Abstract
Primary CNS involvement is very rare in Hodgkin lymphoma. Here we present two cases of spinal cord dissemination. Two women of 40 and 65 years of age presented symptoms of spinal cord injury; imaging showed an intramedullary mass in T10 and T2, respectively, without vertebral involvement and upper diaphragmatic lymph nodes. Lymph-node biopsy confirmed the diagnosis of classical Hodgkin lymphoma in both patients. The first patient received four cycles of chemotherapy (escalated BEACOPP and ABVD) with intrathecal therapy, and the second four cycles of doxorubicin, vinblastine, dacarbazine (AVD) and local irradiation after surgery decompression. Complete metabolic response was obtained at the end of treatment. After 5 and 7 years of follow-up respectively, neurological deficits persisted in both.
Plain language summary
Lymph-node infiltration is the most common presentation in Hodgkin lymphoma at diagnosis. Primary extranodal involvement is rare and spinal cord infiltration exceptional. Back pain, tingling and vesico-sphincter dysfunctions are the main symptoms. 18F-fluorodeoxyglucose (FDG) PET and MRI can detect the location and extension of neurological involvement. We present here two cases of tumoral myelitis and a review of the literature. Local treatment (surgery/radiotherapy) is often administered together with chemotherapy to optimize local control and to avoid long-term sequelae.
Primary CNS lymphoma accounts for 5% of extranodal lymphomas. Diffuse large B-cell lymphoma is the most common type (90–95% of cases), with a predominance of brain and meningeal presentation [Citation1,Citation2]. Other histological subtypes are Burkitt lymphoma and T-cell lymphoma [Citation1,Citation2]. CNS Hodgkin lymphoma is exceptional, and myelitis is mainly due to secondary effects of radiotherapy and/or chemotherapy [Citation3–5]. Here we describe two cases of extranodal Hodgkin lymphoma with suspended cord involvement (i.e., without contiguous bone involvement), and present a brief review of the literature.
Case reports
First case report
Presentation
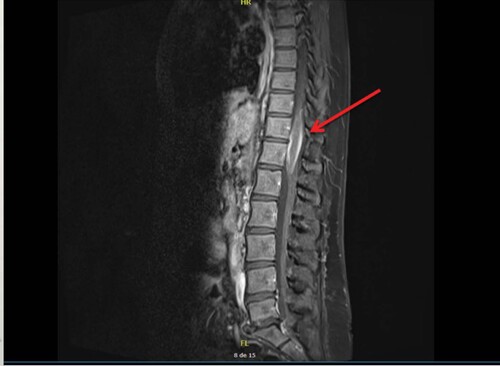
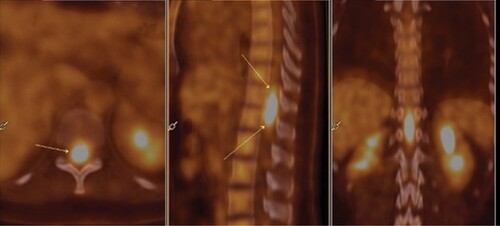
A 40-year-old woman, without any personal or family history, presented with cervical lymphadenopathy and lumbar pain in May 2015. She had a grade 1 motor and sensory deficit of the lower limbs with limited vesico-sphincter dysfunctions. She had no fever or night sweats. The spinal MRI revealed a 6 cm spinal cord tumor without vertebral lesion from the T10 to the L1 levels (). 18F-fluorodeoxyglucose (FDG) PET imaging showed pathological uptakes in the cervical, axillary and mediastinal lymph nodes and an intramedullary spinal cord signal of 4 cm between the T10 and L1 levels with a 9.82 Standard Uptake Value (SUVmax) (). Brain MRI and fundus did not show any other localization.
Diagnosis
The cervical lymph-node biopsy confirmed the diagnostic of nodular sclerosing Hodgkin lymphoma. Bone marrow biopsy was negative, and the lactate dehydrogenase levels were normal. Local exploration was limited to the cerebral fluid because of the risk of neurological worsening. An excess of proteins was noted (3.9 g/l) and 670 cells per mm3 were detected. The flow cytometry analysis revealed a T-reactive lymphocyte population and B/T cell clonality tests were negative. PCR for EBV was negative for blood and cerebrospinal fluid.
Treatment
The patient received two cycles of escalated BEACOPP and four cycles of ABVD with four intrathecal injections of cytarabine, Depo-medrol and methotrexate.
Outcome
Complete metabolic response was obtained at the end of treatment and a grade 1 sensitive deficit of the lower limbs persisted at 5 years of follow-up.
Second case report
Presentation
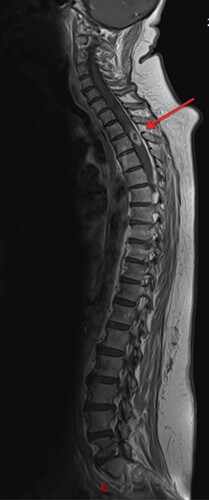
A 65-year-old woman experienced an acute T2 spinal cord compression in January 2013 with paraplegia and vesico-sphincter dysfunctions. MRI showed a tumor lesion suspended in the spinal cord opposite the T2 vertebra and without contiguous bone involvement (). The extension workup did not show any other neurological involvement.
Diagnosis
Spinal decompression surgery was performed and EBV+ classical Hodgkin lymphoma infiltration was diagnosed on biopsies made during surgery; the subtype could not be determined (unclassified form). Right axillary lymph nodes were noted on 18F-FDG PET imaging (4.3 SUVmax), and bone marrow exploration was negative.
Treatment
The treatment consisted of four cycles of AVD, without bleomycin because of the patient’s age, and a 30 Gy vertebral irradiation.
Outcome
Complete metabolic response was rapidly obtained after two cycles and confirmed after four cycles treatment. The patient is still alive at 7 years from the end of treatment and paraplegia persists as a sequel.
Review of the literature
Spinal cord involvement without bone infiltration is very rare in Hodgkin lymphoma, but 13 case reports were published between 1985 and August 2021 and are summarized in [Citation6–18]. These 13 cases were retrieved from PubMed using the following keyword search: (‘myelitis’,‘tumoral myelitis’, ‘neurological localization of Hodgkin lymphoma’, ‘spinal cord infiltration’, ‘primary extranodal Hodgkin lymphoma’). Cases with associated vertebral involvement and not published in English or French were excluded.
Table 1. Literature review.
The spinal involvement was isolated (= without lymph-node infiltration) in nine out of 13 cases. A total of 11 patients had a single spinal cord lesion and two had two locations (cerebral localization for the first and a second spinal cord compression level for the second) [Citation16,Citation17]. The median age of the patients was 32 (12–74) years and the sex ratio H/F was 1.1. The level of the spinal lesions varied from patient to patient as follows: two patients had a cervical spinal lesion, four a cervicodorsal lesion, one a dorsal lesion, four a lumbar lesion and two a lumbosacral lesion. All patients had various symptoms, including paresthesia, low back pain, motor or sensory deficits, vesico-sphincter dysfunctions and dizziness. The median time between neurological symptoms and Hodgkin lymphoma diagnosis was 1.5 (1–5) months. Just one patient presented myelitis 13 years after the diagnosis of Hodgkin lymphoma [Citation8].
The diagnosis was confirmed by lymph-node biopsy, exploration of cerebral fluid, or spinal cord biopsy when it was possible and safe; spinal cord biopsy was performed in 11 cases, with confirmation of Hodgkin lymphoma diagnosis in nine patients and a lumbar punction suggested in the two last patients (Hodgkin lymphoma infiltration in one) [Citation6,Citation8], and lymph-node biopsy was performed in two patients after non contributory lumbar punction and spinal cord biopsy [Citation6,Citation18]. The nodular sclerosis form was the most represented subtype (6/13), the mixed cellularity and lymphocyte predominant subtypes were documented in only two patients [Citation13,Citation15], and in four patients the histologic subtype was not specified [Citation8,Citation10,Citation11,Citation14]. Surgery was first performed in ten patients as follows: six for a spinal cord decompression [Citation8,Citation9,Citation14,Citation15,Citation18] and four for diagnosis (biopsy only) [Citation10,Citation12,Citation16,Citation17]. After surgery, five patients received a combination of chemotherapy and radiotherapy [Citation7,Citation9,Citation12,Citation13,Citation18], three only chemotherapy [Citation10,Citation14,Citation15] and two only radiotherapy [Citation16,Citation17]. Three patients were not operated; one patient received chemotherapy and radiotherapy [Citation6], one patient only intrathecal chemotherapy [Citation8] and the last received no treatment [Citation11]. Explorations of cerebral fluid were performed during the surgery in two patients, without identification of Hodgkin lymphoma cells [Citation13,Citation16].
ABVD or ABVD-like was the most used regimen (7/13), with a median cycle number of 6 per patient (3–8). The median intensity dose of radiotherapy was 30 Gy (21–45), and only one patient received more than 36 Gy (patient treated in 1987) [Citation6]. The response rate was evaluable in 11 patients as follows: spinal MRI was performed in seven patients [Citation6,Citation7,Citation9,Citation13,Citation15–18], a combined body scan in one patient [Citation7] and 18F-fluorodeoxyglucose (FDG) PET in one patient [Citation15] and only a 18F-FDG PET in one patient [Citation18]. The type of evaluation was not specified for three patients [Citation10,Citation12,Citation14], and two patients died early from their lymphoma [Citation8,Citation11]. Complete response was obtained in the majority of the patients (9/11), and partial response in one patient [Citation18]. Concerning neurological symptoms, complete resolution was achieved in one patient [Citation17], hypoesthesia or mild lower extremity weakness persisted after treatment in four patients [Citation7,Citation9,Citation12,Citation16], one patient presented a vincristine-related peripheral neuropathy [Citation6], two died early [Citation8,Citation11], and this information was not specified for five patients [Citation10,Citation13–15,Citation18].
Discussion & conclusion
Primary spinal cord involvement is exceptional in lymphoma and occurs in less than 1% of all CNS sites of disease [Citation19]. Diffuse large B-cell lymphoma is the most common histological type, but several cases of neurological Hodgkin lymphoma have already been described. Standards of care are well established for CNS diffuse large B-cell lymphoma, but not for Hodgkin disease [Citation20]. Considering its high sensitivity to detect extranodal involvement, 18F-fluorodeoxyglucose PET is sufficient to detect early neurological infiltration and MRI to evaluate column involvement. The blood–brain barrier (BBB) protects the brain from pathogen invasion or inflammation and is composed of endothelial cells, astrocytes and pericytes, with close contact with the brain microenvironment (microglia) [Citation21]. The BBB has long been considered an immunological sanctuary, but it is a permeable and dynamic barrier that allows constant communication between the blood and the brain. Disruption of this barrier can occur in several circumstances, such as stroke disease, tumor, Alzheimer’s disease, Parkinson’s disease, infection [Citation21]. In the case of solid tumors, the metastatic cells migrate to the brain by hematogenous route in the cerebral capillaries, and then infiltrate the cerebral parenchyma by extravasation (melanoma, breast, lung and kidney cancer) [Citation21,Citation22]. In lymphomas, dissemination occurs mainly through the lymphatic system, but recent studies have shown the presence of circulating tumor DNA, which may explain diffuse extranodal localizations [Citation23].
In the case of Hodgkin lymphoma, Reed–Sternberg cells are rarely detected in the cerebrospinal fluid, and thus lumbar punction may not be indicated in these cases and a non specific excess of protein is often documented [Citation6,Citation13,Citation16]. Histologic documentation with a surgical biopsy, is highly recommended if the neurological lesion is unique and symptomatic. Laminectomy is often performed to establish the diagnosis and decompress the spinal cord at the same time. Recent work has been reported on the feasibility of detecting circulating tumor DNA from cerebrospinal fluid, which could avoid high-risk surgery [Citation24].
Extranodal Hodgkin lymphoma is considered as stage IV disease or advanced-stage with a higher risk of relapse than early-stage [Citation25]. ABVD and escalated BEACOPP are the two main chemotherapy regimens (6–8 cycles), and early evaluation by PET is recommended after two cycles of induction to adapt the end of treatment and to minimize toxicity (escalating strategy with BEACOPP if PET is positive after two cycles of ABVD or deescalating strategy with ABVD if PET is negative after BEACOPP) [Citation25–28]. The 5-year progression free survival varies from 74 to 95% [Citation22–24]. The addition of the anti-CD30 immunoconjugate brentuximab vedotin to AVD (bleomycin not permitted because of pulmonary fibrosis risk) is feasible and safe with a 2-year progression free survival of 81% [Citation28]. There is no standard of care for neurological localization. Methotrexate and cytarabine are the two main drugs able to cross the BBB. There are few methotrexate-based treatment regimens for Hodgkin lymphoma and cytarabine is more often offered to relapsed patients [Citation29–31].
In the review of the literature, patients were treated with a standard regimen (ABVD or ABVD-like regimen) with a high level of complete response (9/13). The BBB is probably altered in these patients, allowing the diffusion of usual drugs [Citation30]. The use of radiotherapy in addition to surgery to increase local control, is also questionable. A 30–36 Gy radiotherapy is usually proposed to patients with residual lesions or initial bulky disease in nodal and extranodal Hodgkin lymphoma [Citation32]. For patients with neurological localization, management must be multidisciplinary. Several molecules are known to cross the BBB such as temozolomide, carmustine, lomustine, cisplatin, ifosfamide, etoposide, procarbazine, vincristine, irinotecan, cyclophosphamide and doxorubicin at low concentration [Citation33]. Conventional chemotherapy regimens seem to be effective; the BEACOPP regimen would include more molecules crossing the barrier than the ABVD. Intrathecal chemotherapy injections are not clearly recommended. Surgery should be reserved for patients with severe spinal cord compression and/or for diagnosis if there is no other tumoral target. Radiotherapy could be proposed to patients in partial remission after chemotherapy and preservation of neurological functions should be a priority. 3D neuroimaging is the first step to define the tumor and clinical target volume, and combination of pretherapeutic MRI and PET is very useful to determine the therapeutic irradiation field. These patients could also benefit from advanced radiotherapy technology (stereotactic body radiotherapy), and the maximum delivered dose would be discussed with radiotherapists [Citation32,Citation34]. Despite a good disease control, most of patients present with long-term neurological symptoms (e.g., dysesthesia, sensory and/or motor deficits, chronic pain and dizziness). Personalized rehabilitation programs should be offered to each patient during and after the conventional treatment.
Spinal cord infiltration is rare in Hodgkin lymphoma and symptoms often precede diagnosis by a few weeks.
Liquid biopsy could be a diagnostic help for non-operable patients and a new biomarker.
Surgery and irradiation could provide a good local control in addition to chemotherapy.
BEACOPP regimen seems to be the best choice, regarding the number of molecules crossing the blood–brain barrier. Radiotherapy can increase local control in case of incomplete response.
Management should be multidisciplinary.
Prognosis remains good if the treatment is administered early.
Author contributions
K Le Dû worked on the literature review and wrote the manuscript. C Rossi revised the final version. The other authors transmitted imaging and histologic results and were involved in the preparation and revisions of the manuscript, and approved the final version of the manuscript for submission.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
All the data were de-identified and both patients gave informed consent after oral medical information. Their agreement was recorded in the medical file.
Acknowledgments
The authors thank the patients and their families for their participation. The authors would like to thank Enago (www.enago.com) for the English language review.
References
- LauwMIS, LucasCHG, OhgamiRS, WenKW. Primary central nervous system lymphomas: a diagnostic overview of key histomorphologic, immunophenotypic, and genetics features. Diagnostics10(12), 1076 (2020).
- OstromQT, GittlemanH, XuJet al.CBTRUS Statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro. Oncol.18, v1–v75 (2016).
- GerstnerER, AbreyLE, SchiffDet al.CNS Hodgkin lymphoma. Blood112(5), 1658–1661 (2008).
- FermeC, EghbaliH, MeerwaldtJHet al.Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N. Engl. J. Med.357(19), 1916–1927 (2007).
- GatcombeH, LawsonJ, PhuphanichS, CrockerI. Treatment related myelitis in Hodgkin’s lymphoma following stem cell transplant, chemotherapy and radiation: a case report and review of the literature. J. Neurooncol.79(3), 293–298 (2006).
- LydingJM, TsengA, NewamanA, CollinsS, SheaW. Intramedullary spinal cord metastasis in Hodgkin’s lymphoma. Cancer60(8), 1741–1744 (1987).
- HigginsSA, PeschelRE. Hodgkin’s disease with spinal cord compression. A case report and a review of the literature. Cancer75(1), 94–98 (1995).
- JardinF, StamatoullasA, FruchartC, D’AnjouJ, ClementJF, TillyH. Atteinte de la moelle épinière et envahissement meningé lors d’une maladie de Hodgkin. A propos d’un cas et revue de la littérature. Rev. Med. Intern.20(3), 267–271 (1999).
- RiffaudL, AdnM, BrassierG, MorandiX. Acute cauda equina compression revealing Hodgkin’s disease: a case report. Spine28(14), 270–272 (2003).
- HeranNS, YongRL, HeranMS, YipS, FairholmD. Primary intradural extra-arachnoid hodgkin lymphoma of the cervical spine. Case report. J. Neurosurg. Spine5(1), 61–64 (2006).
- KilaniB, AmmariL, TiouiriH, KanounF, BenRomdhane K, BenChaabane T. Transverse myelitis revealing Hodgkin disease. Presse Med.35(4 Pt 1), 615–617 (2006).
- Al-KhayatH, Al-BakerO, GroofAet al.Cervical radiculopathy secondary to Hodgkin’s lymphoma. Sur. Neurol.67(5), 540–543 (2007).
- SamadianM, VahidiS, KhormaeeF, AshrafH. Isolated primary spinal epidural Hodgkin’s disease in a child. Pediatr. Neurol.40(6), 480–482 (2009).
- ChotaiN, DuttaR. Primary intradural Hodgkin’s lymphoma in lumbosacral spine: a rare location. Clin. Neuroradiol.20(4), 247–249 (2010).
- YamanO, OzdemirN, SevinIE, OzerFD, UnluogluS. Primary spinal epidural Hodgkin’s lymphoma. J. Surg. Case Rep.2013(10), rjt090 (2013).
- MartinezDL, GujratiM, GeoffroyF, TsungAJ. Isolated CNS Hodgkin’s lymphoma: implications for tissue diagnosis. CNS Oncol.3(6), 383–387 (2014).
- WilliamsonTJ, WangM, ClarkJ, WilliamsJ, DrndaA. Primary intradural Hodgkin lymphoma of the cornus medullaris and cauda equina: case report. CNS Oncol.9(3), 52 (2020).
- BloxhamN, CrossJ, GarnettMet al.Hodgkin lymphoma presenting with spinal cord compression: challenges for diagnosis and initial management. Pediatr. Dev. Pathol.24, 10935266211033269 (2021).
- FerreriAJM. Therapy of primary CNS lymphoma: role of intensity, radiation, and novel agents. Hematology Am. Soc. Hematol. Educ. Program.2017(1), 565–577 (2017).
- GrommesC, RunbensteinJL, DeAngelisLM, FerreriAJM, BatchelorTT. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro-Oncol.21(3), 296–305 (2019).
- KeaneyJ, CampbellM. The dynamic blood-brain barrier. FEBS J.282(21), 4067–4079 (2015).
- SprowlsSA, ArsiwalaTA, BumgarnerJRet al.Improving CNS delivery to brain metastases by blood-tumor barrier disruption. Trends Cancer5(8), 495–505 (2019).
- CirilloM, CraigAFM, BorchmannS, KurtzD. Liquid biopsy in lymphoma: molecular methods and clinical applications. Cancer Treat. Rev.91, 102106 (2020).
- YanW, XuT, ZhuH, YuJ. Clinical applications of cerebrospinal fluid circulating tumor DNA as a liquid biopsy for central nervous system tumors. Onco. Targets Ther.13, 719–731 (2020).
- MomotowJ, BorchmannS, EichenauerDA, EngertA, SasseS. Hodgkin lymphoma review on pathogeneis, diagnosis, current and future treatment approaches for adult patients. J. Clin. Med.10(5), 1125 (2021).
- BorchmannP, GoergenH, KobeCet al.PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised Phase III trial by the German Hodgkin Study Group. Lancet390(10114), 2790–2802 (2017).
- CasasnovasRO, BouabdallahR, BricePet al.Positron emission tomography-driven strategy in advanced Hodgkin lymphoma: prolonged follow-up of the ALH 2011 Phase III lymphoma Study Association Study. J. Clin. Oncol.40(10), 1091–1101 (2022).
- EichenauerDA, PlütschowA, KreisslSet al.Incorporation of Brentuximab vedotin into first-line treatment of advanced classical Hodgkin’s lymphoma: final analysis of a Phase II randomised trial by the German Hodgkin Study Group. Lancet Oncol.18(12), 1680–1687 (2017).
- GobbiPG, FedericoM. What has happened to VBM (vinblastine, bleomycin and methotrexate) chemotherapy for early-stage Hodgkin lymphoma?Crit. Rev. Oncol. Hematol.82(1), 18–24 (2012).
- JahnkeK, DoolittleND, MuldoonLL, NeuweltEA. Implications of the blood–brain barrier in primary central nervous system lymphoma. Neurosurg. Focus21(5), E11 (2006).
- GallaminiA, RossiA, PattiCet al.Consolidation radiotherapy could be safely omitted in advanced Hodgkin lymphoma with large nodal mass in complete metabolic response after ABVD: final analysis of the randomized GITIL/FIL HD0607 trial. J. Clin. Oncol.38(33), 3905–3913 (2020).
- SpechtL, YahalomJ, IllidgeTet al.Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int. J. Radiat. Oncol. Biol. Phys.89(4), 854–862 (2014).
- ShahN, MohammadAS, SaralkarPet al.Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Phamacol. Res.132, 47–68 (2018).
- ChangJH, ShinJH, YamadaYJet al.Stereotactic body radiotherapy for spinal metastases: what are the risks and how do we minimize them?Spine41(Suppl. 20), S238–S245 (2016).