Abstract
Antibody–drug conjugates have transformed the treatment of HER2+ breast and other cancers. Unfortunately, the CNS remains a sanctuary site for many such patients in part due to poor macromolecule penetration across the blood–brain tumor barrier. Trastuzumab deruxtecan (T-DXd), a high-payload antibody–drug conjugate, was recently found to improve progression-free survival in HER2+ breast cancer patients versus prior-generation trastuzumab emtansine, prompting us to evaluate CNS activity in a woman with brain-only metastatic disease. T-DXd achieved complete response despite heavy pretreatment. Three persistent, previously-irradiated lesions were biopsy-proven to represent treatment effect. Subsequent recurrence occurred upon treatment holiday; partial response was observed with rechallenge. This case suggests T-DXd is active in HER2+ breast cancer brain metastases and supports further prospective evaluation.
Brain metastases (BrM) are rapidly emerging as the key bottleneck in the treatment of HER2+ breast cancer (BC) given improving survival substantially driven by HER2-directed antibody and antibody–drug conjugate (ADC)-based regimens, and their differential activity intra-versus extracranially owing to constraints imposed by the blood–brain barrier [Citation1–4]. As such, BrM affect up to half of HER2+ BC patients and portend severely worse prognosis including disability and shorter survival, with CNS burden of disease a significant predictor of survival in HER2+ BC [Citation5,Citation6]. In this context, BrM treatment in patients with oligometastatic CNS spread relies heavily upon local therapies including surgical resection and targeted radiotherapy, which themselves cannot prevent continued CNS progression; the role of systemic therapy in brain-only metastatic BC is largely unknown, with opinion split on the use of systemic anticancer therapy after local therapy [Citation7].
Emerging data suggest potential activity for trastuzumab-based ADC efficacy in HER2+ BrM, which may be explained by signaling-independent cytotoxicity and tumor-specific bystander killing [Citation8]. For example, in a post hoc analysis of the KAMILLA trial of trastuzumab emtansine (T-DM1), 49% of patients with RECIST-measurable BrM that had not undergone prior local therapy saw a reduction in the sum of major diameters of ≥30% [Citation9], and in the EMILIA trial, patients with trastuzumab-resistant advanced BC with BrM saw improved overall survival with T-DM1 over capecitabine/lapatinib, posited to be in part due to improved CNS activity of this agent. However, the CNS remains an increasingly common site of relapse given the efficacy of T-DM1 in preventing extracranial but not intracranial relapse; for example 5.9% of T-DM1-treated patients in the adjuvant KATHERINE trial relapsed in the brain [Citation10].
[fam-]Trastuzumab deruxtecan-nxki (T-DXd) is a high-payload ADC FDA approved in late 2019 and early 2020 for unresectable/metastatic HER2+ BC and metastatic gastric and gastroesophageal adenocarcinomas that have received prior trastuzumab-based treatment. Owing to its unique and advantageous pharmaceutical properties, this ADC has shown unprecedented activity not only in traditional HER2+ cancers but has also been shown to have activity in HER2-low (defined as IHC1+ or IHC2+/ISH- by the American Society of Clinical Oncology/College of American Pathologists guidelines) [Citation11,Citation12] BC and a variety of HER2-mutant solid cancers [Citation13–17]. Small molecule tyrosine kinase inhibitors with CNS activity, such as tucatinib and neratinib, have also made an impact in HER2+ BC patients with and without BrM and are being evaluated for leptomeningeal metastasis, but the CNS ramifications of such next-generation ADCs is increasingly important [Citation18–20].
We report the case of a patient with rapid, multifocal and durable BrM response to T-DXd, with pathologic confirmation, demonstrating its potential use in this feared and difficult-to-treat setting. Remarkably in this same case, brain metastasis progression was eventually noted following a treatment holiday, but this progression was reversed with T-DXd rechallenge.
Case report
A 31-year-old woman was diagnosed in 2012 with a clinical T1cN1 infiltrating ductal cancer of the left breast, ER90%, PR5-10% and HER2+ (IHC3+). She underwent neoadjuvant therapy on a clinical trial regimen consisting of paclitaxel and neratinib (limited to 7 weeks owing to hepatotoxicity) followed by paclitaxel plus trastuzumab then standard ddAC chemotherapy. She then underwent lumpectomy followed by radiation. She completed 1 year of adjuvant trastuzumab and 2 years of adjuvant goserelin and tamoxifen followed by anastrazole (for a total of 5 years of adjuvant endocrine therapy). Upon limited metastatic recurrence to the GI tract in 2018, while still on endocrine therapy (biopsy-confirmed, ER-, PR-, AR 30% and HER2 3+), she was started on combination paclitaxel plus trastuzumab and pertuzumab first line therapy (without adjuvant endocrine therapy) but 4 months later developed new presentation of CNS-restricted disease with multiple supratentorial metastases and numerous infratentorial lesions, with spread along the cerebellar folia concerning for leptomeningeal disease (more than 25 independent metastatic foci). For this, she was switched to T-DM1 therapy, with complete response in the GI; given a lack of CNS benefit she underwent stereotactic radiosurgery to 19 lesions at doses of 18-27-Gy in 1–3 fractions plus posterior fossa radiotherapy (36 Gy in 12 fractions), with a second course of SRS to one growing parietal lesion. Following continued progression of some of these lesions, she underwent salvage whole brain irradiation (30 Gy in 12 fractions, with partial posterior fossa sparing), resulting in multifocal response. She subsequently received treatment with capecitabine, trastuzumab and neratinib and she required bevacizumab for radiation necrosis. Over the following year, she again suffered from progressive CNS disease (16 independent metastatic foci), and despite a trial of tucatinib-based therapy she continued to have worsening CNS involvement, which ultimately led to the initiation of T-DXd. CNS response was graded using MRI with 1 mm slice thickness and RANO-BM criteria [Citation21]. By the 8-week MRI, 9/12 measurable (≥5 mm) lesions saw CR, and the remaining three, while enlarged, were subsequently biopsy-proven to represent radiation necrosis. All four non target lesions completely responded, and no new lesions manifested, for a CNS overall best response of CR ( & ).
Side-by-side comparison of post contrast, T1-weighted MRIs done before (left-sided panels) and 6 weeks after initiation of T-DXd. Each arrow indicates a metastasis that resolved after treatment initiation.
TDXd: Trastuzumab deruxtecan.
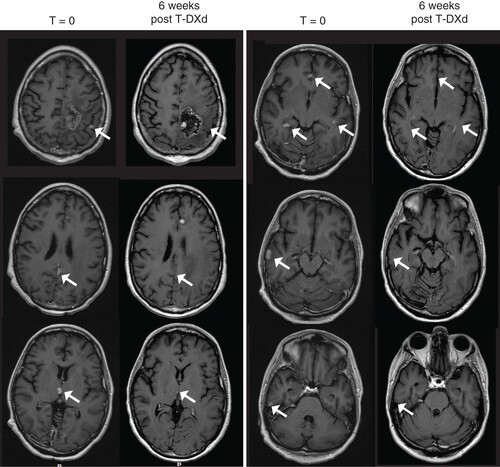
Table 1. Metastasis evolution after 2 months of initial (1A) and subsequent (1B) T-DXd administration.
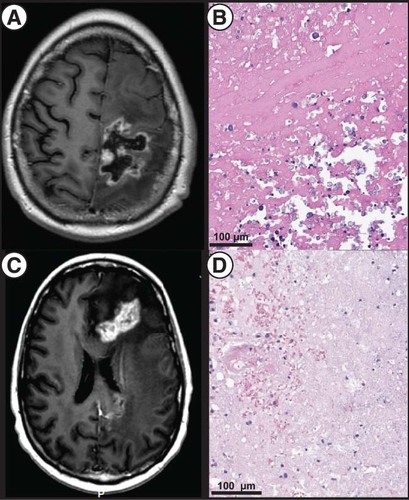
Sampling was performed 2 months later on the symptomatic and enlarging dominant left frontoparietal lesion for palliation and diagnosis (owing to right sided weakness with dyscoordination, in the setting of significant surrounding vasogenic edema refractory to steroids and increased perfusion on dynamic contrast enhanced MR imaging, concerning for disease progression, A); pathologic examination identified necrotic changes without viable tumor (B). She experienced significant symptomatic improvement and tolerated corticosteroid wean. About 4 months later, she suffered from right-sided symptoms referable to edema associated with two now-confluent left frontal lesions (C). Advanced neuroimaging was equivocal for recurrent disease versus necrosis in the setting of elevated plasma volume on dynamic contrast enhancement, and stereotactic biopsy followed by laser-interstitial thermal therapy were performed for diagnostic and therapeutic purposes targeted at either diagnostic possibility. Pathology again showed no viable tumor (D). She experienced symptomatic relief and her dexamethasone requirements reduced considerably. Throughout this time, T-DXd was continued.
(A) T1-weighted, post contrast MRI showing prominent size of a dominant left frontoparietal lesion. (B) Postoperative pathology showing necrotic and fibrinous material and, at upper right, dystrophic mineralization without evidence of active tumor (H&E staining). (C) T1-weighted, post contrast MRI showing a dominant, bilobed left frontal lesion with surrounding vasogenic edema responsible for the patient’s symptoms and treated via LITT. (D) Pathology of the targeted lesion showing tissue necrosis with few mononuclear cells (H&E).
LITT: Laser-interstitial thermal therapy.
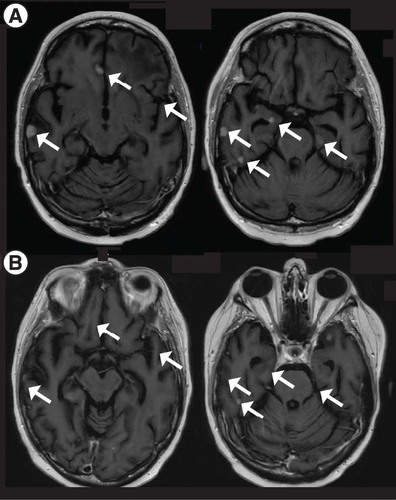
Following this latter intervention, T-DXd was held given concern for grade 1 drug-related pneumonitis as determined on chest CT showing ground-glass opacities (after a total initial course of 7 months, with durability of response), for which prednisone was administered. The patient, however, had normal pulmonary function tests and respiratory status; these findings improved but did not completely resolve. About 6 months after her last T-DXd dose, surveillance imaging showed progression of numerous lesions, with expected contraction of the treated left frontal lesion (A; 12 independent metastatic foci). Given the lack of pulmonary symptoms, a decision was made to restart T-DXd; 2 months and 2 cycles later, surveillance imaging again showed PR with 52% reduction in the sum of target-lesion diameters, stable non target lesions and no new BrM (B & B). T-DXd was continued for 4 months and again subsequently held for concern for pulmonary toxicity.
Post contrast T1-weighted MRI showing (A) progression of CNS disease in the setting of a T-DXd holiday and (B) improvement of most lesion upon T-DXd reinstatement (2 months after (A)). Each arrow indicates a metastasis that resolved after treatment initiation.
TDXd: Trastuzumab deruxtecan.
Discussion
Identification of HER2-directed therapies with CNS activity is a key priority for breast and other HER2-expressing cancers. Recent promise has come from new agents like the small molecule kinase inhibitor tucatinib, which has shown to extend CNS PFS to 9.9 months (versus 4.2 in the control arm, p < 0.001) and overall survival (24.7 vs 19.2, p = 0.004) in patients with HER2+ metastatic BC previously treated with trastuzumab, pertuzumab and T-DM1 with or without BrM in the HER2CLIMB trial, where tucatinib or placebo were added in a randomized, double-blind fashion to trastuzumab and capecitabine [Citation22,Citation23]. There has also been enthusiasm surrounding T-DXd, an antibody–drug conjugate, consisting of HER2-targeting trastuzumab conjugated to the topoisomerase I inhibitor, deruxutecan. T-DXd was recently found in DESTINY-Breast03 to confer significantly improved PFS for patients with HER2+ metastatic BC compared with T-DM1 in the second line setting (after trastuzumab + taxane therapy), with a PFS hazard ratio of 0.284 (p = 7.8 × 10-22), and estimated 12-month survival of 94.1% (T-DXd) versus 85.9% (T-DM1; p = 0.0072), without drug-related deaths [Citation24]. Its use is predicted to become increasingly widespread owing to this, and to ASCO guidelines recommending continuation of systemic therapy in HER2+ BC patients developing BrM without systemically progressive disease [Citation3,Citation25]. This agent has several features that suggest the potential for increased brain tumor activity over other ADCs, including a higher drug–antibody ratio than T-DM1 (8 vs 4) and a membrane-permeable payload allowing for bystander-tumor cytotoxicity regardless of HER2 expression heterogeneity (attributed largely to the cathepsin-based cleavable linker technology) [Citation26,Citation27].
Early reports suggesting CNS activity include a cohort analysis of 14 patients with evaluable BrM treated with T-DXd 5.4 mg/kg in DESTINY-Breast01 (of which 12 also had brain RT prior to enrollment), with CNS response rate of 50%. Among the larger cohort of 24 BrM patients, overall response, median PFS, median duration of response and rate of CNS recurrence were similar to those without BrM at baseline (8/24 with progression, two in the brain in the baseline BrM subgroup; and 40/160 with progression, two in the brain in the baseline non-BrM subgroup) [Citation28]. Subgroup analysis from DESTINY-Breast03 showed T-DXd treatment was associated with substantial intracranial response and CNS disease reduction, with intracranial ORR of 63.9 versus 33.4% for T-DM1 [Citation24]. The ongoing DEBBRAH (NCT04420598) and TUXEDO-1 (NCT04752059) studies are directly testing the potential of T-DXd in patients with untreated or active HER2+ BrM, and include limited cohorts of BC patients with HER2 low disease and those with leptomeningeal carcinomatosis; interim TUXEDO-1 results, which also required prior trastuzumab and pertuzumab treatment, demonstrated 78.6% intracranial response rate in 14 patients (most of whom progressed after prior local therapy), with a median follow-up of 11 months [Citation29]. The DESTINY-Breast12 trial will also prospectively evaluate patients with and without BrM for CNS outcomes (NCT04739761).
In this report, we present the case of a patient with diffuse BrM which developed in the late-line setting including after multiple radiation regimens prior to T-DXd initiation. Remarkably, these responses occurred despite the selective pressures of prior local and systemic therapies, and progressive disease upon treatment holiday was reversible, with subsequent partial response. Severe radiation necrosis requiring surgical intervention pathologically confirmed local disease control (i.e., no viable tumor present) at two time points and locations (2 and 6 months following initiation of T-DXd), with further radiographic improvement at both sites up to 1 year following treatment.
Conclusion
This case is the first published report to our knowledge of CNS response to T-DXd, which was durable, reversible, multifocal and pathologically-confirmed and suggests that further investigation into T-DXd treatment as an earlier-line therapy in patients with HER2+ BrM is warranted, including potentially prior to consideration of local therapies. While the treatment mainstay for BrM is SRS or other forms of irradiation (and in cases of oligometastasis with large and symptomatic tumors, palliative resection plus adjuvant irradiation) [Citation30,Citation31] an increasing menu of CNS-active cancer-directed therapies are altering this paradigm. Larger-scale and more focused analyses are needed to establish efficacy and correlates of response, and critically, to pseudoprogression. In this patient’s case, two interventions were required for palliation and diagnosis of large and symptomatic lesions, given the relatively low specificity of even advanced imaging (e.g., FDG-PET and dynamic contrast-enhanced MR) for discriminating between recurrent disease and radiation necrosis, which require vastly different treatments [Citation32–35]. Such information, and understanding parameters that may influence differential sensitivity and adverse events, for example prior treatments, tumor size and pre-existing necrosis, are critical as these treatments become more widely used, and in light of evidence that the combination of T-DM1 plus stereotactic radiosurgery results in clinically significant radiation necrosis [Citation36]. As such, this case highlights a potential new safety signal for BrM that undergo both SRS and ADC treatment that requires further attention as more patients undergo such multimodality treatment. Narrowing such knowledge gaps for patients with both parenchymal brain and leptomeningeal metastasis are anticipated to improve outcomes for this critical and difficult-to treat patient population.
HER2+ breast cancer patients have an expanding menu of treatment options, including antibody–drug conjugates, which have undefined CNS activity.
The CNS is increasingly the site of refractory disease for patients with breast cancer, warranting improved understanding of CNS outcomes for HER2-targeting agents.
Early CNS outcomes with trastuzumab deruxtecan (T-DXd) suggest brain metastasis activity including in lesions previously treated with local therapy.
The presented case demonstrates CNS complete response to T-DXd in a patient heavily pretreated with both systemic anticancer and local ablative therapies, with regression upon treatment holiday and partial response with rechallenge.
Two enlarging brain metastases in this setting were pathologically-proven radiation necrosis, raising the possibility that combinatorial therapy may raise the risk of this phenomenon, as seen with other antibody–drug conjugates.
Further prospective study is needed to better delineate long-term CNS outcomes, and predictors of response and relapse, with T-DXd and other HER2-targeting therapies.
Financial & competing interests disclosure
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. At the time of research execution and publication, all authors are affiliated with Memorial Sloan Kettering Cancer Center. The authors of this research deny any conflicts of interest regarding this study, and make the following disclosures: NS Moss: Consulting fees for advisory board participation: AstraZeneca; trial funding: GT Medical Technologies. BD Santomasso: Consulting fees for advisory board participation: Janssen, Legend, BMS, Incyte, In8bio. S Modi: Research funding: Genentech, Daiichi Sankyo, AstraZeneca, Seagen; Consultant/Advisory/Speaking honoraria: Genentech, Daiichi Sankyo, AstraZeneca, Seagen, Macrogenics, Novartis, Glaxo-Smith Kline, Zymeworks. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained appropriate Institutional Review Board approval or have followed the principles outlined in the Declaration of Helsinki for all human investigations. In addition, informed consent has been obtained from the participants involved. This work is compliant with CARE guideline.
Acknowledgments
The authors would like to thank MK Rosenblum for neuropathologic review.
Additional information
Funding
References
- DuchnowskaR, LoiblS, JassemJ. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treat. Rev.67, 71–77 (2018).
- HosonagaM, SayaH, ArimaY. Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev.39(3), 711–720 (2020).
- RamakrishnaN, TeminS, ChandarlapatySet al.Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO Clinical Practice Guideline update. J. Clin. Oncol.36(27), 2804–2807 (2018).
- RostamiR, MittalS, RostamiP, TavassoliF, JabbariB. Brain metastasis in breast cancer: a comprehensive literature review. J. Neurooncol.127(3), 407–414 (2016).
- SimmonsC, RaysonD, JoyAAet al.Current and future landscape of targeted therapy in HER2-positive advanced breast cancer: redrawing the lines. Ther. Adv. Med. Oncol.14, 17588359211066677 (2022).
- SperdutoPW, MeskoS, LiJet al.Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J. Clin. Oncol.38(32), 3773–3784 (2020).
- VogelbaumMA, BrownPD, MessersmithHet al.Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J. Clin. Oncol.40(5), 492–516 (2022).
- SwainSM, BaselgaJ, MilesDet al.Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann. Oncol.25(6), 1116–1121 (2014).
- MontemurroF, DelalogeS, BarriosCHet al.Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial(☆). Ann. Oncol.31(10), 1350–1358 (2020).
- von MinckwitzG, HuangCS, ManoMSet al.Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med.380(7), 617–628 (2019).
- FarshidG, BilousM, MoreyAet al.ASCO/CAP 2018 breast cancer HER2 testing guidelines: summary of pertinent recommendations for practice in Australia. Pathology51(4), 345–348 (2019).
- WolffAC, HammondMEH, AllisonKHet al.Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline focused update. J. Clin. Oncol.36(20), 2105–2122 (2018).
- LiBT, MicheliniF, MisaleSet al.HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov.10(5), 674–687 (2020).
- ModiS, ParkH, MurthyRKet al.Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a Phase Ib study. J. Clin. Oncol.38(17), 1887–1896 (2020).
- ShitaraK, BangYJ, IwasaSet al.Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med.382(25), 2419–2430 (2020).
- CutsemEV, BartolomeoMD, SmythEet al.Primary analysis of a phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectable or metastatic gastric or gastroesophageal junction (GEJ) cancer who progressed on or after a trastuzumab-containing regimen. Ann. Oncol.32, S1283–S1346 (2021).
- HorisawaN, AdachiY, TakatsukaDet al.The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer29(2), 234–241 (2022).
- SauraC, OliveiraM, FengYHet al.Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥2 HER2-directed regimens: Phase III NALA trial. J. Clin. Oncol.38(27), 3138–3149 (2020).
- MurthyRK, LoiS, OkinesAet al.Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med.382(7), 597–609 (2020).
- Stringer-ReasorEM, O’BrienBJ, Topletz-EricksonAet al.Pharmacokinetic (PK) analyses in CSF and plasma from TBCRC049, an ongoing trial to assess the safety and efficacy of the combination of tucatinib, trastuzumab and capecitabine for the treatment of leptomeningeal metastasis (LM) in HER2 positive breast cancer. J. Clin. Oncol.39(15), 1044 (2021).
- LinNU, LeeEQ, AoyamaHet al.Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol.16(6), e270–278 (2015).
- CuriglianoG, MuellerV, BorgesVet al.Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann. Oncol.33(3), 321–329 (2022).
- LinNU, BorgesV, AndersCet al.Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J. Clin. Oncol.38(23), 2610–2619 (2020).
- HurvitzS, KimS-B, ChungW-Pet al.Trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Cancer Res.82, GS3-01 (2022).
- ModiS. Trastuzumab deruxtecan in previously treated HER2-positive metastatic breast cancer: plain language summary of the DESTINY-Breast01 study. Future Oncol.17(26), 3415–3423 (2021).
- OgitaniY, AidaT, HagiharaKet al.DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res.22(20), 5097–5108 (2016).
- OgitaniY, HagiharaK, OitateM, NaitoH, AgatsumaT. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci.107(7), 1039–1046 (2016).
- JerusalemGHM, ParkYH, YamashitaTet al.Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: a subgroup analysis of the DESTINY-Breast01 trial. J. Clin. Oncol.39, 526 (2021).
- BartschR, BerghoffAS, FurtnerJet al.Trastuzumab-deruxtecan (T-DXd) in HER2-positive breast cancer patients (pts) with active brain metastases: primary outcome analysis from the TUXEDO-1 trial. Ann. Oncol.33, 194 (2022).
- MahajanA, AhmedS, McAleerMFet al.Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, Phase III trial. Lancet Oncol.18(8), 1040–1048 (2017).
- BanderED, YuanM, ReinerASet al.Durable 5-year local control for resected brain metastases with early adjuvant SRS: the effect of timing on intended-field control. Neurooncol. Pract.8(3), 278–289 (2021).
- NewmanWC, GoldbergJ, GuadixSWet al.The effect of surgery on radiation necrosis in irradiated brain metastases: extent of resection and long-term clinical and radiographic outcomes. J. Neurooncol.153(3), 507–518 (2021).
- WilcoxJA, BrownS, ReinerASet al.Salvage resection of recurrent previously irradiated brain metastases: tumor control and radiation necrosis dependency on adjuvant re-irradiation. J. Neurooncol.155(3), 277–286 (2021).
- LanglebenDD, SegallGM. PET in differentiation of recurrent brain tumor from radiation injury. J. Nucl. Med.41(11), 1861–1867 (2000).
- OkuchiS, Rojas-GarciaA, UlyteAet al.Diagnostic accuracy of dynamic contrast-enhanced perfusion MRI in stratifying gliomas: a systematic review and meta-analysis. Cancer Med.8(12), 5564–5573 (2019).
- StumpfPK, CittellyDM, RobinTPet al.Combination of trastuzumab emtansine and stereotactic radiosurgery results in high rates of clinically significant radionecrosis and dysregulation of aquaporin-4. Clin. Cancer Res.25(13), 3946–3953 (2019).
