Abstract
3D printing technology has enabled unprecedented flexibility in the design and manufacturing of complex objects, which can be utilized in personalized and programmable medicine. The aim of this study is to evaluate the potential of 3D printing by digital light processing to fabricate drug-loaded systems with special designs and unique drug-release characteristics, which otherwise are not possible to fabricate by conventional pharmaceutical manufacturing methods. Oral dosage forms of pH responsive hydrogels were 3D printed using acrylic acid monomer, cross-linker (polyethylene glycol diacrylate) and photoinitiator (2,4,6-trimethylbenzoyl-diphenylphosphine oxide [TPO] nanoparticles). Sulforhodamine B, a pH independent fluorescent dye, was used to model a small molecule hydrophilic drug. The printed structures exhibited pH responsive swelling and the effect of pH and tablets’ surface area were studied on drug release. The tablets showed higher swelling and faster drug release at higher pH, making them a promising system for enhancing drug absorption in the intestine. Structures with large surface area and complex structures showed enhanced swelling and faster drug release and vice versa.
3D printing or additive manufacturing is a process of making a solid object from a digital model. 3D printing is achieved using an additive process where successive layers of materials are laid down in different shapes by a printing process [Citation1,Citation2]. Currently, 3D printing is provoking a great interest and is becoming a very useful method in a variety of fields such as tissue engineering [Citation3], medical devices [Citation4,Citation5] pharmaceutics [Citation6–8], dentistry [Citation9], aerospace [Citation10], construction [Citation11] and automotive [Citation12]. This is due to some clear advantages such as rapid manufacturing with unprecedented flexibility in material composition, special geometries, complex microstructure, surface texture, product performance (release kinetics) and capability of fabricating parts directly from computer aided design models. Nowadays, the main 3D printing technologies are based on the following three approaches: the first is selective polymerization of photo-sensitive monomer by UV [Citation13]. The second is selective sintering or binding of particles in powder (binder jetting) [Citation14] and the third technology is selective deposition of filaments or cutting sheets of paper/nylon [Citation15]. Each approach requires suitable materials and tailoring the printing composition (‘ink’) for optimal performance according to the printing technology.
Recent advances in pharmaceutical oral formulations are focused on the development of drug delivery systems that can improve drug availability and absorption in the gastrointestinal track [Citation16]. A more recent effort is being invested on developing oral formulations with programmable properties that can be used for personalized medicine [Citation17,Citation18]. Such systems demand tight control over the dose, composition and bioavailability, than that achieved by conventional dosage forms [Citation19]. Bioavailability of drugs depends on the drug absorption, which can be modulated by the use of selected excipients and forms of drug-delivery systems. Conventional manufacturing of pharmaceutical dosage forms (e.g., tablets, capsules) is typically restricted to certain designs and materials, and is being commonly used in generic form of tablets or capsules with or without enteric coating [Citation20].
The first US FDA approved 3D printed tablet, levetiracetam (Spritam™, Aprecia Pharmaceuticals, OH, USA), demonstrates the use of 3D printing by binder jetting for making rapidly disintegrating structures for high-dose epileptic medications [Citation21]. The current 3D printing of drugs is focused on powder-based and melt-based freeform fabrication methods. These have been used to prepare dosage forms with varying geometries and surface areas by the selective deposition method [Citation22]; multiple drug and multiple release kinetics by fused deposition modeling [Citation23]; and floating drug-delivery systems by binder jetting [Citation24].
One of the great promises in the field of drug delivery system are smart hydrogels [Citation19]. Hydrogels are 3D cross-linked network of hydrophilic polymers which can absorb large amount of water or biological fluids by swelling. The hydrogels may respond to chemical and physical stimuli such as temperature, pressure, pH, ionic strength and magnetic or electric field [Citation25]. Due to their biocompatibility and swelling capability in different stimuli, they can be used for drug-delivery systems for controlled drug release [Citation26–28]. In oral formulation, the enhancement of bioavailability is a central issue. Therefore drugs that are sensitive to stomach conditions, in other words, the high enzymatic activity and low pH are often administered within an enteric coated tablet, which protects the drug and prevents premature drug release in the stomach while enabling a maximal absorption in the small intestine. Therefore by using smart hydrogels, it is possible to minimize drug release in the stomach while enhancing its release in the intestinal pH [Citation29]. The aim of this research is fabrication by 3D printing and investigation of pH responsive hydrogel tablets with complex structures, and controlled release of drugs by digital light processing (DLP) printing technology. The technology is based on photopolymerization, enabled by DLP for curing photoreactive polymers. The localized polymerization is performed within a bath filled with a polymerizable ink, usually by proper focusing of UV light. DLP allows high-resolution, low printing cost and large build size [Citation30].
We have developed a hydrogel formulation that could function as a model for 3D printed controlled drug delivery system. Hydrogel tablets with complex structures were printed by using acrylic acid as the monomer and polyethylene glycol diacrylate (PEGDA) as a cross-linker, resulting in pH responsive drug-delivery system.
Materials & methods
Materials
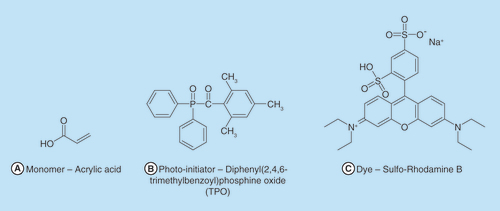
Acrylic acid was purchased from Acros (Belgium). TPO photo-initiator diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide was obtained from BASF (Germany). The surfactant Brij 58 and the dye Sulforhodamine B were purchased from Sigma Aldrich (Merck, Germany). PEGDA was acquired from Sartomer-Arkema ( azuewah, France). Sodium hydrogen phosphate (Merck-Germany), sodium chloride (Bio-Lab, Israel), potassium phosphate monobasic (Sigma Aldrich, Merck-Germany) and hydrochloric acid (J. T Baker, Avantor, USA) were used for the dissolution medium. Triple distilled water (TDW) was obtained from NANOpure®-DIamondTM (TDW; 0.0055 μS.cm-1; Barnsted system, IA, USA).
Methods
Preparation of aqueous ink containing Sulforhodamine B as a drug model
Aqueous inks containing Sulforhodamine B as a drug model were prepared in three steps:
Dissolving the following components by magnetic stirring: TDW (58% w/w), acrylic acid (38% w/w), PEGDA (2% w/w) and TPO nanoparticles powder (2% w/w). The TPO nanoparticles were prepared by lyophilizing clear solutions containing 23.75% w/w Brij 58, 1.25% w/w TPO, 25% TDW and 50% 2-propanol. Lyophilization was performed using laboratory-scale benchtop freeze-drying system (Labconco Freezone 2.5, MI, USA). The solution (30 ml sample in a 100 ml round bottom flask) was lyophilized at a temperature of -47 ± 3°C and absolute pressure of approximately 0.470 mbar. The samples were kept in these conditions for 24 h [Citation13].
Preparation of Sulforhodamine B stock solution: 0.02 g of Sulforhodamine B powder was dissolved in 10 g of step A solution by magnetic stirrer in order to have a 0.2% (w/w) concentration of Sulforhodamine B in the aqueous ink.
Preparation of the printed aqueous ink containing Sulforhodamine B: 83 g of the aqueous ink (step A) (99% w/w) was mixed with 0.83 g of Sulforhodamine B stock solution (step B), (1% w/w). Chemical structures of the relevant molecules are presented in .
3D printing of drug-loaded, pH responsive hydrogel tablets
A predesigned hydrogel model was 3D printed using a 3D printer (Pico 2, Asiga, Australia). This printer operates by stereolithography system with digital mirror device and UV-LED light source (385 nm). 3D printing of the hydrogel was performed at a rate of 3 s per layer (100 μm layer thickness with 3 s irradiation to each layer). Each structure (build size 16 × 16 × 5 mm/24 × 24 × 10 mm) was printed within 20–40 min.
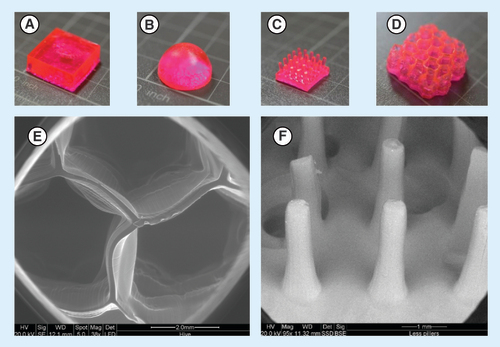
The 3D printed hydrogels were lyophilized for 24 h in order to remove all the water and then the dry mass was recorded. The structures were then observed under an environmental scanning electron microscope (Quanta 200 FEG, Holland) to visualize gross morphology.
Measurement of swelling index
In order to measure the swelling of the hydrogel, the 3D printed dried samples were first weighed (Wi) and then immersed in buffers of different pH at 37 ± 0.5°C. After each predetermined time interval, the hydrogels were removed from the medium, blotted dry in order to gently remove excess water and weighed again (Wt). The swelling index of the printed hydrogel was determined from the ratio of the swollen mass and dry mass.
Measurement of drug release
The in vitro dissolution tests were performed with USP apparatuses 2 (ERWEKA® and VanKel® ERWEKA, Germany), equipped with paddles rotating at 50 rpm. The tests were run in triplicates on the four tablets described in . A volume of 900 ml dissolution medium (phosphate buffer pH 7.4 or 1.2) was maintained at 37°C and 200 μl was collected in a 96-well black plate at 0, 1, 2, 4, 8 and 24 h without replacing the volume removed. The samples were quantified using fluorescence measurements by a microplate reader (Synergy-HT, Biotek, USA) at excitation/emission of 530/590 nm according to a pre-prepared calibration curve. The percentage of Sulforhodamine B dissolved during each collection time was calculated relative to the amount of Sulforhodamine B loaded within each tablet. Due to the removal of water from the tablet after printing, only the solid content of the printing solution was taken into consideration.
Kinetic models
Drug release data from all shapes were fitted to various kinetic models to evaluate the release pattern. The correlation of the release to each kinetic model was calculated and the coefficient of determination (R2) was obtained. The different kinetic models examined were zero order, Korsmeyer–Peppas and Higuchi model given by equations below [Citation31].
Where Mt/M∞ is the ratio between the cumulative amounts of drug released (up to 60% release only at Krosmeyer–Peppas model) at time t (Mt) and infinite time (M∞), K0, Km and KH are the zero order, Krosmeyer–Peppas and Higuchi model release constants, respectively, and n is the release exponent indicative of the drug release mechanism. An n value ≤ 0.45 indicates Fickian mechanism that occurs mainly due to diffusion. For n ≥ 0.89, the release rate is independent of time and the mechanism is mainly controlled by erosion. Intermediate values (i.e., 0.45 ≤ n ≤ 0.89) represent a non-Fickian or anomalous transport and suggest mechanism of release influenced from both erosion and drug diffusion [Citation32].
Cells viability assay
In order to evaluate the safety of the tablet materials and their effect on intestinal epithelium cells, Caco2, viability, the tablets were incubated for 72 h in 50 ml of double deionized water (DDW) under gentle stirring. The condition medium was collected, diluted and supplemented to the cell medium for 72-h incubation. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide viability assay was performed according to manufacturer instructions and optical absorption was measured by plate reader (570 nm) [Citation33].
Results & discussion
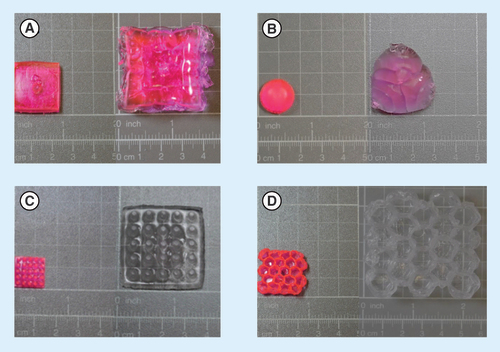
Preliminary experiments were conducted in order to evaluate the optimal composition of the 3D aqueous ink, for selection of the optimal cross-linker, photoinitiator and monomer type. We searched for such a monomer which can exhibit very low solubility in acidic conditions thus can pass through the stomach without releasing the drug which is loaded within the hydrogel [Citation34–36]. The photoinitiator (PI) nanoparticles were used based on our previous demonstration of 3D printing in water [Citation13]. The acrylic acid was selected due to its ability to swell at a certain pH after polymerization [Citation37]. Sulforhodamine B, a pH-independent fluorescent dye, was used to model a small molecule hydrophilic drug in the release experiments. After optimizing the printing parameters, four different shapes of tablets were printed and evaluated for their substance release and swelling. The fabricated structures are shown in and are referred as: A – box, B – hemisphere, C – 5 × 5 and D – hive.
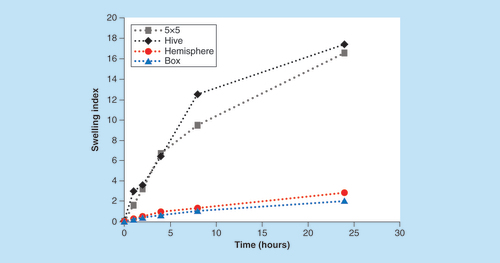
In general, when the printed objects were immersed in phosphate buffer (pH 7.4), they swelled about 3- to 15-times of their initial size (), depending on the shape of the delivery system. Each tablet structure had a different release profile as could be expected from the reports about correlation between swelling characteristics and drug release behavior of pH responsive hydrogels and the reports on tablets with different shapes. For example, Sohail et al. reported that drug release from pH-sensitive polyvinylpyrrolidone–acrylic acid hydrogels, prepared by a mold, was increased by increasing the pH of the medium and acrylic acid content in the hydrogels [Citation38–40]. Goyanes et al. reported the effect of geometry on drug release from 3D printed tablets fabricated by using hot melt extrusion 3D printing. It was found that the drug release from tablets was dependent on the ratio of surface area to volume, indicating the possibility of controlling the drug release by tailoring the shape of the tablets, such as a sphere, pyramid, cube and cylinder [Citation22]. In the present study, by utilization of the DLP printing approach, we were able to evaluate both the shape and the swelling of objects with complex geometries, on the release and swelling behavior.
(A) Box. (B) Hemisphere. (C) 5 × 5. (D) Hive.
Measurement of swelling indices
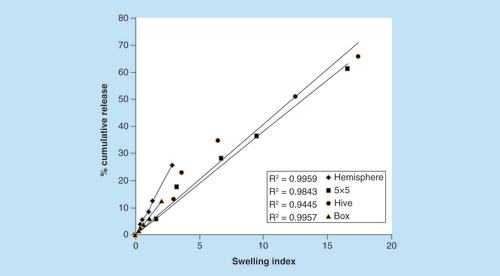
Under the assumption that the main release mechanism is governed by the swelling ability of the 3D printed tablets, the swelling index of tablets with each of the shapes was measured under the same sink conditions. The calculated surface areas of the four shapes are presented in ascending order; hemisphere, 5 × 5, box and hive: 6.2, 9.1, 22.4 and 89.6 cm2, respectively. In order to accurately predict the swelling abilities, the tablets were weighted and the surface area was normalized according to the average weight of each shape. Normalized surface area of hive, 5 × 5, hemisphere and box were; 58.2, 20.1, 4.22 and 4.15 cm2/g, respectively. As shown in , the swelling index of hive and 5 × 5 tablets (17.4 and 16.6, respectively) is significantly higher than the hemisphere and the box shapes (2.8 and 2, respectively) as expected. We speculated that shapes presenting higher swelling abilities would show correlated higher Sulforhodamine B release.
pH-dependent drug release
Sulforhodamine B was selected as a drug model for hydrophilic small molecules due to its high solubility in all tested pH ranges and its pH independent absorption over the range of pH between 3 and 10 [Citation41]. The release mechanism of Sulforhodamine B was found to be governed by the pH dependent swelling properties [Citation42]. The effect of pH on release from the 3D tablets was evaluated by measuring the cumulative release at pH 1.2 and 7.4. The results shown in indicate a major difference in release from the hive and 5 × 5 shapes, compared with the hemisphere and box shapes.
(A) Hemisphere. (B) 5 × 5. (C) Box. (D) Hive.
Full lines represent pH 7.4 release while dotted lines represent 1.2 pH release ±SD (n = 9).
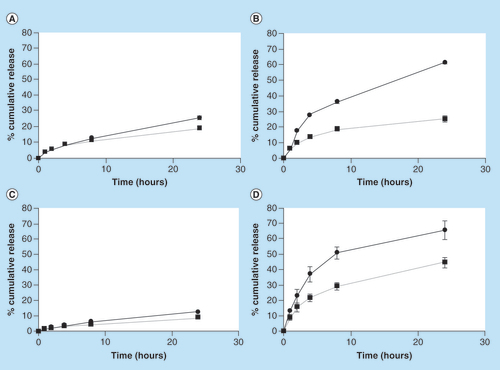
After 24 h, the release from shapes 5 × 5, hive, box and hemisphere in pH = 7.4 was: 61, 65.7, 12.3 and 25.6%, respectively, and in pH = 1.2 was: 25, 44.4, 8.5 and 18.9%, respectively. The ratio between these values, referred to as responsiveness index (RI) was calculated for each shape; 5 × 5 shape presented in B has shown the largest RI of 2.4 after 24 h. Hive shape has demonstrated a RI of 1.48. Both box and hemisphere shapes exhibit similar low RI of 1.4 and 1.3, respectively. Using such differences in the extent of drug release could potentially facilitate the fine-tuning of drug release profile in vivo.
Cross-linking effect
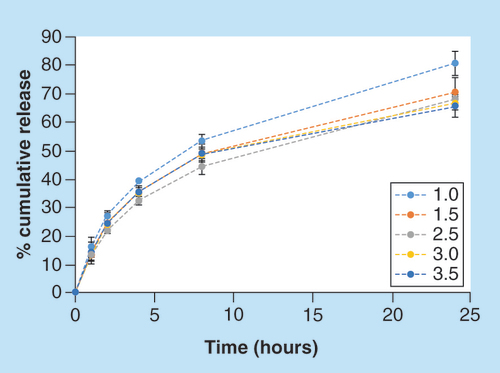
The effect of the cross-linker concentration on the release (pH 7.4) was also evaluated. Tablets with the 5 × 5 shape were printed at the same printing conditions, while using solutions with various cross-linker concentrations (1–3.5% w/w). As shown in , the tablet which was printed with the lowest concentration of cross-linker (1% w/w), yielded the highest cumulative release, 80.5% after 24 h. Tablets containing 1.5, 2.5, 3 and 3.5 cross-linker (% w/w) demonstrated cumulative release percentage of 70.5, 67.7, 66.6 and 65.5, respectively, indicating a reverse correlation between the cross-linker concentration and the cumulative release: the higher the cross-linker concentration, the lower the cumulative release. This finding is in agreement with previous reports on the release from cross-linked hydrogels [Citation43,Citation44].
Release & swelling correlation
In order to better understand the relation between swelling and drug release, we compared the percentage of cumulative Sulforhodamine B release in all four shapes at pH 7.4 () to the swelling index at each time interval (). Data are calculated for up to 60% release, in accordance with the kinetics model of Korsmeyer–Peppas. We have evaluated the correlation between the ability of each tablet to expand with its ability to release the drug, shown as the cumulative release of Sulforhodamine B as a function of swelling index (). Hive and 5 × 5 shapes have reached the highest values of both release and swelling, while box and hemisphere reached the lowest values. The correlation was evaluated by R2, which range from 0.944 to 0.995, thus indicating a direct linear association between release and swelling [Citation45,Citation46]. These results clearly show the important effect of tablet design on the ability to control the extent and duration of drug release.
Kinetics models
Mathematical models of drug release are used to evaluate the mechanism in which a drug is discharged from the tablet. The data acquired from the dissolution tests were analyzed in order to find the most representative kinetic model. As shown in , all four shapes demonstrated poor coefficient of determination (R2) in zero order kinetic model. Korsmeyer–Peppas model was found to be best fitted for box, hemisphere and hive (0.996, 0.998 and 0.987, respectively). Nonetheless, 5 × 5 shape presented a better fit toward Higuchi's models (0.977) assuming Fickian diffusion is the rate limiting step and the predominant release mechanism. All four shapes exhibited a value of n in the range of 0.45 < n < 0.89, representing a non-Fickian diffusion mechanism. Such behavior is related to the response rate in which the polymer structure alters post absorption of the surrounding molecules [Citation47]. Anomalous diffusion mechanism obtained here is in accordance with polyacrylic acid glassy character at 37°C [Citation48] and extensive swelling of glassy polymers often abstained from following Fickian concentration-dependent release. Instead, glassy polymer tends to react slowly to changes in their environment. System parameters such as stress, temperature, medium type and concentration are time-dependent resulting in an anomalous release.
Table 1. Parameters obtained from various kinetic models.
Tablet biocompatibility & cell viability
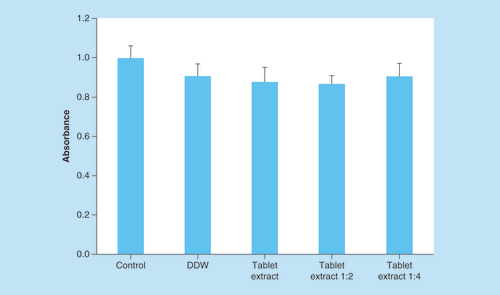
The biocompatibility and potential toxicity effect of the tablets and their condition media were studied in human epithelial colorectal adenocarcinoma cells, Caco2 cells, which are the acceptable model for intestinal absorption studies. shows viability level after 72 h of treatment normalized to the untreated cells. Cells supplemented with DDW (control) reached values of 0.9 OD and served as a reference for treated cells. The tablets condition media and dilutions of 1:2 and 1:4 reached values of 0.87, 0.86 and 0.90 OD, respectively. Statistical tests were performed showing no significant difference between treated and untreated cells, suggesting that there is no cytotoxic effect ().
DDW served as a control and viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. ±SD (n = 9).
DDW: Double-deionized water.
As presented in this research, 3D printed drug-loaded hydrogels can be used for targeted and delayed drug release in the small intestine as they can pass intact through the acidic environment of the stomach with minimal drug release. Hydrogels of polyacrylic acid exhibit very low solubility in acidic conditions due the carboxylic acid groups, which remain unionized at low pH. As the pH of the surrounding media increases, these groups in tablet matrix begin to ionize, thus resulting in increased hydrophilicity and solubility. These structures can be used to protect drugs from the acidic conditions of the stomach, as well as to prevent gastric irritation that is caused by a variety of drugs.
Conclusion
In this study, we have demonstrated the utilization of 3D printing to improve performance of classic solid dosage forms by offering new ways to control drug release as function of pH and surface area, while controlling geometry parameters. We have shown a good correlation between the swelling of the tablets and the release of a model drug, meaning we can tune the drug release by designing specific shapes with the desired surface area. It is expected that advancing this technology would eventually allow to provide an important means for personalized drug delivery by controlling critical parameters for drug action and absorption.
Future perspective
Utilization of various 3D printing technologies for additive manufacturing of drug delivery systems is expected to lead to exciting and unique products for the pharmaceutical industry. Future products will take advantage of the possibilities to form delivery systems with complex geometries that would enable formation of programmable and personal medicine. For example, printed multidrug tablets that are composed of compartments that can open up and release specific drugs at a specific time or pH and tablets that can float in the stomach for a prolonged time due to presence of printed channels filled with air. Future applications will depend on progress in rapid printing in large scale and availability of printing materials approved for medical applications.
Utilization of 3D printing to improve performance of classical solid dosage forms by offering new ways to control drug release as function of pH and surface area, while controlling complex geometry parameters.
The swelling index of 3D printed structures can be controlled through the structure of the printed delivery systems.
For each geometry, the release increases with the swelling index of the printed object.
Kinetic models suggest that the drug release from 3D printed tablet is mostly governed by tablet swelling as well as diffusion from the tablet surface.
3D printed drug-loaded hydrogels can be used for targeted and delayed drug release in the small intestine, as they can pass intact through the acidic environment of the stomach with minimal drug release.
Biocompatibility and potential toxicity effect of the tablets were studied in human epithelial colorectal adenocarcinoma cells, Caco2 cells) and showing no significant difference between treated and untreated cells, suggesting that there is no cytotoxic effect.
Advancing this technology would enable providing important means for personalized and programmable drug-delivery systems.
Acknowledgements
The authors would like to express their gratitude to E Blayvas for performing the E-SEM measurements.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Jones N . Three-dimensional printers are opening up new worlds to research. Nature487, 22–23 (2012).
- Moulton SE , WallaceGG. Three-dimensional fabricated polymer-based drug delivery systems. Jconrel.193, 27–34 (2014).
- Murphy SV , AtalaA. 3D bioprinting of tissues and organs. Nature Biotech.32(8), 773–785 (2014).
- Letourneau CA , DaviesCTet al. 3D printing of medical devices: when a novel technology meets traditional legal principles. Reed Smith (2015).
- Researchers use 3D printers to create custom medical implants. Phys. Org. (2014). https://phys.org/news/2014-08-3d-printers-custom-medical-implants.html.
- Yu DG , ZhuLM, Branford-WhiteCJ, YangXL. Three-dimensional printing in pharmaceutics: promises and problems. J. Pharm. Sci.97(9), 3666–3690 (2008).
- Goole J , AmighiK. 3D printing in pharmaceutics: a new tool for designing customized drug-delivery systems. Ijpharm.499, 376–394 (2016).
- Goyanes A , ChangH, SedoughDet al. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Ijpharm496, 414–420 (2015).
- Professional grade 3D printer microlay dentalfab . 3D printing for digital dentistry. microlay.com/dentalfab.
- 3D printing takes off with aeronautics & aerospace. sculpteo.com/blog/2015/11/25/3d-printing-takes-off-with-aerodinamics-aerospace.
- Top 10.3D printed construction innovations. https://3dprintingindustry.com/news/top-10–3d-printed-construction-innovations-83578/.
- Nadgorny M , XiaoZ, ChenC, ConnalL. Three-dimensional printing of pH-responsive and functional polymers on an affordable desktop printer. Am. Chem. Soc.8(42), 28946–28954 (2016).
- Pawar AA , SaadaG, CoopersteinIet al. High-performance 3D printing of hydrogels by water-dispersible photoinitiator nanoparticles. Sci. Adv.2(4), e1501381; 1–7 (2016).
- Weiland S , PetzoldtF. Binder jet 3D-printing for metal additive manufacturing: applications and innovative approaches. Ceramic Forum Inter.10, E26–E30 (2016).
- Davies MJ , CostleyE, RenJ, GibbonsP, KondorA, NaderiM. On drug–base incompatibilities during extrudate and fused deposition 3D printing. J. 3D Print. Med.1(1), 31–47 (2017).
- Kalepu S , ManthinaM, PadavalaV. Oral lipid-based drug-delivery systems: an overview. Acta Pharm. Sinica B.3(6), 361–372 (2013).
- Alhnan MA , OkwuosaTC, SadiaM, WanKW, AhmedW. Emergence of 3D printed dosage forms: opportunities and challenges. Pharm. Res. (2016).
- Alomari M , MohamedFH, BasitAW, GaisfordS. Personalized dosing: printing a dose of one's own medicine. Ijpharm.494, 568–577 (2015).
- Sandeep C , HarikumarSL, Kanupriya. Hydrogels: a smart drug delivery system. IJRPC2(3), 603–614 (2012).
- Miller DS , ParsonsAM, BreslandJet al. A simple and inexpensive enteric-coated capsule for delivery of acid-labile macromolecules to the small intestine. J. Zhejiang Uni-Sci B.16(7), 586–592 (2015).
- FDA approves the first 3D printed drug (2015). https://www.spritam.com/#/patient.
- Goyanes A , Robles-MartinezP, BuanzA, BasitAW. Effect of geometry on drug release from 3D printed tablets. Ijpharm494, 657–663 (2015).
- Goyanes A , WangJ, BuanzAet al. 3D printing of medicines: engineering novel oral devices with unique design and drug release characteristics. Am. Chem. Soc.12, 4077–4084 (2015).
- Zhang XF , YuDG, ZhuLM, Branford-WhiteC, DowdenS, YangXL. Sustained-release floating drug delivery systems prepared using three-dimensional printing. IEEE (2009).
- Krishna Rao KSV , HaCS. pH sensitive hydrogels based on acryl amides and their swelling and diffusion characteristics with drug delivery behavior. Polym. Bull.62, 167–181 (2009).
- Ahmed EM . Hydrogel: preparation, characterization and applications: a review. J. Adv. Res.6, 105–121 (2015).
- Peppas NA , BuresP, LeobandungW, IchikawaH. Hydrogels in pharmaceutical formulations. Euro. J. Pharm. Bio.50, 27–46 (2000).
- Hoffman AS . Hydrogels for biomedical applications. Adv. DD Rev.54, 3–12 (2002).
- Rizwan M , YahyaR, HassanAet al. pH sensitive hydrogels in drug delivery: brief history, properties, swelling and release mechanism, material selection and applications. Polym.9, 137 (2017).
- Patel DK , Hosein SakheiA, LayaniM, ZhangB, GeQ, MagdassiS. Highly stretchable and UV curable elastomers for digital light processing-based 3D printing. Adv. Mater.29(15), 1–7 (2017).
- Dash S , MurthyPM, NathL, ChowdhuryP. Kinetic modeling on drug release from controlled drug delivery systems. Acta. Pol. Pharm.67(3), 217–223 (2010).
- Siahi-Shadbad MR , Asare-AddoK, AzizianK, HassanzadehD, NokhodchiA. Release behavior of propranolol HCl from matrix tablets containing psyllium powder in combination with hydrophilic polymers. AAPS PharmSciTech.12(4), 1176–1182 (2011).
- Riss TL , MoravecRA, NilesALet al. Cell viability assays: assay guidance manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences2–3 (2013).
- Almeida H , AmaralMH, LobaoP. Temperature and pH stimuli-responsive polymers and their applications in controlled and self-regulated drug-delivery. JAPS2(6), 1–10 (2012).
- Karolewicz B . A review of polymers as multifunctional excipients in drug dosage form technology. SpharmJ.24(5), 525–536 (2016).
- Swift T , SwansonL, GeogheganM, RimmerS. The pH-responsive behavior of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter.12, 2542–2549 (2016).
- Kraus K , TiekeB. pH- and temperature-responsive hydrogels of acrylic acid, n-isopropylacrylamide and a nonionic surfmer: phase behavior, swelling properties and drug release. Colloid Polym. Sci.292(3), 3127–3135 (2014).
- Sohail K , KhanIU, ShahzadY, HussainT, RanjhaNM. pH-sensitive polyvinylpyrrolidone-acrylic acid hydrogels: impact of material parameters on swelling and drug release. Pharm. Sci.50(1), 173–184 (2014).
- Bartil T , BaunekhelM, CedricC, JeromeR. Swelling behavior and release properties of pH-sensitive hydrogels based on methacrylic derivatives. Acta Pharm.57, 301–314 (2007).
- Krishna Rao KSV , HaCS. pH sensitive hydrogels based on acryl amides and their swelling and diffusion characteristics with drug delivery behavior. Polym. Bull.62, 167–181 (2009).
- Coppeta J , RogersC. Dual emission laser-induced fluorescence for direct planar scalar behavior measurements. Exp. Fl.25, 1–15 (1998).
- De SK , AluruNR, JohnsonB, CroneWC, BeebeDJ, MooreJ. Equilibrium swelling and kinetics of pH-responsive hydrogels: models, experiments and simulations. JMS11(5), 544–555 (2002).
- Wong RSH , AshtonM, DodouK. Effect of cross-linking agent concentration on the properties of unmedicated hydrogels. Pharmaceutics7, 305–319 (2015).
- Maitra J , ShuklaVK. Cross-linking in hydrogels: a review. Am. J. Polymer Sci.4(2), 25–31 (2014).
- Sriamornsak P , ThirawongT, WeerapolY. Swelling and erosion of pectin matrix tablets and their impact on drug release behavior. Eur. J. Pharm. Biopharm.67, 211–219 (2007).
- Wan LCS , HengPWS, WongLF. Relationship between swelling and drug release in a hydrophilic matrix. Drug Dev. Ind. Pharm.19(10), 1201–1210 (1993).
- Crank J . The mathematics of diffusion. Ox. Sci. Pub.11, 254–256 (1979).
- Kricheldorf Hans R , OskarNuyken, GrahamS. Handbook of Polymer Synthesis (Second Edition). Chapter 4, page 279.