SUMMARY
Metastatic breast cancer is considered incurable, with a 5-year survival of approximately 23%. However, sequential chemotherapy regimens produce clinical benefit for patients, including prolonged progression-free and overall survival times. Eribulin is a convenient (<5 min infusion) and novel treatment that has shown activity and efficacy and tolerability in advanced breast cancer patients in Phase II and Phase III trials. Eribulin has also shown encouraging results as first-line treatment, either alone in patients with HER2- disease or in combination with trastuzumab in patients with HER2+ disease, in Phase II trials.
• Eribulin is a convenient (<5 min infusion) and novel treatment, which has established activity and efficacy in metastatic breast cancer patients in Phase II and Phase III trials. Eribulin has also shown encouraging results in Phase II trials as first-line treatment, either alone in patients with HER2- disease or in combination with trastuzumab in patients with HER2+ disease.
• Eribulin has an acceptable safety/tolerability profile comparable to other chemotherapy regimens. Common side effects (≥25%) included neutropenia, anemia, asthenia/fatigue, alopecia, peripheral neuropathy, nausea and constipation.
• Eribulin has demonstrated a lower incidence of severe neuropathy compared with ixabepilone and paclitaxel and the incidence of grade 3/4 neutropenia with eribulin was comparatively less than with ixabepilone, vinorelbine and docetaxel as observed from Phase III trial cumulative data.
• In addition, eribulin also has a low probability of drug–drug interactions in the clinical setting, easy administration as bolus, low hypersensitivity chances and full tolerability in renal dysfunction patients.
• Eribulin’s place in the treatment paradigms for breast cancer is still evolving, based upon ongoing Phase II and III efficacy and tolerability data in breast cancer. The available data and real-world experience establish eribulin as an attractive single agent for use in metastatic breast cancer beyond first-line usage.
• Eribulin should be further tested as first-line treatment in advanced breast cancer, in the adjuvant and neoadjuvant setting, in combination with a variety of chemotherapies, including biologic treatments, as well as other cancer types to accurately determine its place in therapy.
Background
Breast cancer is the most commonly diagnosed malignancy and the second leading cause of cancer mortality among women [Citation1]. Approximately 5% of all women have metastatic breast cancer (MBC) at the time of initial diagnosis [Citation2,Citation3]. Moreover, MBC is incurable, with a 5-year survival of approximately 23% [Citation4]. In women with early-stage breast cancer, approximately 20% will develop distant metastases within 5 years of initial diagnosis and 20% will develop recurrence over 10 years following adjuvant systemic treatment [Citation5].
Current chemotherapy recommendations for MBC include both single-agent and combination regimens. Notably, there is no compelling evidence demonstrating that combination therapy is superior to sequential single-agent therapy [Citation6]; the risk of toxicity increases with combination therapy. According to recent National Comprehensive Cancer Network guidelines, preferred single agents for MBC include anthracycline, taxane, antimetabolites (capecitabine and gemcitabine), and other microtubule-disrupting agents (vinorelbine and eribulin) [Citation6]. Agents are used sequentially until patients experience progression or intolerable toxicity; many MBC patients derive benefit from multiple lines of therapy.
Indications & usage
In November 2010, the US FDA approved eribulin mesylate for the treatment of patients with MBC who have previously received more than two chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting [Citation7,Citation8]. Approval was based on favorable results from EMBRACE study, a Phase III multicenter, international, open-label, randomized clinical trial, where eribulin demonstrated statistically and clinically significant improvements in overall survival (OS) compared with a single-agent therapy selected by their physician [Citation9]. In 2014, eribulin was approved in the EU for patients with locally advanced breast cancer or MBC who have progressed after at least one chemotherapeutic regimen for advanced disease; prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting, unless patients were not suitable for these treatments.
Dosage & administration
The recommended dose of eribulin mesylate is 1.4 mg/m2 intravenously (iv.) over 2–5 min on days 1 and 8 of a 21-day cycle [Citation7]. In patients with mild hepatic impairment (Child–Pugh A), the recommended dose is 1.1 mg/m2, 0.7 mg/m2 for those with moderate hepatic impairment (Child–Pugh B), and 1.1 mg/m2 for moderate renal impairment (creatinine clearance of 30–50 ml/min), all administered iv. over 2–5 min on days 1 and 8 of a 21-day cycle [Citation7].
Clinical pharmacology
• Eribulin: mechanism of action, pharmacokinetics & pharmacodynamics
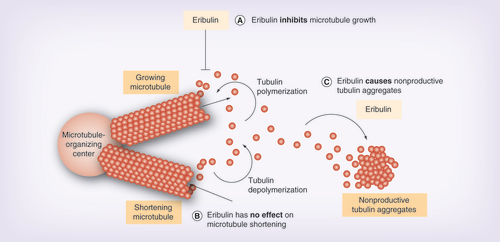
Eribulin is a structurally modified synthetic analog of halichondrin B, a natural product isolated from the marine sponge Halichondria okadai, and is a nontaxane inhibitor of microtubule dynamics. Notably, eribulin has a unique a mechanism of action compared with taxanes, epothilones and vinca alkaloids [Citation10–13]. Eribulin binds to only the growing + ends, inhibiting the microtubule growth phase without affecting the shortening phase, and causing tubulin sequestration into nonproductive aggregates ( [Citation14]) [Citation11–13,Citation15]. The effects of eribulin on microtubule dynamics during interphase result in formation of abnormal mitotic spindles, thereby suppressing the metaphase/anaphase transition [Citation11].
(A) Eribulin inhibits microtubule growth. (B) Eribulin has no effect on microtubule shortening. (C) Eribulin causes nonproductive tubulin aggregates.
Image courtesy of EISAI (NJ, USA).
Eribulin mesylate has triphasic elimination with a rapid distribution phase in plasma, slow elimination and low renal excretion [Citation16]. The pharmacokinetics of eribulin mesylate is linear, with a mean elimination half-life of approximately 40 h [Citation7]. The human plasma protein binding of eribulin mesylate at concentrations of 100–1000 ng/ml ranges from 49 to 65%. Eribulin mesylate exposure after multiple dosing is comparable to that following a single dose; no accumulation of eribulin mesylate is observed with weekly administration. Unchanged eribulin mesylate was the major circulating species in plasma following administration to patients. Eribulin mesylate is eliminated primarily in feces unchanged. After administration, approximately 82% of the dose was eliminated in feces and 9% in urine, mostly as unchanged eribulin [Citation7]. Eribulin also has a low probability of drug–drug interactions [Citation17–20], low risk of hypersensitivity [Citation21], and has been well tolerated in patients with renal dysfunction [Citation22].
Clinical evidence
• Overview of published Phase II clinical trials
In three open-label, single-arm, Phase II studies, the efficacy and tolerability of eribulin in pretreated patients with MBC (prior treatment with an anthracycline and a taxane) was assessed. Patients received eribulin mesylate (1.4 mg/m2) as a 2–5-min iv. infusion () [Citation23–25]. Vahdat et al. reported objective response rates (ORRs; by independent review; all partial responses [PRs]) of 11.5% (95% CI: 5.7–20.1) and a clinical benefit rate (CBR; PR plus stable disease [SD] ≥6 months) of 17.2% (95% CI: 10.0–26.8) [Citation23]. Cortes et al. demonstrated ORRs of 9.3% (95% CI: 6.1–13.4; all PRs), the SD rate was 46.5%, and CBR (complete response [CR] + PR + SD + 6 months) was 17.1% (95% CI: 12.8–22.1) [Citation24]. In a Japanese population, Aogi et al. reported an ORR of 21.3% (95% CI: 12.9–31.8; all PRs), SD occurred in 30 patients (37.5%), and a CBR (CR + PR + SD ≥6 months) of 27.5% (95% CI: 18.1–38.6); patients in this study were less heavily pretreated (median of three prior chemotherapy regimens, and over 75% of patients had three or fewer prior therapies) than patients in the other Phase II studies (median of four prior chemotherapy regimens, and fewer than 40% had three or fewer prior therapies), which may explain the higher ORR in this study [Citation25]. Median duration of response (DOR) was 3.9 months (95% CI: 2.8–4.9), progression-free survival (PFS) was 3.7 months (95% CI: 2.0–4.4), and OS was 11.1 months (95% CI: 7.9–15.8) [Citation25]. In all three Phase II trials, the most common treatment-related grade 3/4 toxicities were neutropenia, leukopenia, fatigue and peripheral neuropathy [Citation23–25].
First-line treatment: locally recurrent or metastatic HER2- & HER+ breast cancer
A recent Phase II, single-arm study assessed the clinical effects of eribulin mesylate (1.4 mg/m2, 2–5 min iv. on days 1 and 8 of a 21-day cycle) as first-line treatment in 56 patients with locally recurrent or metastatic HER2- breast cancer [Citation26]. ORR was 29% (95% CI: 17.3–42.2), CBR was 52% (95% CI: 38.0–65.3) and median DOR was 5.8 months (95% CI: 4.67–10.6). Median PFS was 6.8 months (95% CI: 4.44–7.59). In total, 36 patients (64%) had grade 3/4 treatment-related adverse events (AEs); the most common were neutropenia (50%), leukopenia (21%) and peripheral neuropathy (20%) [Citation26].
In another, single-arm, Phase II trial (n = 52), the effects of eribulin mesylate (1.4 mg/m2 iv. on days 1 and 8 of each 21-day cycle) plus trastuzumab (8 mg/kg iv. on day 1, followed by 6 mg/kg of trastuzumab on day 1 of each subsequent cycle) as first-line therapy in patients with locally recurrent or metastatic HER2+ breast cancer were evaluated [Citation27]. The ORR was 71.2% (95% CI: 56.9–82.9) with median time to response of 1.3 months (95% CI: 1.2–1.4), DOR of 11.1 months (95% CI: 6.7–17.8) and PFS of 11.6 months (95% CI: 9.1–13.9). The most common grade 3/4 treatment-emergent AEs were neutropenia (38.5%), peripheral neuropathy (26.9%, all grade 3), fatigue (7.7%) and febrile neutropenia (7.7%). The combination of eribulin plus trastuzumab as first-line therapy for locally recurrent or metastatic HER2+ breast cancer resulted in a substantial tumor response with long DOR and acceptable safety profile [Citation27].
Results for both Phase II trials in previously untreated advanced breast cancer (HER+ and HER-) are presented in .
Phase II feasibility studies
Two recent single-arm, Phase II trials assessed the feasibility of using eribulin as part of adjuvant combination treatment [Citation28,Citation29]. In one study, 67 women with stage I–II, HER2-, estrogen receptor-positive (ER+) breast cancer received eribulin mesylate (1.4 mg/m2 iv., 2–5 min on days 1 and 8 of a 21-day cycle) plus capecitabine as adjuvant treatment in postmenopausal ER+ early-stage breast cancer [Citation28]. The combination was considered feasible for further study if >80% of evaluable patients were able to achieve the target relative dose intensity of 85% (one-sided 95% lower CI >70%). The treatment regimen met the primary end point with a feasibility rate of 81.3% (95% lower CI: 71.4%) and a relative dose intensity of 90.6% [Citation28].
Another Phase II, single-arm study in 55 patients evaluated adjuvant treatment of patients with early-stage breast cancer with eribulin mesylate (1.4 mg/m2 iv., 2–5 min on days 1 and 8 of a 21-day cycle) plus mandated pegfilgrastim (6 mg subcutaneously on day 2 every 14 days) following dose-dense doxorubicin (60 mg/m2) and cyclophosphamide (day 1, every 14 days) [Citation29]. Preliminary results show a feasibility rate of 70% (32/46); 14 patients had a delay or reduction while on study (nonfeasible); however, the majority were still able to complete the full treatment regimen. The feasibility rate was 85% (n = 39/46) when growth factors were implemented after the event [Citation29].
Phase II clinical trial safety
In Phase II MBC studies reported here, eribulin had a manageable tolerability profile and the most common drug-related AEs were neutropenia, fatigue, alopecia, nausea and anemia [Citation23,Citation25–29]. In a pooled analysis of two Phase II trials, treatment with eribulin was associated with a low incidence of peripheral neuropathy overall and severe peripheral neuropathy, which was limited to grade 3 only () [Citation23,Citation24,Citation30].
Since peripheral neuropathy is a common toxicity associated with tubulin-targeted chemotherapeutic agents, in a recent randomized Phase II study, Vahdat et al. compared the incidence of peripheral neuropathy with eribulin versus ixabepilone in MBC patients [Citation31]. The primary objective was to assess the incidence of neuropathy; the study was designed to detect a difference in neuropathy rate of 35% for eribulin versus 63% for ixabepilone. Patients with locally recurrent/MBC (n = 104) who received prior taxane therapy, one prior chemotherapy for advanced disease, and had pre-existing neuropathy grade 0 (n = 51) or one (n = 53) were randomized to receive 1.4 mg/m2 eribulin mesylate (2–5 min iv. on days 1 and 8 of a 21-day cycle) or 40 mg/m2 ixabepilone (3 h iv. on day 1 of a 21-day cycle). Incidences of peripheral neuropathy and treatment-emergent neuropathy (all grades) with eribulin (n = 51) versus ixabepilone (n = 50) were 31.4 versus 44.0% (p = 0.16) and 33.3 versus 48.0%, respectively (). Although not statistically significant, neuropathy AEs were less common with eribulin than ixabepilone. Eribulin was associated with longer time to onset of treatment-emergent neuropathy and fewer discontinuations due to neuropathy and other toxicities. Compared with ixabepilone, fewer patients receiving eribulin discontinued treatment due to neuropathy (3.9 vs 18.0%). Time to onset of neuropathy was 35.9 and 11.6 weeks for eribulin and ixabepilone, and time to resolution was 48 versus 10 weeks, respectively [Citation31].
• Overview of Phase III clinical trials
EMBRACE trial
The EMBRACE study was a Phase III global, multicenter, randomized (2:1), open-label study (n = 762) of eribulin mesylate (1.4 mg/m2 2–5 min iv. on days 1 and 8 of a 21-day cycle) versus treatment of physician’s choice (TPC) in women with pretreated locally recurrent or metastatic breast cancer who had received two-to-five prior chemotherapies, which included an anthracycline and a taxane [Citation9]. The primary objective was met, showing a significant increase in OS for eribulin compared with TPC (hazard ratio [HR]: 0.81; 95% CI: 0.66–0.99; p = 0.041), with 274 (54%) deaths in the eribulin group and 148 (58%) deaths in the TPC group (). By independent review, eribulin PFS (median) was 3.7 (95% CI: 3.3–3.9) versus 2.2 months (95% CI: 2.1–3.4) for TPC (HR: 0.87; 95% CI: 0.71–1.05; p = 0.137) (). In the EMBRACE study, the AEs were similar to those found in the Phase I and II trials; the most common were asthenia and neutropenia [Citation9].
TPC: Treatment of physician’s choice.
Reproduced with permission from [Citation9] © Elsevier (2011).
![Figure 2. Kaplan–Meier of EMBRACE overall survival. TPC: Treatment of physician’s choice.Reproduced with permission from [Citation9] © Elsevier (2011).](/cms/asset/19c7ef38-e6af-4de4-8513-0ea273f1507a/ibmt_a_12322894_f0002.jpg)
EMBRACE: effects of dose modification
The goal for chemotherapy is to administer the full studied dose; however, for patients experiencing an AE, the impact of dose modification requires exploration [Citation32]. Data from patients receiving eribulin in the EMBRACE trial were analyzed. The primary end point was the effect of dose modification on duration of therapy. Overall, 462 patients had an AE that was treatment-related. A total of 204 patients (44.2%) had dose modification (delay only [61.8%], reduction only [17.6%] and both [20.6%]), and 258 (55.8%) did not have dose modification.
The median PFS was also longer in the dose-modification cohort (130 vs 92 days based on independent review). However, there were no statistically significant correlation between PFS and dose modification after adjusting for the length of treatment exposure using time-dependent and landmark approaches. Delaying or reducing the dose of eribulin in patients who experience AEs may allow patients to remain on therapy longer [Citation32].
Eribulin versus capecitabine
In this Phase III, open-label, randomized, multicenter study, the clinical effects of eribulin versus capecitabine in patients with locally advanced disease or MBC, previously treated with anthracyclines and taxanes, were assessed [Citation33]. Patients were randomized 1:1 to eribulin mesylate (1.4 mg/m2 iv.on days 1 and 8 of a 21-day cycle) or capecitabine (2.5 g/m2/day administered orally in divided doses twice daily on days 1–14 of a 21-day cycle). Eligible patients had received prior therapy including an anthracycline and taxane, and were receiving study drug as first-, second- or third-line therapy for advanced disease. The co-primary end points of this study were OS and PFS. Secondary end points included ORR, quality of life measured using the European Organisation for Research and Treatment of Cancer questionnaire, DOR, 1-, 2- and 3-year survival, and safety. The study was stratified for geographic region and HER2 status.
Of 1102 patients, 554 were randomized to eribulin and 548 capecitabine (375 and 380 patients were HER2-, respectively); the median age was 54.0 years (range: 24–80 years). The median number of treatment cycles was six for eribulin and five for capecitabine. Response rates were not significantly different between eribulin and capecitabine by independent and investigator review (). Median OS was 15.9 and 14.5 months (HR: 0.879; 95% CI: 0.770–1.003; p = 0.056), and PFS (independent review) was 4.1 and 4.2 months (HR: 1.079; 95% CI: 0.932–1.250; p = 0.305) for eribulin and capecitabine, respectively (A). One-year survival was 64.4% for eribulin versus 58.0% for capecitabine (p = 0.035). ORRs (independent review) were 11.0 (95% CI: 8.5–13.9) and 11.5% (95% CI: 8.9–14.5; p = 0.849), respectively. In a preplanned subgroup analysis, OS for HER2- patients was 15.9 months for eribulin and 13.5 months for capecitabine (HR: 0.838; 95% CI: 0.715–0.983; p = 0.030) (B) [Citation34]. Survival differences between arms may be confounded by treatments received beyond progression, including HER2-directed therapy in the HER2+ subgroup. Numerical and statistical differences for 1-year survival in the eribulin versus capecitabine trial and similar to the EMBRACE study.
(A) OS. (B) OS in HER2- subgroup. Data are for the intent-to-treat population.
*HR Cox model including geographic region and HER2 status as strata.
†p-value from stratified log-rank test based on clinical database.
HR: Hazard ratio; OS: Overall survival.
(A) Reproduced with permission from [Citation33]; (B) Reproduced with permission from [Citation34].
![Figure 3. Eribulin versus capecitabine. (A) OS. (B) OS in HER2- subgroup. Data are for the intent-to-treat population.*HR Cox model including geographic region and HER2 status as strata. †p-value from stratified log-rank test based on clinical database.HR: Hazard ratio; OS: Overall survival. (A) Reproduced with permission from [Citation33]; (B) Reproduced with permission from [Citation34].](/cms/asset/18c9156d-09bc-40e4-b801-3ce015cde810/ibmt_a_12322894_f0003.jpg)
From prespecified exploratory subgroup analyses of OS, patients with only nonvisceral disease (HR: 0.51; 95% CI: 0.33–0.80); with more than two organs involved (HR: 0.75; 95% CI: 0.62–0.90); those who had progressed >6 months after last chemotherapy (HR: 0.70; 95% CI: 0.52–0.95); or those who had received an anthracycline and/or a taxane in the metastatic setting (HR: 0.84; 95% CI: 0.72–0.98), appeared to benefit more from treatment with eribulin compared with capecitabine [Citation34]. No subgroup displayed a trend favoring capecitabine for OS. AEs were consistent with the known side-effect profiles of both drugs.
Eribulin in older women
Data from three studies (two Phase II trials [Citation23,Citation24] and the EMBRACE trial [Citation9]) were pooled for an exploratory analysis of the effect of age on the efficacy and safety of eribulin [Citation35]. The analysis included 827 patients (<50 years, n = 253; 50–59 years, n = 289; 60–69 years, n = 206; ≥70 years, n = 79). Age did not significantly affect the efficacy of eribulin, including OS, PFS, ORR or CBR. The overall incidence of AEs was similar among the age groups, as was the incidence of most individual AEs, including peripheral neuropathy and neutropenia. However, certain AEs (asthenia or fatigue, peripheral edema, and dizziness) had a higher incidence among older patients, while others (nausea and vomiting) had a higher incidence in the youngest cohort. AEs leading to dose reduction, delay or discontinuation increased slightly with age, although the odds ratios were not significantly different.
• Eribulin treatment patterns & outcomes
Real-world analysis of eribulin in a community setting
The clinical activity and administration characteristics of eribulin were assessed in a sample of MBC patients who received eribulin between 15 November 2010 and 31 August 2012, at eight US community oncology settings [Citation36,Citation37]. During the 19-month evaluation period, 90 MBC patients who received eribulin and met the inclusion criteria were identified. The median line of eribulin administration was the fourth and the drug was used as a single agent in 79 out of 90 patients (87.8%). The approved starting dosage of 1.4 mg/m2 on days 1 and 8, every 21 days was used in 81 out of 90 (90%) patients; treatment was continued for a median of four cycles. This translated to a 2.9-month mean duration of treatment (range: 0–14.2 months). Overall, the median number of dose reductions and delays were 1 and 1, respectively. These modest dose reductions/delays translated to a delivered dose intensity that ranged from 87 to 95% over the first eight cycles. The median time to treatment failure (interquartile range) was 4 months (2.3–5.6) in all patients.
Over a median of four cycles of eribulin, approximately 63% of patients reported more than one AE. The most common dose-limiting toxicities were neutropenia, anemia, neurotoxicity/neuropathy and febrile neutropenia. Treatment-related toxicity led to the premature discontinuation of eribulin in only eight out of 90 patients (8.9%). Progressive disease (66.6%) was the most common reason for stopping eribulin [Citation36].
Several additional recent publications have described clinical practice-based studies of eribulin treatment in patients with locally advanced disease or MBC who had received at least two prior chemotherapeutic regimens, including an anthracycline and a taxane [Citation38–40]. In a retrospective observational study in 11 Italian cancer centers (n = 133), the ORR was 21.1%, and CBR was 38.3%; over a median of five cycles, 33.8% of patients had a dose reduction, most often due to neurotoxicity, asthenia or hematological toxicity, and 4.5% of patients had eribulin discontinued due to toxicity (specifically, neurotoxicity or asthenia) [Citation38]. A retrospective study of patients treated in an Italian community hospital setting (n = 27) reported an ORR of 33.3% and CBR of 66.7% [Citation39]. The most commonly reported AEs were asthenia, peripheral sensory neuropathy, nausea and neutropenia; patients received a median of four cycles (median duration of treatment: 2.4 months), with 26% of patients having dose reductions during treatment, 37% having dose delays and 33% having day 8 doses cancelled [Citation39]. A review of data from 25 patients treated with eribulin via the London Cancer Drugs Fund found a PR rate of 16% and CBR of 41%; 64% of patients experienced some toxicity, with neutropenia and neurotoxicity as the most commonly reported AEs [Citation40].
Impact on eribulin treatment modification on treatment duration
In a retrospective cross-sectional study, dosing and treatment patterns using data from the MedAssets health system database were examined [Citation41]. Patients with MBC treated with eribulin in the hospital inpatient or outpatient setting between December 2010 and September 2012 were included. Of the 510 patients in the cohort, 34.1% had either a dose decrease (23.3%) or treatment delay (20.2%) during the course of treatment, with approximately 30% of those with a dose decrease subsequently increasing their dose (). More than 80% of the treatment modifications occurred during the first (21.3%), second (48.3%) or third (11.5%) cycle.
Reproduced with permission from [Citation41].
![Figure 4. Effect of eribulin treatment modification on duration of therapy: type and timing of dose modifications. Reproduced with permission from [Citation41].](/cms/asset/241be88d-4306-4c12-8899-b56097c01c0e/ibmt_a_12322894_f0004.jpg)
Patients with the following side effects were more likely to have a dose modification: neutropenia (40.7 vs 29.6%; p < 0.001), fatigue (46.8 vs 31.9%; p < 0.05), nausea (39.2 vs 26.5%; p < 0.001) and constipation (47.9 vs 32.7%; p < 0.05) [Citation41]. Hormone-positive patients were more likely to have dose modifications (58.6 vs 48.5%; p < 0.05), while no difference was seen in HER2+ patients (19.0 vs 17.0%; p = 0.57) or in age ≥65 years (28.7 vs 26.2%; p = 0.540) between the two cohorts [Citation41].
Conclusion
Eribulin is a convenient and novel treatment, which has established activity and efficacy in MBC including an OS advantage in heavily pretreated refractory breast cancer patients in Phase III trials. Eribulin should be further tested as first-line treatment in locally recurrent or metastatic breast cancer, and in combination with a variety of chemotherapies including biologic treatments to accurately determine the optimal place in therapy for eribulin. Additionally, feasibility studies have set the stage for the exploration of eribulin in the adjuvant and neoadjuvant setting for earlier-stage breast cancer. Research should mandate correlative studies to identify potential biomarkers predictive of both efficacy and toxicity. Finally, the mechanism of action of eribulin lends itself to study in a broad variety of solid tumors in comparison to the standard of therapy including ongoing trials in advanced non-small-cell lung cancer, prostate cancer, urothelial cancer, soft tissue sarcomas, and platinum-sensitive ovarian, fallopian and peritoneal cancers.
Table 1. Published Phase II trials for eribulin as monotherapy in advanced breast cancers.
Table 2. Summary of Phase II results: eribulin as first-line treatment in patients with previously untreated advanced HER+ and HER- breast cancer.
Table 3. Phase II neurotoxicity profile of eribulin.
Table 4. Incidence of neuropathy and sensitivity analysis of neuropathy based on a narrow definition of peripheral neuropathy by Common Terminology Criteria for Adverse Events grade.
Table 5. EMBRACE progression-free survival and best overall tumor response by independent review.
Table 6. Eribulin versus capecitabine: response rates.
Financial & competing interests disclosure
LS Schwartzberg is a consultant/advisor for and has received funding from Eisai Inc. and Helsinn. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing and editorial support was provided by Leonard Lionnet, PhD, of MedVal Scientific Information Services. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP2 Guidelines.” Funding to support the preparation of this manuscript was provided by Eisai Inc.
Additional information
Funding
References
- Jemal A , SiegelR, XuJ, WardE. Cancer statistics, 2010. CA Cancer J. Clin.60(5), 277–300 (2010).
- Johnson RH , ChienFL, BleyerA. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA309(8), 800–805 (2013).
- National Cancer Institute . SEER Stat fact sheets: breast. http://seer.cancer.gov/statfacts/html/breast.html.
- American Cancer Society . Breast cancer facts & figures 2011–2012. www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-030975.pdf.
- Brewster AM , HortobagyiGN, BroglioKRet al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J. Natl Cancer Inst.100(16), 1179–1183 (2008).
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)®: breast cancer. Version 3 2013. National Comprehensive Cancer Network, PA, USA (2013).
- Halaven® (eribulin mesylate) injection (prescribing information). Eisai Inc., NJ, USA (2013).
- US FDA . FDA approves new treatment option for late-stage breast cancer. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm233863.htm.
- Cortes J , O’ShaughnessyJ, LoeschDet al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a Phase 3 open-label randomised study. Lancet377(9769), 914–923 (2011).
- Preston JN , TrivediMV. Eribulin: a novel cytotoxic chemotherapy agent. Ann. Pharmacother.46(6), 802–811 (2012).
- Jordan MA , KamathK, MannaTet al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther.4(7), 1086–1095 (2005).
- Okouneva T , AzarenkoO, WilsonL, LittlefieldBA, JordanMA. Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol. Cancer Ther.7(7), 2003–2011 (2008).
- Smith JA , WilsonL, AzarenkoOet al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry49(6), 1331–1337 (2010).
- Swami U , ChaudharyI, GhalibMH, GoelS. Eribulin – a review of preclinical and clinical studies. Crit. Rev. Oncol. Hematol.81(2), 163–184 (2012).
- Jordan MA , KamathK. How do microtubule-targeted drugs work? An overview. Curr. Cancer Drug Targets7(8), 730–742 (2007).
- Gourmelon C , FrenelJS, CamponeM. Eribulin mesylate for the treatment of late-stage breast cancer. Expert Opin. Pharmacother.12(18), 2883–2890 (2011).
- Zhang ZY , KingBM, PelletierRD, WongYN. Delineation of the interactions between the chemotherapeutic agent eribulin mesylate (E7389) and human CYP3A4. Cancer Chemother. Pharmacol.62(4), 707–716 (2008).
- Devriese LA , Mergui-RoelvinkM, WandersJet al. Eribulin mesylate pharmacokinetics in patients with solid tumors receiving repeated oral ketoconazole. Invest. New Drugs31(2), 381–389 (2013).
- Devriese LA , WitteveenPE, WandersJet al. Pharmacokinetics of eribulin mesylate in patients with solid tumours receiving repeated oral rifampicin. Br. J. Clin. Pharmacol.75(2), 507–515 (2013).
- Zheng W , SeletskyBM, PalmeMHet al. Structure–activity relationships of synthetic halichondrin B analog E7389: in vitro susceptibility to PgP-mediated drug efflux. Presented at: Proceedings of the 94th Annual Meeting of the American Association for Cancer Research.44(2), Abstract 5751 (2003).
- Goel S , MitaAC, MitaMet al. A Phase I study of eribulin mesylate (E7389), a mechanistically novel inhibitor of microtubule dynamics, in patients with advanced solid malignancies. Clin. Cancer Res.15(12), 4207–4212 (2009).
- Synold TW , Tsao-WeiDD, QuinnDIet al. Phase I and pharmacokinetic (PK) study of eribulin (E7389) in patients (pts) with renal dysfunction (RD) and advanced urothelial cancer (UC): a California Cancer Consortium Trial [abstract]. J. Clin. Oncol.28(Suppl. 15), Abstract 2527 (2010).
- Vahdat LT , PruittB, FabianCJet al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol.27(18), 2954–2961 (2009).
- Cortes J , VahdatL, BlumJLet al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J. Clin. Oncol.28(25), 3922–3928 (2010).
- Aogi K , IwataH, MasudaNet al. A Phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann. Oncol.23(6), 1441–1448 (2012).
- McIntyre K , O’ShaughnessyJ, SchwartzbergLet al. Phase 2 study of eribulin mesylate as first-line therapy for locally recurrent or metastatic human epidermal growth factor receptor 2-negative breast cancer. Breast Cancer Res. Treat.146(2), 321–328 (2014).
- Wilks S , PuhallaS, O’ShaughnessyJet al. Phase 2, multicenter, single-arm study of eribulin mesylate plus trastuzumab as first-line therapy for locally recurrent or metastatic HER2-positive breast cancer. Clin. Breast Cancer doi:10.1016/j.clbc.2014.04.004 (2014) ( Epub ahead of print).
- Smith JW , RegeJ, ManiarH, SongJ, CoxD, O’ShaughnessyJ. Eribulin mesylate (Erib) plus capecitabine (X) for adjuvant treatment in post-menopausal estrogen receptor-positive (ER+) early-stage breast cancer: Phase II, multicenter, single-arm study [abstract]. J. Clin. Oncol.31(Suppl. 15), Abstract 563 (2013).
- Traina TA , HudisC, FornierMet al. Adjuvant treatment of early-stage breast cancer with eribulin mesylate following dose-dense doxorubicin and cyclophosphamide: preliminary results from a Phase 2, single-arm feasibility study [abstract]. Cancer Res.72(Suppl. 24), 199S (Abstract P1–13–11) (2012).
- Cortes J , MonteroAJ, GluckS. Eribulin mesylate, a novel microtubule inhibitor in the treatment of breast cancer. Cancer Treat. Rev.38(2), 143–151 (2012).
- Vahdat LT , GarciaA, VogelCLet al. Comparison of the incidence of peripheral neuropathy (PN) with eribulin versus ixabepilone (IXA) in metastatic breast cancer (MBC) patients: a randomized Phase II study [abstract]. J. Clin. Oncol.30(Suppl. 27), Abstract 121 (2012).
- Faria C , LiX, PowersA, VahdatLT. Effects of dose modification of eribulin mesylate in patients with locally recurrent or metastatic breast cancer [abstract]. J. Clin. Oncol.31(Suppl. 26), Abstract 157 (2013).
- Kaufman PA , AwadaA, TwelvesCet al. A Phase III, open-label, randomized, multicenter study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with anthracyclines and taxanes [abstract]. Cancer Res.72(Suppl. 24), 109s (Abstract S6–6) (2012).
- Kaufman PA , CortesJ, AwadaAet al. A Phase III, open-label, randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer (MBC) previously treated with anthracyclines and taxanes: subgroup analyses [abstract]. J. Clin. Oncol.31(Suppl. 15), Abstract 1049 (2013).
- Muss H , CortesJ, VahdatLTet al. Eribulin monotherapy in patients aged 70 years and older with metastatic breast cancer. Oncologist19(4), 318–327 (2014).
- Dranitsaris G , TripathyD, BeegleNL, KalbererTL, CoxJD, FariaC. Real-world analysis of eribulin in metastatic breast cancer (MBC): an assessment of time to treatment failure (TTF) in a community oncology setting. [abstract]. J. Clin. Oncol.31(Suppl. 26), Abstract 174 (2013).
- Dranitsaris G , BeegleN, KalbererT, BlauS, CoxD, FariaC. A comparison of toxicity and health care resource use between eribulin, capecitabine, gemcitabine, and vinorelbine in patients with metastatic breast cancer treated in a community oncology setting. J. Oncol. Pharm. Pract. doi:1078155214525369 (2014) ( Epub ahead of print).
- Gamucci T , MichelottiA, PizzutiLet al. Eribulin mesylate in pretreated breast cancer patients: a multicenter retrospective observational study. J. Cancer5(5), 320–327 (2014).
- Poletti P , GhilardiV, LivraghiL, MilesiL, RotaCE, TondiniC. Eribulin mesylate in heavily pretreated metastatic breast cancer patients: current practice in an Italian community hospital. Future Oncol.10(2), 233–239 (2014).
- Ramaswami R , O’CathailSM, BrindleyJH, SilcocksP, MahmoudS, PalmieriC. Activity of eribulin mesylate in heavily pretreated breast cancer granted access via the Cancer Drugs Fund. Future Oncol.10(3), 363–376 (2014).
- Faria C , BelkK, CraverC, McBrideA. Impact of treatment modification on treatment duration in metastatic breast cancer patients treated with eribulin mesylate [abstract]. Value-Based Cancer Care4(3), 26 (2013).
