Summary
Thermography uses the pattern of heat measured on the skin surface to image the breast. This review examines the evidence regarding thermography for breast cancer screening, diagnosis and risk prediction. A systematic literature search was conducted and qualitative and quantitative data were extracted and summarized. Seventeen studies (nonrandomized trials) met the inclusion criteria. Four studies reported thermography accuracy for screening (60,802 women): median sensitivity was 47% (range 25–70%) and the median false-positive rate was 31% (range 21–38%). Twelve studies reported thermography accuracy (9887 women) in the context of a known clinical or mammographic abnormality: median sensitivity was 59% (range 25–97%). Two studies found no association between abnormal thermography and an increased 5-year risk of developing breast cancer. The sensitivity of thermography in breast cancer detection is low and the evidence does not support thermography as an alternative to screening mammography or for the investigation of symptoms.
▪ Thermography is a noninvasive imaging test using a digital infrared scanner to generate a ‘heat map‘ of the breast. It aims to detect breast cancer and is based on the assumption that the skin overlying a breast cancer may have a higher temperature than the skin overlying normal breast tissue or benign breast changes.
▪ This work systematically examines the evidence on breast thermography in relation to its proposed uses as:
– A screening test for breast cancer detection in asymptomatic women;
– A diagnostic test to assist in the investigation of women with breast symptoms, clinical findings or abnormalities on a mammogram or ultrasound;
– A tool to assess the risk of future breast cancer.
▪ This review did not identify any randomized trials of breast thermography. Across studies, the median sensitivity of thermography was 47% for screening and 59% for diagnosis in women with symptoms or known mammographic abnormalities. In both screening and diagnostic settings, the false-positive rate of thermography was high (median 31% for screening and 29% for diagnosis).
▪ There was no evidence to support thermography for the assessment of future breast cancer risk.
▪ Overall, the evidence does not support the use of thermography for any of its proposed indications. As thermography is often marketed directly to consumers, it is important that health professionals are able to provide evidence-based breast care advice to their patients.
The concept of thermography (infrared thermal imaging or digital infrared thermal imaging) as a breast imaging modality holds appeal. From a biological perspective, a test that detects the heat generated by increased blood flow into a tumor is scientifically plausible. From a consumer perspective, there is a strong demand for an imaging test that is painless and does not require exposure to radiation.
In 1956, Lawson described changes in surface temperature related to an underlying breast cancer, and in the following year, he proposed thermography as a technology to translate these temperature changes to images by using a camera to detect the infrared radiation emitted from the skin, and proposed that such technology could translate into an accurate test for breast cancer Citation[1,2]. Since then, more than 55 years have passed and thermographic technology has advanced, with the development of digital imaging and computer-aided diagnostic methods. Despite these technologic advances, it remains unclear whether there has been significant improvement in test accuracy in the more recent clinical studies of thermography Citation[3,4] compared with much earlier studies Citation[5,6]. Most studies have reported the sensitivity of thermography to be less than 50% Citation[3–5,7,8] and lower than the sensitivity of clinical breast examination (CBE) Citation[5,6,8].
Thermography was used in conjunction with mammography and CBE in the Breast Cancer Detection and Demonstration Project (BCDDP) in the 1970s and 1980s Citation[5,7]. The thermography component of screening was discontinued in 1978 due to low sensitivity. Thermography has largely been abandoned by the medical community and warnings against its use have been issued by government agencies in several countries around the world (including the US FDA) Citation[101–103]. Yet thermography remains available and is directly promoted to consumers as a safe alternative to mammographic screening in private clinics in several territories, including the USA, Australasia and the UK Citation[9].
Thermography, as it is currently available, is a noninvasive imaging test that generally uses a digital infrared scanner to generate a ‘heat map‘ of the breast. It is based on the assumption that the skin overlying a breast cancer may have a higher temperature than the skin overlying normal breast tissue or benign breast changes. Prior to the procedure, the patient waits in the imaging room with the breasts exposed for approximately 15 min for the body to reach a ‘temperature equilibrium‘ in the conditions of the room. The images are then acquired by placing the patient in a sitting position in front of the infrared camera. The image is produced and is analyzed by a reader looking at the image to identify areas of asymmetry or focal areas of increased temperature. Computer-aided interpretation may also be used. Sometimes, additional images are acquired following a period of cooling (when cool air is blown across the skin of the breast) and the images are compared with the baseline images.
The aim of this study was to systematically review the published literature in order to describe the accuracy of thermography in the assessment of breast cancer risk, screening (in asymptomatic women) and diagnosis (women with breast symptoms or abnormal clinical findings). The study aimed to appraise the quality of studies and to explore reasons for reported differences in test accuracy in these different clinical settings.
Methods
▪ Identification of studies
This systematic review considered literature published from 1980 onwards. Studies were identified by searching MEDLINE (Ovid, January 1980–June 2012) and the Cochrane Database of Systematic Reviews, using the search terms ‘breast cancer‘, ‘thermography‘, ‘digital infrared‘, ‘imaging‘, ‘risk‘, ‘diagnosis‘ and ‘screening‘. Reference lists from eligible studies were searched and relevant studies reviewed. Abstracts were evaluated and full-text articles of eligible abstracts were examined to identify studies meeting predetermined inclusion criteria.
▪ Eligibility
Included studies were: English language primary research studies from January 1980 to June 2012 reporting data on test accuracy in women undergoing breast thermography for cancer screening (asymptomatic women), diagnosis (women with symptoms or clinical/mammographic abnormalities) or to assess the risk of future cancer. For eligibility, studies must have included data on sensitivity (defined as number true positives divided by the sum of true positives and false negatives) as a minimum accuracy measure; additional data on the false-negative rate was considered desirable. Studies were ineligible if they did not provide the minimum criterion as defined above, if they were review articles or articles solely describing variations on technology/technique or exploring mathematical algorithms, or if they reported a series of fewer than 25 patients. Studies prior to 1980 were not included as it was considered that the technology used in thermography and the comparator tests prior to this time are no longer in use, limiting the clinical relevance of older studies.
▪ Review of studies & data extraction
Abstracts and relevant full-text articles were reviewed and data were extracted by the authors according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines Citation[10]. Where there was uncertainty or discrepancy, consensus was reached following discussion between authors. Studies with unclear eligibility or data reporting were discussed and resolved by consensus. Quality variables that included the study design, selection of subjects (consecutive or not), blinding of reporting and the reference standard were extracted. Using information from these variables that helped judge the likelihood of bias or substantial limitations, each study was assigned an overall quality category (based on quality variables, with consensus reached between both authors for each individual study) Citation[104]. There were five categories used to allocate a quality score (adapted from recommendations on test reviews): good, fair–good, fair, poor–fair and poor Citation[104]. Descriptive data (indication for test and technology used) and quantitative data (cancer prevalence and accuracy or detection data) were extracted and summarized in evidence tables.
▪ Statistical methods
Summary statistics (median and range) were used to describe the studies, subjects and accuracy. Due to the heterogeneity of various parameters across studies (population, technology used and reference standards), a formal meta-analysis was not attempted. Several diagnostic studies reported accuracy for different thermography reporting or interpretation methods; for these studies, the mean sensitivity for the study was used in the calculation of the median sensitivity across studies.
Results
▪ Studies
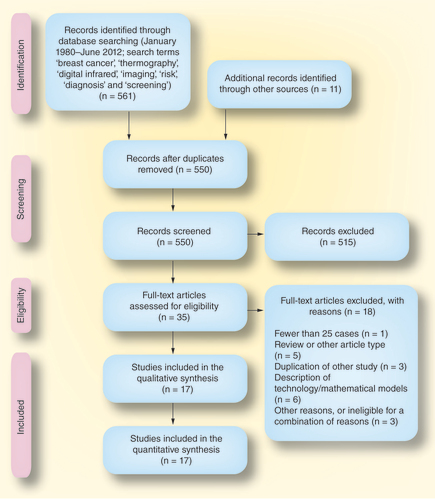
The literature search and process of screening studies is outlined in (based on the PRISMA recommendations) Citation[10]. The search identified 561 studies; 17 studies met the eligibility criteria and were included in the review Citation[3–8,11–21]. There were four studies of thermography as a screening test Citation[5,7,13,21] (three prospective Citation[7,13,21] and one retrospective Citation[5]), 12 studies of thermography as a diagnostic test Citation[3,4,6,8,11,12,14,16–20] (nine prospective Citation[3,4,6,11,16–20] and three retrospective Citation[8,12,14]) and one prospective study of thermography as a predictor of future cancer risk Citation[15]. One of the screening studies also provided data on the future risk of breast cancer associated with a positive thermogram Citation[21]. No randomized controlled trials were identified.
▪ Thermography as a screening test
Four studies reported the accuracy of screening thermography in 60,802 women Citation[5,7,13,21]. Data on the age of the samples were incomplete. Three of the studies were conducted during the 1960–1970s (two as part of the BCDDP program), but were published within the timeframe of our search. The average quality rating of these studies was fair–good.
The median sensitivity for thermography alone was 47% (range 25–70%) . Sensitivity was highest (70%) in the largest study (nearly 40,000 women); however, this sensitivity should be considered in the context of a cancer detection rate (CDR) of 1.8/1000 screens for a prevalent screening round (see also the ‘Discussion‘ section) Citation[13]. The lowest screening sensitivity was reported in the BCDDP Michigan study, when thermography had a sensitivity of 25% in its incidence screening rounds (50% in the prevalence round) Citation[5]. Two studies reported results for CBE Citation[7,13]; in Haberman et al., thermography had a sensitivity of 70% (higher than CBE [58%]) Citation[13]. By contrast, Gohagan et al. (a study with a higher quality rating) found a thermography sensitivity of 44% and showed that thermography sensitivity was lower than the sensitivity of both CBE (47%) and mammography (68%) Citation[7]. Across all of the studies, the combination of thermography and CBE showed a median sensitivity of 79% (range 67–79%) Citation[7,13].
The CDR was reported for thermography alone in one of the four screening studies, and was 3.5/1000 screens Citation[21].
The median false-positive rate of thermography in the screening setting was 31% (range 21–38%).
▪ Thermography as a diagnostic test
Twelve studies reported the accuracy of thermography as a diagnostic test in 9887 women with a symptom, a clinical finding or an abnormality on mammography (Supplementary Table) Citation[3,4,6,8,11,12,14,16–20]. The median of the mean or median age of samples across studies was 51 years. The diagnostic studies were generally more recent than the screening studies. The average quality rating of these studies was poor–fair. The main factors affecting study quality were the lack of blinding of readers of the thermography to the results of other tests, usually CBE and mammography, and/or the selection of subjects with known suspicious lesions or masses on mammography and/or CBE requiring biopsy.
The median sensitivity for thermography alone in the diagnostic setting (12 studies) was 59% (range 25–97%) (Supplementary Table 1). When the studies with a quality rating lower than ‘fair‘ were excluded, median sensitivity dropped to 31% (range 25–47%, data from four studies Citation[3,8,12,19]. When the studies with a quality rating lower than ‘fair‘ were excluded, median sensitivity dropped to 31% (range 25–47%, data from four studies Citation[3,8,12,19]). The highest sensitivities (95% Citation[11] and 97% Citation[18]) were seen in two studies that selected patients with known suspicious clinical or mammographic findings. In these studies, the sensitivity/specificity pairs showed that while the thermography sensitivity was remarkably high (unlike the majority of the other studies and unlike the average estimate), the specificity was extremely low (12% Citation[11] and 14% Citation[18]), showing that thermography has limited ability to discriminate between cancer and benign change, even in highly selected clinical series Citation[11,18].
Five diagnostic studies also reported results for CBE in direct comparison with thermography; median CBE sensitivity was 61% (range 51–86%) Citation[6,8,12,14,19]. In three of these studies, CBE had higher sensitivity than thermography Citation[8,12,19].
Two studies reported the sensitivity of thermography according to tumor size Citation[12,17]. These showed much lower sensitivity in T1 tumors (26–37%) compared with larger (T2–4) tumors (up to 82%) Citation[12,17]. The median false positive rate of thermography in the diagnostic setting was 29% (range 8–86%).
The median false-positive rate is consistent across both screening (median 31%) and diagnostic studies (29%), suggesting that, on average, thermography will be falsely positive in nearly a third of women.
▪ Thermography as a predictor of risk
Thermography was examined as a predictor of future breast cancer risk in two studies Citation[15,21]. One of these was designed as a study to assess thermography in population screening and it integrated follow-up to determine future risk Citation[21]. These were both prospective studies that included 20,772 women undergoing thermography, with minimal losses to follow-up (follow-up in >99% in Moskowitz Citation[15] and in >96% in Williams et al.Citation[21]). Neither study found an abnormal thermogram to be associated with an increase in the 5-year risk of developing breast cancer Citation[15,21].
Discussion
This review did not identify any randomized trials of thermography in breast imaging and the quality of the included studies was generally low, especially the studies of thermography in breast diagnosis. In addition, there was much heterogeneity among the studies with regard to study design (including blinding of reporting and use of comparator tests) and the thermography technology that was being tested. These limitations are considered in the interpretation of the findings of this review.
There were relatively few studies of thermography as a screening test but these studies were generally of higher quality than the diagnostic studies. These studies clearly indicate that the sensitivity of thermography in screening was low (median 47%) Citation[5,7,13,21] and the only study to report a CDR for thermography as a standalone screening test showed a CDR of 3.5/1000 Citation[21]. To provide context, in comparison, a first screening round using mammography screening generally results in a CDR above 5–6/1000 screens Citation[105]. Haberman et al. (the lowest-quality study) found the highest sensitivity (70%), but reported a CDR for combined thermography and CBE of only 1.4/1000 screens for a prevalent population screening round Citation[13]. This very low rate suggests that some, or probably many, cancers were not identified in the study population Citation[13], which would substantially overestimate the reported thermography sensitivity. Gohagan et al. found a sensitivity for thermography of 44% and found it to be lower than for mammography (68%) and even lower than CBE (47%) Citation[7].
In the studies of thermography in the diagnostic setting, and despite the various design limitations that are highlighted in the evidence tables and the ‘Results‘ section (which would give an overestimated accuracy for several studies Citation[11,14,18,20]), median sensitivity was 56% (range 25–97%) Citation[3,4,6,8,11,12,14,16–20]. Thermography sensitivity was lowest for the smaller tumors (15–32% for T0/T1 tumors in one study Citation[17] and 26–55% in another Citation[12]), which are precisely those that are associated with a survival benefit related to early detection and that screening aims to detect. Importantly, some of the relatively recent diagnostic studies (Supplementary Table 1) proposed thermography as an adjunct test for suspicious mammographic and/or clinical findings Citation[4,11,18,20] proposed thermography as an adjunct test for suspicious mammographic and/or clinical findings Citation[4,11,18,20], yet failed to compare thermography to other established adjunctive breast imaging tests (e.g., ultrasound or MRI), further limiting the clinical implications and value of the reported data. Therefore, we cannot infer from these studies how thermography as an adjunct test compares with currently accepted adjunct tests. A notable exception is the study from Kontos et al., which reported on thermography relative to mammography and ultrasound separately, and found that the sensitivity of thermography alone (25%) was much lower than the sensitivity of mammography alone (84%) or of ultrasound alone (88%) Citation[3].
In the studies comparing thermography with CBE, thermography sensitivity often approximated (or was slightly lower than) CBE. In the one study with an unusually high CBE sensitivity (relative to other diagnostic studies reporting CBE data in Supplementary Table 1), Ciatto et al. reported that the sensitivity of CBE alone was 86%, while that of thermography alone was 47% Citation[12]), Ciatto et al. reported that the sensitivity of CBE alone was 86%, while that of thermography alone was 47% Citation[12]. The CBE sensitivity from studies of symptomatic women with relatively large tumors Citation[12] does not reflect CBE sensitivity in asymptomatic women when CBE is used for early detection of breast cancer (range of 52–59% in contemporary screening settings) Citation[22,23].
In both screening and diagnostic settings, the false-positive rate of thermography was high (31% for screening and 29% for diagnosis). This compares with rates of 3–7% and generally less than 10% for mammographic screening worldwide (depending on prevalent or incident rounds) Citation[24,25,105]. The use of thermography as a standalone screening test would result in additional expense and anxiety for women undergoing the test, and this cannot be justified as a trade-off for the purposes of screening, as the sensitivity is so low.
Conclusion & future perspective
In conclusion, thermography has never been evaluated in a randomized controlled trial and there is heterogeneity among studies assessing this technology. The sensitivity of thermography is low (47% as a screening test and 59% as a diagnostic test). This is substantially lower than the sensitivity of mammography. The CDR of the test is unacceptably low and the false-positive rate is high by current screening standards. The evidence, therefore, does not support the use of thermography as an alternative to screening mammography or as a tool for the investigation of symptomatic women; the evidence strongly suggests that thermography should not be used as a tool for the early detection of breast cancer. The continued marketing of thermography to consumers as an alternative breast screening test, particularly without informed consent regarding thermography‘s inferior accuracy (and high false-positive rate) has the potential to undermine evidence-based breast-screening programs and, importantly, has the potential to harm women through the provision of screening that has a limited ability to detect breast cancer.
With improvements in technology and the continued consumer desire for low-cost, radiation-free screening tests, there may be future potential to reconsider thermography as a breast imaging modality. Its performance would need to be assessed in rigorous clinical trials, and it appears unlikely that thermography will form part of breast practice in the foreseeable future.
Table 1. Studies of thermography as a screening test: quality appraisal and accuracy (ordered by year of publication)†.
Table 2. Studies of thermography as a predictor of breast cancer risk in asymptomatic women: quality appraisal and risk outcomes (ordered by year of publication).
Financial & competing interests disclosure
This work is supported by the Australian National Health and Medical Research Council (Program Grant 633003 to the Screening and Test Evaluation Program). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Supplemental Material
Download MS Word (210 KB)Additional information
Funding
References
- Lawson R . Implications of surface temperatures in the diagnosis of breast cancer. Can. Med. Assoc. J.75(4),309–311 (1956).
- Lawson R . Thermography; a new tool in the investigation of breast lesions. Can. Serv. Med. J.8(8),517–524 (1957).
- Kontos M , WilsonR, FentimanI. Digital infrared thermal imaging (DITI) of breast lesions: sensitivity and specificity of detection of primary breast cancers. Clin. Radiol.66(6),536–539 (2011).
- Wishart GC , CampisiM, BoswellMet al. The accuracy of digital infrared imaging for breast cancer detection in women undergoing breast biopsy. Eur. J. Surg. Oncol. 36(6),535–540 (2010).
- Threatt B , NorbeckJM, UllmanNS, KummerR, RosellePF. Thermography and breast cancer an analysis of a blind reading. Ann. NY Acad. Sci.335, 501–527 (1980).
- Goldberg IM , SchickPM, PilchY, ShabotMM. Contact plate thermography: a new technique for diagnosis of breast masses. Arch. Surg.116(3),271–273 (1981).
- Gohagan JK , RodesND, BlackwellCWet al. Individual and combined effectiveness of palpation, thermography, and mammography in breast cancer screening. Prev. Med. 9(6),713–721 (1980).
- Negri S , BonettiF, CapitanioA, BonzaniniM. Preoperative diagnostic accuracy of fine-needle aspiration in the management of breast lesions: comparison of specificity and sensitivity with clinical examination, mammography, echography, and thermography in 249 patients. Diagn. Cytopathol.11(1),4–8 (1994).
- Lovett KM , LiangBA. Risks of online advertisement of direct-to-consumer thermography for breast cancer screening. Nat. Rev. Cancer11(12),827–828 (2011).
- Liberati A , AltmanDG, TetzlaffJet al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6(7),e1000100 (2009).
- Arora N , MartinsD, RuggerioDet al. Effectiveness of a noninvasive digital infrared thermal imaging system in the detection of breast cancer. Am. J. Surg. 196(4),523–526 (2008).
- Ciatto S , PalliD, Rosselli del TurcoM, CatarziS. Diagnostic and prognostic role of infrared thermography. Radiol. Med.74(4),312–315 (1987).
- Haberman JD , LoveTJ, FrancisJE. Screening a rural population for breast cancer using thermography and physical examination techniques: methods and results – a preliminary report. Ann. NY Acad. Sci.335, 492–500 (1980).
- Keyserkingk JR , AhlgrenPD, YuE, BelliveauN. Infrared imaging of the breast: initial reappraisal using high resolution digital technology in 100 successive cases of stage I and II breast cancer. Breast J.4, 245–251 (1998).
- Moskowitz M . Thermography as a risk indicator of breast cancer. Results of a study and a review of the recent literature. J. Reprod. Med.30(6),451–459 (1985).
- Ng EY , UngLN, NgFC, SimLS. Statistical analysis of healthy and malignant breast thermography. J. Med. Eng. Technol.25(6),253–263 (2001).
- Ohashi Y , UchidaI. Applying dynamic thermography in the diagnosis of breast cancer. IEEE Eng. Med. Biol. Mag.19(3),42–51 (2000).
- Parisky YR , SardiA, HammRet al. Efficacy of computerized infrared imaging analysis to evaluate mammographically suspicious lesions. AJR Am. J. Roentgenol. 180(1),263–269 (2003).
- Sterns EE , CurtisAC, MillerS, HancockJR. Thermography in breast diagnosis. Cancer50(2),323–325 (1982).
- Wang J , ChangKJ, ChenCYet al. Evaluation of the diagnostic performance of infrared imaging of the breast: a preliminary study. Biomed. Eng. Online 9, 3 (2010).
- Williams KL , PhillipsBH, JonesPA, BeamanSA, FlemingPJ. Thermography in screening for breast cancer. J. Epidemiol. Community Health44(2),112–113 (1990).
- Bobo JK , LeeNC, ThamesSF. Findings from 752081 clinical breast examinations reported to a national screening program from 1995 through 1998. J. Natl Cancer Inst.92(12),971–976 (2000).
- Sankaranarayanan R , RamadasK, TharaSet al. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J. Natl Cancer Inst. 103, 1476–1480 (2011).
- Houssami N , TreshamJJ, FritschiL, WylieLE. BreastScreen-based mammography screening in women with a personal history of breast cancer, western Australian study. Med. J. Aust.195(8),460–464 (2011).
- Sim MJH , SivaSP, RamliIS, FritschiL, TreshamJ, WylieEJ. Effect of false-positive screening mammograms on rescreening in western Australia. Med. J. Aust.196(11),693–695 (2012).
Websites
- US FDA . FDA safety communication: breast cancer screening – thermography is not an alternative to mammography (2 June 2011). www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm257259.htm
- National Health and Medical Research Council . Statement on thermal imaging for early breast cancer detection (2012). www.nhmrc.gov.au/media/releases/2012/nhmrc-warns-thermography-not-demonstrated-effective-breast-cancer-detection
- National Breast and Ovarian Cancer Centre . Statement on the use of thermography to detect breast cancer (2010). www.health.qld.gov.au/breastscreen/breastscreen/downloads/media/Thermography%20NBOCC%20Statement.pdf
- Agency for Healthcare Research and Quality . Assessing individual study limitations (risk of bias) as a domain of quality (paper 5). Methods guide for medical test reviews (November 2010). http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=998&pageaction=displayproduct
- National Quality Management Committee of BreastScreen Australia . BreastScreen Australia National Accreditation Standards (2008). www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/br-accreditation/$File/standards.pdf