Abstract
Historically biomedical research has examined genetic influences on mental health but these approaches have been limited, likely due to the broad heritability of brain-related disorders (e.g., 30–90%). Epigenetic modifications, such as DNA methylation, are environmentally sensitive mechanisms that may play a role in the origins and progression of mental illness. Recently, genome-wide disruptions of 5-hydroxymethylcytosine (5hmC) were associated with the development of early and late onset mental illnesses such as autism and Alzheimer’s disease, bringing new hope to the field of psychiatry. Here, we review the recent links of 5hmC to mental illness and discuss several putative functions of 5hmC in the context of its promising clinical relevance.
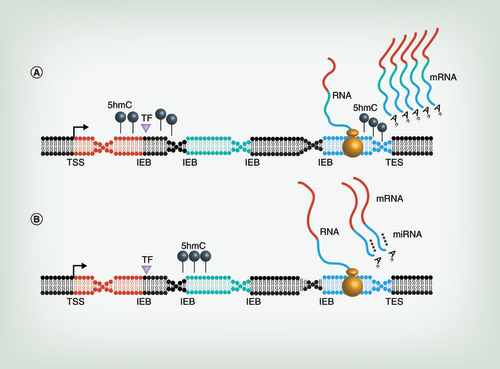
When located near TF binding motifs (purple triangle), 5hmC (black lollipops) may regulate gene expression levels, resulting in either up- (A) or down- (B) regulation. When located at IEB, 5hmC (black lollipops) may regulate alternative splicing of transcripts, resulting in a shift from full-length transcripts (A) to splice isoforms (B). Exons are colored red, green, and blue. Intergenic and intragenic regions are gray. When located near sequences complementary to miRNAs seed regions, 5hmC (black lollipops) may have a latent function in repelling (A) or attracting (B) miRNAs as the DNA is being transcribed into RNA. Group of four asterisks represent miRs. Together, these studies implicate 5hmC in the regulation of transcriptional and translational abundance and diversity.
5hmC: 5-hydroxymethylcytosine; IEB: Intron/exon boundaries; TES: Transcription end site; TF: Transcription factor; TSS: Transcription start site.
A fundamental problem in psychiatry is that diagnoses are based on subjective measures that are primarily acquired through behavioral assessments. Modern genetics has held out hope that one could identify new genetic markers of mental illness early in childhood that could aid the subjective measures of behavior used today. New laboratory tests for these markers would then indicate to psychiatrists the most promising drug for each person or direct pharmaceutical companies to more specific personalized drug development. However, even though complete mapping of the human genome was announced in 2003, the genetic approach has had little impact on improving the lives of individuals struggling with mental illness. This outcome is partially caused by the broad range of heritability in mental illness (i.e., 30–90%) but also is due to a failure in molecular psychiatry that has overlooked a crucial molecular step that causes variations in the expression of key genes in the brain. Past approaches have largely ignored how environmental stimuli can affect the unique amount of gene expression in the brain of each person. Thus, the use of this personalized information provides new hope that one day it will be possible to develop a precise molecular-based treatment, which will change the way mental health is diagnosed and treated today.
Epigenetic mechanisms are environmentally sensitive molecular modifications that are heritable but do not change the underlying DNA sequence. Much effort has been put forth toward understanding two specific epigenetic mechanisms: histone modifications, which alter gene expression through the restructuring of chromatin proteins, and DNA methylation, which modulate gene expression through the addition of a methyl group to specific sites within the DNA sequence. The most studied epigenetic mechanism in the mammalian genome is DNA methylation, which is the addition of a methyl group on the fifth carbon of cytosine (e.g., 5-methylcytosine [5mC]). This DNA mark functions in genomic imprinting, X-chromosome inactivation, chromatin structure and gene silencing [Citation1–5]. For a more definitive and technical perspective of 5mC, please refer to Box 1. Human studies also support a role for 5mC in the development of mental pathologies including bipolar disorder, schizophrenia and major depressive disorder, often resulting in concomitant changes in gene expression [Citation6–10]. It was recently shown that 5mC can be oxidized to 5-hydroxymethylcytosine (5hmC) and that this modification is environmental sensitive [Citation11], stable [Citation12] and highly enriched in the brain [Citation13–17]. While the molecular role(s) of 5hmC remain elusive, primarily due to the dearth of 5hmC functional studies, its unique enrichment in the brain [Citation18] leads many to believe that 5hmC functions are independent from those of 5mC, and that 5hmC may be a critical molecular building block in mental health that is disrupted in disease. For a more definitive and technical perspective of 5hmC, please refer to Box 1. It is noteworthy that traditional DNA methylation detection methods utilizing sodium bisulfite treatment cannot distinguish between the methylated and hydroxymethylated forms of cytosine, meaning that past studies that utilized such methods report a composite of 5mC and 5hmC but have attributed any findings solely to 5mC. This review will provide substantial evidence to support the independent importance of 5hmC in mental health and the development of mental illness and present several putative molecular functions of 5hmC that may shed light on its promising clinical relevance. With recent findings implicating a unique role for 5hmC in developmental brain disorders, 5hmC brings a new frontier to the field of psychiatry.
Development, aging & neurodegeneration
5hmC is essential for proper mammalian development, beginning with an early role in the distinction between embryonic stem cells (ESCs) and neural progenitor cells (NPCs) [Citation33]. Here, the transition from ESC to NPC requires a genome-wide reduction of 5hmC, which is facilitated by a dramatic change in the expression of the modulatory proteins required for oxidizing 5mC to 5hmC (TET1-3; Box 1) [Citation34]. These findings link 5hmC and TET expression with ESC differentiation and suggest that aberrant 5hmC levels may impact neurogenesis. Consistent with this link, embryos completely lacking TET1 and TET3 experience lower 5hmC and higher 5mC levels, which results in more variable expression of important neurodevelopmental genes including those in the cholesterol biosynthesis pathway that lead to the development of holoprosencephaly, a cephalic disorder characterized by the failure of the two brain hemispheres to properly form [Citation35]. To our knowledge, this is the only study that has been able to link deficits of 5hmC with phenotypic alterations in embryos. While these studies demonstrate a role for 5hmC in neurodevelopment during embryogenesis, 5hmC continues to be abundant postnatally and undergoes age-dependent accumulation throughout early life development and into adulthood [Citation36–38]. However, environmental stimuli, such as caloric restriction, can alter this age-associated accumulation of 5hmC, as caloric restricted mice showed an age-dependent reduction of 5hmC in hippocampal and cerebellar tissue [Citation39]. Together, these findings suggest that neurodevelopmental age and environment strongly influence 5hmC dynamics in the brain, which in turn can play a modulatory role in the expression of key genes involved in neurodevelopment.
The emerging link between 5hmC and brain development, and its accumulation with age, have led researchers to examine this epigenetic mark in neurodegenerative diseases [Citation40]. Indeed, age-associated genes that acquire 5hmC are associated with pathways related to neurodegenerative diseases [Citation25]. For example, depletion of 5hmC was found in the hippocampus, cerebellum and entorhinal cortex of patients suffering from Alzheimer’s disease (AD) [Citation41,Citation42], a type of dementia that usually develops slowly and gets worse over time. On the other hand, enrichment of 5hmC in the frontal and midtemporal gyrus was positively correlated with hallmarks of AD, including neurofibrillary tangles (NFT), amyloid beta and ubiquitin load [Citation43]. Together, these findings suggest that 5hmC may be driving the primary molecular components of AD progression within distinct regions of the brain. Notably, AD-associated levels of 5hmC can be detected at preclinical stages as well as at later stages of AD [Citation44], indicating that 5hmC may act as a viable biomarker of AD onset and progression. However, studies employing nuclear labeling or ELISA were unable to find significant alterations in 5hmC associated with AD [Citation45], suggesting that higher resolution methods are required to identify AD-associated changes in 5hmC. Connections between 5hmC and other neurodegenerative disorders including Huntington’s, ataxia-telangiectasia and Fragile X-associated tremor/ataxia syndrome have recently surfaced and we will describe the link to 5hmC of each of these disorders below.
Huntington’s disease (HD) is characterized by deficits in cognitive, psychiatric and motor stability that is typically caused by a CAG trinucleotide expansion in the HTT gene [Citation46]. In addition to the mutation in the HTT gene, previous studies have suggested a role for epigenetic mechanisms in HD, such as altered histone modifications [Citation47] and DNA methylation of a locus closely linked to the HTT gene known as DS495 [Citation48]. These epigenetic connections led to the recent investigation of the genome-wide distribution of 5hmC in a mouse model of HD, which found a deficiency of 5hmC in the mouse striatum and cortex tissues [Citation49]. This altered distribution of 5hmC was generally associated with pathways involved in neuronal development/differentiation, axonal guidance and neuronal function/survival. A specific example included a 5hmC reduction on the ADORA2A gene, a G-protein-coupled receptor that decreases its expression in HD patients, which revealed that 5hmC may play a role in the pathological expression of ADORA2A found in HD patients [Citation50]. Together, these results implicate the loss of 5hmC as a potential molecular mechanism underlying the cause of neuronal cell death associated with HD.
Ataxia-telangiectasia, a devastating neurodegenerative disorder that shows high cell specificity in its development, is caused by a mutation in the ATM gene [Citation51]. Despite this monogenic etiology, alterations in 5hmC were recently found in repeated sequences and regulatory elements of DNA from ATM-deficient Purkinje cells from mice and humans [Citation52]. This 5hmC distribution is likely the result of TET1 responding to DNA damage, which is a hallmark of ATM-deficient neuronal cells. To test whether TET1 activity is related to Purkinje cell degeneration and behavioral deficits in mice, adenoviral particles encoding either human wild-type TET1 (TET1-WT) or kinase dead mutant TET1 (TET-KD) were infected into wild-type and ATM-deficient mouse cerebellum slices. Cerebellum samples lacking TET1 expression that were infected with TET1-WT showed no activation of CASPASE-3, a cell death marker, while infection with TET1-KD activated CASPASE-3. These findings suggest that TET1-mediated 5hmC production is essential for Purkinje cell viability and the prevention of ataxia-telangiectasia-like symptoms in mice, supporting the concept that vulnerability to neurodegeneration is linked to aberrant changes of 5hmC in neuronal cells.
Fragile X-associated tremor/ataxia syndrome (FXTAS), a late-onset neurodegenerative disorder, is one of the characterized Fragile-X disorders that are caused by a CGG expansion in the 5’UTR of the FMR1 gene [Citation53,Citation54]. Genome-wide analysis of 5hmC in the cerebellum of a FXTAS mouse model (rCGG mice) revealed an overall reduction in 5hmC at 16 weeks of age when compared with age-matched controls [Citation55]. Despite the overall reduction of 5hmC, these mice have an increase of 5hmC in repetitive sequences as well as in cerebellum-specific enhancers. Differential 5hmC between the rCGG and control mice were predominantly found in transcription factor (TF) binding sites that are located in genes essential for neuronal development. Finally, ribosomal profiling revealed that the differential 5hmC-associated genes often exhibited altered ribosomal processing in the rCGG mice, suggesting that 5hmC may somehow influence translational changes. In summary, this study links 5hmC to the etiology of FXTAS and implicates a role for 5hmC in TF binding and in regulating ribosomal processing of mature RNA transcripts.
Despite that the above disease associated disruptions in 5hmC represent changes across age; these examples clearly demonstrate a role for 5hmC in proper development and in the onset and progression of neurodegenerative diseases. These developmental and neurodegenerative disease-associated changes in 5hmC often arise within distinct cell-types and brain regions, supporting cell and tissue-specific development of these diseases. Since these studies have been largely descriptive, it will be imperative to determine the functional mechanism(s) played by 5hmC if we are to modify it toward healthy outcomes.
Sensitivity to environmental stimuli
The abundance and distribution of 5hmC is sensitive to environmental stimuli. Mice exposed to enriched environment show reduced 5hmC abundance in the hippocampus primarily on genes involved in axon guidance. These alterations were also associated with increased learning and memory, suggesting that environmental enrichment might modulate the dynamics of 5hmC in the hippocampus and contribute to improved learning and memory [Citation56]. In contrast, our group found increased 5hmC abundance on the glucocorticoid receptor gene (Nr3c1) in the hippocampus of mice stressed for 30 min and allowed to recover for 1 h [Citation57]. Genome-wide 5hmC analysis of these same mice revealed that short-term stress induced genome-wide disruptions of 5hmC and confirmed an overall increase in 5hmC following stress. Similar to the FXTAS mouse model described above, altered 5hmC was found in several TF binding sites of genes. Here these genes also were differentially expressed and have known roles in neurogenesis and neurological activities [Citation29], suggesting that in response to stress the function of 5hmC may be to influence TF binding to provide appropriate levels of gene expression needed to cope with the stress. The fact that 5hmC changes were found within 1 h following a short stress highlights the potential for rapid changes of 5hmC within the brain. It will be interesting to examine the long-term effects of short stress (i.e., more than 1 h after exposure) or how chronic stress alters 5hmC levels. Many believe 5hmC to be important in long-term consequences of mental health, yet this study indicates that alterations in 5hmC can occur rapidly and may impact the expression of key genes related to the origins of mental illnesses. While environmental stressors have been shown to alter gene expression [Citation58], a definitive molecular culprit has been slow to emerge, perhaps 5hmC is a promising candidate.
Environmental stimuli affects brain regions other than the hippocampus. For example, mice exposed to repeated administrations of cocaine have increased 5hmC in the nucleus accumbens, primarily in coding regions and enhancer sequences of genes involved in drug addiction [Citation31]. Notably, 5hmC changes persisted for a minimum of 1 month after cocaine exposure in only a small subset of loci, suggesting that these epigenetic changes are largely reversible. In addition, modulation of 5hmC may also mediate behavioral adaptations. For example, fear extinction, a form of reverse learning, results in dramatic 5hmC changes in the prefrontal cortex of mice [Citation59]. These studies also supported unique molecular roles for the TET enzymes, as TET3, but not TET1 mediated the increased gene expression that was associated with rapid behavioral adaptation. Interestingly, another group found that mice lacking the expression of Tet1 exhibited impaired memory extinction, coupled with long-term synaptic depression and down-regulation of neuronal activity-related genes [Citation60]. Thus, while TET3 may solely facilitate the accumulation of 5hmC in the prefrontal cortex of mice during rapid behavior adaptation in response to fear, TET1 governs alterations in 5hmC on synaptic plasticity genes during behavioral adaptation in response to stressful environmental exposures.
Taken together, these studies open up the possibility that 5hmC may function in the development of environmentally sensitive neuronal dysfunction. It will be of great interest to investigate the role of each TET enzyme coupled with the rapid and stable dynamics of 5hmC at different developmental time points to understand its role in synaptic plasticity, neuronal development, the maintenance of mental health and the onset of mental illness.
Early life adversity, 5hmC & the origins of psychiatric disorders
Several studies indicate that early life experiences have a profound impact on brain development and subsequent adult behavior [Citation61–64]. Recent evidence indicates that the epigenome is a potential molecular mechanism governing the long-lasting effects of early life stress on brain and behavior. One such example involves rhesus macaques that were deprived of early life maternal interactions. As adults, these monkeys have altered 5hmC in the prefrontal cortex on promoters of genes related to neurological functions and psychological disorders (e.g., DRD3, 5-HTT, and GABRA2) [Citation65]. Since these 5hmC disruptions were detected during adulthood, early life changes in 5hmC also can be stable throughout development and may represent the origins of developmental brain disorders such as schizophrenia, bipolar disorder and autism.
Schizophrenia, bipolar disorder & major depressive disorder
Schizophrenia (SCZ) and bipolar disorder (BD) are psychiatric disorders with shared and distinct clinical and genetic features. In both disorders, stressful events increase the risk for onset and relapse mainly through the dysregulation of the hypothalamus–pituitary–adrenal (HPA) axis. Although many genes are linked to HPA dysfunction, the majority of SCZ and BD cases cannot be explained by genetics alone and the epigenome likely has a role in the etiology of these disorders. Consistent with this hypothesis, rodent models exposed to prenatal stress exhibit long-lasting neurological, endocrinological and behavioral changes that are thought to mirror the development of SCZ [Citation66]. These models show increased DNA methylation (5mC + 5hmC) in GABAergic interneurons primarily in CpG-rich regions of GABAergic genes [Citation67,Citation68]. Together, these studies demonstrated that prenatal stressors can alter DNA methylation levels in the brain and implicate a role for DNA methylation in the origins of SCZ and mood disorders [Citation69]. In humans, an independent role for 5hmC in SCZ and BD patients is characterized by increased 5hmC abundance and TET1 expression, but not TET2 or TET3, in the inferior parietal lobule (IPL) [Citation70]. Remarkably, TET1 was not altered in the cerebellum of these patients, suggesting that 5hmC may be involved in the development of psychosis through the inferior parietal lobule, but not the cerebellum, perhaps shedding light on the tissue-specific development of SCZ and BD. The increases of 5hmC in these patients were associated with reduced expression of biologically relevant genes including GAD67 and APOBEC3A, which is an enzyme with critical roles in the active DNA demethylation pathway. In contrast, patients only suffering from severe major depressive disorder have significantly decreased levels of 5hmC [Citation71], suggesting that 5hmC has unique functions among closely related brain disorders. Together, these findings suggest a common etiology in psychosis, one that includes early life adversity and genome-wide changes in 5hmC.
Autism
The autism spectrum disorders (ASD) encompass a broad range of behaviorally related and neurodevelopmental disorders with a high prevalence in children. Notably, only ∼20% of ASD cases show a genetic etiology [Citation72,Citation73]. Prenatal factors shown to increase the risk of ASD in offspring [Citation74] include environmental influences such as multiple births, in vitro fertilization and parental exposure to common drug treatments (e.g., antiepileptic drugs [e.g., valporate] or folic acid) [Citation75]. Together, these findings effectively open the door for contributions from epigenetic modifications such as 5hmC to have an underlying role in the ASD etiology. Consistent with this hypothesis, developmentally specific changes in 5hmC are highly enriched in known autism genes [Citation76]. Moreover, we recently profiled 5hmC in a mouse model of autism that has behavioral and molecular alterations such as deficits in social interactions and GABAergic signaling [Citation77,Citation78]. This study revealed that this mouse model harbored differential 5hmC on a remarkable number of established human autism genes, suggesting that 5hmC may be influencing the observed autistic-like phenotype in these mice [Citation79]. Since these findings were observed post-symptomatically in adult mice, it is unclear if the altered 5hmC represents a cause or a consequence of having autistic-like behaviors. Thus, these findings warrant a deeper investigation of this mouse model at earlier developmental time points.
Early life stressors have been associated with the development of mental illness, but the extent to which a gene x environment interaction contributes to the development of autism has yet to be tested. However, prenatal stress reduced anxiety-like behaviors and altered gene expression in mice heterozygous for the 5-HTT gene [Citation80]. Interestingly, these findings were more pronounced in female offspring, suggesting a gender-specific development of these altered behaviors. This study demonstrates a gene × environment interaction and while this study did not examine 5hmC, the 5hmC-related data discussed in this review supports a 5hmC contribution to these altered behaviors and gene expression. Thus, it will be interesting to examine whether early life stress causes an autism-prone individual (e.g., heterozygote for an autism risk allele) to develop full-blown autism phenotype, and if 5hmC plays a role in this process. If so, do these changes in 5hmC mirror those found in the homozygous mutants. This type of study could show that early life stress affects outcomes in offspring that may have a genetic predisposition to autism, a likely scenario for many mental disorders.
Putative functions/clinical utilities of 5hmC
While many studies have shown that disruptions in 5hmC are linked with mental illness, the precise molecular function(s) of 5hmC remains unknown. Since 5hmC is generally associated with the transcription of neuronal genes, it likely has a role in the regulation of gene expression. Since 5hmC is enriched in post-mitotic neurons, it most likely has an impact on proper neurodevelopment by regulating expression of genes responsible for neuronal propagation, development, and maintenance.
Several studies have revealed that altered 5hmC in differentially expressed genes is proximal to TF binding motifs [Citation29,Citation55]. Therefore, 5hmC may regulate TF binding to DNA, resulting in either up- or down-regulation of gene expression (). 5hmC also has been associated with the regulation of alternative splicing of transcripts after cocaine-administration [Citation31]. Here there was an overall greater change in isoform transcript levels, compared with full-length transcript levels [Citation81]. An increase in 5hmC also was found in splice sites associated with upregulated spliced isoforms and a decrease of 5hmC in splice sites associated with downregulated spliced isoforms; hence, again coupling 5hmC changes in the brain with alternative splicing () [Citation31]. Together, these studies implicate 5hmC in the regulation of transcriptional abundance and diversity.
5hmC may also affect the binding of miRNAs (miRs). We recently discovered that altered 5hmC in differentially expressed genes is proximal to sequences that are complementary to seed regions of several miRs [madrid, alisch, unpublished data]. While miRs are generally thought to bind to mRNA and not DNA, 5hmC may have a co-transcriptional function in attracting or repelling miRs as the DNA is being transcribed into RNA (). Interestingly, chronic variable stress on male mice alters miR content in sperm, and offspring from these sperm have a reduction in HPA axis stress responsivity [Citation82]. Thus, miRs and 5hmC may work together in response to early life stressors by altering gene expression and physiological sensitivity to adversities, which may contribute to the origins of mental illnesses.
The instability of 5hmC levels following prenatal and/or acute stress underscores the potential for 5hmC as a novel biomarker in the diagnosis of mental health. In addition, the presence/absence of these epigenetic modifications is reversible; thus, they may become relevant in therapeutic interventions [Citation83], especially if methods to selectively modulate 5hmC in vivo are developed at the nucleotide level. Finally, 5hmC has gender-specific profiles, which is of interest because the development of several psychiatric disorders are seemingly gender-specific. For example, females show an increased risk in developing anxiety and depression while males show a disposition to development of autism and attention deficit hyperactivity disorder [Citation84,Citation85]. A recent study found gender-specific 5hmC on genes with ontological terms correlating with organ morphogenesis, system development and development of anatomical structures [Citation30], suggesting that 5hmC may differentially influence the development of organs (e.g., the brain) among the genders. Together, these factors must be considered for clinical application and treatment endeavors.
In reality, our understanding of 5hmC functions is obscure and in its infancy. These molecular roles are likely to contribute to a broad spectrum of cellular functions from lifelong neurogenesis to cell death. Environmentally sensitive molecular mechanisms, such as 5hmC, in the brain have become a significant focus of neuroscience research because of growing evidence that they are critical to the development of psychiatric disorders. Thus, in the coming years it will be of great interest to unravel these molecular functions of 5hmC that are contributing to developmental brain disorders.
Conclusion
Many of the studies discussed here suggest that mental illness is associated with disruptions in 5hmC throughout the genome. Since 5hmC is present embryonically and accumulates throughout life, primarily on genes associated with development, these studies support a role for 5hmC in developmental brain disorders. In addition, finding that 5hmC levels also can rapidly change at certain loci to reflect environmental stimuli and that these changes also can become stable, opens new perspectives in the study of epigenetic mechanisms underlying mental health and the origins of mental illness. The growing number of links between 5hmC and psychiatry disorders leads many to suspect a functional role for 5hmC in mental health. While it is still unknown whether these mental illness-related disruptions in 5hmC are causative or correlative to the outcome, defining the molecular function(s) of 5hmC will likely reveal the clinical relevance and should lead to the development of new, more specific drug therapies with more personalized therapeutic options for those with mental illness. Together, these studies highlight the emerging role of 5hmC in mental health and disease and bring new hope to the field of psychiatry.
Future perspective
This review has highlighted the new hope that the study of 5hmC brings to psychiatry. While traditional molecular genetics has been limited in revealing the etiology of mental illness, 5hmC may provide a viable molecular mechanism contributing to the onset and progression of such disorders and diseases. A more comprehensive understanding of 5hmC, both functionally and as a biomarker, may provide a more objective measurement toward a more precise diagnosis of patients with mental illness. Ultimately, the development of methods to modulate 5hmC at the nucleotide level in genes contributing to mental illness may direct pharmaceutical companies in more fruitful directions of drug development for the treatment of mental illness. One such approach that might help achieve this nucleotide-specific modulation of 5hmC within the genome is to tether clustered, regularly interspaced short palindromic repeats (CRISPR) segments of DNA with either DNA methyltransferases or TET enzymes to selectively methylate or hydroxymethylate specific regions of the genome, respectively. Such a method would allow for specific modulation of 5hmC on genes that are known to have altered of 5hmC in mental illnesses, potentially allowing for personalized treatment and prevention of psychiatric disorders.
5hmC in the context of mental illness is new and rapidly growing, suggesting that the future of such research is bright and prospectively fruitful. Our group expects that in the coming years disruptions of 5hmC will be examined in a cell-specific fashion, allowing researchers to study 5hmC at a much higher resolution. Future cell-specific examinations of genome-wide 5hmC in specific neural circuits that contribute to behavioral outcomes will provide an objective marker for a much quicker more reliable diagnosis that will lead to personalized treatments for those at high risk of developing a full-blown mental disorder.
5mC
5mC is a modified version of cytosine that involves the addition of a methyl group to the fifth carbon of cytosine and is nearly exclusive at CpG dinucleotide sites. This modification is catalyzed by DNMTs, which utilize S-adenosyl-l-methionine as the methyl donor [Citation19]. Three active DNMTs have been identified in mammals and they have distinct functions. DNMT3a and DNMT3b have an affinity toward unmethylated CpG sites, while DNMT1 preserves methylation, as it shows preference toward hemimethylated CpG sites [Citation20]. While CpG rich-regions, known as CpG islands, and gene promoters have a significant reduction in 5mC levels, the X chromosome has an overabundance of 5mC [Citation21,Citation22]. 5mC regulates processes such as gene silencing, X-chromosome inactivation, genomic imprinting and chromatin structure [Citation23]. The ‘gold-standard’ in the detection of 5mC is sodium bisulfite sequencing, which converts unmethylated cytosine to uracil that are read as thymine following PCR [Citation24]
5hmC
5hmC is a modified version of cytosine that involves the addition of a hydroxy group to the methyl group of 5mC. This modification is catalyzed by the TET family of enzymes [Citation25]. An approximately tenfold abundance of 5hmC is seen in brain tissues, other tissues of the central nervous system and embryonic stem cells, compared with peripheral tissue [Citation26]. 5hmC levels are reduced in CpG islands and intronic sequences, but are enriched in gene promoters, the 5’-UTR, exons, and distal regulatory regions [Citation26]. Notably, mammals have a disproportionate reduction of 5hmC on chromosome 18 and the X chromosome [Citation27]. While an increase in transcription levels has been correlated with 5hmC enrichment [Citation26], alternatively spliced transcripts display a reduced 5hmC on excised exons as compared with constitutive exons, suggesting that 5hmC may be playing a role in the production of isoform transcripts [Citation28]. Neuronal- and synapse-related genes have a significant increase in 5hmC levels [Citation28]. The function of 5hmC has yet to be discovered, yet several putative functions are discussed in this review, including the regulation of transcription factor binding [Citation29], gender-specific development [Citation30] and transcript diversity through interactions with the spliceosome [Citation31]. Since traditional sodium bisulfite sequencing cannot distinguish between 5hmC and 5mC, Tet1-assisted bisulfite sequencing has become an established method for 5hmC detection [Citation32]
5hmC: 5-hydroxymethylcytosine; 5mc: 5-methylcytosine.
State of psychiatry
Diagnosis is based on subjective measures that are primarily acquired through behavioral assessments.
Genetic influences often sought to explain the etiology of mental health.
The broad heritability and heterogeneity of mental illness has limited the success of molecular genetics to determine its etiology.
Crucial molecular mechanisms that cause variations in the expression of key genes in the brain are currently ignored by molecular psychiatry, which is contributing to misdiagnoses and ineffective treatments: a fundamental problem in the field of psychiatry.
Epigenetics & 5-hydroxymethylcytosine
Environmentally sensitive modifications that are heritable and often induce stable changes in gene expression.
5-hydroxymethylcytosine (5hmC) is a recently identified modification of cytosine that shows significant enrichment in brain over peripheral tissues and on genes known to have neuronal and synaptic functions.
5hmC is present embryonically and accumulates throughout life, suggesting a potential lifelong role in development.
The presence/absence of 5hmC modifications are reversible.
5hmC in mental health
5hmC disruptions have been associated with the development of early and late onset mental illnesses such as autism and Alzheimer’s disease.
Although linked to mental health, disruptions of 5hmC are not currently known to be causative or correlative of mental stability.
Function(s) of 5hmC remain elusive, warranting deeper investigations aimed at unlocking the full potential of 5hmC as a therapeutic measure of mental health.
Potential biomarker in the personalized diagnosis of mental illness.
Potential functions of 5hmC
Current understanding of 5hmC function(s) is obscure and in its infancy.
Alterations of gene expression may be through transcription factor binding, miRNA binding and/or alternative splicing of transcripts to generate isoforms.
Gender-specific distributions, which may explain gender-specific dispositions to mental illness (e.g., depression in females and autism in males).
Future perspective
The development of personalized therapeutic manipulations of 5hmC to prevent onset or progression of mental illness through methods such as CRISPR to target specific genomic loci.
The development of cell-specific profiling of specific neural circuits that contribute to behavioral outcomes will provide an objective marker for a much quicker and more reliable diagnosis that will lead to personalized treatments for those at high risk of developing a full-blown mental illness.
The study of 5hmC brings new hope to the field of psychiatry through its promising clinical relevance.
Disclaimer
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Financial & competing interests disclosure
This work was supported in part by National Science Foundation under Grant No. 1400815 (A Madrid), NARSAD Young Investigator Grant from the Brain & Behavioral Research Foundation #22669 (LA Papale), the University of Wisconsin-Madison Department of Psychiatry, University of Wisconsin Vilas Life Cycle Professorships #133AAA2989, University of Wisconsin Graduate School #MSN184352 (all to RS Alisch). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Bird A . DNA methylation patterns and epigenetic memory . Genes Dev.16 ( 1 ), 6 – 21 ( 2002 ).
- Sharma RP , GavinDP , GraysonDR . CpG methylation in neurons: message, memory, or mask?Neuropsychopharmacol.35 ( 10 ), 2009 – 2020 ( 2010 ).
- Han JA , AnJ , KoM . Functions of TET proteins in hematopoietic transformation . Mol. Cells38 ( 11 ), 925 – 935 ( 2015 ).
- Suzuki MM , BirdA . DNA methylation landscapes: provocative insights from epigenomics . Nat. Rev. Genet.9 ( 6 ), 465 – 476 ( 2008 ).
- Robertson KD . DNA methylation and human disease . Nat. Rev. Genet.6 ( 8 ), 597 – 610 ( 2005 ).
- Abdolmaleky HM , ChengKH , FaraoneSVet al. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder . Hum. Mol. Genet.15 ( 21 ), 3132 – 3145 ( 2006 ).
- Poulter MO , DuL , WeaverICet al. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes . Biol. Psychiatry64 ( 8 ), 645 – 652 ( 2008 ).
- Kuratomi G , IwamotoK , BundoMet al. Aberrant DNA methylation associated with bipolar disorder identified from discordant monozygotic twins . Mol. Psychiatry13 ( 4 ), 429 – 441 ( 2008 ).
- Kappeler L , MeaneyMJ . Epigenetics and parental effects . Bioessays32 ( 9 ), 818 – 827 ( 2010 ).
- Weaver IC , CervoniN , ChampagneFAet al. Epigenetic programming by maternal behavior . Nat. Neurosci.7 ( 8 ), 847 – 854 ( 2004 ).
- Wu H , ZhangY . Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation . Genes Dev.25 ( 23 ), 2436 – 2452 ( 2011 ).
- Penn NW , SuwalskiR , O’RileyC , BojanowskiK , YuraR . The presence of 5-hydroxymethylcytosine in animal deoxyribonucleic acid . Biochem. J.126 ( 4 ), 781 – 790 ( 1972 ).
- Kriaucionis S , HeintzN . The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain . Science324 ( 5929 ), 929 – 930 ( 2009 ).
- Sun W , ZangL , ShuQ , LiX . From development to diseases: the role of 5hmC in brain . Genomics104 ( 5 ), 347 – 351 ( 2014 ).
- Wang J , TangJ , LaiM , ZhangH . 5-hydroxymethylcytosine and disease . Mutat. Res. Rev. Mutat. Res.762167 – 175 ( 2014 ).
- He YF , LiBZ , LiZet al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA . Science333 ( 6047 ), 1303 – 1307 ( 2011 ).
- Ito S , D’AlessioAC , TaranovaOV , HongK , SowersLC , ZhangY . Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification . Nature466 ( 7310 ), 1129 – 1133 ( 2010 ).
- Kraus TF , KilincS , SteinmaurerM , StieglitzM , GuibourtV , KretzschmarHA . Profiling of methylation and demethylation pathways during brain development and ageing . J. Neural. Transm. (Vienna)123 ( 3 ), 189 – 203 ( 2016 ).
- Cheng X . Structure and function of DNA methyltransferases . Annu. Rev. Biophys. Biomol. Struct.24 , 293 – 318 ( 1995 ).
- Okano M , BellDW , HaberDA , LiE . DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development . Cell99 , 247 – 257 ( 1999 ).
- Sharp AJ , StathakiE , MigliavaccaEet al. DNA methylation profiles of human active and inactive X chromosomes . Genome Res.21 , 1592 – 1600 ( 2011 ).
- Ioshikhes IP , ZhangMQ . Large-scale human promoter mapping using CpG islands . Nat. Genet.26 ( 1 ), 61 – 63 ( 2000 ).
- Irier HA , JinP . Dynamics of DNA methylation in aging and Alzheimer’s disease . CellBiol.31 ( Suppl. 1 ), S42 – S48 ( 2012 ).
- Frommer M , McDonaldLE , MillarDSet al. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands . Proc. Natl Acad. Sci. USA89 ( 5 ), 1827 – 1831 ( 1992 ).
- Song CX , SzulwachKE , FuYet al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine . Nat. Biotechnol.29 ( 1 ), 68 – 72 ( 2011 ).
- Branco MR , FiczG , ReikW . Uncovering the role of 5-hydroxymethylcytosine in theepigenome . Nat. Rev. Genet.13 , 7 – 13 ( 2012 ).
- Chopra P , PapaleLA , WhiteATet al. Array-based assay detects genome-wide 5-mC and 5-hmC in the brains of humans, non-human primates, and mice . BMC Genomics15 , 131 ( 2014 ).
- Khare T , PaiS , KonceviciusKet al. 5-hmC in the brain is abundant in synaptic genes and shows differences at the exon–intron boundary . Nat. Struct. Mol. Biol.19 ( 10 ), 1037 – 1043 ( 2012 ).
- Li S , PapaleLA , ZhangQet al. Genome-wide alterations in hippocampal 5-hydroxymethylcytosine links plasticity genes to acute stress . Neurobiol. Dis.86 , 99 – 108 ( 2016 ).
- Gross JA , PacisA , ChenGGet al. Characterizing 5-hydroxymethylcytosine in human prefrontal cortex at single base resolution . BMC Genomics16 , 672 ( 2015 ).
- Feng J , ShaoN , SzulwachKEet al. Role of Tet1 and 5-hydroxymethylcytosine in cocaine action . Nat. Neurosci.18 ( 4 ), 536 – 544 ( 2015 ).
- Yu M , HonGC , SzulwachKEet al. Tet-assisted bisulfite sequencing of 5-hydroxymethylcytosine . Nat. Protoc.7 ( 12 ), 2159 – 2170 ( 2012 ).
- Phase IMA , QiuR , WuXet al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in mammalian neurogenesis . Cell Rep.3 ( 2 ), 291 – 300 ( 2013 ).
- Tan L , XiongL , XuWet al. Genome-wide comparison of DNA hydroxymethylation in mouse embryonic stem cells and neural progenitor cells by a new comparative hMeDIP-seq method . Nucleic Acids Res.41 ( 7 ), e84 ( 2013 ).
- Kang J , LienhardM , PastorWAet al. Simultaneous deletion of the methylcytosine oxidases Tet1 and Tet3 increases transcriptome variability in early embryogenesis . Proc. Natl Acad. Sci. USA112 ( 31 ), E4236 – E4245 ( 2015 ).
- Szulwach KE , LiX , LiYet al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging . Nat. Neurosci.14 ( 12 ), 1607 – 1616 ( 2011 ).
- Chen H , DzitoyevaS , ManevH . Effect of aging on 5-hydroxymethylcytosine in the mouse hippocampus . Restor. Neurol. Neurosci.30 ( 3 ), 237 – 245 ( 2012 ).
- Zampieri M , CiccaroneF , CalabreseR , FranceschiC , BurkleA , CaiafaP . Reconfiguration of DNA methylation in aging . Mech. Ageing Dev.151 , 60 – 70 ( 2015 ).
- Chouliaras L , Van Den HoveDL , KenisGet al. Age-related increase in levels of 5-hydroxymethylcytosine in mouse hippocampus is prevented by caloric restriction . Curr. Alzheimer Res.9 ( 5 ), 536 – 544 ( 2012 ).
- Al-Mahdawi S , VirmouniSA , PookMA . The emerging role of 5-hydroxymethylcytosine in neurodegenerative diseases . Front. Neurosci.8 , 397 ( 2014 ).
- Chouliaras L , MastroeniD , DelvauxEet al. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients . Neurobiol. Aging34 ( 9 ), 2091 – 2099 ( 2013 ).
- Condliffe D , WongA , TroakesCet al. Cross-region reduction in 5-hydroxymethylcytosine in Alzheimer’s disease brain . Neurobiol. Aging35 ( 8 ), 1850 – 1854 ( 2014 ).
- Coppieters N , DieriksBV , LillC , FaullRL , CurtisMA , DragunowM . Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain . Neurobiol. Aging35 ( 6 ), 1334 – 1344 ( 2014 ).
- Bradley-Whitman MA , LovellMA . Epigenetic changes in the progression of Alzheimer’s disease . Mech. Ageing Dev.134 ( 10 ), 486 – 495 ( 2013 ).
- Lashley T , GamiP , ValizadehN , LiA , ReveszT , BalazsR . Alterations in global DNA methylation and hydroxymethylation are not detected in Alzheimer’s disease . Neuropathol. Appl. Neurobiol.41 ( 4 ), 497 – 506 ( 2015 ).
- Walker FO . Huntington’s disease . Lancet369 ( 9557 ), 218 – 228 ( 2007 ).
- Lee J , HwangYJ , KimKY , KowallNW , RyuH . Epigenetic mechanisms of neurodegeneration in Huntington’s disease . Neurotherapeutics10 ( 4 ), 664 – 676 ( 2013 ).
- Reik W , MaherER , MorrisonPJ , HardingAE , SimpsonSA . Age at onset in Huntington’s disease and methylation at D4S95 . J. Med. Genet.30 ( 3 ), 185 – 188 ( 1993 ).
- Wang F , YangY , LinXet al. Genome-wide loss of 5-hmC is a novel epigenetic feature of Huntington’s disease . Hum. Mol. Genet.22 ( 18 ), 3641 – 3653 ( 2013 ).
- Villar-Menendez I , BlanchM , TyebjiSet al. Increased 5-methylcytosine and decreased 5-hydroxymethylcytosine levels are associated with reduced striatal A2AR levels in Huntington’s disease . Neuromolecular Med.15 ( 2 ), 295 – 309 ( 2013 ).
- McKinnon PJ . ATM and ataxia telangiectasia . EMBO Rep.5 ( 8 ), 772 – 776 ( 2004 ).
- Jiang D , ZhangY , HartRP , ChenJ , HerrupK , LiJ . Alteration in 5-hydroxymethylcytosine-mediated epigenetic regulation leads to Purkinje cell vulnerability in ATM deficiency . Brain138 ( Pt 12 ), 3520 – 3536 ( 2015 ).
- Hagerman PJ , HagermanRJ . Fragile X-associated tremor/ataxia syndrome . Ann. NY Acad. Sci.1338 , 58 – 70 ( 2015 ).
- Santoro MR , BraySM , WarrenST . Molecular mechanisms of fragile X syndrome: a twenty-year perspective . Annu. Rev. Pathol.7 , 219 – 245 ( 2012 ).
- Yao B , LinL , StreetRCet al. Genome-wide alteration of 5-hydroxymethylcytosine in a mouse model of fragile X-associated tremor/ataxia syndrome . Hum. Mol. Genet.23 ( 4 ), 1095 – 1107 ( 2014 ).
- Irier H , StreetRC , DaveRet al. Environmental enrichment modulates 5-hydroxymethylcytosine dynamics in hippocampus . Genomics104 ( 5 ), 376 – 382 ( 2014 ).
- Li S , PapaleLA , KintnerDBet al. Hippocampal increase of 5-hmC in the glucocorticoid receptor gene following acute stress . Behav. Brain Res.286 , 236 – 240 ( 2015 ).
- Cirelli C , FaragunaU , TononiG . Changes in brain gene expression after long-term sleep deprivation . J. Neurochem.98 ( 5 ), 1632 – 1645 ( 2006 ).
- Li X , WeiW , ZhaoQYet al. Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation . Proc. Natl Acad. Sci. USA111 ( 19 ), 7120 – 7125 ( 2014 ).
- Rudenko A , DawlatyMM , SeoJet al. Tet1 is critical for neuronal activity-regulated gene expression and memory extinction . Neuron79 ( 6 ), 1109 – 1122 ( 2013 ).
- Roth TL , SweattJD . Epigenetic marking of the BDNF gene by early-life adverse experiences . Horm. Behav.59 ( 3 ), 315 – 320 ( 2011 ).
- Labonte B , SudermanM , MaussionGet al. Genome-wide epigenetic regulation by early-life trauma . Arch. Gen. Psychiatry69 ( 7 ), 722 – 731 ( 2012 ).
- McGowan PO , SasakiA , D’AlessioACet al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse . Nat. Neurosci.12 ( 3 ), 342 – 348 ( 2009 ).
- Oberlander TF , WeinbergJ , PapsdorfM , GrunauR , MisriS , DevlinAM . Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses . Epigenetics3 ( 2 ), 97 – 106 ( 2008 ).
- Massart R , SudermanM , ProvencalNet al. Hydroxymethylation and DNA methylation profiles in the prefrontal cortex of the non-human primate rhesus macaque and the impact of maternal deprivation on hydroxymethylation . Neuroscience268139 – 148 ( 2014 ).
- Koenig JI , ElmerGI , ShepardPDet al. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia . Behav. Brain Res.156 ( 2 ), 251 – 261 ( 2005 ).
- Guidotti A , DongE , TuetingP , GraysonDR . Modeling the molecular epigenetic profile of psychosis in prenatally stressed mice . Prog. Mol. Biol. Transl. Sci.128 , 89 – 101 ( 2014 ).
- Matrisciano F , TuetingP , DalalIet al. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice . Neuropharmacol.68 , 184 – 194 ( 2013 ).
- Brown AS . The environment and susceptibility to schizophrenia . Prog. Neurobiol.93 ( 1 ), 23 – 58 ( 2011 ).
- Dong E , GavinDP , ChenY , DavisJ . Upregulation of TET1 and downregulation of APOBEC3A and APOBEC3C in the parietal cortex of psychotic patients . Transl. Psychiatry2 , e159 ( 2012 ).
- Tseng PT , LinPY , LeeYet al. Age-associated decrease in global DNA methylation in patients with major depression . Neuropsychiatr. Dis. Treat.10 , 2105 – 2114 ( 2014 ).
- Gaugler T , KleiL , SandersSJet al. Most genetic risk for autism resides with common variation . Nat. Genet.46 ( 8 ), 881 – 885 ( 2014 ).
- Bulik-Sullivan B , FinucaneHK , AnttilaVet al. An atlas of genetic correlations across human diseases and traits . Nat. Genet.47 ( 11 ), 1236 – 1241 ( 2015 ).
- Gardener H , SpiegelmanD , BukaSL . Prenatal risk factors for autism: comprehensive meta-analysis . Br. J. Psychiatry195 ( 1 ), 7 – 14 ( 2009 ).
- Rogers EJ . Has enhanced folate status during pregnancy altered natural selection and possibly Autism prevalence? A closer look at a possible link . Med. Hypotheses71 ( 3 ), 406 – 410 ( 2008 ).
- Wang T , PanQ , LinLet al. Genome-wide DNA hydroxymethylation changes are associated with neurodevelopmental genes in the developing human cerebellum . Hum. Mol. Genet.21 ( 26 ), 5500 – 5510 ( 2012 ).
- Penagarikano O , LazaroMT , LuXHet al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism . Sci. Transl. Med.7 ( 271 ), 271ra – 278 ( 2015 ).
- Penagarikano O , AbrahamsBS , HermanEIet al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits . Cell147 ( 1 ), 235 – 246 ( 2011 ).
- Papale LA , ZhangQ , LiS , ChenK , KelesS , AlischRS . Genome-wide disruption of 5-hydroxymethylcytosine in a mouse model of autism . Hum. Mol. Genet.24 ( 24 ), 7121 – 7131 ( 2015 ).
- Van Den Hove DL , JakobSB , SchrautKGet al. Differential effects of prenatal stress in 5-Htt deficient mice: towards molecular mechanisms of gene × environment interactions . PLoS ONE6 ( 8 ), e22715 ( 2011 ).
- Feng J , WilkinsonM , LiuXet al. Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens . Genome Biol.15 ( 4 ), R65 ( 2014 ).
- Rodgers AB , MorganCP , BronsonSL , RevelloS , BaleTL . Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation . J. Neurosci.33 ( 21 ), 9003 – 9012 ( 2013 ).
- Szyf M . Epigenetics, a key for unlocking complex CNS disorders? Therapeutic implications . Eur. Neuropsychopharmacol.25 ( 5 ), 682 – 702 ( 2015 ).
- Nolen-Hoeksema S . Sex differences in unipolar depression: evidence and theory . Psychol. Bull.101 ( 2 ), 259 – 282 ( 1987 ).
- Wooten GF , CurrieLJ , BovbjergVE , LeeJK , PatrieJ . Are men at greater risk for Parkinson’s disease than women?J. Neurol. Neurosurg. Psychiatry75 ( 4 ), 637 – 639 ( 2004 ).
