Abstract
Fabrication of 3D scaffolds with patient-specific designs, high structural and component complexity, and rapid on-demand production at a low-cost by printing technique has attracted ever-increasing interests in tissue engineering. Cell-laden 3D bioprinting offers good prospects for future organ transplantation. Compared with nonbiological 3D printing, cell-laden 3D bioprinting involves more complex factors, including the choice of printing materials, the strategy of gelling, cell viability and technical challenges. Although cell-populated 3D bioprinting has so many complex factors, it has proven to be a useful and exciting tool with wide potential applications in regenerative medicine to generate a variety of transplantable tissues. In this review, we first overview the bioprinting materials, gelling strategies and some major applications of cell-laden 3D bioprinting, with main focus on the recent advances and current challenges of the field. Finally, we propose some future directions of the cell-populated 3D bioprinting in tissue engineering and regenerative medicine.
In this review, we first overview the bioprinting materials, gelling strategies and some major applications of cell-populated 3D bioprinting, with main focus on the recent advances and current challenges of the field. Finally, we propose some future directions of the cell-laden 3D bioprinting in tissue engineering and regenerative medicine.
Cell-laden 3D bioprinting
Currently, there has been an ever-increasing demand for organ transplants [Citation1]. However, donor organ scarcity remains a big clinical challenge in transplantation. For example, less than a third of waiting patients can receive matched organs from donors in the USA [Citation2]. One of the most promising techniques to alleviate the organ shortage crisis is tissue engineering, which can fabricate the replacement for the lost or damaged tissues and organs [Citation3]. Therefore, developing 3D scaffolds with precise structure is critical in tissue engineering applications.
In 1986, 3D printing (3DP) was first described by CW Hull. In his method, which he named ‘sterolithography,’ materials can be printed in layers to form a solid 3D structure, curing with ultraviolet light rapidly [Citation4]. 3DP, a new type of rapid inkjet printing technology, shows significant promise for the fabrication of complex composite objects or devices at very high speed and precision via a layer-by-layer ink jetting approach [Citation5,Citation6]. In addition to stereolithography, 3DP techniques include fused deposition modeling [Citation7], selective laser sintering [Citation8], digital light processing [Citation9], 3DP [Citation10], laminated object manufacturing [Citation11] and polyjet [Citation12]. Klein and colleagues [Citation13] made the use of titanium powder and biological ceramic to form artificial mandible with 3DP technology, undergoing the first artificial mandible replacement surgery. Zopf et al. [Citation14] fabricated a customized, bioresorbable tracheal splint based on a computed tomographic image of the patient's airway and laser-based 3DP, and then implanted the artificial splint into an infant with tracheobronchomalacia. They found that the infant did not have respiratory distress at birth of 35 weeks, appearing to be in normal health. These results demonstrated that 3DP technology has been applied for organ transplantation in clinic. However, due to the limitation of the printing materials, 3DP technology is mainly used to print inanimate artificial joints and prosthesis with metal, plastic, ceramic, etc. [Citation15]. Thus-obtained simple implants provide mostly a temporary structural support matrix for tissue regeneration, and cannot serve as substitutes for bioactive organs.
The goal of tissue engineering is to repair or completely replace damaged tissues with artificially fabricated substitutes having improved biological functions through rebuilding and remodeling. Therefore, development of strategies that utilize living cells for bioactive organ construction is important for tissue engineering. 3D bioprinting, derived from combination of cell biology, materials science, 3DP technique and medicine, holds much promise to realize the aim aforementioned. Thus-fabricated scaffolds have a 3D porous structure similar to extracellular matrix (ECM), leading to significantly improved cell–cell contact, cell–matrix interactions and cell density [Citation16]. For instance, Kievit and colleagues demonstrated that 3D porous chitosan–alginate scaffolds dramatically promoted the proliferation and enrichment of the CD133+ cancer stem cell population. By 15 days after cell seeding, they found, the cancer stem cells (CSC) fraction on chitosan-alginate (CA) scaffolds increased from 0.3 to 42%, and the total number of U-87 MG CD133+ CSCs on CA scaffolds reached 2188-fold while those grown as monolayers showed little change [Citation17]. Compared with traditional 3DP, the main characteristics of 3D bioprinting technology are the use of biocompatible and biodegradable biomaterials, and the mild gelling process good for maintaining the cell viability. 3D bioprinting generates bioactive scaffold for organ and tissue transplant, and therefore has a great potential in tissue engineering and regenerative medicine.
Unlike conventional 3DP techniques that have been used to print cell-free scaffolds, bioprinting is a quite different approach that involves simultaneous depositing of living cells with the materials (bioinks). There are two approaches for the combination of cells and scaffolds in 3D bioprinting. One is to seed the cells sparsely and homogenously in the prefabricated 3D scaffolds, which has been extensively investigated for many years [Citation18]. With this method, the seeded cells exhibit intense interaction with biomaterials, resulting in high cell activity, nice cell adhesion, proliferation and differentiation on the 3D scaffolds [Citation19,Citation20]. However, this method cannot control the cell density and distribution accurately, and fail to generate appropriate ECM microenvironment. Without a proper ECM microenvironment, the stem cells cannot function properly as in the organs or tissues. The other approach is to print high-water content biomaterials and living cells synchronously to form organ or macrotissues, which is named ‘cell-laden 3D bioprinting.’ The distinct advantage of this newly developed printing technology is its ability to simultaneously and rapidly deposit live cells, growth factors along with biomaterial scaffolds in a precisely controlled way to form a bioactive construct that mimics the native tissue architecture and functions. This technology has a great potential in tissue engineering, as various functional tissues can be fabricated with appropriate structures and cell compositions in a wide range of sizes, a high-throughput and highly reproducible fashion (). For those reasons, the development and subsequent applications of the cell-laden 3D bioprinting have attracted ever-increased interests from biomedical researchers during the past 5 years.
Hydrogels, with similar structure, composition and mechanical properties to the ECM of the natural soft tissue, have been explored as the main candidate materials for cell-laden 3D bioprinting at present [Citation21]. The hydrogel scaffolds used in cell-laden 3D bioprinting should meet five basic requirements:
The bioprinting hydrogels must be cytocompatible, weakly immunogenic and have nontoxic by-products of degradation that can be metabolized and secreted without eliciting detrimental local or systemic effects;
Hydrogel scaffolds can be rapidly formed in situ by cross-linking via covalent or noncovalent interactions. And the gelling process is gentle enough to maintain the viability of the printed cells;
The mechanic performance of the hydrogel scaffolds can be tuned to fit the damaged or injured tissues or organs, and are stable enough to avoid internal collapse during and after the printing process;
The 3D printer should be carefully designed to keep the viability and functions of the printed cells in the scaffolds, and less affected by the dispensing pressure and nozzle diameter during the printing process;
The hydrogel scaffolds are easy for postprocessing, such as easy for dynamic culture to maintain the cell viability, and convenient for cell activity detection (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide [MTT], Alamar Blue and BrdU incorporation). In this review, we will give an overview of the materials, printing strategy and applications of cell-laden 3D bioprinting, with a main focus on the recent advances of the field.
Finally, the major challenges facing the field are discussed, and some possible future directions of the cell-populated 3D bioprinting are suggested.
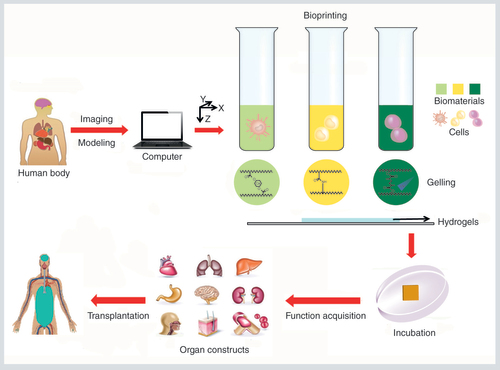
The human body defects are scanned and the architectural information is imported into the computers allowing on-demand printing. Then, several cell-laden bioinks are printed through the nozzle system by chemical or physical cross-linking, and deposited in spatially predefined locations. The cells in the bioprinted scaffolds proliferate, migrate and mature into functional organs. These biomimetic organs could be ultimately used for tissue engineering.
Materials for cell-laden 3D bioprinting
Cell-laden 3D bioprinting provides not only a medium to deliver cells to the target location but also a physiological environment to support and regulate cell proliferation and differentiation [Citation22]. Hydrogel, which could be cross-linked as a 3D hydrophilic network, can absorb and maintain large quantities of water without dissolution, mimicking the native tissue microenvironment in vivo, such as the interstitial fluid. Therefore, a large number of hydrogels have been developed for tissue engineering and regenerative medicine [Citation23]. Hydrogel is highly permeable to oxygen, nutrients and other water-soluble compounds, and has been explored extensively for fabrication of tissue constructs by cell-populated 3D bioprinting [Citation24]. Although many types of hydrogels have been prepared in the past, most of them are not suitable for cell-populated 3D bioprinting. The selection of appropriate hydrogels for use in cell-laden 3D bioprinting depends on several factors, such as printability, biocompatibility, mechanical properties and biomimicry. To be used in cell-laden 3D bioprinting, materials must form hydrogels via suitable cross-linking mechanisms to facilitate rapid bioink deposition, must be biocompatible for transplantation over long-term period of time and must have suitable swelling characteristics and short-term stability. Short-term stability is required to maintain initial mechanical properties, ensuring that tissue structures such as pores, channels and networks do not collapse during and postprinting. Furthermore, the chosen materials must facilitate cellular attachment, proliferation and functions [Citation25].
Hydrogels currently used in the field of cell-populated 3D bioprinting are predominantly based on either natural polymers (e.g., alginate, collagen, fibrin, gelatin and hyaluronic acid [HA], to name a few, often isolated from animal or human tissues) [Citation22,Citation26] or synthetic macromolecules (e.g., pluronic and poly(ethylene glycol) [PEG]) [Citation22,Citation27]. The major advantages of natural hydrogels over the synthetic polymers-based ones for 3D bioprinting are their cell friendliness, similarity to human ECM, inherent bioactivity and even improvement of the differentiation of the cultured stem cells. But the main defects of natural hydrogels are their poor mechanical performance and processibility. Such shortcomes can be overcome by development of synthetic hydrogels, the structure and physiochemical properties of which can be tailored precisely to suit specific applications. Major challenges in the use of synthetic polymers include their poor biocompatibility, toxic degradation products and loss of mechanical properties during degradation. Even so, synthetic hydrogels are still attractive for 3D bioprinting, since their structure and properties can be fine-tuned to meet the requirements for cell-laden 3D bioprinting.
Alginate
Alginate is an anionic polysaccharide consisting of β-D-mannuronate (M) and α-L-guluronate (G) residues. M and G residues are covalently linked together in different sequences or blocks [Citation28]. In the presence of divalent cations, alginate solution could rapidly cross-link between the G blocks of adjacent polymer strands. Thus, the mechanical strength and dissolution time of alginate hydrogel scaffold are directly related to the G/M ratio of the gel [Citation29]. Furthermore, alginate hydrogel scaffold is suitable for encapsulating cells, because its high carboxylic acid content causes massive water absorption. However, the good hydrophilia of alginate hydrogel scaffold makes it hard for the cells and proteins to adhere. To address this issue, modification of alginate with other bioactive molecules such as Arg-Gly-Asp Acid (RGD) and dopamine are usually adopted [Citation30,Citation31]. The concentration of alginate influences, to a large extent, the gelation time, porosity and mechanical strength of the formed scaffold. In addition, the concentration of divalent cations significantly affects the printability of alginate [Citation32]. Alginate hydrogels have been used extensively in 3D bioprinting due to their low cost, abundant availability and good biocompatibility with negligible inflammatory effect after implantation in vivo. Alginate hydrogels have significant advantages, but they are derived from a xenogenic source that is far from humans on the phylogenetic tree. Therefore, alginate cannot be considered as biomimetic material for mammalian cells because mammals do not use this polymer in their ECM.
Collagen & gelatin
Collagen, a main structural protein of ECM in various tissues and extracted from animals, causes minimal immunological reaction, and therefore has many applications in biomedical field [Citation33]. For 3D bioprinting, collagen is often denatured. Collagen serves as a major component of the ECM, especially in fibrous connective tissues where it can be the main tissue component. Because of its essential role in native tissue, collagen is an attractive candidate biomaterial for tissue engineering. Collagen-based hydrogel not only improves the adhesion of the encapsulated cells but also enhances cell attachment and growth due to its abundant cell-attachable domains [Citation34]. Moreover, collagen hydrogel has relatively low immunogenicity compared with other naturally derived biomaterials, likely due to the high evolutionary stability of collagen among mammals [Citation35]. Interestingly, collagen hydrogel is thermo- and pH-sensitive, which makes it an ideal bioprinting material. Collagen solution is often prepared at 4°C in an acidic environment. Upon pH to a physiological range (pH 7.4) and incubating the solution at 37°C for 30 min, the hydrogel scaffold will be formed. Several researchers have already explored the feasibility of collagen as bioink to print 3D constructs, and demonstrated that collagen is suitable for cell growth and proliferation [Citation36,Citation37]. Although collagen has been already combined with the existing bioprinting technology to encapsulate cells as 3D matrix to culture various cells, there remain many limitations in bioprinting. The slow gelation process of collagen makes the fabrication of 3D constructs by bioprinting technique very difficult. To address the issue, various hydrogels are developed based on mixture of collagen hydrogel and other polymers that have fast cross-linking kinetics [Citation38]. Therefore, more efforts are desired to use collagen in 3D bioprinting.
Gelatin, a denatured form of collagen, is derived from bones and skins of animals. Gelatin is in a gel state at low temperature by forming a helical structure, while at high temperature it concerts back to a random coil conformation, resulting in a sol state. This feature renders it unsuitable for in vivo application [Citation39]. Therefore, gelatin must be modified chemically or mixed with other cross-linking polymers. The most widely used strategy to induce cross-linking in gelatin hydrogel is introduction of methacrylamide groups to gelatin, followed by UV irradiation [Citation40,Citation41]. However, gelatin is more denatured, leading to less biocompatible, less chemically stable and more inflammatory than fibrous collagen. Therefore, gelatin is more often used as a sacrificial material to transfer cells or construct complex structures, due to its thermal responsive property which makes it easier to be removed after bioprinting [Citation42]. Thus, gelatin can be used in one of three ways in bioprinting applications:
As a hydrogel scaffold after UV cross-linking by modified with methacrylate;
As a component of the hydrogels to tailor the viscosity and gelling time [Citation43];
As a sacrificial material.
In addition, lack of proper mechanical strength is another major drawback of gelatin-derived hydrogel scaffolds. So, 3D bioprinting with single gelatin material is rarely reported. However, combining with other materials for 3D bioprinting is a good choice of gelatin. Fibrin, alginate and other materials were successfully mixed with gelatin to print cell-laden 3D structures [Citation43,Citation44].
Fibrin
Fibrin, as a key protein in blood clotting, is formed by the reaction between fibrinogen and thrombin. Fibrin-based hydrogel scaffold is highly biocompatible, and can promote cell adhesion and proliferation. Therefore, there are also some reports on the applications of fibrin in 3D bioprinting [Citation45]. In the work by Mosesson et al. a solution of fibrinogen monomers was activated to form a polymeric fibrin matrix upon cleavage of fibrinopeptides of their N-terminal ends by the serine protease thrombin [Citation46]. Unlike the slow gelation process of collagen, the gelation process of fibrin can be controlled by adjusting the concentration of thrombin [Citation47], making bioprinting 3D constructs of fibrin possible. However, the formed fibrin scaffold is lack of mechanical strength, thereby limiting the manipulation of scaffold after gelation. Furthermore, the fast degradation of the fibrin hydrogel also significantly impedes its application in long-term cell culture. Cells could produce enzymes by themselves, resulting in fibrinolysis as well as hydrogel dissolution after 1 week. Therefore, adding of protease inhibitors can preserve the matrix for several weeks in vitro [Citation48]. Methods to create stiffer matrices and slow down degradation in vitro have been described, but it is uncertain if it still works when translated to the in vivo environment.
Hyaluronic acid
HA is a linear anionic glycosaminoglycan consisting of repeated units of D-glucuronic acid and N-acetyl-D-glucosamine moieties. HA distributes widely in almost all connective tissues and neural tissues, and it is also an important ECM component of cartilage [Citation49]. Therefore, HA has been extensively used in biomedical field due to its excellent biocompatibility and biodegradability. HA cannot gel by itself, therefore, it needs chemical modification or blending with other functional materials for 3D bioprinting. The commonly used approach for 3D bioprinting with HA is photopolymerization. Pescosolido et al. [Citation50] modified HA with a photosensitive dextran derivative to print 3D structure. HA mixed with hydroxyethyl-methacrylate-modified dextran solution was exposed to UV radiation in the presence of a photoinitiator, causing polymerization of dextran chains to form a hydrogel. HA entangled with the cross-linked dextran network. Chemically modified HA-based hydrogel is very useful for bioprinting application, because it is easy to work with, highly customizable in terms of structure and mechanical properties, and replicate the native ECM well. While the HA hydrogel gelled by itself has poor mechanical performance and quick degradation, cross-linkable HA hydrogel has good mechanical strength and chemical stability against enzymatic degradation.
The above-discussed materials are all derived from nature, which can provide an environment similar to ECM. Naturally derived materials are biocompatible and biodegradable, but their mechanical properties can hardly match the requirements of 3D bioprinting. Synthetic materials, to be discussed below, can be rationally designed and prepared, to solve the problems of natural materials and meet the requirements of 3D bioprinting.
Pluronic F-127
Pluronic F-127 is a kind of nonionic triblock copolymer, and composed of a central hydrophobic chain of polypropylene oxide flanked by two hydrophilic chains of polyethylene oxide [Citation51]. An important property of Pluronic F-127 is its thermoresponsibility. The concentrated solution of pluronic appears as liquid at low temperature, while it will form a hydrogel at high temperature, and the gelation process is reversible [Citation52]. So printing of pluronic needs a heating system around the needle to ensure the transition from liquid to gel state, and a heating plate is also needed to maintain the temperature of the construct after printing. Even at physiological temperature, the structure of pluronic-based hydrogel scaffold cannot hold for a long time, so pluronic is rarely used alone for bioprinting. However, the instability of pluronic hydrogel scaffold is a two-edged sword. Wu et al. [Citation53] used pluronic as sacrificial hydrogel to bioprint channel-type structures. By combining with other functional polymers, pluronic can be printed to form stable 3D structure [Citation54].
Poly(ethylene glycol)
PEG is a polyether compound that has many biomedical applications such as drug delivery and tissue engineering [Citation55]. PEG is a hydrophilic polymer resistant to protein adsorption, and it is usually conjugated with proteins, enzymes and other biomolecules as drug delivery systems to improve the drug delivery efficiency [Citation55]. Nowadays, photopolymerization of PEG-based hydrogel is widely used in 3D bioprinting. Compared with the hydrogels based on naturally derived fibrin, agarose, alginate and collagen, PEG hydrogel has a higher mechanical stiffness [Citation56]. Cui et al. [Citation57] printed articular chondrocytes using PEG dimethacrylate (PEGDMA) hydrogel via a layer-by-layer technique with UV exposure at every layer. In another study, acrylated-RGD was bioprinted with acrylated-PEG followed by UV exposure to solidify the structure [Citation58].
Cellular activities such as proliferation, differentiation, migration and secretion can be modulated through natural polymer-based scaffolds [Citation59]. The composition of ECM affects how cells interact with it, such as the extent of integrin-mediated cell adhesion [Citation60]. These interactions can be imitated by the development of artificial ECM from a mixture of natural ECM components. However, synthetic polymers generally lack the necessary signals for cells to reorganize them to generate functioning tissues [Citation61]. Slow remodeling of the scaffolds and prolonged immune response are major problems with such scaffolds [Citation62]. The interactions of synthetic polymers with cells are generally indirect and limited. To improve cell response, several approaches, such as blending with natural polymers [Citation63], addition of cell responsive segments to the polymer backbone [Citation64] and chemical functionalization of the polymer chains with bioactive agents [Citation65] are developed.
Moreover, the response of 3D-encapsulated cells is closely related to the biophysical and physicochemical properties such as stiffness, composition and structure of the surrounding material. Specifically, stiffness of the scaffold can regulate cell shape and migration [Citation66]. Depending on the composition, a surrounding matrix can deliver biochemical cues that affect focal adhesions and migration of the cells. In addition, the hydrogel structure influences cell morphology and migration. For example, a change in concentration of one component (e.g., collagen monomers) can alter the stiffness and the inner structure of a cell substrate [Citation23]. Köpf and colleagues [Citation67] studied the morphogenesis of human umbilical artery smooth muscle cells (SMC) in agarose-collagen blend hydrogel. They found that cells encapsulated in hybrid hydrogels with varying concentrations of agarose and constant type I collagen content of 0.2% showed different morphologies after cultivation for 4 days. With increasing agarose content, the cell shape varied from a coexistence of spread and round cells to only rounded cells. Frisman et al. [Citation68] considered that cells in the hydrogel with higher cross-linking degree displayed more spindled morphologies and larger areas, the cell area was significantly higher in the less cross-linked hydrogels than in the more cross-linked hydrogels. They also found cell morphology was related to the hydrogel modulus, mainly that cells prefer a spindled morphology in softer hydrogels.
An ideal bioprinting hydrogel would be one that has high biocompatibility and biomimicry, degrades at a rate equal to or similar to that of ECM synthesis by the neotissue, provides appropriate structural and mechanical support during and after printing, and forms stable cross-links in the order of seconds to minutes. However, none of currently available hydrogels meet all of these criteria. Therefore, the major challenge in this regard is to develop combined hydrogel systems or make chemical modification, ensuring that the materials have suitable degradation rate and nontoxic by-products, and that these materials have well-understood and controllable biological effects in the construct.
Strategies for cell-laden 3D bioprinting
Both of the materials and the printing techniques are key to the successful fabrication of organs and tissues via 3D bioprinting. In the above section, we have introduced the major types of materials used for cell-laden 3D bioprinting, in this section we will discuss the technologies widely employed in cell-laden 3D bioprinting. A variety of strategies have been developed for cell-laden 3D bioprinting. Generally, these strategies could be divided into two types, based on their underlying printing principles: physical methods (including temperature-controlled gelling method) [Citation69], and chemical approaches (including laser-assisted gelling, enzymatic cross-linking, ionic cross-linking and covalent cross-linking) [Citation70–72]. Physical cross-linking is usually achieved through a relatively weak and reversible interaction. Chemical cross-linking technique often yields robust hydrogel scaffolds by conjugating of gel precursors through formation of new covalent bond. Either method has its distinct advantages and limitations, which will be discussed in more detail below.
Temperature-controlled gelling method
When the temperature of gel solution decreases or increases to a certain value, which is named utmost critical solution temperature/lowest critical solution temperature, some hydrogels will change from solution state to gel state. For example, the phase of gelatin has a rapid change when the temperature is lower than its utmost critical solution temperature of 20°C [Citation44]. And the hydroxybutyl chitosan will change from solution state to gel state in only 30 s when the temperature is higher than its lowest critical solution temperature of 30°C [Citation73]. These kinds of hydrogels are named thermosensitive hydrogels. Thermosensitive hydrogel forms gel by physical interaction, including entanglements of hydrogel chain, hydrogen bonding or hydrophobic interactions between the hydrogel units. For 3D bioprinting, the gelling temperature is usually selected at 4–37°C, which is safe to the printed cells. During 3D bioprinting, the thermosensitive hydrogel is stored in a cold/thermal printing head and squeezed to generate cylindrical lines of a control size on the thermal/cold printing platform. Kang and colleagues [Citation74] fabricated an artificial ear with thermosensitive hydrogel at 18°C. They used three kinds of thermosensitive materials: gelatin, poly(ε-caprolactone) (PCL) polymer and Pluronic F-127. Gelatin is in liquid state above 37°C, and becomes solid below 20°C; PCL as a supporting material has a low melting temperature of 60°C, and could rapid solidify at 18°C; while Pluronic F-127 as a sacrificial material could be easily printed at low temperature and removed under mild conditions (). Lee and colleagues [Citation75] constructed liver tissue by liver decellularized ECM (dECM) bioink and PCL at 37°C. At low temperature, the liver dECM bioink was in the solution state; however, it underwent cross-linking and entered the gel state after being incubated for 30 min at 37°C. PCL could also be used to improve the mechanical properties of dECM. These results demonstrated that human-scale tissue could be quickly constructed with the temperature-controlled gelling method.
(A) Scheme showing 3D bioprinting technique. (B) Illustration of basic patterning of 3D architecture including multiple cell-populated hydrogels and supporting PCL polymer. (C) CAD/CAM process for automated printing of 3D shape-imitating target tissue or organ. A 3D CAD model developed from medical image data generates a visualized motion program, which includes instructions for XYZ-stage movements and actuating pneumatic pressure to achieve 3D printing.
CAD: Computer-aided design; CAM: Computer-aided manufacturing; PCL: Poly(ε-caprolactone).
Reproduced with permission from [Citation74] © Nature Publishing Group (2016).
![Figure 2. Human-scale tissue constructs. (A) Scheme showing 3D bioprinting technique. (B) Illustration of basic patterning of 3D architecture including multiple cell-populated hydrogels and supporting PCL polymer. (C) CAD/CAM process for automated printing of 3D shape-imitating target tissue or organ. A 3D CAD model developed from medical image data generates a visualized motion program, which includes instructions for XYZ-stage movements and actuating pneumatic pressure to achieve 3D printing.CAD: Computer-aided design; CAM: Computer-aided manufacturing; PCL: Poly(ε-caprolactone).Reproduced with permission from [Citation74] © Nature Publishing Group (2016).](/cms/asset/20db265b-9023-45a3-9df2-26cbe0f203e2/idpm_a_12339811_f0003.jpg)
The major advantages of temperature-controlled gelling approach with thermosensitive hydrogels include:
The gelling speed could be controlled by changing the temperature;
The gelling process is mild, resulting in relatively high cell viability.
It should be mentioned that such thermosensitive hydrogels usually exhibit poor mechanical properties that are not good for 3D bioprinting. A combination of two or more thermosensitive hydrogels is often adopted to improve the mechanical property of thermosensitive hydrogels [Citation74].
Laser-assisted gelling method
Laser-assisted gelling technique is based on laser-induced forward transfer, in which a pulsed laser is used to induce the phase change of materials [Citation4]. Under suitable irradiation conditions, and for liquid presenting a wide range of rheologies, the materials can be deposited in the form of well-defined circular droplets with a high degree of spatial resolution [Citation26]. For laser-assisted gelling, the materials are usually modified with methacrylamide side groups to yield photosensitive derivatives [Citation41,Citation76]. VA-086 [Citation41] and Irgacure 2959 [Citation77,Citation78], which could be initiated by UV light, are often used as photoinitiators. Billiet and colleagues [Citation41] demonstrated that VA-086 enhanced biocompatibility, comparing to the conventional Irgacure 2959. Guillotin and colleagues [Citation79] found that the higher the laser energy was deposited, the bigger the droplet would become. Their experiment also revealed that with fast laser scanning speed (1600 and 800 mm/s), the consecutive droplets were individualized. While slow laser scanning speed (400 and 200 mm/s) resulted in short distance between the printed droplets, achieving a good resolution of cell printing. Ouyang and colleagues [Citation80] compared the difference of methacrylated HA (MeHA) hydrogels formed via prephoto-cross-linking, postphoto-cross-linking and in situ photo-cross-linking approaches, and found that the prephoto-cross-linking resulted in high and inconsistent extrusion force, heterogeneously printed material structure and low cell viability. While the postphoto-cross-linking led to the bioink flow prior to stabilization, which could not maintain the filament structure, even with a higher MeHA concentration and higher UV intensity. However, the in situ photo-cross-linking showed low and consistent extrusion force, uniform printed filament and high-encapsulated cell viability (). Clearly, the in situ photo-cross-linking method is applicable to print a wide variety of photo-cross-linkable hydrogels.
(A) Schematic diagram depicting three different cross-linking strategies (precross-link, postcross-link and in situ cross-link) for bioprinting photo-cross-linkable inks. The in situ cross-linking process involves light exposure through a photopermeable capillary, during continuous extrusion, prior to deposition. (B) Representative images of nozzles with extruded material and printed lattice structure.
Reproduced with permission from [Citation80] © John Wiley and Sons Inc. (2017).
![Figure 3. Laser-assisted gelling method with different strategies. (A) Schematic diagram depicting three different cross-linking strategies (precross-link, postcross-link and in situ cross-link) for bioprinting photo-cross-linkable inks. The in situ cross-linking process involves light exposure through a photopermeable capillary, during continuous extrusion, prior to deposition. (B) Representative images of nozzles with extruded material and printed lattice structure.Reproduced with permission from [Citation80] © John Wiley and Sons Inc. (2017).](/cms/asset/bd0e5ef5-b53c-4927-99ef-7d634dc203a2/idpm_a_12339811_f0004.jpg)
The light-assisted bioprinting could be applied for physiological conditions, and the speed of light-assisted gelling process is very fast (from seconds to minutes) [Citation57]. Light-based bioprinting approach enables the generation of accurate multiscale 3D structures, but this method requires expensive and sophisticated instrumentation, thus largely limiting its wide applications [Citation81]. Especially, UV radiation will cause the damage to the cells to be printed, because UV light may induce chromosomal and genetic instability of the cells exposed to UV radiation, leading to slow growth or death of the cells compared with that of the unirradiated ones [Citation82]. Moreover, some investigators considered that the major problem facing the photo-cross-linking hydrogel is oxygen inhibition. Oxygen will rapidly scavenge the radicals that are required for the cross-linking, causing incomplete or insufficient formation of cross-links to maintain the shape of hydrogel scaffold [Citation83–85]. To solve these problems, Lim and colleagues [Citation86] made the use of a visible-light photoinitiating system, Vis + ruthenium/sodium persulfate, for 3D bioprinting. Compared with the conventionally adopted UV + I2959 system, gelatin–methacryloyl-based hydrogel constructs initiated with Vis + ruthenium/sodium persulfate system yielded higher shape fidelity and better cell viability. This can be considered as a potential alternative approach for laser-assisted 3D bioprinting.
Enzymatic cross-linking method
Many proteases can react with peptide chains of proteins, inducing cross-linking of proteins to form a network. For example, transglutaminase can catalyze the binding reaction between the lysine amino of protein and the hydroxyl amide group of glutamic acid, forming a stable gel via covalently cross-linking between proteins and peptides [Citation87]. In the presence of trace amount of hydrogen peroxide, horseradish peroxidase can form a covalent bond between two tyrosine residues of silk fibroin, resulting in a covalently cross-linked hydrogel [Citation88]. Das and colleagues [Citation89] reported the development of clinically relevant sized tissue analogs by 3D bioprinting, using enzymatic cross-linking of silk fibroin and gelatin by mushroom tyrosinase and sonication cross-linking of silk fibroin. In their strategy, the tyrosinase oxidizes the accessible tyrosine residues of silk and/or gelatin into reactive o-quinone moieties that can either condense with each other or undergo nonenzymatic reactions with available amines of both silk and gelatin. And sonication alters the hydrophobic interaction and accelerates self-assembly of silk fibroin macromolecules to form β-sheet crystals, which physically cross-link the hydrogel.
Most of the hydrogels formed by enzymatic cross-linking method are protein-based hydrogels. And the structure of the enzymatic cross-linking hydrogel scaffold could be controlled by the concentration of proteins and enzymes. The main advantage of these protein-based hydrogels is their good cytocompatibility. However, these hydrogels are soft and fragile, and difficult to maintain their 3D structure [Citation90]. This problem can be overcome by combined use of different natural and/or synthetic polymers in 3D bioprinting.
Ionic cross-linking method
In ionic cross-linking process, hydrogel-based network is produced via adding of cations. The most common ionic cross-linking-type hydrogel is alginate-based hydrogel. When the alginate is in contact with divalent cations, such as Ca2+, Sr2+ or Ba2+ [Citation91], it will tether neighboring G-blocks of alginate chains, forming a reversible electrostatic bridge [Citation92]. Gaetani et al. [Citation93] generated an in vitro tissue with homogenous distribution of human cardiomyocyte progenitor cells (hCMPCs) in the alginate scaffold via tissue printing technology. After printing, the scaffold was cross-linked with CaCl2 solution. The cells showed 92 and 89% viability at 1 and 7 days of culturing, respectively, after printing.
The stability, permeability and mechanical property of alginate hydrogel scaffolds heavily rely on the alginate concentration, deacetylation degree, molecular weight and cross-linking agents [Citation91,Citation94–95]. Shi and colleagues [Citation96] found that the concentrations of alginate solutions (2, 5 and 10%) are vital for their viscoelasticity and interior structure. Alginate solution at high concentration can yield hydrogel having strong ‘G’ at the same frequency, which can influence the printed layers of alginate hydrogel (printed at 1, 3 and 5 layers of 2, 5 and 10% of alginate solution, respectively). And the polymer networks gradually become denser in 2, 5 and 10% alginate hydrogels due to the existing of relatively larger amount of alginate molecular chains. Moreover, the concentration of alginate hydrogel influences the morphology of the bioprinted mouse fibroblast cells (L929). The cells in 2% alginate hydrogel have more space to accommodate and reproduce, while the same cells in 5% bioprinted construct were moderately restrained but could migrate in bleb shapes. The cells in 10% hydrogel were strictly trapped and finally formed spheroids. Furthermore, Ahn and colleagues [Citation70] found that for low concentration of CaCl2 (2.5 wt% CaCl2), the alginate viscosity did not change significantly with exposure time, indicating that the alginate was not highly cross-linked by Ca2+. In contrast, for the high calcium concentration of CaCl2 (10 wt% CaCl2), the viscosity of the alginate solution increased significantly with the exposure time. The results showed that manipulation for the gelation degree of the alginate can be controlled by altering the weight fraction and exposure time of the ion solution.
The ionic cross-linking method is widely applied to fabricate the hydrogel scaffolds in 3D bioprinting. Ionic cross-linking of alginate possesses optimal cell encapsulation properties as the slow gelation process is gentle and not harmful to the cells [Citation97]. The printed cells encapsulated in Ca2+ cross-linked hydrogel scaffold have shown to be protected from stress and pressure involved in extrusion, resulting in high viability rates [Citation98,Citation99]. However, the Ca2+ cross-linked hydrogel scaffold can quickly lose its mechanical properties during in vitro culture (40% within 9 days) [Citation100], due to the instability of the ionic bond. So, bioprinting of Ca2+ cross-linked hydrogel scaffold is not commonly implemented for bone regeneration due to the poor mechanical behavior of the scaffold compared with those of natural bone.
Covalent cross-linking methods
Many hydrogel scaffolds can be constructed by covalent cross-linking approaches, among which are Schiff's base, Michal Addition and Guest-Host gelling methods, to name a few [Citation101–103]. However, very limited work of cell-populated 3D bioprinting has been reported using covalent cross-linking methods for two reasons. One is that due to the limitation of current 3DP equipment, it is difficult for two kinds of hydrogels to form gels quickly during printing process. The other issue with this approach is the significant damage caused to the cells by the covalent gelling process. Recently, Rutz and colleagues [Citation104] reported the formation of a printable hydrogel via covalent cross-linking approach. In their experiment, polymer solutions were lightly cross-linked with a long-chain chemical cross-linker, a homobifunctional PEG ending in two reactive groups (PEGX) via amine-carboxylic acid coupling. When the gel was stabilized, the bioink was printed layer-by-layer with a 3D bioplotter to build well-defined, self-supporting structures. By changing the reactive groups of PEGX, polymers with other functional groups may be cross-linked. Therefore, using this method, hydrogels formed via other cross-linking chemistries, such as click chemistry and Michael addition, are also printed into tissues and organs, which further expand the number of 3D-printable bioinks available.
Applications of cell-laden 3D bioprinting
Biological tissues and organs are complex anisotropic 3D constructs, where a variety of cells and ECMs are orderly arranged to form functional microstructures or devices. In most tissues, vascular networks enable nutrients and oxygen supply for cell proliferation and function [Citation100]. 3D bioprinting technology has been applied in generating tissues for in vitro medical models [Citation105,Citation106] and in vivo transplantation [Citation107–111], thanks to the feasibility of 3D spatial arrangement of various cells, as well as different biomaterials endowed with different physical and chemical properties to mimic the anatomical geometry, mechanical and biological microenvironment of native tissues and organs. In this section, we will give a brief introduction to some typical tissues and organs fabricated by cell-laden 3D bioprinting (Supplementary Table 1).
Vasculature
A large number of cell-populated 3D bioprinting approaches have been developed to create hierarchical vascular networks characterized by varied lumen diameter, wall-thickness, cell composition and cell arrangement [Citation112]. Miller and colleagues [Citation113] succeeded in printing a 3D carbohydrate glass lattice and fabricating a cell-populated monolithic tissue constructed with multilayer interconnected vasculatures by removal of the carbohydrate fibers in culture medium (A). Efficient endothelialization and enhanced cell activity were observed. The merit of their work is that it can be widely combined with various printable materials and additional manufacturing process together. Recently, Koleskya and colleagues [Citation114] codeposited Pluronic F-127 filaments and human mesenchymal stem cells (hMSCs)-laden gelatin/fibrin filaments to form 3D lattice (B). After printing, this 3D scaffold was embedded within fibroblasts-laden gelatin/fibrin matrix. Dissolution of Pluronic F-127 filaments led to the formation of tubular structures in the scaffold, and then the tubular structures were lined with human umbilical vein endothelial cells (HUVECs). During 6-week culture, the stable vasculature scaffold supported hMSCs proliferation, as well as osteogenic differentiation, forming 1-cm thick vascularized osteogenic tissue. Alternatively, some research groups built tubular structures with the assistance of coaxial cross-linking system. In the system, the printing head was composed of two coaxial channels, where alginate was delivered through the outer channel and CaCl2 was delivered through the core channel, then alginate and CaCl2 could be mixed during printing. Jia and colleagues [Citation115] reported the use of coaxial cross-linking system to fabricate cell-populated vascularized constructs based on hybrid bioinks consisting of gelatin methacryloyl, sodium alginate and branched PEG (4-arm PEG-tetra-acrylate [PEGTA]; C). The diameter of the bioprinted tube could be easily tuned by changing printing speed or switching on/off the outermost bioink channel, thereby facilitating the transplant of the highly heterogeneous vasculature at different anatomical sites. Incorporation of PEGTA could enhance the mechanical properties of the printed constructs, and support cell spread as well as migration, forming an integrated multilayered hollow fibers. After 21 days of culture, the expression of CD31 and αSMA could be clearly detected, indicating the development of early-stage vessel. However, the decreased compressive moduli of the constructs were observed after 21 days of culture, implying these constructs may collapse and lose perfusability after 21 days. Gao and colleagues [Citation116] fabricated a multicellular, multilayer and multilevel fluidic channel structure. In their experiment, partially gelled hollow filaments of cell-populated alginate, which were created by coaxial nozzle system, were weaved around a rotary rod moving in the x-axial direction, and the ungelled parts on the outside of the adjacent filaments then progressively cross-linked with each other, followed by the second-layer deposition. Afterward, the rod was removed away, leaving a three-layer conduit consisting of an inner macrochannel for mechanical support and two outer microchannels for nutrient perfusion. The printed cells maintained high viability and homogeneous distribution in different layers. Applying a coaxial nozzle system, Li and colleagues [Citation117] constructed a branched microchannel by cross-linking the peripherally ungelled part of adjacent hollow alginate fibers (D). Although great progresses have been achieved with 3D bioprinting of vascularized constructs, challenge of high-resolution anatomically accurate vascular network at clinically-relevant scale ranges still remains [Citation118].
(A) Schematic diagram showing the formation of an open channel in fibrin hydrogel, where the printed glass filaments were used as a sacrificial template. Endothelial cells could adhere to the open channel wall and sprout (as indicated by arrowheads) from patterned channels. (B) An image of bioprinted vascularized tissues containing interpenetrated sacrificial bioinks (red) and cell bioinks (green). The tubes achieved endothelialization by human umbilical vein endothelial cells (red) and supported human neonatal dermal fibroblasts (green) viability at 45 days. (C) An image of coaxial nozzles. The multilayer hollow tubes with varied diameters could be fabricated by coaxial system and fulfilled by human umbilical vein endothelial cells (green). (D) A Y-shaped tube bioprinted with fibroblasts. A fluorescence photograph showing a large amount of fibroblasts (green) in the tube on day 6 after printing.
(A) Reproduced with permission from [Citation113] © Nature Publishing Group (2012).
(B) Reproduced with permission from [Citation114] © PNAS (2016).
(C) Reproduced with permission from [Citation115] © Elsevier Ltd (2016).
(D) Reproduced with permission from [Citation117] © American Institute of Physics (2016).
![Figure 4. Vascular networks constructed by 3D bioprinting. (A) Schematic diagram showing the formation of an open channel in fibrin hydrogel, where the printed glass filaments were used as a sacrificial template. Endothelial cells could adhere to the open channel wall and sprout (as indicated by arrowheads) from patterned channels. (B) An image of bioprinted vascularized tissues containing interpenetrated sacrificial bioinks (red) and cell bioinks (green). The tubes achieved endothelialization by human umbilical vein endothelial cells (red) and supported human neonatal dermal fibroblasts (green) viability at 45 days. (C) An image of coaxial nozzles. The multilayer hollow tubes with varied diameters could be fabricated by coaxial system and fulfilled by human umbilical vein endothelial cells (green). (D) A Y-shaped tube bioprinted with fibroblasts. A fluorescence photograph showing a large amount of fibroblasts (green) in the tube on day 6 after printing. (A) Reproduced with permission from [Citation113] © Nature Publishing Group (2012). (B) Reproduced with permission from [Citation114] © PNAS (2016). (C) Reproduced with permission from [Citation115] © Elsevier Ltd (2016). (D) Reproduced with permission from [Citation117] © American Institute of Physics (2016).](/cms/asset/6cc40a11-3f1f-4c56-966b-084956adbbba/idpm_a_12339811_f0005.jpg)
Heart
Heart disease is a rampant global burden [Citation119]. Nowadays, the application of bioprinting technology has brought hope to repair damaged myocardium. In an early research performed by Gaetani et al. [Citation93], hCMPCs, a promising cell source with differentiation ability toward cardiac lineage, were incorporated into alginate bioink and subsequently printed into a 3D lattice-shaped structure. The precise and instructive microenvironment supported cell proliferation and increased the expression of early cardiac transcription factors Nkx2.5, GATA-4 and Mef-2c, as well as late cardiac marker TnT. After placing the printed construct on the top of a matrigel layer, hCMPCs migrated to the matrigel layer and formed tube-like structure. However, hCMPCs did not display full cardiac differentiation as indicated by the lack of nicely organized striated phenotype of cardiomyocytes. They further printed a gelatin/HA-based patch harboring hCMPCs [Citation120]. In a mouse model, the hCMPCs in the porous matrix remained high viability and secreted factors, reducing left ventricular remodeling and improving cardiac performance. The hCMPCs showed cardiomyogenic as well as vascular differentiation when exposed to cardiac contractile electrostimulation and biological factors in vivo, suggesting this circumstance can initiate the maturation and function of the bioprinted tissues. Recently, Jang and colleagues [Citation121] printed a prevascularized patch utilizing dECM bioinks (A). During the printing process, dECM bioink I containing cardiac progenitor cells was extruded on a PCL-supporting layer, followed by alternative deposition of dECM bioink II encapsulating MSCs and VEGF. By patterning the dual stem cells mentioned above, improved cardiac functions and reduced cardiac fibrosis were observed, companying by neomuscle as well as capillary formation. This research highlights the need for future study of reasonable arrangement of different cells and their surrounding environment in a complex structures created by biopringting.
(A) Optical image of bioprinted prevascularized cardiac patch after implantation. The two type of stem cells were alternatively patterned in a 3D grid structure. (B) A neural scaffold consisted of 5% w/v alginate, 5% w/v carboxymethylchitosan and 1.5%agarose. The TUJ1 signal of bioprinted cells (21-day differentiation) implied persistent cell viability, with neuronal cell clusters interconnected by neurites (blue: nucleus; red: TUJ1). (C) A hybrid bioink containing gelatin, alginate and fibrinogen was used to fabricate skin constructs. The top image showed 200 μm wide lines printed by this bioink. Pink arrows indicated motion paths of the printing nozzle. Masson's Trichrome staining of bioprinted skin constructs, which recapitulated three typical layers of normal human skin. (D) An image of a composite vertebrae model that was bioprinted by alternative deposition of PCL filaments (white) and cell-populated bioink (blue). Macroscopic image of anatomically shaped vertebrae constructs after 12 weeks of implantation. (E) An immunofluorescence image showing hiPSC-HPC (green) and supporting cells (red) arranged in a hexagona hepatic model. The hiPSC-HPC aggregates positively expressed albumin (red) and E-cadherin (green) on day 7 postprinting, demonstrating active proliferation and metabolic function (blue: nucleus).
(A) Reproduced with permission from [Citation121] © Elsevier Ltd. (2017).
(B) Reproduced with permission from [Citation122] © John Wiley and Sons Inc. (2016).
(C) Reproduced with permission from [Citation123] © John Wiley and Sons Inc. (2017).
(D) Reproduced with permission from [Citation124] © John Wiley and Sons Inc. (2016).
(E) Reproduced with permission from [Citation125] © PNAS (2016).
CPC: Cardiac progenitor cell; hiPSC: Human induced pluripotent stem cells; HPC: Hepatic progenitor cell; MSC: Mesenchymal stem cell; PCL: Poly(ε-caprolactone).
![Figure 5. Heart, neural network, skin, bone, and liver fabricated by 3D bioprinting. (A) Optical image of bioprinted prevascularized cardiac patch after implantation. The two type of stem cells were alternatively patterned in a 3D grid structure. (B) A neural scaffold consisted of 5% w/v alginate, 5% w/v carboxymethylchitosan and 1.5%agarose. The TUJ1 signal of bioprinted cells (21-day differentiation) implied persistent cell viability, with neuronal cell clusters interconnected by neurites (blue: nucleus; red: TUJ1). (C) A hybrid bioink containing gelatin, alginate and fibrinogen was used to fabricate skin constructs. The top image showed 200 μm wide lines printed by this bioink. Pink arrows indicated motion paths of the printing nozzle. Masson's Trichrome staining of bioprinted skin constructs, which recapitulated three typical layers of normal human skin. (D) An image of a composite vertebrae model that was bioprinted by alternative deposition of PCL filaments (white) and cell-populated bioink (blue). Macroscopic image of anatomically shaped vertebrae constructs after 12 weeks of implantation. (E) An immunofluorescence image showing hiPSC-HPC (green) and supporting cells (red) arranged in a hexagona hepatic model. The hiPSC-HPC aggregates positively expressed albumin (red) and E-cadherin (green) on day 7 postprinting, demonstrating active proliferation and metabolic function (blue: nucleus). (A) Reproduced with permission from [Citation121] © Elsevier Ltd. (2017). (B) Reproduced with permission from [Citation122] © John Wiley and Sons Inc. (2016). (C) Reproduced with permission from [Citation123] © John Wiley and Sons Inc. (2017). (D) Reproduced with permission from [Citation124] © John Wiley and Sons Inc. (2016). (E) Reproduced with permission from [Citation125] © PNAS (2016).CPC: Cardiac progenitor cell; hiPSC: Human induced pluripotent stem cells; HPC: Hepatic progenitor cell; MSC: Mesenchymal stem cell; PCL: Poly(ε-caprolactone).](/cms/asset/b2f998b9-9349-4052-875a-ae62d75ec505/idpm_a_12339811_f0006.jpg)
3D bioprinting technology has been used to recapitulate anatomical and mechanical diversity between roots and leaflets of heart valves. In an early research performed by Duan's group [Citation43], the geometries of 3D porcine aortic valve were first scanned by micro-CT, and then the architectural information was imported into a bioprinting system. Afterward, SMC-laden root component and valve interstitial cells (VIC)-laden leaflet component were alternately deposited in a regionally constrained manner (F). After 7 days of culture, SMC distributed in stiff matrix showed increased αSMA level, while VIC located in soft matrix showed increased vimentin level, indicating a fibroblastic phenotype of VIC. However, the printed VIC also expressed αSMA level, which was considered as a biomarker of valve-disease-related myofibroblast phenotype of VIC [Citation126]. In another research, Duan and colleagues [Citation77] mixed MeHA and methacrylated gelatin at different ratios to provide better mechanical and biological cues for VIC proliferation, spreading, as well as maintaining VIC fibroblastic phenotype. After 7 days of culture, VIC within the bioprinted valve leaflets kept over 90% cell viability and actively remodeled the ECM. Moreover, with the decrease in the stiffness of the hydrogel and increase in the ratio of methacrylated gelatin, VIC presented a better fibroblastic phenotype. Even with the current progress, bioprinting of biomechanically and structurally anisotropic heart tissues still needs extensive work [Citation127,Citation128].
Neural network
3D bioprinting technology paves the way to nerve tissue engineering. In an early research, Owens and colleagues [Citation129] used cellular and agarose cylinders with 0.5-mm diameter to print a nerve graft, on which Schwann cell cylinders were surrounded by mouse bone marrow stem cell cylinders [Citation26]. Removal of agarose cylinders resulted in a tubular structure with longitudinal parallel channels. The 3D-bioprinted nerve graft showed an inspiring nerve repair effect, which was comparable to the standard clinical methods applying autologous grafts or collagen tubes. Recently, Lozano and colleagues [Citation130] used a bioink containing a novel gellan gum modified with RGD (RGD-GG) to print a biomimetic-multilayered brain construct, in which primary neural cells were encapsulated in discrete layers. After in vitro culture, the printed cells with high viability formed neuronal networks with axons extending into the neighboring layer, suggesting the feasibility of RGD-GG-based bioprinting in the brain and nerve tissue engineering. Most recently, Gu and colleagues [Citation122] reported bioprinting of 3D neural minitissues with a bioink consisting of polysaccharides alginate/carboxymethylchitosan/agarose and frontal cortical human neural stem cells (hNSCs; B). The composition ratio of the hybrid materials was optimized to not only have an appropriate solution viscosity and gelling behaviors for satisfactory printability but also provide a benign porous and mechanical support for the cell growth and function. The printed hNSCs showed promising differentiation potential into GABAergic neurons, together with glial cells expressing astrocyte and oligodendrocyte lineage markers. When bicuculline was added, hNSCs presented a calcium response. Based on the previous research, the subsequent effort will likely entail the optimization of graft geometry and cellular composition.
Skin
There are some reports on applications of 3D bioprinting technology for skin regeneration. Michael et al. [Citation131,Citation132] deposited 20 layers of NIH3T3 fibroblasts-laden collagen on the top of a stabilizing matrix (Matriderm®) via laser-assisted bioprinting method, and subsequently deposited 20 layers of HaCaT keratinocytes-laden collagen on the top of it. The delivery of this bilayered multicellular constructs into full-thickness skin wounds in mice led to collagen synthesis of fibroblasts, vessel formation and keratinocyte differentiation. Pourchet and colleagues [Citation123] developed a strategy to fabricate biomimetic skin substitutes (C). First, they printed the dermis component with normal human dermal fibroblasts embedded in alginate/fibrinogen matrix. After the maturation of the dermis component, the normal human epidermal keratinocytes were seeded on the dermis component, followed by in vitro culture to generate skin constructs. The bioprinted skin constructs showed typical layers of native epidermis and expressed biomarkers related to skin development, such as cytokeratin 10, filaggrin, loricrin, collagen I, collagen V, as well as laminin 332. Cubo and colleagues [Citation133] applied a printing system equipped with four syringes to cooperatively extrude several bioinks including human fibroblasts, human plasma, CaCl2, as well as human keratinocytes, forming a bilayered dermoepidermal equivalents (∼100 cm2 of the size) within 35 min. This fast and convenient method demonstrated the possibility of producing skin substitutes both with clinically relevant dimensions and high cell viability. In another study, Huang et al. [Citation134] incorporated EGF and dermal homogenates (homogenates from mouse plantar dermis or from dorsal basal dermis) into a bioprinted 3D ECM mimic for regenerating sweat glands. The highly porous structures are beneficial to the proliferation of the printed epidermal progenitors and matrix formation. Compared with the dorsal basal dermis-based ECM mimic, transplantation of the mouse plantar dermis-based ECM mimic enabled a fast functional recovery of the sweat glands in mice after 14 days. This result clearly demonstrated that the ECM mimic created by bioprinting provided physiologically relevant cues for the gland-specific differentiation of epidermal progenitors originated from dorsal basal epidermis. Skardal et al. [Citation38] conducted skin regeneration through direct bioprinting of a hydrogel into defect sites. The morphology and geometry of full-thickness skin wounds in nu/nu mice were acquired and then amniotic fluid-derived stem cell-laden fibrin/collagen precursor was precisely deposited inside the wound site (2.0 × 2.0 cm). After 2 weeks, satisfactory wound closure rates, re-epithelialization and angiogenesis were observed, thanks to not only flexible deposition of various bioinks by multiple-dispensing modules but also biological factors secreted by amniotic fluid-derived stem cells directly exposed to the natural pathological environment [Citation135]. Although significant success has been achieved, much effort has to be paid to explore and evaluate the clinic application of in situ bioprinting for skin repair.
Bone & cartilage
Like in other tissue or organ engineering, 3D bioprinting has emerged as a very useful technology in bone and cartilage regeneration. Daly and colleagues [Citation124] irradiated alginate modified with RGD (RGD-γ alginate) with gamma ray, and found it supported cell invasion and ECM remodeling, resulting in increased chondrogenesis level of MSCs in vitro and improved endochondral bone formation in vivo. They then moved toward coprinting hMSCs-laden RGD-γ alginate along with PCL microfibers as a mechanical support to fabricate a vertebrae-shaped mechanically reinforced cartilaginous template (D). Delivery of this printed construct into mice led to the formation of vascularized bone organ characteristic of trabecular-like endochondral bone with a supporting marrow structure. Recently, a research group led by Abbadessa and colleagues [Citation136] carried out cartilage bioprinting by employing both temperature- and UV photosensitive cross-linking approaches based on hybrid bioinks composed of PEG triblock copolymers and MeHA. Compared with the PEG triblock copolymers alone, incorporation of MeHA into the bioink could significantly improve the printability, mechanical strength and cell viability, leading to the successful formation of a cartilage-like tissue. In another work, Xu’ s group [Citation137] fabricated a 1-mm thick, five-layered cartilage-like tissue by integrating the advantages of electrospun nanofiber layers for enhanced mechanical properties and fibrin-collagen matrices for good biocompatibility. Chondrogenesis was observed, as indicated by the deposition of type II collagen and glycosaminoglycans (GAG)s. In order to mimic the native nanofibrous matrix constitution of medial knee meniscus in vivo, Narayanan and colleagues [Citation138] mixed alginate bioink encapsulating with human adipose-derived stem cells with short polylactic acid nanofiber, and found that the addition of polylactic acid nanofibers could improve the surface topography and mechanical strength of the bioprinted meniscus-type constructs, facilitating cell proliferation and chondrogenic differentiation. Rhee and colleagues [Citation139] demonstrated the feasibility of bioprinting multidomain meniscus constructs using various collagen concentrations. The bioprinted meniscus constructs showed geometric and mechanical anisotropy, which were able to support and maintain fibrochondrocyte viability.
There is an urgent demand for the osteochondral tissue repair. To realize 3D bioprinting of the cartilage part and bone part of osteochondral tissue, Park and colleagues used HA, an important constituent of the cartilage ECM, and Collagen-1 which is dispersed in native bone tissue ECM, to encapsulate chondrocytes and osteoblasts, respectively. They demonstrated the specific biomimetic ECM contributed to the viability and function of each cell type in bioprinted osteochondral tissue models [Citation34]. Levato and colleagues tried to apply the microcarrier technology in 3D bioprinting of osteochondral tissue. The microcarriers enabled the high MSCs density and viability during printing. Moreover, the microcarriers encapsulation increased construct stiffness, improved osteogenic differentiation and bone matrix production by MSCs [Citation140]. A new active mixing printhead, which allows rapid mixing of complex viscoelastic materials at the microscale, has been developed as a flexible tool in local compositions and properties control of materials with potential in bioprinting gradient architecture of cartilage and osteochondral tissues [Citation141]. Applying a multihead tissue/organ-building system, Shim and colleagues [Citation142] used two kinds of bioinks to fabricate a multilayered and cell-rich construct for osteochondral tissue regeneration. The atelocollagen-based bioink with osteoinductive activity was printed onto a support framework as a subchondral bone layer, followed by deposition of the HA-based bioink that possessed chondrogenic induction activity as a superficial cartilage layer. Following transplantation, chondrogenesis in the printed construct was observed indicated by typical morphology of chondrocytes within lacuna and increased collagen-II expression. In the meantime, atelocollagen showed the ability to support the formation of neobone tissue because of its appropriate degradation rate.
Despite huge effort has been made and significant success has been achieved with regards to the bioinks for bone repair, the mechanically enhanced, biocompatible bioinks are urgently needed to engineer hard tissues such as bone and cartilage with functional as well as mechanical similarity to that of human native tissues [Citation143].
Other tissues
3D bioprinting technology can also find applications in liver and ear injury repair and regeneration. Ma and colleagues [Citation125] used digital light-processing technique to fabricate 3D hepatic model-encapsulating human induced pluripotent stem cells-derived hepatic progenitor cells (hiPSC-HPCs), HUVECs and adipose-derived stem cells (E). The three types of cells embedded within hybrid photopolymerizable HA (glycidal methacrylate-HA [GMHA]) and GelMA were arranged in hexagonal pattern, which resembled the morphology of human liver lobule unit. Compared with the 2D monolayer culture or the 3D-HPC-only model, hiPSC-HPCs in the 3D hepatic model showed significant cell communication, elevated liver-specific gene expression, enhanced albumin secretion as well as urea synthesis and increased CYP450 production. Lee and colleagues [Citation144] presented a hybrid construct by alternating parallel patterned PCL lines with cell-laden collagen lines to target liver tissue regeneration in the assistance of multihead tissue/organ-building system. PCL framework prevented the deformation of the formed construct. Hepatocytes, HUVECs and human lung fibroblasts were coencapsulated within the collagen gel. The cell–cell interactions promoted angiogenesis, and facilitated albumin secretion and urea production of hepatocytes.
Lee and colleagues [Citation145] employed a sacrificial layer to build an ear-shaped construct with both auricular cartilage and fat tissue by 3DP. Chondrocytes- and adipocytes- laden alginate bioinks were spatially heterogeneously printed in specific regions and showed mutually independent chondrogenesis and adipogenesis, respectively.
Nowadays, none of the current methods for 3D printing follow natural mechanisms of wound healing or biological development. Recently, Laronda et al. [Citation146] 3D printed a bioprosthetic ovary with hydrogel, and then seeded with follicle. Follicle-seeded scaffolds become highly vascularized and ovarian function is fully restored when implanted in surgically sterilized mice. Moreover, pups are born through the natural mating and thrive through maternal lactation. These results indicated clearly that with the rapid advance of the field, the differences in the properties and performance between the engineered tissues and natural tissues become much smaller than ever before. With the rapid development of bioprinting materials and instrumentation, we believe, 3D bioprinting technology has great potential in the precise and reproducible engineering of fully developed tissues/organs mentioned above, and beyond the ones discussed above.
Conclusion & outlook
Cell-laden 3D bioprinting as a novel transforming approach in the field of tissue engineering has been extensively studied in the past years due to its easy, quick and accurate fabrication of bioactive scaffolds with defined shapes and accurate cell distributions, offering promising alternatives to future donor organ transplantation. However, construction of more complex and composite tissue and organ structures with biological functions similar to that of the native ones is still difficult. So far, there is no report on successful clinical applications of 3D-bioprinted bioactive organs. Several major challenges, we think, still face the field and need to be solved before moving from fundamental research to clinical applications:
First, the development of suitable printing materials is still at the initial stage. In a sense, the materials determine the future applications of 3D bioprinting technique in tissue engineering and regenerative medicine. To be used in 3D bioprinting, the cell-laden hydrogels should maintain physiological tonicity in water, as well as in salt solution, obtaining the optimal cell adhesion to both natural polymers and synthetic polymers. Consequently, novel bioprinting materials with good biocompatibility, biodegradability, biological responsiveness and mechanical performance need to be further designed and synthesized for emulating the complexity and functions of natural extracellular matrices, and matching specific tissues or organs.
Second, the inability of most current 3D bioprinting instrumentation to fabricate complex 3D tissue and organs with precisely and flexibly controlled cell distributions, vascularization and innervation creates another major obstacle, since most existing printing systems can only achieve bioprinting with a single bioink during each deposition process. Moreover, resolution of the 3D bioprinting system is a crucial factor to create complex tissue and organ structures with full biological functions. To reach the goal, it is highly desired to develop multifunctional, smart and integrated 3D bioprinting technology and instrumentation.
Third, to regenerate large-volume tissue and organ structures to be used in clinical practice in the future, transportation of oxygen and nutrients inside 3D structures is a must for the fabricated vascularized 3D tissue and organ structures. Without a vascular structure in the printed 3D tissue and organs, the printed cells cannot survive to regenerate tissue and organs after implantation. Several studies have demonstrated the ability of bioprinting vascular structures. However, incorporating vascular structures into tissue and organ structures has not yet been achieved. Moreover, for the cultivation of complex organ encapsulated with multiple cells, development of culture media used for multiple cell types is also highly desired.
Next, the cell–cell interactions and cell-material interactions during the rapid, tough printing process, especially the cell adhesion, growth and differentiation within the 3D bioprinting scaffold, need to be clarified and well understood. In this context, more efforts should be paid to study and understand the biological events in vitro and in vivo within the printed constructs. For example, the acoustic biopinting, causing low thermal and mechanical stresses on cells, is an effective and biocompatible printing method for patterning single cells or coculturing of cells, which help us to investigate organ development process based on pure RNA or DNA from single cells in vitro [Citation147,Citation148]. Moreover, development of in situ, sensitive and long-term tracking technology to monitor, and evaluate the viability, migration, proliferation and differentiation of the implanted stem cells in vivo is of great significance.
The last but not the least, in order to ensure clinical applications in tissue engineering and regenerative medicine, the bioinks and the bioprinted products should be standardized and meet the governmental regulations, like any other implantable medical devices currently used in clinic. The American Society for Testing and Materials has addressed the standardization of ‘Characterization and Testing of Biomaterial Scaffolds Used in Tissue-Engineered Medical Products’, which provides a useful guidance for biomaterials. As for the 3D bioprinting, the production of printing equipment, the manufacture of biomaterials, biologic factors and cells, the design of 3DP model, the testing of 3D products and the evaluation of implantation process should be conducted with quality control. Recently, the US FDA issued a draft ‘Technical Considerations for Additive Manufactured Devices’, which provides guidance for 3DP manufacturers. In addition, ethical concerns have to be considered for tissue or organs implantation in humans.
3D bioprinting has been developed rapidly, yet still in its infancy. The field faces many challenges with regard to the printing materials, instrumentation and governmental regulations, to name a few, largely limits its clinical applications. With the joint efforts from materials scientists, engineers, biomedical researchers and doctors, we believe, this new additive manufacturing technique will become an increasingly viable strategy in tissue engineering and regenerative medicine, and, will find wide applications in clinic in the near future.
Future perspective
Over the next 5–10 years, more and more new bioprinting methods and materials will be developed, and will provide us more facile printing processes and complex printed constructs with high spatial resolution, tunable mechanical property as well as biocompatibility. Meanwhile, a large-scale and automated system for printed cell culture, amplification and function acquisition in vitro will be established. Additionally, these new advances will expand and deepen our understanding of the molecular mechanism of stem cell origin, differentiation, proliferation and maintenance in the body. Definitely, more efforts will be paid for elucidating the mechanisms of the interactions between adult tissue microenvironment and the printed cells. By integration of the above development of equipment, biomaterials and biology, 3D bioprinting technologies will enable us to achieve more functional organoids for tissue transplantation, drug discovery and other biomedical applications.
Cell-laden 3D bioprinting
The main characteristics of cell-laden 3D bioprinting technology include the use of biocompatible and biodegradable biomaterials, and the mild gelling process good for maintaining the cell survival and function.
The distinct advantage of cell-laden 3D bioprinting technology is its ability to simultaneously deposit live cells, growth factors along with biomaterial scaffolds in a precisely controlled way to mimic the native tissue architecture and functions.
The hydrogel scaffolds used in cell-laden 3D bioprinting should meet several basic requirements: good cytocompatible, fast cross-linking speed, excellent mechanical performance and easy postprocessing.
Materials for cell-laden 3D bioprinting
Discussion of proper hydrogels and their applications.
Natural polymers such as alginate, collagen, fibrin, gelatin and hyaluronic acid are usually used for cell-laden 3D bioprinting due to their cell-friendliness, similarity to human extracellular matrix and bioactivity. However, poor mechanical performance and processibility are their shortcomes.
Synthetic macromolecules such as pluronic and poly(ethylene glycol) have the advantages of controllable physiochemical properties, but lack the necessary signals for cells to reorganize them to generate functioning tissues.
Strategies for cell-laden 3D bioprinting
Five major types of gelling methods and their applications are discussed.
Temperature-controlled gelling approach is mild for cell survival during and after printing, and is able to control the gelling speed by changing the temperature. But the hydrogels used in this approach usually exhibit poor mechanical properties.
Laser-assisted gelling technique is fast and inexpensive with high resolution. However UV radiation will cause the damage to the cells printed.
Enzymatic cross-linking method is safe, and often used for protein-based hydrogel printing. But the hydrogels used for enzymatic cross-linking method are soft and fragile, and thus limited their applications.
The ionic cross-linking method is inexpensive and widely used in 3D bioprinting. However, it is not suitable to print hard tissue.
The covalent cross-linking method is very useful in 3D bioprinting, but limited by the printing equipment. This approach, it is expected, may find more applications in the future with the development of printable bioinks and 3D bioprinters.
Applications of cell-laden 3D bioprinting
Introduction to some typical tissues and organs fabricated by cell-laden 3D bioprinting.
Many types of vascularized constructs have been fabricated with 3D bioprinting. However, challenge of high-resolution anatomically accurate vascular network at clinically relevant scale ranges still remains.
The bioprinted instructive microenvironments improve cardiac progenitor cells proliferation and differentiation, resulting better cardiac functions. However, bioprinting of microenvironments, which recapitulate the anatomical and mechanical diversity between roots and leaflets of heart valves, still needs extensive work.
The biomimetic tubular structures and multilayered constructs were bioprinted to induce the neural network formation and cell function acquisition.
The skin appendages regeneration such as sweat gland repair should be taken into consideration in 3D bioprinting of skin constructs. 3D bioprinting technology paves the way for in situ bioprinting of skin.
Gradient biomaterials could be engineered by 3D bioprinting technology to approximate the zone architecture of cartilage and osteochondral tissues. The hard tissues regeneration is still an unsolved challenge due to the lack of bioprinting materials with proper mechanical properties.
The 3D bioprinting technology enables us to orderly arrange the interaction of multiple types of cells in biomimetic microarchitecture such as microscale hexagonal architecture similar to hexagonal lobule unit of liver to facilitate tissue engineering.
Conclusion & outlook
Novel bioprinting materials need to be further designed and synthesized.
Multifunctional, smart and integrated 3D bioprinting technology and instrumentation should be developed.
Constructing large-volume tissue and organ with vascular structure is good for the transportation of oxygen and nutrients.
Developing in vitro and in vivo tracking method can understand the behavior of cells in 3D bioprinting scaffolds.
Bioinks, bioprinted products and printing equipment should be standardized and meet the governmental regulations.
Financial & competing interests disclosure
This work was sponsored by Key Research Program of the Chinese Academy of Sciences (No. ZDRW-ZS-2016–2–3), and the Ministry of Science and Technology of China (Nos. 2017YFA0104301 and 2016YFC1000809). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/full/10.2217/3dp-2017-0010
References
- Anonymous . Organ donation depends on trust. Lancet387(10038), 2575 (2016).
- Ozbolat IT , YuY. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans. Biomed. Eng.60(3), 691–699 (2013).
- Langer R , VacantiJP. Tissue engineering. Science260(5110), 920–926 (1993).
- Hull CW . Apparatus for production of three-dimensional objects by stereolithography. US 4575330 A ( Google Patents) (1986).
- Ferris CJ , GilmoreKG, WallaceGGet al. Biofabrication: an overview of the approaches used for printing of living cells. Appl. Microbiol. Biotechnol.97(10), 4243–4258 (2013).
- Jones N . Three dimensional printers are opening up new worlds to research. Nature487(7405), 22–23 (2012).
- Anitha R , ArunachalamS, RadhakrishnanP. Critical parameters influencing the quality of prototypes in fused deposition modelling. J. Mater. Process. Tech.118(1–3), 385–388 (2001).
- Tay BY , EvansJRG, EdirisingheMJ. Solid freeform fabrication of ceramics. Int. Mater. Rev.48(6), 341–370 (2003).
- Hornbeck LJ . Digital light processing for high-brightness high-resolution applications. Proc. SPIE.3013, 27–40 (1997).
- Utela B , StortiD, AndersonRet al. A review of process development steps for new material systems in three dimensional printing (3DP). J. Manuf. Process.10(2), 96–104 (2008).
- Mueller B , KochanD. Laminated object manufacturing for rapid tooling and pattern making in foundry industry. Comput. Ind.39(1), 47–53 (1999).
- Singh R . Process capability study of polyjet printing for plastic components. J. Mech. Sci. Technol.25(4), 1011–1015 (2011).
- Klein GT , LuY, WangMY. 3D printing and neurosurgery-ready for prime time. World Neurosurg.80(3–4), 233–235 (2013).
- Zopf DA , HollisterSJ, NelsonMEet al. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med.368(21), 2043–2045 (2013).
- Rengier F , MehndirattaA, von Tengg-KobligkHet al. 3D printing based on imaging data: review of medical applications. Int. J. Comput. Assist. Radiol. Surg.5(4), 335–341 (2010).
- Billiet T , VandenhauteM, SchelfhoutJet al. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials.33(26), 6020–6041 (2012).
- Kievit FM , FlorczykSJ, LeungMCet al. Proliferation and enrichment of CD133+ glioblastoma cancer stem cells on 3D chitosan-alginate scaffolds. Biomaterials.35(33), 9137–9143 (2014).
- Khademhosseini A , LangerR, BorensteinJet al. Microscale technologies for tissue engineering and biology. Proc. Natl Acad. Sci. USA103(8), 2480–2487 (2006).
- Hockaday LA , KangKH, ColangeloNWet al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication.4(3), 035005 (2012).
- Seyednejad H , GawlittaD, KuiperRVet al. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(ε-caprolactone). Biomaterials33(17), 4309–4318 (2012).
- Seliktar D . Designing cell-compatible hydrogels for biomedical applications. Science336(6085), 1124–1128 (2012).
- Murphy SV , AtalaA. 3D bioprinting of tissues and organs. Nat. Biotechnol.32(8), 773–785 (2014).
- Drury JL , MooneyDJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials24(24), 4337–4351 (2003).
- Malda J , VisserJ, MelchelsFPet al. 25th Anniversary article: engineering hydrogels for biofabrication. Adv. Mater.25(36), 5011–5028 (2013).
- Murphy SV , SkardalA, AtalaA. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. A.101(1), 272–284 (2013).
- Mandrycky C , WangZJ, KimKet al. 3D bioprinting for engineering complex tissues. Biotechnol. Adv.34(4), 422–434 (2016).
- O'Connell G , GarciaJ, AmirJ. 3D bioprinting: new directions in articular cartilage tissue engineering. ACS Biomater. Sci. Eng. doi:10.1021/acsbiomaterials.6b00587 (2017) ( Epub ahead of print).
- Rowley JA , MadlambayanG, MooneyDJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials20(1), 45–53 (1999).
- Kuo CK , MaPX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials22(6), 511–521 (2001).
- Lee KY , MooneyDJ. Alginate: properties and biomedical applications. Progr. Polym. Sci.37(1), 106–126 (2012).
- Hou JX , LiC, GuanYet al. Enzymatically crosslinked alginate hydrogels with improved adhesion properties. Polym. Chem.6(12), 2204–2213 (2015).
- Cohen DL , LoW, TsavarisAet al. Increased mixing improves hydrogel homogeneity and quality of three-dimensional printed constructs. Tissue Eng. Part C Methods.17(2), 239–248 (2011).
- Ferreira AM , GentileP, ChionoVet al. Collagen for bone tissue regeneration. Acta Biomater.8(9), 3191–3200 (2012).
- Park JY , ChoiJC, ShimJHet al. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication6(3), 035004 (2014).
- Lynn AK , YannasIV, BonfieldW. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. B. Appl. Biomater.71B(2), 343–354 (2004).
- Smith CM , StoneAL, ParkhillRLet al. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng.10(9–10), 1566–1576 (2004).
- Lee VK , LanziAM, NgoHet al. Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell. Mol. Bioeng.7(3), 460–472 (2014).
- Skardal A , MackD, KapetanovisEet al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med.1(11), 792–802 (2012).
- Gómez-Guillén MC , GimenezB, Lopez-CaballeroMEet al. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocolloid25(8), 1813–1827 (2011).
- Bertassoni LE , CardodoJC, ManoharanVet al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication6(2), 024105 (2014).
- Billiet T , GevaertE, De SchryverTet al. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials35(1), 49–62 (2014).
- Lee W , LeeV, PolioSet al. On-demand three-dimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnol. Bioeng.105(6), 1178–1186 (2010).
- Duan B , HockadayLA, KangKHet al. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A101(5), 1255–1264 (2013).
- Huang J , FuH, WangZet al. BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel for 3D bioprinting. RSC Adv.6(110), 108423–108430 (2016).
- Gruene M , PflaumM, HessCet al. Laser printing of three-dimensional multicellular arrays for studies of cell–cell and cell–environment interactions. Tissue Eng. Part C Methods17(10), 973–982 (2011).
- Mosesson MW , SiebenlistKR, MehDA. The structure and biological features of fibrinogen and fibrin. Ann. NY Acad. Sci.936, 11–30 (2001).
- Godal HC , AbildgaardU. Gelation of soluble fibrin in plasma by ethanol. Scand. J. Haematol.3(5), 342–346 (1966).
- Kupcsik L , AliniM, StoodartMJ. Epsilon-aminocaproic acid is a useful fibrin degradation inhibitor for cartilage tissue engineering. Tissue Eng. Part A.15(8), 2309–2313 (2009).
- Collins MN , BirkinshawC. Hyaluronic acid based scaffolds for tissue engineering – a review. Carbohyd. Polym.92(2), 1262–1279 (2013).
- Pescosolido L , SchuurmanW, MaldaJet al. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules12(5), 1831–1838 (2011).
- Kabanov AV , BatrakovaEV, AlakhovVY. Pluronic (R) block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Rel.82(2–3), 189–212 (2002).
- Vadnere M , AmidonG, LindenbaumSet al. Thermodynamic studies on the gel sol transition of some pluronic polyols. Int. J. Pharm.22(2–3), 207–218 (1984).
- Wu W , DeConinckA, LewisJA. Omnidirectional printing of 3D microvascular networks. Adv. Mater.23(24), H178–H183 (2011).
- Mueller M , BecherJ, SchnabelrauchMet al. Nanostructured pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication7(3), 035006 (2015).
- Lin C , AnsethKS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res.26(3), 631–643 (2009).
- Mann BK , GobinAS, TsaiATet al. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: Synthetic ECM analogs for tissue engineering. Biomaterials22(22), 3045–3051 (2001).
- Cui X , BreitenkampK, FinnMGet al. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A.18(11–12), 1304–1312 (2012).
- Gao G , YonezawaT, HubbellKet al. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J.10(10), 1568–1577 (2015).
- Zorlutuna P , VranaNE, KhademhosseiniA. The expanding world of tissue engineering: the building blocks and new applications of tissue engineered constructs. IEEE Rev. Biomed. Eng.6, 47–62 (2013).
- Zamir E , GeigerB. Molecular complexity and dynamics of cell–matrix adhesions. J. Cell Sci.114(20), 3583–3590 (2001).
- Gomes S , LeonorIB, ManoJFet al. Natural and genetically engineered proteins for tissue engineering. Prog. Polymer Sci.37(1), 1–17 (2012).
- McAllister TN , DusserreN, MaruszewskiMet al. Cell-based therapeutics from an economic perspective: primed for a commercial success or a research sinkhole? Regen. Med. 3(6), 925–937 (2008).
- Sionkowska A . Current research on the blends of natural and synthetic polymers as new biomaterials: review. Prog. Polymer Sci.36(9), 1254–1276 (2011).
- Patterson J , HubbellJA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials31(30), 7836–7845 (2010).
- Edlund U , DanmarkS, AlbertssonAC. A strategy for the covalent functionalization of resorbable polymers with heparin and osteoinductive growth factor. Biomacromolecules9(3), 901–905 (2008).
- Ulrich TA , JainA, TannerKet al. Probing cellular mechanobiology in three-dimensional culture with collagen–agarose matrices. Biomaterials31(7), 1875–1884 (2010).
- Köpf M , CamposDFD, BlaeserAet al. A tailored three-dimensionally printable agarose-collagen blend allows encapsulation, spreading, and attachment of human umbilical artery smooth muscle cells. Biofabrication8(2), 025011 (2016).
- Frisman I , SeliktarD, Bianco-PeledH. Nanostructuring PEG–fibrinogen hydrogels to control cellular morphogenesis. Biomaterials32(31), 7839–7846 (2011).
- Ehrbar M , SalaA, LienemannPet al. Elucidating the role of matrix stiffness in 3D cell migration and remodeling. Biophys. J.110(2), 284–293 (2011).
- Ahn S , LeeH, BonassarLJet al. Cells (MC3T3-E1)-laden alginate scaffolds fabricated by a modified solid-freeform fabrication process supplemented with an aerosol spraying. Biomacromolecules13(9), 2997–3003 (2012).
- Kolesky DB , TrubyRL, GladmanASet al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater.26(19), 3124–3130 (2014).
- Selimovic S , OhJ, BaeHet al. Microscale strategies for generating cell-encapsulating hydrogels. Polymers4(3), 1554–1579 (2012).
- Yang KK , GaoTT, BaoZXet al. Preparation and characterization of a novel thermosensitive nanoparticle for drug delivery in combined hyperthermia and chemotherapy. J. Mater. Chem. B.1(46), 6442–6448 (2013).
- Kang HW , LeeSJ, KoIKet al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol.34(3), 312–319 (2016).
- Lee H , HanW, KimHet al. Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules.18(4), 1229–1237 (2017).
- Du MC , ChenB, MengQYet al. 3D bioprinting of BMSC-laden methacrylamide gelatin scaffolds with CBD-BMP2-collagen microfibers. Biofabrication7(4), 044104 (2015).
- Duan B , KapetanovicE, HockadayLAet al. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater.10(5), 1836–1846 (2014).
- Melhem MR , ParkJ, KnappLet al. 3D printed stem-cell-laden, microchanneled hydrogel patch for the enhanced release of cell-secreting factors and treatment of myocardial infarctions. ACS Biomater. Sci. Eng.3(9), 1980–1987 (2017).
- Guillotin B , SouquetA, CatrosSet al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials31(28), 7250–7256 (2010).
- Ouyang L , HighleyCB, SunWet al. A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable inks. Adv. Mater.29(8), 1604983 (2017).
- Kang LH , ArmstrongPA, LeeLJet al. Optimizing photo-encapsulation viability of heart valve cell types in 3D printable composite hydrogels. Ann. Biomed. Eng.45(2), 360–377 (2017).
- Dahle J , KvamE, StokkeT. Bystander effects in UV-induced genomic instability: antioxidants inhibit delayed mutagenesis induced by ultraviolet A and B radiation. J. Carcinog.4, 11 (2005).
- Melchels FP , DhertWJ, HutmacherDWet al. Development and characterization of a new bioink for additive tissue manufacturing. J. Mater. Chem. B.2(16), 2282–2289 (2014).
- Studer K , DeckerC, BeckEet al. Overcoming oxygen inhibition in UV-curing of acrylate coatings by carbon dioxide inerting, part I. Prog. Org. Coat.48(1), 92–100 (2003).
- Decker C , JenkinsAD. Kinetic approach of oxygen inhibition in ultraviolet- and laser-induced polymerizations. Macromolecules18(6), 1241–1242 (1985).
- Lim KS , SchonBS, MekhileriNVet al. New visible-light photoinitiating system for improved print fidelity in gelatin-based bioinks. ACS Biomater. Sci. Eng.2(10), 1752–1762 (2016).
- Dai XL , MaC, LanQet al. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication8(4), 045005 (2016).
- Compaan AM , ChristensenK, HuangY. Inkjet bioprinting of 3D silk fibroin cellular constructs using sacrificial alginate. ACS Biomater. Sci. Eng.3(8), 1519–1526 (2017).
- Das S , PatiF, ChoiYJet al. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater.11, 233–246 (2015).
- Nakamura M , IwanagaS, HenmiCet al. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication2(1), 014110 (2010).
- Nunamaker EA , OttoKJ, KipkeDR. Investigation of the material properties of alginate for the development of hydrogel repair of dura mater. J. Mech. Behav. Biomed. Mater.4(1), 16–33 (2011).
- Shim JH , LeeJS, KimJYet al. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J. Micromech. Microeng.22(8), 085014 (2012).
- Gaetani R , DoevendansPA, MetzCHGet al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials33(6), 1782–1790 (2012).
- Augst AD , KongHJ, MooneyDJ. Alginate hydrogels as biomaterials. Macromol. Biosci.6(8), 623–633 (2006).
- Park J , LeeSJ, ChungSet al. Cell-laden 3D bioprinting hydrogel matrix depending on different compositions for soft tissue engineering: characterization and evaluation. Mater. Sci. Eng. C.71, 678–684 (2017).
- Shi PJ , LaudeA, YeongWY. Investigation of cell viability and morphology in 3D bio-printed alginate constructs with tunable stiffness. J. Biomed. Mater. Res. Part A.105(4), 1009–1018 (2017).
- Luo YX , LodeA, SonntagFet al. Well-ordered biphasic calcium phosphate-alginate scaffolds fabricated by multi-channel 3D plotting under mild conditions. J. Mater. Chem. B.1(33), 4088–4098 (2013).
- Jia J , RichardsDJ, PollardSet al. Engineering alginate as bioink for bioprinting. Acta. Biomater.10(10), 4323–4331 (2014).
- Neufurth M , WangX, SchroderHCet al. Engineering a morphogenetically active hydrogel for bioprinting of bioartificial tissue derived from human osteoblast-like SaOS-2 cells. Biomaterials35(31), 8810–8819 (2014).
- Shoichet MS , LiRH, WhiteMLet al. Stability of hydrogels used in cell encapsulation: an in vitro comparison of alginate and agarose. Biotechnol. Bioeng.50(4), 374–381 (1996).
- Zhang ZF , LoebusA, de VicenteGet al. Synthesis of poly(ethylene glycol)-based hydrogels via amine-michael type addition with tunable stiffness and postgelation chemical functionality. Chem. Mater.26(12), 3624–3630 (2014).
- Rodell CB , MacArthurJW, DorseySMet al. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv. Funct. Mater.25(4), 636–644 (2015).
- Su WY , ChenYC, LinFH. Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta. Biomater.6(8), 3044–3055 (2010).
- Rutz AL , HylandKE, JakusAEet al. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv Mater.27(9), 1607–1614 (2015).
- Knowlton S , OnalS, YuCHet al. Bioprinting for cancer research. Trends Biotechnol.33(9), 504–513 (2015).
- Peng WJ , DattaP, AyanBet al. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater.57, 26–46 (2017).
- Shafiee A , AtalaA. Printing technologies for medical applications. Trends. Mol. Med.22(3), 254–255 (2016).
- Hsieh FY , HsuSH. 3D bioprinting: a new insight into the therapeutic strategy of neural tissue regeneration. Organogenesis11(4), 153–158 (2015).
- Richards D , JiaJ, YostMet al. 3D bioprinting for vascularized tissue fabrication. Ann. Biomed. Eng.45(1), 132–147 (2017).
- Wang XH , AoQ, TianXHet al. 3D bioprinting technologies for hard tissue and organ engineering. Mater.9(10), 802–824 (2016).
- Ameri K , SamurkashianR, YeghiazariansY. Three-dimensional bioprinting emerging technology in cardiovascular medicine. Circulation135(14), 1281–1283 (2017).
- Zhang YS , YueK, AlemanJet al. 3D bioprinting for tissue and organ fabrication. Ann. Biomed. Eng.45(1), 148–163 (2017).
- Miller JS , StevensKR, YangMTet al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater.11(9), 768–774 (2012).
- Koleskya DB , HomanaKA, Skylar-ScottaMAet al. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl Acad. Sci. USA113(12), 3179–3184 (2016).
- Jia WT , Gungor-OzkerimPS, ZhangYSet al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials106, 58–68 (2016).
- Gao Q , LiuZJ, LinZWet al. 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomater. Sci. Eng.3(3), 399–408 (2017).
- Li S , LiuYY, LiYet al. A novel method for fabricating engineered structures with branched micro-channel using hollow hydrogel fibers. Biomicrofluidics10(6), 064106 (2016).
- Datta P , AyanB, OzbolatIT. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater.51, 1–20 (2017).
- Cheung DY , DuanB, ButcherJT. Current progress in tissue engineering of heart valves: multiscale problems, multiscale solutions. Expert Opin. Biol. Ther.15(8), 1–18 (2015).
- Gaetani R , FeyenDAM, VerhageVet al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials61, 339–348 (2015).
- Jang J , ParkHJ, KimSWet al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials112, 264–274 (2017).
- Gu Q , Tomaskovic-CrookE, LozanoRet al. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Healthcare Mater.5(12), 1429–1438 (2016).
- Pourchet LJ , ThepotA, AlbouyMet al. Human skin 3D bioprinting using scaffold-free approach. Adv. Healthcare Mater.6(4), 1601101 (2017).
- Daly AC , CunniffeGM, SathyBNet al. 3D Bioprinting of developmentally inspired templates for whole bone organ engineering. Adv. Healthcare Mater.5(18), 2353–2362 (2016).
- Ma XY , QuX, ZhuWet al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl Acad. Sci. USA113(8), 2206–2211 (2016).
- Kloxin AM , BentonJA, AnsethHS. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials31(1), 1–8 (2010).
- Duan B . State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann. Biomed. Eng.45(1), 1–15 (2016).
- Jana S , LermanA. Bioprinting a cardiac valve. Biotechnol. Adv.33(8), 1503–1521 (2015).
- Owens CM , MargaF, ForgacsGet al. Biofabrication and testing of a fully cellular nerve graft. Biofabrication5(4), 045007 (2013).
- Lozano R , StevensL, ThompsonBCet al. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials67, 264–273 (2015).
- Michael S , SorgH, PeckCet al. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE8(3), e57741 (2013).
- Koch L , DeiwickA, SchlieSet al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng.109(7), 1855–1863 (2012).
- Cubo N , GarciaM, del CañizoJFet al. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication9(1), 015006 (2017).
- Huang S , YaoB, XieJFet al. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta. Biomater.32, 170–177 (2016).
- Ozbolat IT . Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol.33(7), 395–400 (2015).
- Abbadessa A , MouserVHM, BlokzijlMMet al. A synthetic thermosensitive hydrogel for cartilage bioprinting and its biofunctionalization with polysaccharides. Biomacromolecules17(6), 2137–2147 (2016).
- Xu T , BinderKW, AlbannaMZet al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication5(1), 015001 (2013).
- Narayanan LK , HuebnerP, FisherMBet al. 3D-bioprinting of polylactic acid (PLA) nanofiber-alginate hydrogel bioink containing human adipose-derived stem cells. ACS Biomater. Sci. Eng.2(10), 1732–1742 (2016).
- Rhee S , PuetzerJL, MasonBNet al. 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater. Sci. Eng.2(10), 1800–1805 (2016).
- Levato R , VisserJ, PlanellJAet al. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication6(3), 035020 (2014).
- Ober TJ , ForestiD, LewisJA. Active mixing of complex fluids at the microscale. Proc. Natl Acad. Sci. USA112(40), 12293–12298 (2015).
- Shim JH , JangKM, HahnSKet al. Three-dimensional bioprinting of multilayered constructs containing human mesenchymal stromal cells for osteochondral tissue regeneration in the rabbit knee joint. Biofabrication8(1), 014102 (2016).
- Park SH , JungCS, MinBH. Advances in three-dimensional bioprinting for hard tissue engineering. Tissue Eng. Regen. Med.13(6), 622–635 (2016).
- Lee JW , ChoiYJ, YongWJet al. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication8(1), 015007 (2016).
- Lee JS , HongJM, JungJWet al. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication6(2), 024103 (2014).
- Laronda MM , RutzAL, XiaoSet al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun.8, 15261 (2017).
- Demirci U , MontesanoG. Single cell epitaxy by acoustic picolitre droplets. Lab on A Chip7(9), 1139–1145 (2007).
- Fang Y , FramptonJP, RaghavanSet al. Rapid generation of multiplexed cell cocultures using acoustic droplet ejection followed by aqueous two-phase exclusion patterning. Tissue Eng. Part C Methods18(9), 647–657 (2012).
