on microfluidics to enable biotechnology for improving public health, only few companies have been using microfluidic devices as a part of their solutions. The hindering factors in commercialization of microfluidic devices include production efficiency, integration difficulties, cost and acquiring regulatory approval. Considering these factors, 3D printing is a more user-friendly fabrication technique and a reasonable commercialization route compared with plastic/PDMS molding. 3D printing can print in materials with different required properties at acceptable resolution for many microfluidics applications. To support the advances of 3D printed microfluidics, academic efforts should be matched with industrial point of view. Industrial collaborators could discuss the potential of successful product and provide feedback on consumer's requirements and expectations to academic researchers and researchers could focus on designing and providing an integrated microfluidic solution with a specific application.
By using 3D printers in the near future, the microfluidic innovators will not need to establish a company to commercialize their designs and inventions. They will benefit from 3D printing service companies to iteratively design fully functional microfluidic device, and when ready, the devices will be available online for sale and can be ordered for fabrication by costumers. In this way, the microfluidic designers can put their efforts on the invention of new designs and leave all the fabrication and supply to the 3D printing service companies.
3D printing fabrication technique has its own limitations. For example, current commercialized 3D printers have limitations in resolution; therefore, devices with features smaller than resolution limit cannot be fabricated using 3D printers. The limitations of material, the resolution and the cost of 3D fabrication are likely to improve. Therefore, we conclude that 3D printing has the potential to be used instead of soft lithography and injection molding for the fabrication of microfluidic devices. We foresee a cloud-based microfluidics software for marketing microfluidic components, designed by a large number of designers where the designs are available for purchasing, integration and fabrication by 3D printing services companies.
Financial & competing interests disclosure
S Tasoglu acknowledges the American Heart Association Scientist Development Grant (15SDG25080056) and the University of Connecticut Research Excellence Program award for financial support of this research. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Au AK , LeeW, FolchA. Mail-order microfluidics: evaluation of stereolithography for the production of microfluidic devices. Lab on a Chip14(7), 1294–1301 (2014).
- Amin R , KnowltonS, HartAet al. 3D-printed microfluidic devices. Biofabrication8(2), 022001 (2016).
- Yenilmez B , KnowltonS, YuCH, HeeneyMM, TasogluS. Label-free sickle cell disease diagnosis using a low-cost, handheld platform. Adv. Mater. Technol.1(5), 1600100 (2016).
- Yenilmez B , KnowltonS, TasogluS. Self-contained handheld magnetic platform for point of care cytometry in biological samples. Adv. Mater. Technol.1(9), 1600144 (2016).
- Knowlton S , YuCH, ErsoyF, EmadiS, KhademhosseiniA, TasogluS. 3D-printed microfluidic chips with patterned, cell-laden hydrogel constructs. Biofabrication8(2), 025019 (2016).
- Knowlton S , YenilmezB, TasogluS. Towards single-step biofabrication of organs-on-a-chip via 3D printing. Trends in Biotechnology34(9), 685–688 (2016).
- Markets and Markets . Microfluidics market worth $7.5 billion by 2020. www.marketsandmarkets.com/PressReleases/microfluidics.asp.
- Yole Development . Back to the news after fundraising, new collaborations and acquisitions, microfluidic players are in position: now, the game can start. www.yole.fr/Microfluidic_Applications_Players.aspx.
- Chin CD , LinderV, SiaSK. Commercialization of microfluidic point-of-care diagnostic devices. Lab on a Chip12(12), 2118–2134 (2012).
- Sackmann EK , FultonAL, BeebeDJ. The present and future role of microfluidics in biomedical research. Nature507(7491), 181–189 (2014).
- Knowlton SM , SencanI, AytarYet al. Sickle cell detection using a smartphone. Scientific Reports5, 15022 (2015).
- Knowlton S , YuCH, JainN, GhiranIC, TasogluS. Smart-phone based magnetic levitation for measuring densities. PloS One10(8), e0134400 (2015).
- Amin R , KnowltonS, YenilmezB, HartA, JoshiA, TasogluS. Smart-phone attachable, flow-assisted magnetic focusing device. RSC Advances6(96), 93922–93931 (2016).
- Volpatti LR , YetisenAK. Commercialization of microfluidic devices. Trends Biotechnol.32(7), 347–350 (2014).
- Au AK , HuynhW, HorowitzLF, FolchA. 3D-printed microfluidics. Angew. Chem. Int. Ed. Engl.55(12), 3862–3881 (2016).
- Waheed S , CabotJM, MacdonaldNPet al. 3D printed microfluidic devices: enablers and barriers. Lab on a Chip16(11), 1993–2013 (2016).
- Ho CMB , NgSH, LiKHH, YoonYJ. 3D printed microfluidics for biological applications. Lab on a Chip15(18), 3627–3637 (2015).
- Lee KG , ParkKJ, SeokSet al. 3D printed modules for integrated microfluidic devices. RSC Advances4(62), 32876–32880 (2014).
- Wang X , PapautskyI. Size-based microfluidic multimodal microparticle sorter. Lab on a Chip15(5), 1350–1359 (2015).
- Goral VN , TranE, YuenPK. A pump-free membrane-controlled perfusion microfluidic platform. Biomicrofluidics9(5), 054103 (2015).
- Cheng Y , YuY, FuFet al. Controlled fabrication of bioactive microfibers for creating tissue constructs using microfluidic techniques. ACS Appl. Mater. Interfaces8(2), 1080–1086 (2016).
- Au AK , BhattacharjeeN, HorowitzLF, ChangTC, FolchA. 3D-printed microfluidic automation. Lab on a chip15(8), 1934–1941 (2015).
- Gong H , WoolleyAT, NordinGP. High density 3D printed microfluidic valves, pumps, and multiplexers. Lab on a Chip16, 2450–2458 (2016).
- Keating SJ , GariboldiMI, PatrickWG, SharmaS, KongDS, OxmanN. 3D printed multimaterial microfluidic valve. PLoS One11(8), e0160624 (2016).
- Morgan AJ , San JoseLH, JamiesonWDet al. Simple and versatile 3D printed microfluidics using fused filament fabrication. PloS One11(4), e0152023 (2016).
- Alessandri K , FeyeuxM, GurchenkovBet al. A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human Neuronal Stem Cells (hNSC). Lab on a Chip16(9), 1593–1604 (2016).
- Patrick WG , NielsenAA, KeatingSJet al. DNA assembly in 3D printed fluidics. PloS One10(12), e0143636 (2015).
- Folch A . First SL-prints of PDMS. www.linkedin.com/pulse/first-sl-prints-pdms-albert-folch.
