SUMMARY
A cancer diagnosis during pregnancy presents a challenge to practitioners and patients. Often diagnosis is delayed. There are limited prospective case series and scant long-term neonatal and maternal data on which to base treatment plans. Also, the majority of pregnancy-associated case series include women diagnosed with breast cancer up to 1 year postpartum. The later-diagnosed group has a poorer prognosis and should be evaluated separately. To avoid attributing breast masses to pregnancy-related changes, masses should be evaluated as if the patient were not pregnant. Mammography, MRI, ultrasound, mastectomy and lumpectomy, axillary dissection, sentinel lymph node biopsy and even chemotherapy during the second and third trimesters can be considered for the pregnant patient with breast cancer.
• The diagnosis of breast cancer is often delayed during pregnancy.
• Termination of a pregnancy complicated by breast cancer has not been shown to improve maternal survival. This is true even for patients diagnosed with estrogen receptor-positive breast cancer.
• The human fetus tolerates anthracycline-based chemotherapy during the second and third trimesters.
• Safety of taxane use during pregnancy is increasingly described in the literature.
• As taxane use increases in pregnancy, patients diagnosed early in pregnancy may not be required to perform mastectomy, since taxane treatment can be used during the interval between anthracycline-based chemotherapy and postpartum radiation.
• Sentinel node biopsy using radioactive technetium-99 alone can be safely performed to avoid full axilllary dissection.
• Iatrogenic early preterm deliveries are discouraged.
Cancer is diagnosed approximately once per 1000 pregnancies, with 3500 cases estimated annually [Citation1]. As women delay starting or completing their childbearing to older maternal ages, this incidence is expected to increase. Delayed first birth is itself a risk factor for breast cancer. Approximately 1:3000 pregnancies is complicated by breast cancer [Citation1]. As a result of a delicate balance among maternal well-being, fetal safety and limited data, a cancer diagnosis during pregnancy presents a challenge to both healthcare practitioners and patients. For patients who choose to continue their pregnancies, the delay in initiating treatment to prevent fetal exposure until after delivery may be detrimental to their health and long-term survival. The biggest challenge in understanding the influence of pregnancy on the prognosis of breast cancer is that the majority of case series is not only retrospective, but also includes ‘pregnancy associated breast cancer’, which includes patients diagnosed with breast cancer during pregnancy and patients diagnosed up to 1 year postpartum. There is much data portending a worse prognosis for women diagnosed with breast cancer within the first year following a pregnancy and these cases should be evaluated separately.
The diagnosis and treatment of breast cancer during pregnancy raise several challenges that will be addressed in this review. Delays in diagnosis or unnecessary alterations of treatment standards will jeopardize the disease-free and overall survival of pregnant women with breast cancer.
Delayed diagnosis due to pregnancy
Pregnant patients with breast masses often experience a delay in diagnosis of breast cancer. Masses can be obscured by the cystic changes of pregnancy and patient discomfort can make palpation difficult. Newly found breast masses should not be attributed to normal physiologic changes during pregnancy. The axillae should be included in examination of the breasts. There is a common hesitancy for biopsies to be performed in pregnancy. The average diagnostic delay for pregnant patients with a breast mass is 2–6 months [Citation2,Citation3]. A delay in diagnosis may increase the risk of nodal involvement (up to 0.9% with 1-month delay) [Citation4]. Ultrasound is the first method to evaluate a mass in pregnancy, to distinguish solid versus cystic lesions [Citation1]. Features suggestive of malignancy include architectural distortion, an echogenic halo, parallel orientation and lack of posterior enhancement. Benign lesions are usually round or oval in shape and have posterior acoustic enhancement but this may also occur in high-grade tumors, hormone negative tumors and tumors in young patients [Citation5]. Sensitivity and specificity of ultrasound are not altered by pregnancy. At any gestational age, biopsies can be safely performed for suspicious masses but the pathologist should be alerted to the hyperproliferative changes of the pregnant breast. Often a fine needle aspiration is nondiagnostic for this reason; instead, a core or excision biopsy is recommended.
Doses of ionizing radiation less than 0.1 Gy do not have fetal effects [Citation6]. The fetal radiation exposure from mammography with abdominal shielding is low (0.004 Gy), but the change in density, increased water and decreased fat content of the breasts in pregnancy may hinder the mammographic appearance of a mass. Despite these limitations, mammography is still recommended to detect microcalcifications, architectural distortion or focal asymmetry, masses or multifocal disease. As in nonpregnant women, a negative mammogram in the setting of a palpable mass should not discourage a biopsy. Mammography has a sensitivity of approximately 86% during pregnancy [Citation1]. depicts the evaluation of a breast mass found during pregnancy. Contrast-enhanced MRI is not generally recommended to evaluate the breasts during pregnancy because gadolinium-based contrast agents are required, and the pregnant patient is also required to lie prone, which can be difficult during late gestation. Although there are no reports of teratogenic effects of gadolinium-based contrast agents in humans, there are also no prospective controlled studies evaluating the fetal effects of gadolinium-based contrast agents in humans.
Prognosis of breast cancer during pregnancy
Women diagnosed during pregnancy with breast cancer stages I and IIA have similar survival rates compared with nonpregnant women [Citation3,Citation7–12]. Age at diagnosis may affect cancer aggressiveness in pregnant woman [Citation13–15]. Johansson reported the difference in mortality between pregnancy-associated breast cancer (PABC) and non-PABC was more pronounced among women older than 35 years and among women with PABC diagnosed within 1 year postpartum. Women diagnosed within the first 2–3 years after a pregnancy have a worse prognosis, yet the majority of the case–control series includes women diagnosed up to 1 year postpartum. Azim et al. concluded that the prognosis of breast cancer arising in the postpartum period was significantly associated with poor overall survival compared with patients diagnosed during pregnancy, in a recent meta-analysis of 30 published studies on breast cancer diagnosed during pregnancy or postpartum [Citation14].
Amant et al. reported on breast cancer survival for patients diagnosed exclusively during pregnancy [Citation16]. At a mean of 61 months after diagnosis, disease-free and overall survival was not statistically different for 311 pregnant patients (all younger than 45) stages I–III compared with 865 controls, matched for age and treatment. This remained true when limiting controls to nulliparous patients. Multivariate analysis was adjusted for such prognostic indicators as age at diagnosis, stage, tumor grade and histologic type, estrogen, progesterone receptor and HER2 positivity and treatment. Overall survival at a mean of 61 months was comparable to that of 865 nonpregnant patients matched (not 1:1, but both groups included women with stages I–II) for age, stage, grade and histologic type of tumor, hormonal receptors, HER2/neu status and type of treatment. There was no statistical difference in disease-free survival, recurrence (HR: 1.34; 95% CI: 0.93–1.91; p = 0.14) or overall survival (HR: 1.19; 95% CI: 0.73–1.93; p = 0.51). Pregnancy was not a factor in recurrence risk or survival on multivariate analysis [Citation16,Citation17].
Histology & receptors
As in nonpregnant women, the predominant histological type is invasive ductal (70–90%), followed by invasive lobular carcinoma [Citation3,Citation18–22]. Age, rather than pregnancy, determines the biologic features of breast cancer. The rate of estrogen receptor-negative cancers in PABC [Citation3,Citation19,Citation20] and the HER2/neu status are comparable to nonpregnant premenopausal women with breast cancer [Citation21–24]. Because the occurrence of breast cancer during pregnancy is uncommon and because of the high levels of estrogens and progestins associated with pregnancy, ligand-binding assays could cause false-negative receptor results [Citation23].
Investigating spread of disease
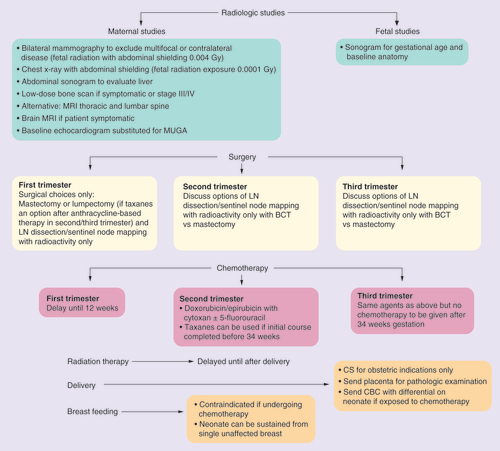
The decision to perform radiographic studies during pregnancy should be limited to those that will affect management before delivery. These include bilateral mammography (fetal exposure: 004 Gy) to exclude multifocal or bilateral disease, chest x-ray to exclude pulmonary metastases (fetal exposure: 0001 Gy) and liver ultrasound to exclude liver metastases. Bone scan, brain MRI and PET scan are delayed until postpartum unless patients are symptomatic during the pregnancy [Citation17]. For suspected metastases to bone, a noncontrast skeletal MRI can be considered.
Termination of pregnancy
Studies of pregnant women diagnosed with breast cancer fail to show a survival benefit with pregnancy termination [Citation8,Citation25–28]. Some studies showed a trend toward an improved survival for patients who continued their pregnancies compared with those who terminated [Citation27], but stage is not reported in the majority of publications so it is unclear if a bias exists in which only women with a better prognosis continued their pregnancies. In a study about the psychological impact of a cancer diagnosis during pregnancy, potential variables relating to long-term distress on maternal surveys including the Basic Symptom Inventory, and Impact of Events Scales, included if they had been advised to terminate the pregnancy [Citation29].
Treatment of breast cancer during pregnancy
The goals of cancer treatment are the same for pregnant and nonpregnant women: to control cancer locally and prevent systemic spread. Beadle et al. reported that any treatment intervention during pregnancy provided a trend toward improved overall survival compared with delaying evaluation and treatment until postpartum (78.8 vs 44.6%; p = 0.68) [Citation30]. Loibl et al. agree that delaying treatment for fetal indications did not afford a survival advantage [Citation24]. For patients diagnosed prior to 12 weeks (chemotherapy would not be an option due to fetal organogenesis), surgery should be performed and adjuvant chemotherapy begun after 12 weeks gestation.
Surgical options
Surgical options during pregnancy are similar to those offered to nonpregnant women with consideration of tumor size and location. As in nonpregnant women, there is no survival advantage of mastectomy compared with lumpectomy [Citation31]. Patients electing breast conservation surgery should be aware that radiation therapy will be delayed until postpartum. To fill the time lapse between surgery during pregnancy and postpartum radiation, often chemotherapy is given until 34 weeks. Patients need to discuss this timing with their physicians to be certain that the delay of radiation until postpartum will not compromise their treatment goals. Surgeries performed after 24 weeks gestation should include fetal monitoring and staffing by anesthesiologists familiar with the physiologic changes during pregnancy including delayed gastric emptying, lower CO2 levels and the need for left uterine displacement to decrease aorto-caval compression as a consequence of the supine position after 20 weeks of pregnancy. Patients diagnosed early in the second trimester can be offered breast conservation surgery since chemotherapy can be used until 34 weeks to treat the cancer while radiotherapy is delayed until postpartum.
For the best cosmetic results, reconstruction is delayed, but spacers can be placed and expanded during pregnancy. During prolonged surgical procedures, compression devices should be placed on bilateral lower extremities to decrease the risk of thromboembolism in pregnancy. Sentinel lymph node biopsy for staging of the regional lymph nodes can be performed safely so that pregnant patients do not necessarily have to undergo full axillary dissection [Citation32–34]. The same day approach is preferred in pregnancy, using the radioactive tracer, technetium-99, rather than dye. Fetal exposure to the radioactivity is minimal and outweighed by the benefits of avoiding unnecessary axillary dissection. Lymphazurin dye carries a risk for anaphylaxis and the use of methylene blue is not recommended in pregnancy due to the fetal risks of methemoglobin, although this was mainly reported when used with amniotic cavity injection [Citation35,Citation36].
Chemotherapy during pregnancy: agent choices & neonatal outcomes
In discussion of neonatal outcomes after treatment for maternal cancer during pregnancy, one must account for the gestational age at delivery, as preterm infants will incur more medical issues and long-term consequences compared with infants delivered closer to term. Chemotherapy is not recommended during organogenesis in the first 12 weeks of pregnancy. The majority of case series of pregnant patients treated with chemotherapy provides information on the neonate at birth or up to 18 months of age at most. Long-term developmental and overall health details are limited.
Berry et al. treated 23 pregnant patients with breast cancer with doxorubicin (50 mg/m2), cyclophosphamide (500 mg/m2) and 5-fluorouracil (1000 mg/m2) for a median of four cycles. No congenital anomalies occurred. One patient went into spontaneous preterm labor just 2 days after treatment and delivered an infant with transient leukopenia without infectious consequences. Premature infants often have leukopenia due to increased regulatory T-cell values and diminished IL-7, so the effect may not have been secondary to chemotherapy exposure [Citation37]. Hahn expanded this series to 57 women/children pairs and surveyed parents/guardians about their children’s health and development. All but three children were developmentally appropriate and all were reported to be healthy. Two children were reported to have educational needs and one child was diagnosed with Down’s Syndrome [Citation38].
In 2001, Aviles et al. performed detailed evaluations of 84 children exposed in utero to chemotherapy. Although not specifically for breast cancer, regimens used often included anthracyclines and cyclophosphamide. The 84 children were born to mothers with hematological malignancies (29 acute leukemia, 26 Hodgkin’s disease and 29 malignant lymphoma) who received chemotherapy during pregnancy, including 38 during the first trimester. Children were examined for physical health, growth and development and hematological, cytogenetic, neurological, psychological and learning disorders. With a median follow-up of 18.7 years (range: 6–29 years) learning and educational performance were normal, and no congenital, neurological or psychological abnormalities were observed nor malignancies diagnosed [Citation39]. Amant assessed behavior, general health, cognition, hearing and growth in 70 children exposed to chemotherapy for maternal breast cancer during pregnancy. Echocardiograms were performed in children exposed to anthracyclines. All cardiac performance was within the normal range, but subtle differences in cardiac dimensions required additional study. Children with neurocognitive issues were concentrated in the group born at a preterm gestational age [Citation40].
The majority of case series included treatment during pregnancy with anthracyclines and cyclophosphamide with or without 5-fluorouracil. Taxanes were often postponed until postpartum. Rouzier et al. compared the pathological response of 48 patients with PABC to non-PABC as a marker of chemosensitivity, and found no difference [Citation41]. Evidence regarding fetal tolerance to taxane is accumulating [Citation42–55]. In Amant’s collaborative study, 169 women received taxane therapy during pregnancy and authors reported no increased malformations [Citation16].
In 2012, birth weight, gestational age at delivery, rate of growth restriction, congenital anomalies and incidence of maternal and neonatal neutropenia were compared between 15 cases treated with taxanes during pregnancy and 123 patients with breast or ovarian cancer not exposed to taxanes. There were no statistically significant differences between the two groups [Citation6]. Rouzier et al. found a better response when taxanes were added to anthracycline-based regimens during pregnancy [Citation41]. If anthacycline-based chemotherapy is completed too early for a safe delivery, consideration should be given to treating with taxanes rather than delaying until postpartum or performing an iatrogenic preterm delivery to avoid exposure.
Other challenges with treating breast cancer during pregnancy include the unanswered issue of whether dose-dense therapy is safe to use. Giving anthracyclines/cyclophosphamide ± 5-fluorouracil every 2 weeks will allow more time to treat with taxane therapy before delivery. There was no difference in gestational age at delivery, spontaneous preterm birth rate, congenital anomalies, maternal or neonatal neutropenia, birth weight or incidence of neonatal growth restriction for ten children exposed to dose-dense chemotherapy during pregnancy compared with 99 children exposed to conventional timing of cycles during pregnancy [Citation56]. Peccatori et al. reported that weekly epirubicin during pregnancy appears safe and effective with low fetal toxicity [Citation57].
A significant challenge in treating pregnant patients with breast cancer is that pharmacokinetic studies are limited. The literature cannot currently answer whether chemotherapy should be given based on prepregnancy, ideal or current body weight. There are no guidelines to advise if increased dosing of chemotherapy should be employed to account for the physiologic changes that may decrease free drug levels. Such changes include expanded plasma volume, increased protein binding, increased glomerular filtration rate and increased activity of maternal liver enzymes. In support of this suspicion, pregnant women report less nausea and vomiting while receiving chemotherapy during pregnancy compared with their experience with identical regimens postpartum. Van Calsteren et al. reported that for anthrayclines and taxanes given during pregnancy, maximum concentration and area under the curve were lower [Citation58]. At the current time, it is recommended that chemotherapy doses during pregnancy be based on height and actual, not ideal or prepregnancy maternal weight [Citation16].
In the less common case of estrogen receptor-positive breast cancer during pregnancy, the use of tamoxifen or other anti-estrogens including aromatase inhibitors is not recommended [Citation59]. Herceptin or trastuzumab is also contraindicated during pregnancy due to reports of reversible fetal oligohydramnios [Citation60–69]. If a patient conceives while taking trastuzumab early in gestation, this can be discontinued and the ongoing pregnancy maintained [Citation70].
Radiation
Radiation for breast cancer is delayed until after delivery. Fetal radiation exposure would be unacceptably high for the standard dose of radiation for breast cancer. Fetal exposure can range from 3.9 to 15 rad during the first trimester when the fundus of the uterus is farthest from the breast, to up to 200 rad toward the end of pregnancy [Citation71].
Breast cancer during pregnancy: delivery recommendations
Iatrogenic preterm deliveries can have long-term medical complications for the infant and are discouraged. Chemotherapy during pregnancy should be completed or temporarily halted by 34 weeks gestation to allow at least 3 weeks between treatment and spontaneous labor. This will decrease the fetal risks for transient neutropenia at birth. If postpartum treatment including additional chemotherapy, radiation or surgery is planned, consideration of a ‘late preterm’ induction of labor at 35–37 weeks can be considered. (For a 35-week induction of labor, the latest chemotherapy would be given by 32 weeks). Mode of delivery, in other words, vaginal delivery or cesarean section, should be determined by obstetrical indications. Chemotherapy can resume within days after an uncomplicated vaginal birth or 7 days after a cesarean section.
Conclusion & future perspective
The evaluation of a palpable mass found during pregnancy should be pursued as if the patient is not pregnant. Although some articles suggest pregnancy carries a worse prognosis, not entirely due to delays in diagnosis, termination of the pregnancy has not been shown to afford a survival benefit. The chemotherapy agents used and the timing of treatment will depend on the patient’s pregnancy’s gestational age at diagnosis, with the plan to complete or discontinue chemotherapy by 34 weeks, allowing 3 weeks prior to delivery. Iatrogenic premature deliveries prior to 35 weeks are discouraged. Neonatal outcomes appear favorable after second and third trimester chemotherapy.
Pharmacokinetic studies in pregnant women are needed to advise oncologists of the correct dose of chemotherapy to be prescribed during pregnancy. Other future studies can provide more detailed long-term followup on the children exposed in utero as fetuses especially as the less-studied taxane exposures increase during pregnancy.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
Acknowledgements
Thanks to G Simpkins for the preparation and editing of this manuscript.
References
- Aha BY , KimHH, MoonWKet al. Pregnancy- and lactation-associated breast cancer: mammographic and sonographic findings. J. Ultrasound Med.22, 491–497 (2003).
- Woo JC , YuT, HurdTC. Breast cancer in pregnancy: a literature review. Arch. Surg.138, 91–98 (2003).
- Ishida T , YokoeT, KasumiFet al. Clinicopathologic characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: analysis of case–control study in Japan. Jpn J. Cancer Res.83, 1143–1149 (1992).
- Nettleton J , LongJ, KuhanD, WuR, ShaefferJ, El-MahdiA. Breast cancer during pregnancy: quantifying the risk of treatment delay. Obstet. Gynecol.87, 414–418 (1996).
- Wojcinski S , StefanidouN, HillemansP, DegenhardtF. The biology of malignant breast tumors has an impact on the presentation in ultrasound: an analysis of 315 cases. BMC Womens Health13, 47 (2013).
- Prenatal Radiation Exposure: A Fact Sheet for Physicians (CDC) .www.bt.cdc.gov/radiation/prenatalphysician.asp.
- Petrek JA , DukoffR, RogatkoA. Prognosis of pregnancy-associated breast cancer. Cancer67, 869–872 (1991).
- Zemlickis D , LishnerM, DegendorferPet al. Maternal and fetal outcomes after breast cancer in pregnancy. Am. J. Obstet. Gynecol.166, 781–787 (1992).
- Halaska MJ , PentheroudakisG, StrnadPet al. Presentation, management and outcome of 32 patients with pregnancy-associated breast cancer: a matched controlled study. Breast J.15, 461–467 (2009).
- Ibrahim EM , EzzatAA, BaloushA, HussainZH, MohammedGH. Pregnancy-associated breast cancer: a case–control study in a young population with a high-fertility rate. Med. Oncol.17, 293–300 (2000).
- Zhang J , LiuG, WuJet al. Pregnancy-associated breast cancer: a case control and long-term follow-up study in China. J. Exp. Clin. Cancer Res.22, 23–27 (2003).
- Smith LH , DanielsenB, AllenME, CressM. Cancer associated with obstetric delivery: results of linkage with the California Cancer Registry. Am. J. Obstet. Gynecol.189, 1128–1135 (2003).
- Nugent P , O’ConnellTX. Breast cancer and pregnancy. Arch. Surg.120, 1221–1224 (1985).
- Azim HA Jr , SantoroL, Russell-EduW, PentheroudakisG, PavlidisN, PeccatoriFA. Prognosis of pregnancy-associated breast cancer: a meta-analysis of 30 studies. Cancer Treat. Rev.38, 834–842 (2012).
- Johansson AL , AnderssonTM, HsiehCCet al. Stage at diagnosis and mortality in women with pregnancy-associated breast cancer (PABC). Breast Cancer Res. Treat.139, 183–192 (2013).
- Amant F , von MinckwitzG, HanSNet al. Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J. Clin. Oncol.31, 2532–2540 (2013).
- Amant F , DeckersS, Van CalsterenKet al. Breast cancer in pregnancy: recommendations of an international consensus meeting. Eur. J. Cancer46, 3158–3168 (2010).
- Pavlidis N , PentheroudakisG. The pregnant mother with breast cancer: diagnostic and therapeutic management. Cancer Treat. Rev.31, 439–447 (2005).
- Bonnier P , RomainS, Dilhuydy, JMet al. Influence of pregnancy on the outcome of breast cancer: a case-control study. Societe Francaise de Senologie et de Pathologie Mammaire Study Group. Int. J. Cancer72, 720–727 (1997).
- Shousha S . Breast carcinoma presenting during or shortly after pregnancy and lactation. Arch. Pathol. Lab. Med.124, 1053–1060 (2000).
- Middleton LP , AminM, GwynK, TheriaultR, SahinA. Breast carcinoma in pregnant women: assessment of clinicopathologic and immunohistochemical features. Cancer98, 1055–1060 (2003).
- Reed W , SandstadB, HolmR, NeslandJM. The prognostic impact of hormone receptors and c-erbB-2 in pregnancy-associated breast cancer and their correlation with BRCA1 and cell cycle modulators. Int. J. Surg. Pathol.11, 65–74 (2003).
- Elledge RM , CioccaDR, LangoneG, McGuireWL. Estrogen receptor, progesterone receptor, and HER-2/neu protein in breast cancers from pregnant patients. Cancer71, 2499–2506 (1993).
- Loibl S , von MinckwitzG, GwynKet al. Breast carcinoma during pregnancy. International recommendations from an expert meeting. Cancer106, 237–46 (2006).
- Psyrri A , BurtnessB. Pregnancy-associated breast cancer. Cancer J.11, 83–95 (2005).
- King RM , WelchJS, MartinJKJr, CoulamCB. Carcinoma of the breast associated with pregnancy. Surg. Gynecol. Obstet.160, 228–232 (1985).
- Clark RM . Breast cancer and pregnancy: the ultimate challenge. Clin. Oncol.1, 11–18 (1989).
- Jacobs IA , ChangCK, SaltiGI. Coexistence of pregnancy and cancer. Am. Surg.70, 1025–1029 (2004).
- Henry M , HuangLN, SprouleBJ, CardonickE. The psychological impact of a cancer diagnosis during pregnancy: determinants of long term distress. Psychooncology21(4), 444–450 (2014).
- Beadle BM , WoodwardW, MiddletonLPet al. The impact of pregnancy on breast cancer outcomes in women < or =35 years. Cancer115, 1174–1184 (2009).
- Kuerer HM , GwynK, AmesFC, TheriaultRL. Conservative surgery and chemotherapy for breast carcinoma during pregnancy. Surgery131, 108–110 (2002).
- Gropper AB , CalvilloKZ, DominiciLet al. Sentinel lymph node biopsy in pregnant women with breast cancer. Ann. Surg. Oncol.21(8), 2506–2511 (2014).
- Gentilini O , CremonesiM, ToescaAet al. Sentinel lymph node biopsy in pregnant patients with breast cancer. Eur. J. Nucl. Med. Mol. Imaging37, 78–83 (2010).
- Pandit-Taskar N , DauerLT, MontgomeryL, St GermainJ, ZanzonicoPB, DivgiCR. Organ and fetal absorbed dose estimates from 99mTc-sulfur colloid lymphoscintigraphy and sentinel node localization in breast cancer patients. J. Nucl. Med.47, 1202–1208 (2006).
- Cinar H , KocaB, KesiciogluTet al. Isosulfan blue-induced anaphylactic reaction during sentinel lymph node biopsy in breast cancer. Breast21(2), 220–222 (2012).
- Crooks J . Haemolytic jaundice in a neonate after intra-amniotic injection of methylene blue. Arch. Dis. Child.57, 872–873 (1982).
- Berry DL , TheriaultRL, HolmesFAet al. Management of breast cancer during pregnancy using a standardized protocol. J. Clin. Oncol.17, 855–861 (1999).
- Hahn KM , JohnsonPH, GordonNet al. Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer107, 1219–1226 (2006).
- Aviles A , NeriN. Hematological malignancies and pregnancy: a final report of 84 children who received chemotherapy in utero. Clin. Lymphoma2(3), 173–177 (2001).
- Amant F , Van CalsterenK, HalaskaMet al. Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: an observational study. Lancet Oncol.13, 256–264 (2012).
- Rouzier R , WerkoffG, UzanCet al. Pregnancy-associated breast cancer is as chemosensitive as non-pregnancy-associated breast cancer in the neoadjuvant setting. Ann. Oncol.22, 1582–1587 (2011).
- Laurentiis M , CancelloG, D’AgostinoDet al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J. Clin. Oncol.26, 44–53 (2008).
- Martin M , PienkowskiT, MackeyJet al. Adjuvant docetaxel for node-positive breast cancer. N. Engl. J. Med.352, 2302–2313 (2005).
- Sparano JA , WangM, MartinoSet al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med.358, 1663–1671 (2008).
- Jones S , HolmesFA, O’ShaughnessyJet al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J. Clin. Oncol.27, 1177–1183 (2009).
- Cardonick E , BhatA, GilmandyarD, SomerR. Maternal and fetal outcomes of taxane chemotherapy in breast and ovarian cancer during pregnancy: case series and review of the literature. Ann. Oncol.30, 16–23 (2012).
- Sood AK , ShahinMS, SoroskyJI. Paclitaxel and platinum chemotherapy for ovarian carcinoma during pregnancy. Gynecol. Oncol.83, 599–600 (2001).
- Mendez LE , MuellerA, SalomE, González-QuinteroVH. Paclitaxel and carboplatin chemotherapy administered during pregnancy for advanced epithelial ovarian cancer. Obstet. Gynecol.102, 1200–1202 (2003).
- Rouzi AA , SahlyNN, SahlyNF, AlahwalMS. Cisplatinum and docetaxel for ovarian cancer in pregnancy. Arch. Gynecol. Obstet.280, 823–825 (2009).
- Chun KC , KimDY, KimJH, KimYM, KimYT, NamJH. Neoadjuvant chemotherapy with paclitaxel plus platinum followed by radical surgery in early cervical cancer during pregnancy: three case reports. Jpn J. Clin. Oncol.40, 694–698 (2010).
- García-González J , CuevaJ, LamasMJ, CurielT, GrañaB, López-LópezR. Paclitaxel and cisplatin in the treatment of metastatic non-small-cell lung cancer during pregnancy. Clin. Transl. Oncol.10, 375–376 (2008).
- Kim JH , KimHS, SungCW, KimKJ, KimCH, LeeKY. Docetaxel, gemcitabine, and cisplatin administered for non-small cell lung cancer during the first and second trimester of an unrecognized pregnancy. Lung Cancer59, 270–273 (2008).
- Azim HA , ScarfoneG, PeccatoriFA. Carboplatin and weekly paclitaxel for the treatment of advanced non-small cell lung cancer (NSCLC) during pregnancy. J. Thorac. Oncol.4, 559–560 (2009).
- Mir O , BerveillerP, GoffinetFet al. Taxanes for breast cancer during pregnancy: a systematic review. Ann. Oncol.21, 425–6 (2010).
- Serkies K , We˛GrzynowiczE, JassemJ. Paclitaxel and cisplatin chemotherapy for ovarian cancer during pregnancy: case report and review of the literature. Arch. Gynecol. Obstet.283(Suppl. 1), 97–100 (2011).
- Cardonick E , GilmandyarD, SomerR. Maternal and neonatal outcomes of dose-dense chemotherapy for breast cancer in pregnancy. Obstet. Gynecol.120(6), 1267–1272 (2012).
- Peccatori FA , AzimHAJr, ScarfoneGet al. Weekly epirubicin in the treatment of gestational breast cancer. Breast Cancer Res. Treat.115, 591–594 (2009).
- Van Calsteren K , VerbesseltR, OttevangerNet al. Pharmacokinetics of chemotherapeutic agents in pregnancy: a preclinical and clinical study. Acta Obstetr. Gynecol. Scand.89(10), 1338–45 (2010).
- Azim HA Jr , Del MastroL, ScarfoneGet al. Treatment of breast cancer during pregnancy: regimen selection, pregnancy monitoring and more… Breast 20, 1–6 (2011).
- Sekar R , StonePR. Trastuzumab use for metastatic breast cancer in pregnancy. Obstet. Gynecol.110, 507–510 (2007).
- Shrim A , Garcia-BournissenF, MaxwellC, FarineD, KorenG. Favorable pregnancy outcome following Trastuzumab (Herceptin) use during pregnancy – case report and updated literature review. Reprod. Toxicol.23, 611–613 (2007).
- Bader AA , SchlembachD, TamussinoKF, PristauzG, PetruE. Anhydramnios associated with administration of trastuzumab and paclitaxel for metastatic breast cancer during pregnancy. Lancet Oncol.8, 79–81 (2007).
- Waterston AM , GrahamJ. Effect of adjuvant trastuzumab on pregnancy. J. Clin. Oncol.24, 321–322 (2006).
- Fanale MA , UyeiAR, TheriaultRL, AdamK, ThompsonRA. Treatment of metastatic breast cancer with trastuzumab and vinorelbine during pregnancy. Clin. Breast Cancer6, 354–356 (2005).
- Watson WJ . Herceptin (trastuzumab) therapy during pregnancy: association with reversible anhydramnios. Obstet. Gynecol.105, 642–643 (2005).
- Azim HA Jr , PeccatoriFA, LiptrottSJ, CataniaC, GoldhirschA. Breast cancer and pregnancy: how safe is trastuzumab?Nat. Rev. Clin. Oncol.6, 367–370 (2009).
- Beale JM , TuohyJ, McDowellSJ. Herceptin (trastuzumab) therapy in a twin pregnancy with associated oligohydramnios. Am. J. Obstet. Gynecol.201, e13–e14 (2009).
- Weber-Schoendorfer C , SchaeferC. Trastuzumab exposure during pregnancy. Reprod. Toxicol.25, 390–391; author reply 392 (2008).
- Pant S , LandonMB, BlumenfeldM, FarrarW, ShapiroCL. Treatment of breast cancer with trastuzumab during pregnancy. J. Clin. Oncol.26, 1567–1569 (2008).
- Zagouri F , SergentanisTN, ChrysikosD, PapadimitriouCA, DimopoulosMA, BartschR. Trastuzumab administration during pregnancy: a systematic review and meta-analysis. Breast Cancer Res. Treat.137, 349–357 (2013).
- Antypas C , SandilosP, KouvarisJet al. Fetal dose evaluation during breast cancer radiotherapy. Int. J. Radiat. Oncol. Bio. Phys.40, 995–999 (1998).