Abstract
Aim: This meta-analysis aimed to investigate the clinical value of calprotectin in rheumatoid arthritis (RA) patients. Methods: The data regarding blood calprotectin levels in RA patients were retrieved from PubMed, Embase, Web of Science and Cochrane databases. Results: Thirty-one eligible articles were included. Calprotectin was increased in RA patients compared with healthy controls (mean difference [MD] = 1.48, 95% CI: 1.16–1.79). Calprotectin was positively associated with C-reactive protein (correlation coefficient [CC] = 0.58, 95% CI: 0.53–0.63) and disease activity score (CC = 0.48, 95% CI: 0.38–0.58) in RA patients. Interestingly, calprotectin showed an increased trend in RA responders compared with nonresponders, but without statistical significance (MD = 0.38, 95% CI: -0.09–0.85). Conclusion: Blood calprotectin relates to disease risk, inflammation and activity in RA patients.
Plain language summary
This meta-analysis included published data about blood calprotectin levels in rheumatoid arthritis (RA) patients. After integrated analyses, it was observed that blood calprotectin levels were higher in RA patients compared with healthy subjects; calprotectin was positively related to systemic inflammation and disease activity in RA patients. However, blood calprotectin levels failed to predict treatment outcomes in RA patients.
Rheumatoid arthritis (RA), a common autoimmune disease characterized by synovitis, systemic inflammation, joint destruction and extra-articular involvement, affects approximately 1% of the total population worldwide [Citation1–3]. The treatment options for RA were limited before the late 20th century and mainly included traditional drugs such as NSAIDs, glucocorticoids and conventional disease-modifying antirheumatic drugs (DMARDs). Along with rapid biological technology advancement, biologics (e.g., TNF inhibitor [TNFi], IL-6 inhibitor, biosimilars) and small-molecule compounds (e.g., tofacitinib) were also developed and greatly improved outcomes of RA [Citation4–6]. However, a proportion of patients remain who fail to respond or easily flair. Therefore, to better manage RA disease activity and treatment outcomes, more feasible biomarkers have been discovered, such as ACPA, 14-3-3 η and so on [Citation7,Citation8].
Calprotectin, a heterocomplex consisting of S100A8/A9 proteins, also named myeloid-related protein 8/14, exhibits potency as an inflammatory biomarker reflecting disease risk and activity in several autoimmune inflammatory diseases such as inflammatory bowel disease, spondyloarthritis and RA [Citation9–12]. Specifically, blood calprotectin is observed to be increased in RA patients compared with other rheumatologic disease controls and healthy controls (HCs), which also relates to systemic inflammation and disease activity of RA [Citation11,Citation13,Citation14]. Interestingly, it has also been reported that high blood calprotectin levels predict RA treatment response [Citation15]. However, another study found that blood calprotectin levels fail to predict RA treatment response [Citation16]. Since the comprehensive role of calprotectin in RA has not been systemically analyzed, it is necessary to perform a meta-analysis to assemble the data. Therefore, this meta-analysis included 31 related studies and aimed to investigate aberrant levels of calprotectin and their relation to inflammation, activity and treatment response in RA patients.
Methods
Data sources & search strategy
A literature search in PubMed, Embase, Web of Science and Cochrane databases up to November 2021 was carried out to retrieve published studies that evaluated calprotectin levels in RA patients and HCs, the correlation of calprotectin with RA activity or the relationship between calprotectin and treatment response. The following keywords in different combinations were adopted in the literature retrieval: ‘calprotectin’, ‘CLP’, ‘S100A8/A9’, ‘migration inhibitory factor-related protein of 8 and 14 kDa’, ‘MRP-8/MRP-14’, ‘MRP-8/14’, ‘leukocyte L1 protein’, ‘rheumatoid arthritis’ and ‘RA’. In addition to database retrieval, the cited references in the retrieved articles were also reviewed to identify omitted studies. This meta-analysis was implemented in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the Meta-analysis of Observational Studies in Epidemiology guidelines [Citation17].
Screening of studies
Preliminary screening was performed based on the title of the retrieved articles. The abstract and full text of the preliminarily qualified works were acquired and further reviewed for eligibility based on the following criteria: patients with a diagnosis of RA; published cohorts (retrospective or prospective), case-control, cross-sectional or longitudinal studies aimed at investigating calprotectin levels in RA patients and HCs, the correlation of calprotectin with RA activity, or/and the relationship between calprotectin and treatment response; at least one of the following outcome-related data were available in full text or abstract: serum or plasma calprotectin levels in RA patients and HCs; the correlation coefficient of calprotectin with Disease Activity Scores for 28 joints (DAS28) based on erythrocyte sedimentation rate (ESR); the correlation coefficient of calprotectin with CRP; baseline serum or plasma calprotectin levels of RA patients who achieved European League Against Rheumatism (EULAR) response and those who failed to achieve EULAR response. Reviews, case reports and studies with overlapping or unavailable data were ineligible for inclusion. A total of 31 eligible articles were in included in this meta-analysis [Citation11–16,Citation18–42].
Data extraction
Each eligible study was separately reviewed by two data collectors and the following data were collected: first author's name, publication year, country (region), study type, number of RA patients and HCs, serum or plasma calprotectin levels in RA patients and HCs, correlation coefficients between calprotectin and CRP or DAS28 and baseline serum or plasma calprotectin levels of RA patients who achieved EULAR response and those who failed to achieve EULAR response. After collection, the resulting datasets were checked. If there was nonconformity in both datasets, a review of the original article was performed again, and a consensus was reached after discussion.
Meta-analysis
R 3.6.3 software packages (https://cran.r-project.org/bin/windows/base/old/3.6.3/) were applied for meta-analysis. The continuous variables for meta-analysis are expressed as standardized mean differences (MDs) with 95% CIs. For correlation analysis, pooled correlation coefficients with 95% CIs were imputed. The inconsistency index (I2) was used for the assessment of heterogeneity among enrolled studies, and an I2>50% indicated that there was significant heterogeneity among studies. The pooled results are shown in the random-effects model due to significant heterogeneity. Considering there was significant heterogeneity among pooled studies, a sensitivity analysis was carried out by omitting each study in the meta-analysis, which evaluated the influence of each study on the pooled results. Publication bias was determined by the Egger regression test and Begg and Mazumdar test for meta-analysis.
Results
Study flow
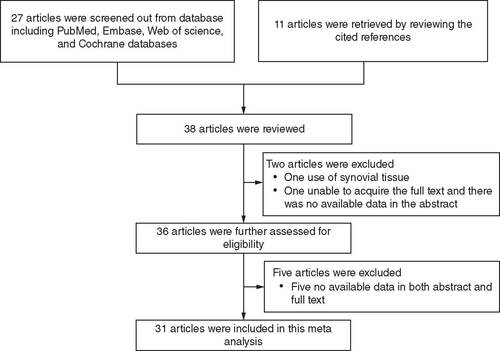
Twenty-seven articles were screened from databases (including PubMed, Embase, Web of Science and Cochrane) and another 11 articles were retrieved by reviewing the cited references. A total of 38 articles were reviewed, among which two articles were excluded (one use of synovial tissue, one unable to acquire the full text and there was no available data in the abstract). Subsequently, 36 articles were further assessed for eligibility, among which five articles were excluded due to no available data in both abstract and full text. Finally, a total of 31 articles were included in this meta-analysis (). Key information on the included articles is summarized in Supplementary Table 1. The PRISMA checklist for this study is shown in Supplementary Table 2.
Abnormal calprotectin levels in RA patients
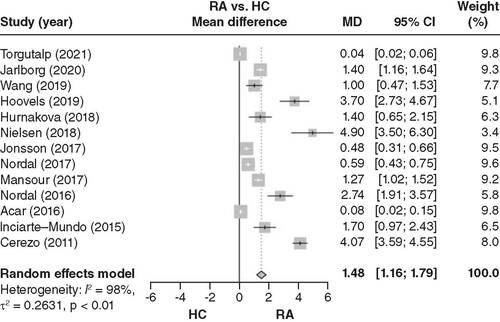
Thirteen articles were included in the pooled analysis of calprotectin levels between RA patients and HCs. Blood calprotectin levels were increased in RA patients compared with HCs (MD = 1.48; 95% CI: 1.16–1.79; ).
Correlation of calprotectin levels with inflammation & activity in RA patients
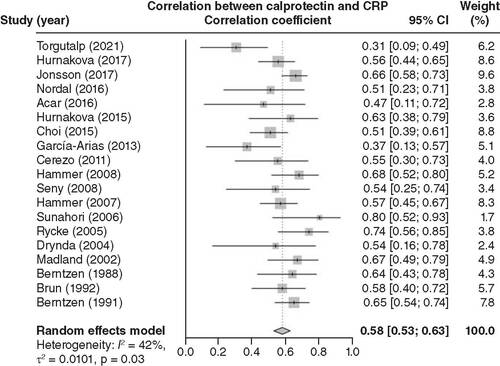
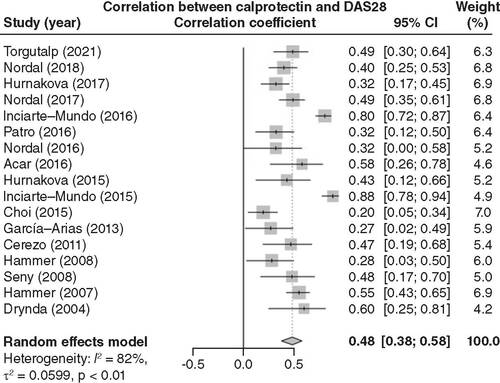
Nineteen articles were included in the pooled analysis of the correlation between calprotectin levels and inflammation in RA patients. Blood calprotectin levels were positively associated with CRP levels (correlation coefficient = 0.58; 95% CI: 0.53–0.63; ). In addition, 17 articles were included in the pooled analysis of the correlation between calprotectin levels and disease activity in RA patients. Blood calprotectin levels were positively linked with DAS28 scores (correlation coefficient = 0.48; 95% CI: 0.38–0.58; ).
After a pooled analysis of 19 related articles, blood calprotectin level was positively related to CRP in RA patients.
CRP: C-reactive protein; RA: Rheumatoid arthritis.
After a pooled analysis of 17 related articles, blood calprotectin levels were positively correlated with DAS28 scores in RA patients.
DAS28: Disease Activity Score for 28 joints; RA: Rheumatoid arthritis.
Linkage of calprotectin levels with treatment response in RA patients
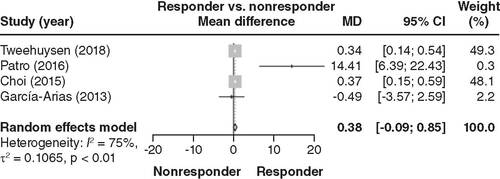
Only four articles had data about calprotectin levels and treatment response in RA patients and were therefore included in the pooled analysis. Blood calprotectin levels showed an increased trend in responders compared with nonresponders, but without statistical significance (MD = 0.38; 95% CI: -0.09–0.85; ).
Sensitivity analysis & publication bias
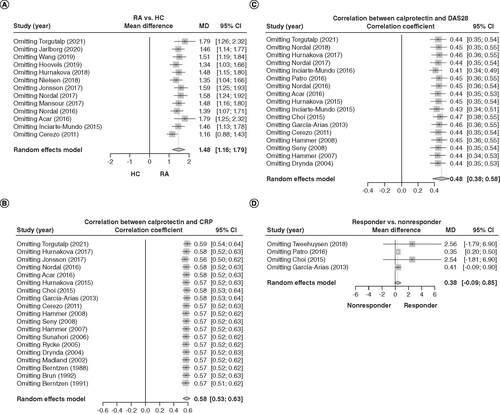
Omitting each study did not change the findings regarding increased calprotectin levels in RA patients versus HCs (A), positive correlation between calprotectin levels and CRP in RA patients (B) or positive association between calprotectin levels and DAS28 scores in RA patients (C). However, omitting Patro's 2016 study [Citation26] resulted in calprotectin levels being significantly increased in responders versus nonresponders (D).
(A) Sensitivity analysis of calprotectin levels between rheumatoid arthritis patients and healthy controls. (B) Sensitivity analysis of the correlation between calprotectin levels and C-reactive protein. (C) Sensitivity analysis of the correlation between calprotectin levels and disease activity scores for 28 joints. (D) Sensitivity analysis of calprotectin level between RA responders and nonresponders.
RA: Rheumatoid arthritis.
In terms of publication bias, the Begg and Mazumdar test suggested data on the correlation between calprotectin among RA patients and HCs included publication bias, while other data did not have publication bias (). The Egger test indicated that analysis regarding all data in the meta-analysis did not demonstrate publication bias.
Table 1. Publication bias in key assessments.
Subgroup analyses based on serum or plasma samples
Both serum calprotectin (MD = 1.56; 95% CI: 1.20–1.93) and plasma calprotectin (MD = 1.18, 95% CI: 0.52–1.83) were elevated in RA patients compared with HCs (A); serum calprotectin (correlation coefficient = 0.53; 95% CI: 0.45–0.61) and plasma calprotectin (correlation coefficient = 0.63; 95% CI: 0.59–0.67) were positively associated with CRP levels (B). Furthermore, serum calprotectin (correlation coefficient = 0.49; 95% CI: 0.35–0.61) and plasma calprotectin (correlation coefficient = 0.48; 95% CI: 0.36–0.59) were positively associated with DAS28 scores (C). Finally, in studies evaluating treatment response, only serum calprotectin was explored, therefore, the relation between serum calprotectin and response (D) was the same as the relation between blood calprotectin and response; that is, serum calprotectin also exhibited an increasing trend in responders compared with nonresponders, but without statistical significance (MD = 0.38; 95% CI: -0.09–0.85; D).
(A) Separate analyses of the differences in serum or plasma calprotectin between rheumatoid arthritis patients and healthy controls. (B) Separate analyses of the correlation between serum or plasma calprotectin and C-reactive protein. (C) Disease activity scores for 28 joints. (D) Treatment response.

Discussion
Calprotectin serves as a potential biomarker relating to risk of several autoimmune inflammatory diseases [Citation9,Citation10,Citation43]. For instance, calprotectin is increased in ulcerative colitis and Crohn's disease patients compared with age/gender-matched HCs [Citation9]; calprotectin is also elevated in patients with systemic lupus erythematosus compared with matched controls [Citation43]. In terms of RA, calprotectin levels are also increased [Citation11,Citation12,Citation18,Citation19]. To make a comprehensive evaluation, the current meta-analysis pooled 13 articles evaluating calprotectin levels between RA patients and HCs, then confirmed that blood calprotectin levels were higher in RA patients compared with HCs. The possible explanations were: calprotectin reflected higher systemic inflammation, the latter was a key feature of RA, so calprotectin exhibited an increased trend in RA patients compared with HCs [Citation14,Citation22]; the presence of high amounts of calprotectin as a consequence of local production in the synovium and subsequent release in the blood would also contribute to the higher blood calprotectin in RA patients. Therefore, calprotectin cannot be considered a systemic biomarker of inflammation.
Interestingly, calprotectin measurement is also applied as a tool for monitoring disease activity and flares in autoimmune inflammatory diseases [Citation14,Citation27,Citation28,Citation44,Citation45]. For example, calprotectin level positively links with CRP level, endoscopic score, symptom score in ulcerative colitis patients, as well as CRP level in Crohn's disease patients [Citation44]; it also positively relates to CRP, ESR and Bath ankylosing spondylitis disease activity index (BASDAI) score in ankylosing spondylitis patients [Citation45]. In terms of RA management, calprotectin level is also positively associated with CRP and DAS28 scores in RA patients [Citation14,Citation27,Citation28]. The current meta-analysis pooled 19 and 17 articles relating to calprotectin's correlation with RA inflammation and disease activity, respectively, and showed that blood calprotectin levels were positively related to CRP levels and DAS28 scores in RA patients. The possible explanations were:
Calprotectin directly bound granulocytes, monocytes and macrophages, then promoted the secretion of proinflammatory cytokines such as IL-1, IL-6, TNF-α and so on, therefore calprotectin is positively related to inflammation [Citation46];
Calprotectin stimulated synovium inflammation via mediating M1-like macrophages and canonical Wnt signaling [Citation47], and it closely linked with RA synovitis [Citation48], therefore, calprotectin was positively linked with DAS28 scores in RA patients.
The current study was also designed to analyze the correlation between calprotectin and the Clinical Disease Activity Index (CDAI) in RA patients; while among 31 included articles, six assessed the CDAI score, and after a full-text search, the correlation coefficient for calprotectin and CDAI was only available in two articles [Citation14,Citation22], which showed that serum calprotectin was positively related to CDAI score (correlation coefficient = 0.279) [Citation14] and plasma calprotectin was positively linked with CDAI score (correlation coefficient = 0.32) [Citation22]. Since only two articles were available and the sample types differed, the pooled analysis is not meaningful, therefore we did not perform it.
Apart from the supervisory role of calprotectin, it is also reported to estimate treatment response in autoimmune inflammatory diseases [Citation15,Citation45]. For instance, the decrement of calprotectin during the first month shows potency to predict 6-month treatment response in ankylosing spondylitis patients [Citation45]. In RA, a previous study found that pretreatment calprotectin levels are increased in RA responders compared with RA nonresponders treated using a TNFi [Citation15]; however, another study found that pretreatment calprotectin levels were not related to treatment response to TNFi [Citation31]. These findings are conflicting. To clarify this issue, the current meta-analysis pooled four articles reporting a linkage between calprotectin levels and treatment response and observed that blood calprotectin levels showed an increased trend in responders compared with nonresponders, but without statistical significance. Therefore, further multicenter, large sample studies to explore this issue are necessary in the future.
The current meta-analysis provides comprehensive information about the potency of calprotectin as a biomarker for RA management, while some limitations should be mentioned. First, the detection kit for calprotectin differs among studies, which may cause bias in the results. Second, the features of enrolled patients varied among studies, which could cause bias as well. Third, most of the analyzed studies had small sample sizes (20/31 studies had a sample size <100 RA patients). Fourth, the source of samples differing among included studies (serum or plasma), which may cause bias, although subgroup analyses by serum or plasma calprotectin were performed separately for clarification and this limitation was unavoidable.
Conclusion
This meta-analysis shows that calprotectin relates to disease risk, inflammation and activity in RA patients. It may also predict treatment response to some extent, but this issue needs further validation.
Results regarding the clinical implications of blood calprotectin levels in rheumatoid arthritis (RA) are conflicting and need further investigation.
This meta-analysis included 31 related studies and aimed to investigate the relationship between blood calprotectin levels and disease activity and response in RA.
Blood calprotectin levels were increased in RA patients compared with healthy controls.
Blood calprotectin levels were positively associated with C-reactive protein levels in RA patients.
Blood calprotectin levels were positively linked with Disease Activity Scores for 28 joints in RA patients.
Blood calprotectin levels showed an increasing trend in responders compared with nonresponders, but without statistical significance.
The publication bias was low according to the Begg and Mazumdar and Egger tests.
Conclusively, calprotectin is related to disease risk, inflammation and activity in RA patients and might also predict treatment response to some extent. This issue needed further investigation.
Author contributions
J Zeng contributed to the study conception and design. Material preparation, data collection and analysis were performed by X Liu and J Liu. The first draft of the manuscript was written by P Wu. L Yang commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary table 1
Download MS Word (22.2 KB)Supplementary Table 2
Download MS Word (37 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/bmm-2022-0216
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Weyand CM , GoronzyJJ. The immunology of rheumatoid arthritis. Nat. Immunol.22(1), 10–18 (2021).
- Tanaka Y . Rheumatoid arthritis. Inflamm. Regen.40, 20 (2020).
- van der Woude D , vander Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol.32(2), 174–187 (2018).
- Singh N , LiCI. Impact of rheumatoid arthritis and biologic and targeted synthetic disease modifying antirheumatic agents on cancer risk and recurrence. Curr. Opin. Rheumatol.33(3), 292–299 (2021).
- Camean-Castillo M , Gimeno-BallesterV, Rios-SanchezE, Fenix-CaballeroS, Vazquez-RealM, Alegre-DelRey E. Network meta-analysis of tofacitinib versus biologic treatments in moderate-to-severe rheumatoid arthritis patients. J. Clin. Pharm. Ther.44(3), 384–396 (2019).
- Romano C , EspositoS, FerraraR, CuomoG. Tailoring biologic therapy for real-world rheumatoid arthritis patients. Expert Opin. Biol. Ther.21(5), 661–674 (2021).
- Atzeni F , TalottaR, MasalaIF, BongiovanniS, BoccassiniL, Sarzi-PuttiniP. Biomarkers in rheumatoid arthritis. Isr Med. Assoc. J.19(8), 512–516 (2017).
- Wei JC , LeongPY, LiuGY. Chaperone/scaffolding/adaptor protein 14-3-3eta (eta): a diagnostic marker of rheumatoid arthritis. Int. J. Rheum. Dis.23(11), 1439–1442 (2020).
- Mori A , MitsuyamaK, SakemiRet al. Evaluation of serum calprotectin levels in patients with inflammatory bowel disease. Kurume Med. J.66(4), 209–215 (2021).
- Romand X , PacletMH, CourtierAet al. Serum calprotectin is increased in early axial spondyloarthritis with sacroiliitis and objective signs of inflammation: results from the DESIR cohort. Joint Bone Spine88(1), 105068 (2021).
- Torgutalp M , YaylaME, ErogluDSet al. Serum calprotectin is indicating clinical and ultrasonographic disease activity in rheumatoid arthritis, even with normal C-reactive protein levels. Mediterr. J. Rheumatol.32(1), 56–65 (2021).
- Jarlborg M , CourvoisierDS, LamacchiaCet al. Serum calprotectin: a promising biomarker in rheumatoid arthritis and axial spondyloarthritis. Arthritis Res. Ther.22(1), 105 (2020).
- Nordal HH , FagerholMK, HalseAK, HammerHB. Calprotectin (S100A8/A9) should preferably be measured in EDTA-plasma; results from a longitudinal study of patients with rheumatoid arthritis. Scand. J. Clin. Lab. Invest.78(1–2), 102–108 (2018).
- Hurnakova J , HulejovaH, ZavadaJet al. Relationship between serum calprotectin (S100A8/9) and clinical, laboratory and ultrasound parameters of disease activity in rheumatoid arthritis: a large cohort study. PLOS ONE12(8), e0183420 (2017).
- Tweehuysen L , den BroederN, van HerwaardenNet al. Predictive value of serum calprotectin (S100A8/A9) for clinical response after starting or tapering anti-TNF treatment in patients with rheumatoid arthritis. RMD Open4(1), e000654 (2018).
- Choi IY , GerlagDM, HereniusMJet al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann. Rheum. Dis.74(3), 499–505 (2015).
- Hutton B , SalantiG, CaldwellDMet al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med.162(11), 777–784 (2015).
- Wang Y , LiangY. Clinical significance of serum calprotectin level for the disease activity in active rheumatoid arthritis with normal C-reactive protein. Int. J. Clin. Exp. Pathol.12(3), 1009–1014 (2019).
- Van Hoovels L , Vander CruyssenB, BogaertL, Vanden Bremt S, BossuytX. Pre-analytical and analytical confounders of serum calprotectin as a biomarker in rheumatoid arthritis. Clin. Chem. Lab. Med.58(1), 40–49 (2019).
- Hurnakova J , HulejovaH, ZavadaJet al. Serum calprotectin may reflect inflammatory activity in patients with active rheumatoid arthritis despite normal to low C-reactive protein. Clin. Rheumatol.37(8), 2055–2062 (2018).
- Nielsen UB , BruhnLV, EllingsenT, Stengaard-PedersenK, HornungN. Calprotectin in patients with chronic rheumatoid arthritis correlates with disease activity and responsiveness to methotrexate. Scand. J. Clin. Lab. Invest.78(1–2), 62–67 (2018).
- Jonsson MK , SundlisaeterNP, NordalHHet al. Calprotectin as a marker of inflammation in patients with early rheumatoid arthritis. Ann. Rheum. Dis.76(12), 2031–2037 (2017).
- Nordal HH , BrokstadKA, SolheimM, HalseAK, KvienTK, HammerHB. Calprotectin (S100A8/A9) has the strongest association with ultrasound-detected synovitis and predicts response to biologic treatment: results from a longitudinal study of patients with established rheumatoid arthritis. Arthritis Res. Ther.19(1), 3 (2017).
- Mansour HE , AbdullrhmanMA, MobasherSAet al. Serum calprotectin in rheumatoid arthritis: a promising diagnostic marker, how far is it related to activity and sonographic findings? J. Med. Ultrasound 25(1), 40–46 (2017).
- Inciarte-Mundo J , Victoria HernandezM, Ruiz-EsquideVet al. Serum calprotectin versus acute-phase reactants in the discrimination of inflammatory disease activity in rheumatoid arthritis patients receiving tumor necrosis factor inhibitors. Arthritis Care Res. (Hoboken)68(7), 899–906 (2016).
- Patro PS , SinghA, MisraR, AggarwalA. Myeloid-related protein 8/14 levels in rheumatoid arthritis: marker of disease activity and response to methotrexate. J. Rheumatol.43(4), 731–737 (2016).
- Nordal HH , BrunJG, HordvikM, EidsheimM, JonssonR, HalseAK. Calprotectin (S100A8/A9) and S100A12 are associated with measures of disease activity in a longitudinal study of patients with rheumatoid arthritis treated with infliximab. Scand. J. Rheumatol.45(4), 274–281 (2016).
- Acar A , GuzelS, SarifakiogluBet al. Calprotectin levels in patients with rheumatoid arthritis to assess and association with exercise treatment. Clin. Rheumatol.35(11), 2685–2692 (2016).
- Hurnakova J , ZavadaJ, HanovaPet al. Serum calprotectin (S100A8/9): an independent predictor of ultrasound synovitis in patients with rheumatoid arthritis. Arthritis Res. Ther.17, 252 (2015).
- Inciarte-Mundo J , Ruiz-EsquideV, HernandezMVet al. Calprotectin more accurately discriminates the disease status of rheumatoid arthritis patients receiving tocilizumab than acute phase reactants. Rheumatology (Oxford)54(12), 2239–2243 (2015).
- Garcia-Arias M , Pascual-SalcedoD, RamiroSet al. Calprotectin in rheumatoid arthritis: association with disease activity in a cross-sectional and a longitudinal cohort. Mol. Diagn. Ther.17(1), 49–56 (2013).
- Andres Cerezo L , MannH, PechaOet al. Decreases in serum levels of S100A8/9 (calprotectin) correlate with improvements in total swollen joint count in patients with recent-onset rheumatoid arthritis. Arthritis Res. Ther.13(4), R122 (2011).
- Hammer HB , HaavardsholmEA, KvienTK. Calprotectin (a major leucocyte protein) is associated with the levels of anti-CCP and rheumatoid factor in a longitudinal study of patients with very early rheumatoid arthritis. Scand. J. Rheumatol.37(3), 179–182 (2008).
- de Seny D , FilletM, RibbensCet al. Monomeric calgranulins measured by SELDI-TOF mass spectrometry and calprotectin measured by ELISA as biomarkers in arthritis. Clin. Chem.54(6), 1066–1075 (2008).
- Hammer HB , OdegardS, FagerholMKet al. Calprotectin (a major leucocyte protein) is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann. Rheum. Dis.66(8), 1093–1097 (2007).
- Sunahori K , YamamuraM, YamanaJet al. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res. Ther.8(3), R69 (2006).
- De Rycke L , BaetenD, FoellDet al. Differential expression and response to anti-TNFalpha treatment of infiltrating versus resident tissue macrophage subsets in autoimmune arthritis. J. Pathol.206(1), 17–27 (2005).
- Drynda S , RingelB, KekowMet al. Proteome analysis reveals disease-associated marker proteins to differentiate RA patients from other inflammatory joint diseases with the potential to monitor anti-TNFalpha therapy. Pathol. Res. Pract.200(2), 165–171 (2004).
- Madland TM , HordvikM, HagaHJ, JonssonR, BrunJG. Leukocyte protein calprotectin and outcome in rheumatoid arthritis. A longitudinal study. Scand. J. Rheumatol.31(6), 351–354 (2002).
- Berntzen HB , MuntheE, FagerholMK. The major leukocyte protein L1 as an indicator of inflammatory joint disease. Scand. J. Rheumatol. Suppl.76, 251–256 (1988).
- Brun JG , HagaHJ, BoeEet al. Calprotectin in patients with rheumatoid arthritis: relation to clinical and laboratory variables of disease activity. J. Rheumatol.19(6), 859–862 (1992).
- Berntzen HB , FagerholMK, OstensenM, MowinckelP, HoyeraalHM. The L1 protein as a new indicator of inflammatory activity in patients with juvenile rheumatoid arthritis. J. Rheumatol.18(1), 133–138 (1991).
- Haga HJ , BrunJG, BerntzenHB, CerveraR, KhamashtaM, HughesGR. Calprotectin in patients with systemic lupus erythematosus: relation to clinical and laboratory parameters of disease activity. Lupus2(1), 47–50 (1993).
- Carlsen K , MalhamM, HansenLFet al. Serum calprotectin in adolescents with inflammatory bowel disease-a pilot investigation. J. Pediatr. Gastroenterol. Nutr.68(5), 669–675 (2019).
- Hu H , DuF, ZhangS, ZhangW. Serum calprotectin correlates with risk and disease severity of ankylosing spondylitis and its change during first month might predict favorable response to treatment. Mod. Rheumatol.29(5), 836–842 (2019).
- Kessel C , HolzingerD, FoellD. Phagocyte-derived S100 proteins in autoinflammation: putative role in pathogenesis and usefulness as biomarkers. Clin. Immunol.147(3), 229–241 (2013).
- van den Bosch MH , BlomAB, SchelbergenRFet al. Alarmin S100A9 induces proinflammatory and catabolic effects predominantly in the M1 macrophages of human osteoarthritic synovium. J. Rheumatol.43(10), 1874–1884 (2016).
- Sakellariou G , LombardiG, VitoloBet al. Serum calprotectin as a marker of ultrasound-detected synovitis in early psoriatic and rheumatoid arthritis: results from a cross-sectional retrospective study. Clin. Exp. Rheumatol.37(3), 429–436 (2019).