Abstract
Aim: To explore the relationship between inflammatory markers and prolonged postoperative ileus (PPOI), and to establish a nomogram for predicting PPOI. Patients & methods: The data of 229 patients were analyzed retrospectively. Univariate and multivariate logistic regression analysis were used to analyze the risk factors affecting the occurrence of PPOI. The predictive model of PPOI was established and verified internally. Results: Postoperative PPOI occurred in 87 (38.0%) of all 229 patients. Our study showed that age, preoperative neutrophil–lymphocyte ratio and changes in neutrophil–lymphocyte ratio were independent risk factors for PPOI. Conclusion: The nomograms established based on these independent risk factors have good predictive efficacy and may be able to guide clinicians to individualize the diagnosis and treatment.
Introduction
According to the International Agency for Research on Cancer’s (IARC) 2020 report, colorectal cancer is the third most common cancer worldwide and the second leading cause of cancer-related deaths [Citation1]. Although multidisciplinary treatments, such as surgery, chemotherapy and radiotherapy, are available, surgical intervention remains the primary curative approach for colorectal cancer [Citation2]. Postoperative ileus (POI) is a gastrointestinal dysfunction that commonly occurs after abdominal surgery, characterized by symptoms such as nausea and vomiting, abdominal distention and delayed defecation [Citation3]. POI is considered a normal physiological response to surgery, typically disappearing within 3 days, but may persist without remission or recur, a condition called prolonged postoperative ileus (PPOI) [Citation4]. PPOI is one of the most prevalent complications following colorectal cancer surgery, with a previously reported incidence of 3–30% [Citation5]. The occurrence of PPOI will result in prolonged hospitalization, increased hospitalization costs and a significant burden on the healthcare system. There is still a lack of internationally recognized standard definitions for PPOI. In 2013, Vather et al. proposed a concept based on a systematic evaluation, PPOI was diagnosed if patients met two or more of the following five criteria on postoperative day 4 (POD 4) or later: 1) nausea or vomiting; 2) inability to tolerate oral diet in the previous 24 h; 3) failure to regain flatus in the previous 24 h; 4) abdominal distention; and 5) radiologic confirmation [Citation6]. In this study, Vather et al. conducted a comprehensive systematic review following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). They collected the literature that considered POI as the primary end point of abdominal surgery between 1996 and 2011 (including only randomized controlled trials, systematic evaluations and meta-analyses that studied POI in humans. There were no language restrictions. Conference abstracts were not included). From an initial pool of 3234 publications, 52 relevant articles were meticulously chosen, and the respective POI definitions employed in these studies were thoroughly extracted and analyzed. Furthermore, based on these extracted definitions, Vather et al. conducted a global survey to gather insights from renowned experts who had contributed to the field through their scholarly work. Through a meticulous integration of data from the systematic review and the results obtained from the global survey, the authors formulated the precise definition of PPOI as presented above. Based on these definitions, a series of studies have been conducted by many international teams in the prediction and treatment of PPOI in the past [Citation5,Citation7,Citation8].
Early recovery of bowel function and prevention of PPOI are vital aspects of clinical practice guidelines for enhanced recovery after surgery (ERAS) in elective colorectal surgery [Citation9]. The concept of ERAS, first proposed by Kehlet et al. [Citation10], aims to reduce surgical stress, enhance postoperative function recovery and reduce the incidence of postoperative complications through perioperative management, which has been widely adopted in colorectal cancer surgery [Citation11]. However, effective treatment for PPOI is still lacking. Therefore, identifying patients at high risk of PPOI at an early stage and adopting individualized interventions is crucial [Citation7]. Consequently, exploring clinical indicators capable of predicting the occurrence of PPOI is of paramount importance.
Previous studies have demonstrated that several factors, such as age and a history of chronic diseases, can cause chronic inflammation and lead to the development of colorectal cancer [Citation12]. Various hematological markers, such as the neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), lymphocyte–monocyte ratio (LMR), systemic immune-inflammation index (SII) and prognostic nutritional index (PNI), have been considered prognostic indicators for various malignancies [Citation13–16]. Recently, dynamic changes in NLR and SII (ΔNLR and ΔSII, respectively) have also been proposed as better prognostic indicators in patients with colorectal cancer [Citation17,Citation18]. Previous research has indicated that inflammatory response is one of the main reasons for the development of POI and that a variety of inflammatory cells, such as neutrophils, play an important role in the development of POI [Citation19,Citation20]. However, the relationship between perioperative inflammatory indicators of colorectal cancer surgery, early postoperative inflammatory indicators and their dynamic changes and PPOI is still uncertain. Inflammatory indicators, such as NLR, can be derived from routine blood tests, which have the advantage of being less invasive and readily obtainable. Therefore, our team aimed to link inflammatory indicators with PPOI in colorectal cancer, determine the correlation between inflammatory indicators and PPOI, and establish a high-performance prediction model for predicting and preventing the development of PPOI at an early stage.
The purpose of this study was to investigate the risk factors for the development of PPOI in patients undergoing radical resection for colorectal cancer, to determine the independent relationship between the aforementioned inflammatory indicators and PPOI, and to establish a nomogram for predicting the development of PPOI.
Patients & methods
Patients
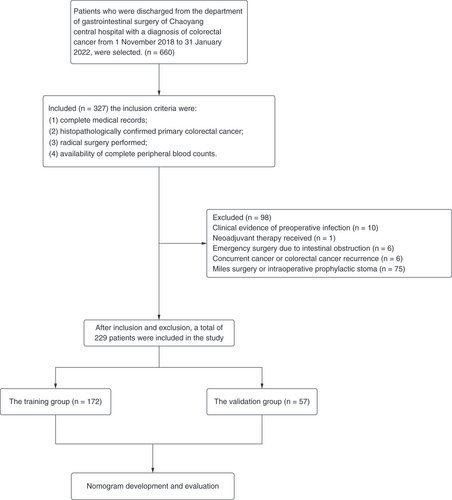
The data of 660 patients who were discharged from the Department of Gastrointestinal Surgery of Chaoyang Central Hospital with a diagnosis of colorectal cancer from 1 November 2018 to 31 January 2022 were selected. The inclusion criteria were: 1) complete medical records; 2) histopathologically confirmed primary colorectal cancer; 3) radical surgery performed; 4) availability of complete peripheral blood counts. The exclusion criteria were: 1) clinical evidence of preoperative infection; 2) presence of hematologic disorders; 3) neoadjuvant therapy received; 4) emergency surgery due to intestinal obstruction; 5) concurrent cancer or colorectal cancer recurrence; 6) use of immunosuppressive drugs; 7) miles surgery or intraoperative prophylactic stoma. After inclusion and exclusion, a total of 229 patients were included in the study. The flowchart is shown in .
PPOI diagnostic criteria
The diagnostic criteria in this study were based on the PPOI concept proposed by Vather et al. in 2013. PPOI was diagnosed if patients met two or more of the following five criteria on POD 4 or later: 1) nausea or vomiting; 2) inability to tolerate oral diet in the previous 24 h; 3) failure to regain flatus in the previous 24 h; 4) abdominal distention; and 5) radiologic confirmation [Citation6].
Calculation of inflammatory indicators
This study included various previously reported inflammatory indicators [Citation13–18,Citation20–22], such as NLR, PLR, LMR, SII, PNI, ΔNLR (postoperative NLR – preoperative NLR) and ΔSII (postoperative SII – preoperative SII). NLR was calculated as neutrophil count/lymphocyte count; PLR was calculated as platelet count/lymphocyte count; LMR was calculated as lymphocyte count/monocyte count; SII was calculated as platelet count × neutrophil count/lymphocyte count; PNI was calculated as 10 × albumin (g/dl) + 0.005 × total lymphocyte count (per mm3); ΔNLR was calculated as postoperative NLR – preoperative NLR; and ΔSII was calculated as postoperative SII – preoperative SII.
Data collection
Patient demographic and clinical data, such as age, gender, BMI, tumor location, tumor American Joint Committee on Cancer stage, surgical method (cases with intermediate open abdomen were classified as open group), smoking history, history of previous abdominal surgery, underlying diseases (including hypertension, diabetes and coronary heart disease), preoperative blood routine and postoperative day 1 blood routine and blood biochemical indexes, were collected, and the relevant inflammatory indicators were calculated. This study was approved by the Ethics Committee of Chaoyang Central Hospital (approval number: 2023 [Citation6]).
Statistical analyses
Data that met normality were expressed as mean ± standard deviation and analyzed using a t-test, while data that did not meet normality were expressed as median and interquartile range and analyzed using the Mann–Whitney rank sum test. Preoperative and postoperative levels of albumin, NLR, PLR, LMR, SII and PNI were analyzed using receiver operating characteristic (ROC) curve analysis. Optimal cutoff values for each parameter were determined using Youden index, and the data were dichotomized based on these cutoff values and represented as categorical variables. Categorical data were expressed as frequencies and percentages, and the Chi-square test was used for analysis. Parameters with statistical significance in the univariate analysis were further subjected to multivariate binary logistic regression analysis. IBM SPSS Statistics 25 software was used for the analyses. p < 0.05 was considered statistically significant in all analyses. The dataset was randomly split into training and validation sets in a 75:25 ratio. A nomogram based on the results of multiple-factor analysis was constructed using R version 4.2.2 software and validated. The predictive performance of the nomogram was evaluated by calculating the area under the receiver operating characteristic curve (AUC). A calibration curve was constructed to compare the predicted probabilities from the nomogram with the actual probabilities.
Results
Patient demographic data & optimal cut-off values for each indicator
Based on the inclusion and exclusion criteria, a total of 229 patients were enrolled in the study, comprising 134 males (58.5%) and 95 females (41.5%), with a mean age of 65.52 years. Among them, 136 (59.4%) were rectal patients, 41 (17.9%) were left hemicolectomy patients and 52 (22.7%) were right hemicolectomy patients. A total of 178 patients (77.7%) underwent laparoscopic radical resection for rectal cancer, while 51 patients (22.7%) underwent open surgery. PPOI occurred in 87 patients, with an incidence rate of 38.0%. The demographic data of the other patients are shown in Supplementary Table 1.
ROC curves were used to analyze the preoperative and postoperative routine blood and biochemical indexes, and the cutoff value with the highest sensitivity and specificity was selected for each index. The cutoff values for the preoperative indexes were 43.74 g/l for albumin, 4.71 for NLR, 182.66 for PLR, 5.76 for LMR, 476.63 for SII and 43.75 for PNI. For the postoperative indexes, the cutoff values were 35.19 g/l for albumin, 10.10 for NLR, 263.97 for PLR and 1.31 for LMR. shows the cutoff values and their corresponding sensitivity and specificity.
Table 1. Cutoff values of each inflammatory marker and their corresponding sensitivity and specificity.
Univariate & multivariate analysis of PPOI
Univariate analysis revealed that age ≥70 years, open surgery, preoperative NLR ≥4.71, preoperative LMR <5.76, postoperative NLR ≥10.10, postoperative SII ≥2426.55, postoperative PLR ≥263.97, postoperative LMR <1.31 and ΔNLR were significantly associated with PPOI. Furthermore, multifactorial logistic regression analysis demonstrated that age ≥70 years (OR: 1.903; 95% CI: 1.017–3.560; p = 0.044), preoperative NLR ≥4.71 (OR: 6.084; 95% CI: 1.816–20.382; p = 0.003) and ΔNLR (OR: 1.181; 95% CI: 1.052–1.325; p = 0.005) were independent risk factors for the development of PPOI after radical surgery in patients with colorectal cancer.
Development & validation of a nomogram predicting the PPOI
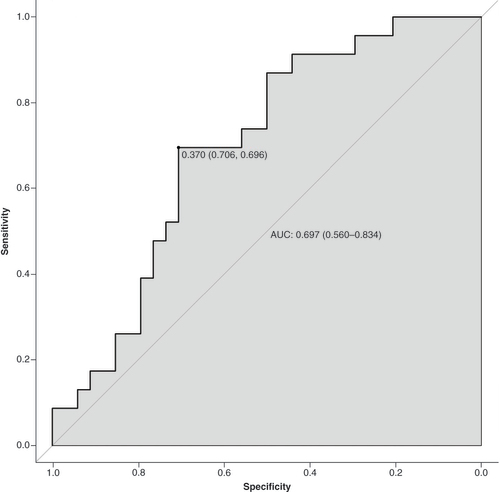
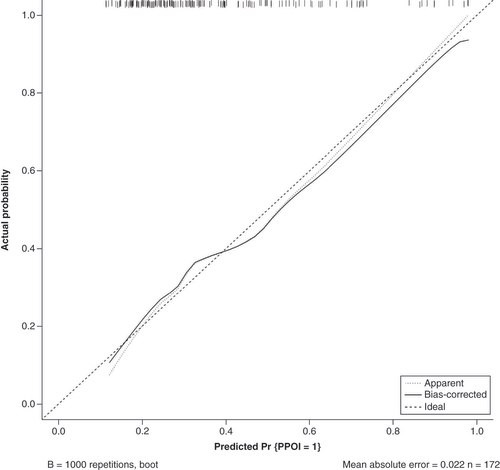
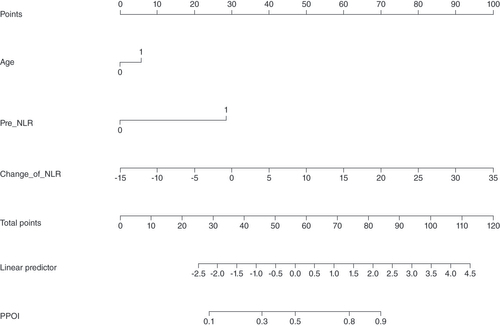
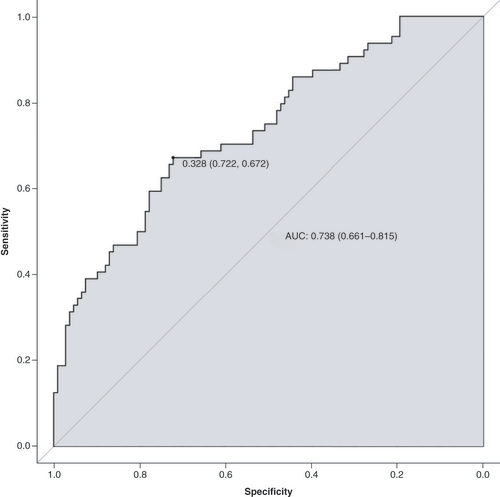
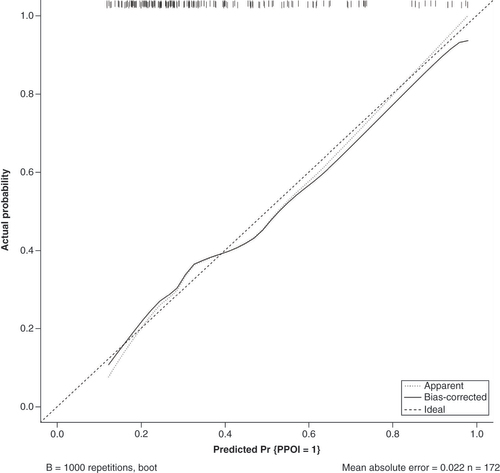
The data were randomly divided into a training group (n = 172) and a validation group (n = 57) according to a 75% and 25% split; the baseline analysis between the two groups is shown in Supplementary Table 2. Except for BMI (p = 0.015), there were no significant differences in baseline demographic and clinicopathological characteristics between the two groups (Supplementary Table 2). A nomogram to predict PPOI () and ROC curves () and calibration curves () were developed in the training group using three independent risk factors identified by multifactorial logistic regression analysis with an AUC value of 0.738 (95% Cl: 0.661–0.815). Internal validation was performed in the validation group and ROC curves (), and calibration curves () were plotted with an AUC value of 0.697 (95% Cl: 0.560–0.834). This indicates that the PPOI column line plot has good predictive efficacy; the calibration curve shows good agreement between predictions and actual observations.
A straight line was drawn vertically from the axis of each variable toward the ‘points’ scale. The points for each variable were summed together to generate a total point score, which is projected on the bottom line to obtain the individual predictive risk of PPOI.
pre-NLR: Preoperative neutrophil-lymphocyte ratio; ΔNLR: Change of NLR, postoperative NLR – preoperative NLR.
AUC: Area under the receiver operating characteristic curve; ROC: Receiver operating characteristic curve.
The apparent line represents actual nomogram performance. The bias-corrected line represents the bootstrap-corrected performance of the nomogram. The diagonal line is an ideal model, indicating 100% predictive power.
Discussion
Colorectal cancer is one of the most common malignant tumors, and according to the International Agency for Research on Cancer in 2020, the incidence of colorectal cancer ranks third in all malignant tumors with 10.0%, and the mortality rate ranks second with 9.4% [Citation1]. Currently, the treatment of colorectal cancer is still mainly surgical. PPOI is one of the most common postoperative complications of colorectal cancer that seriously affects the recovery process of patients.
Identifying the risk factors associated with PPOI is crucial for early detection and prevention. Our study identified age ≥70 years, preoperative NLR ≥4.71 and high ΔNLR as independent predictors of PPOI. In addition, we developed a nomogram to predict the occurrence of PPOI, which demonstrated a good predictive efficacy, as indicated by an AUC value of 0.738 (95% Cl: 0.661–0.815). Furthermore, the calibration curve indicated a satisfactory agreement between predicted and observed outcomes.
Previous reports have reported a range of 3–30% for the incidence of PPOI [Citation5]. However, in our study we observed a higher incidence of 38.0%, with PPOI occurring in 87 out of 229 patients who underwent radical resection for colorectal cancer. This is higher than the reported incidence in the previous literature. For example, Liang et al. reported a PPOI incidence of 21.54% (67 out of 311 patients) in those diagnosed with gastric or colorectal cancer [Citation5]. Vather et al. reported an incidence of 26.9% (88 out of 327 patients) in those who underwent elective surgery for colorectal cancer [Citation23]. The difference in incidence may be due to the imperfect implementation of the ERAS protocol at our center and the fact that 22.3% of patients still underwent surgery in an open fashion, which led to a higher rate of PPOI occurrence.
In this study, ROC curves and the Youden index were were used to analyze the metrics included in the study in order to select the cutoff values with the highest sensitivity and specificity. Supplementary Table 1 shows the cutoff values of each index and their corresponding sensitivity and specificity. Among these, the preoperative NLR had a cutoff value of 4.71, with a sensitivity of 0.172 and a specificity of 0.944. In a 2020 report, Mungan et al. established a cutoff value of 3.92 (sensitivity 0.375, specificity 0.802) for NLR [Citation24]. The authors considered that the varying nonstandardized study populations led to the NLR cutoff value’s high specificity and low sensitivity. Our research team consider that the low sensitivity was mainly due to the fact that NLR is calculated from the ratio of neutrophils to lymphocytes, and there are many factors that can lead to an increase in NLR in a clinical setting. By setting a cutoff value of 4.71, we achieved high specificity in diagnosing PPOI by excluding most of the clinic’s factors that affect NLR, although sensitivity was compromised. It is important to acknowledge that this was a review, single-center study with a limited sample size, and the calculated cutoff value’s low sensitivity might be attributed to data bias. Consequently, its clinical applicability might be somewhat affected. To gain a more robust and generalizable understanding, a larger scale, multicenter and prospective study will be essential in the future.
In this study, age ≥70 years was identified as an independent risk factor for developing PPOI after radical resection for colorectal cancer. This may be attributed to the fact that older individuals have a poorer nutritional status and gastrointestinal motility function compared with younger individuals, which necessitates a longer recovery period [Citation25]. In addition, elderly individuals tend to have a higher prevalence of comorbid diseases such as hypertension, diabetes mellitus and coronary heart disease [Citation26]. Therefore, perioperative care for elderly patients aged ≥70 years should be more alert to the risk of PPOI, such as nutritional risks, fluctuations in blood pressure and blood glucose levels, and the presence of underlying cardiopulmonary diseases, with individualized interventions aimed at carefully selecting and implementing ERAS protocols.
The tumor microenvironment, especially the inflammatory response, plays a crucial role in tumor development and may be associated with systemic inflammation [Citation27]. Recently, the NLR has been recognized as an indicator of poor prognosis in various solid tumors, including colorectal, breast and lung cancers [Citation13,Citation28,Citation29]. In this study, a preoperative NLR ≥4.71 was identified as an independent risk factor for the development of PPOI following radical resection for colorectal cancer. Previous studies have reported that tumors secrete various growth factors, such as TNF-α, granulocyte colony-stimulating factor, IL-1 and IL- 6, which can increase the number of neutrophils at the tumor site [Citation30]. Neutrophils can promote tumor growth and metastasis by remodeling the extracellular matrix, releasing reactive oxygen species, nitric oxide and arginase to inhibit T-lymphocyte responses, and increase mutation rates [Citation31]. An increased number of neutrophils and suppression of T lymphocytes elevate NLR. Hence, patients with larger tumors and advanced staging often exhibit increased infiltration of inflammatory cells, such as neutrophils, at the tumor site. This enhanced inflammatory response may be reflected, to some extent, by an elevated preoperative NLR, manifested as preoperative NLR ≥4.71. Such patients may present with poorer preoperative bowel status, characterized by intestinal edema or adhesions, which can lead to more difficult surgeries, more intraoperative bowel irritation and extended surgical duration. Consequently, these factors contribute to a higher incidence of postoperative PPOI. For patients with preoperative NLR ≥4.71, clinicians should be vigilant and adopt a more cautious perioperative preparation strategy, avoid redundant operations during surgery and reduce the irritation to the intestine, so as to reduce the incidence of PPOI.
Previous studies have demonstrated that dynamic changes in NLR, defined as ΔNLR (postoperative NLR/preoperative NLR), are an independent prognostic factor in patients with stage II or III colorectal cancer who underwent radical resection [Citation17]. Similarly, Chen et al. reported that dynamic changes in SII, defined as ΔSII (postoperative SII–preoperative SII), are an independent prognostic factor in patients undergoing radical resection for colorectal cancer [Citation18]. In our study, we investigated the relationship between ΔNLR and ΔSII and the development of PPOI after radical resection for colorectal cancer. Our results indicate that ΔNLR is an independent risk factor for PPOI. ΔNLR reflects the changes in systemic inflammation in patients before and after surgery and the effect of surgery on inflammatory status. Sui et al. reported in 2022 that neutrophils are closely associated with POI, and increased neutrophil infiltration was observed in a model of POI induced by intestinal manipulation [Citation19]. The mechanism may be related to the activation of intestinal resident macrophages by intestinal manipulation or surgical trauma, leading to the release of multiple cytokines, including IL-10, which promotes leukocyte aggregation and inhibits gastrointestinal function by affecting intestinal muscles and nerves [Citation19]. IL-10 can also influence neutrophil migration to the site of trauma by regulating neutrophil chemokine expression. IL-10 deficiency reduces neutrophil exudation into the intestinal wall and improves paralytic intestinal obstruction [Citation19]. The article also mentioned JAK1 and MAPK2 as being associated with neutrophil infiltration [Citation19]. Based on these multiple pathways, which may lead to elevated postoperative NLR and subsequently elevated ΔNLR, a high ΔNLR can reflect to some extent that the surgical procedure is more irritating to the intestinal tract, and that the surgery is more difficult and prolonged, and that such patients may have a higher incidence of PPOI.
We developed and internally validated a nomogram based on the identified independent risk factors. The AUC values for the training and validation groups were 0.738 (95% CI: 0.661–0.815) and 0.697 (95% CI: 0.560–0.834), respectively, and the calibration curves also showed positive agreement. The calibration curves further affirmed the nomogram’s accuracy. In comparison, Sugawara et al. constructed a nomogram to predict PPOI after major abdominal surgery based on smoking history, surgical approach and open surgery usage, yielding an AUC value of 0.71 (95% CI: 0.66–0.77) [Citation32]. Similarly, Lin et al. developed a nomogram considering age, gender, surgical access and intraoperative fluid overload, achieving AUCs of 0.779 (95% CI: 0.736–0.822) for the training group and 0.791 (95% CI: 0.677–0.905) for the external validation group [Citation7], surpassing the AUC values reported in our study. The higher AUC values in the studies by Lin et al. might be attributed to their larger sample size (1254 cases), which mitigates potential biases. Nevertheless, our study incorporated NLR and ΔNLR as objective clinical indicators, enhancing the precision and objectivity of PPOI prediction. Our nomogram may serve as an early warning system for PPOI after radical resection of colorectal cancer. It can aid in the stratification of postoperative patients for different interventions based on their risk levels, accelerate the postoperative recovery process and achieve precise diagnosis and treatment.
For patients with colorectal cancer, we recommend conducting blood collection for NLR assessment upon admission, before any clinical intervention, to gain crucial baseline data. Based on the patient’s age and preoperative NLR, individualized perioperative interventions should be implemented. These interventions may encompass active nutritional support, meticulous blood pressure and blood glucose control, and comprehensive management of other underlying medical conditions. Particularly, patients with elevated preoperative NLR levels may exhibit poorer bowel status, necessitating a judicious surgical plan guided by additional tests and imaging. Furthermore, we advocate postoperative day 1 as an essential checkpoint for blood collection and ΔNLR calculation. By utilizing our nomogram, we can accurately gauge each patient’s risk of developing PPOI. Early preventive measures should be promptly initiated, including gum chewing, abdominal warm compresses and early mobilization to mitigate the occurrence of PPOI and promote a smooth postoperative recovery. By following this comprehensive approach, we aim to improve patient outcomes and optimize the management of colorectal cancer patients undergoing surgery.
However, this study has several limitations. First, this is a review research with a small sample size. The clinical data collected may be biased, and some variables may not have been collected. Second, the nomograms were created based on data from our center alone, which may introduce bias. Third, the use of medications for pain control, particularly opioids, was not considered, which may have an impact on PPOI. In the future, the nomogram can be further improved by designing prospective, multicenter and large-sample experiments, incorporating more clinical factors that may affect the PPOI and developing clinical indicators with both specificity and sensitivity in order to improve the predictive efficacy.
Conclusion
Age, preoperative NLR and ΔNLR are independent risk factors for PPOI. The nomogram established based on independent risk factors has a good predictive performance and may guide clinicians to individualize the diagnosis and treatment.
Colorectal cancer is one of the common tumors worldwide, and prolonged postoperative ileus (PPOI) is one of the common complications after colorectal cancer surgery.
Various inflammatory biomarkers (neutrophil–lymphocyte ratio, platelet–lymphocyte ratio, lymphocyte–monocyte ratio, systemic immune-inflammation index, prognostic nutritional index (PNI) and dynamic changes in neutrophil–lymphocyte ratio and systemic immune-inflammation index) have been considered prognostic indicators for various malignancies.
The relationship between various inflammatory markers and PPOI remains uncertain.
In this study, receiver operating characteristic curves and Youden index were were used to analyze the metrics included in the study in order to select the cutoff values with the highest sensitivity and specificity.
Age, preoperative NLR and dynamic changes in NLR have been identified as independent risk factors for PPOI.
The nomogram constructed using these risk factors have demonstrated improved predictive performance, and could potentially assist clinicians in making individualized diagnoses and treatment plans.
This study still has certain limitations, such as a relatively small sample size and its review nature being single center.
Further research is warranted in the future, incorporating additional parameters to enhance the predictive efficacy.
Author contributions
All authors have substantial contributions to conception and design, or acquisition of data, analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the published version.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Supplemental Table 1
Download MS Word (33 KB)Acknowledgments
The authors thank all the participants in this study.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/bmm-2023-0355
Financial disclosure
This work was supported by Science and Technology Project for Youth of Chaoyang Central Hospital, Liaoning Province, China. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Sung H , Ferlay J , Siegel RL , Laversanne M , Soerjomataram I , Jemal A , Bray F . Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
- Shinji S , Yamada T , Matsuda A et al. Recent advances in the treatment of colorectal cancer: a review. J. Nippon. Med. Sch. 89(3), 246–254 (2022).
- Wells CI , Milne TGE , Seo SHB et al. Post-operative ileus: definitions, mechanisms and controversies. ANZ J. Surg. 92(1–2), 62–68 (2022).
- Wolthuis AM , Bislenghi G , Lambrecht M et al. Preoperative risk factors for prolonged postoperative ileus after colorectal resection. Int. J. Colorectal. Dis. 32(6), 883–890 (2017).
- Liang WQ , Zhang KC , Li H et al. Preoperative albumin levels predict prolonged postoperative ileus in gastrointestinal surgery. World J. Gastroenterol. 26(11), 1185–1196 (2020).
- Vather R , Trivedi S , Bissett I . Defining postoperative ileus: results of a systematic review and global survey. J. Gastrointest. Surg. 17(5), 962–972 (2013).
- Lin Z , Li Y , Wu J , Zheng H , Yang C . Nomogram for prediction of prolonged postoperative ileus after colorectal resection. BMC Cancer 22(1), 1273 (2022).
- Mao H , Milne TGE , O’Grady G , Vather R , Edlin R , Bissett I . Prolonged postoperative ileus significantly increases the cost of inpatient stay for patients undergoing elective colorectal surgery: results of a multivariate analysis of prospective data at a single institution. Dis. Colon. Rectum. 62(5), 631–637 (2019).
- Gustafsson UO , Scott MJ , Hubner M et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 43(3), 659–695 (2019).
- Bardram L , Funch-Jensen P , Jensen P , Crawford ME , Kehlet H . Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 345(8952), 763–764 (1995).
- Ni X , Jia D , Chen Y , Wang L , Suo J . Is the Enhanced Recovery After Surgery (ERAS) program effective and safe in laparoscopic colorectal cancer surgery? A meta-analysis of randomized controlled trials. J. Gastrointest. Surg. 23(7), 1502–1512 (2019).
- Mármol I , Sánchez-de-Diego C , Pradilla Dieste A , Cerrada E , Rodriguez Yoldi MJ . Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 18(1), 197 (2017).
- Qian C , Cai R , Zhang W et al. Neutrophil-lymphocyte ratio and circulating tumor cells counts predict prognosis in gastrointestinal cancer patients. Front. Oncol. 11, 710704 (2021).
- Hu B , Yang XR , Xu Y et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 20(23), 6212–6222 (2014).
- Noh GT , Han J , Cho MS et al. Impact of the prognostic nutritional index on the recovery and long-term oncologic outcome of patients with colorectal cancer. J. Cancer Res. Clin. Oncol. 143(7), 1235–1242 (2017).
- Chan JC , Chan DL , Diakos CI et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann. Surg. 265(3), 539–546 (2017).
- Ashizawa N , Furuya S , Katsutoshi S et al. Clinical significance of dynamic neutrophil-lymphocyte ratio changes in patients with colorectal cancer. Anticancer Res. 40(4), 2311–2317 (2020).
- Chen Q , Wu H , Guo X et al. The change of systemic immune-inflammation index independently predicts survival of colorectal cancer patients after curative resection. Mediators Inflamm. 2020, 4105809 (2020).
- Sui C , Tao L , Bai C et al. Molecular and cellular mechanisms underlying postoperative paralytic ileus by various immune cell types. Front. Pharmacol. 13, 929901 (2022).
- Wehner S , Vilz TO , Stoffels B , Kalff JC . Immune mediators of postoperative ileus. Langenbecks Arch. Surg. 397(4), 591–601 (2012).
- Kim Y , Kim YM , Kim JH , Youn YH , Kim JW , Park H . Peri-operative inflammatory marker as a predictive factor for prolonged post-operative ileus after gastrectomy for gastric cancer. J. Neurogastroenterol. Motil. 27(4), 588–595 (2021).
- Yatabe S , Eto K , Haruki K et al. Signification of systemic immune-inflammation index for prediction of prognosis after resecting in patients with colorectal cancer. Int. J. Colorectal. Dis. 35(8), 1549–1555 (2020).
- Vather R , Josephson R , Jaung R , Robertson J , Bissett I . Development of a risk stratification system for the occurrence of prolonged postoperative ileus after colorectal surgery: a prospective risk factor analysis. Surgery 157(4), 764–773 (2015).
- Mungan İ , Dicle ÇB , Bektaş Ş et al. Does the preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer? Mil. Med. Res. 7(1), 9 (2020).
- Liang WQ , Zhang KC , Cui JX et al. Nomogram to predict prolonged postoperative ileus after gastrectomy in gastric cancer. World J. Gastroenterol. 25(38), 5838–5849 (2019).
- Lin Z , Li Y , Wu J , Zheng H , Yang C . Nomogram for prediction of prolonged postoperative ileus after colorectal resection. BMC Cancer 22(1), 1273 (2022).
- Templeton AJ , McNamara MG , Šeruga B et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J. Natl Cancer Inst. 106(6), dju124 (2014).
- Duan J , Pan L , Yang M . Preoperative elevated neutrophil-to-lymphocyte ratio (NLR) and derived NLR are associated with poor prognosis in patients with breast cancer: a meta-analysis. Medicine (Baltimore) 97(49), e13340 (2018).
- Tsukioka T , Izumi N , Komatsu H et al. Elevation of neutrophil-to-lymphocyte ratio is a significant poor prognostic factor in completely resected centrally located lung squamous cell carcinoma. In Vivo 36(5), 2303–2307 (2022).
- Zou ZY , Liu HL , Ning N , Li SY , DU XH , Li R . Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol. Lett. 11(3), 2241–2248 (2016).
- Azab B , Shah N , Radbel J et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med. Oncol. 30(1), 432 (2013).
- Sugawara K , Kawaguchi Y , Nomura Y et al. Perioperative factors predicting prolonged postoperative ileus after major abdominal surgery. J. Gastrointest. Surg. 22(3), 508–515 (2018).