Abstract
Aim: First-degree relatives (FDR) of individuals with Type 2 diabetes (T2D) feature restricted adipogenesis, which render them more vulnerable to T2D. Epigenetics may contribute to these abnormalities. Methods: FDR pre-adipocyte Methylome and Transcriptome were investigated by MeDIP- and RNA-Seq, respectively. Results:Methylome analysis revealed 2841 differentially methylated regions (DMR) in FDR. Most DMR localized into gene-body and were hypomethylated. The strongest hypomethylation signal was identified in an intronic-DMR at the PTPRD gene. PTPRD hypomethylation in FDR was confirmed by bisulphite sequencing and was responsible for its upregulation. Interestingly, Ptprd-overexpression in 3T3-L1 pre-adipocytes inhibited adipogenesis. Notably, the validated PTPRD-associated DMR was significantly hypomethylated in peripheral blood leukocytes from the same FDR individuals. Finally, PTPRD methylation pattern was also replicated in obese individuals. Conclusion: Our findings indicated a previously unrecognized role of PTPRD in restraining adipogenesis. This abnormality may contribute to increase FDR proclivity toward T2D.
Subcutaneous adipose tissue (SAT) has been identified as a key player in the evolution toward Type 2 diabetes (T2D) [Citation1]. Limited SAT expandability induces hypertrophy and alters the secretion profile in mature adipose cells, causes low-grade chronic inflammation, ectopic fat accumulation and peripheral insulin resistance [Citation2]. In humans, SAT hypertrophy results from an attenuated differentiation capacity of resident pre-adipocytes rather than lack of precursor cells [Citation3]. Importantly, the increased adipose cell size predicts T2D even independent of obesity [Citation4].
First-degree relatives of Type 2 diabetics (FDR) have up to tenfold increased risk of developing diabetes [Citation5]. Healthy FDR also feature dysfunctional SAT and show signs of inflammation and macrophage infiltration accompanied by adipocyte hypertrophy, due to the impaired commitment and/or differentiation of SAT-resident pre-adipocytes [Citation3,Citation4,Citation6]. These findings indicated that, in FDR, diabetes risk is associated to reduced ability to recruit new adipocytes in response to stimuli promoting adipose tissue expansion, inducing ectopic accumulation of fat, insulin resistance and rendering these individuals more vulnerable to T2D development.
The molecular mechanisms responsible for the restricted adipogenesis in FDR remain unclear. Despite intensive efforts to identify risk loci contributing to limited adipose tissue expandability [Citation7], no genotype accounting for this defect in most FDR individuals has been so far identified. Environmental cues, which are also shared within the family groups, may attenuate the individual adipogenic potential leading to resident fat cell hypertrophy [Citation8“10]. Accumulating evidence now identifies the epigenome as a signal integrator active at the interface between the environment and the function of genes whose alterations impact on evolution toward T2D [Citation11]. Indeed, epigenetic changes may convert environmental cues into phenotypic traits by reprogramming transcription and contributing to disease development and/or transmission [Citation12]. In addition, recent human studies in both SAT specimen [Citation13,Citation14] and SAT-derived pre-adipocytes [Citation15,Citation16], have revealed the important role of DNA methylation in the functional regulation of genes determining adipogenesis [Citation2,Citation13“17]. Thus, the hypothesis that epigenetic dysregulation impacts on FDR risk of T2D by impairing adipogenesis in the SAT deserves to be mechanistically investigated. There is presently no information on Methylome profile diversities in adipocyte precursor cells from healthy individuals who are FDR and subjects with no family history of T2D.
In the present work, we report the first genome-wide DNA methylation profile in adipocyte precursor cells from the SAT stromal vascular fraction (SVF) of healthy FDR and matched individuals with no T2D familiarity. These studies have established that, in FDR individuals, the excess risk of T2D is associated with hypomethylation events affecting major molecular pathways involved in transduction of signals controlling adipogenesis. Our data revealed a previously undescribed role of PTPRD in regulating adipogenesis.
Materials &methods
Study participants
Discovery cohort
Individuals with one ascertained first-degree relative with T2D (n = 9; FDR) and 11 subjects with no family history of T2D (control [CTRL]) were selected from the European Network on Functional Genomics of Type 2 diabetes (EUGENE2) consortium [Citation18]. The recruitment and clinical phenotyping of the subjects have been previously described [Citation18]. The participants included in this study were healthy nondiabetic offspring of parents with T2D. For inclusion, one of the parents had to have T2D and the other parent normal glucose tolerance evaluated by an oral glucose tolerance test (OGTT) or lack of any family history of T2D. The study was approved by the appropriate institutional review board.
Abdominal SAT biopsies were obtained by needle aspiration from the paraumbilical region. Following careful dissection, adipose tissue specimens were digested with collagenase for 45 min at 37°C. After digestion, the suspension was centrifuged in two phases: an upper (fat cells) and a lower (SVF cells) phase. The isolated fat cells were filtered through a 250 μm nylon mesh, washed four-times with fresh medium for removal of collagenase. Cell size of isolated adipocytes was measured as described in [Citation19]. The isolated SVF cells were washed twice and the erythrocytes were lysed with 155 mmol/l NH4Cl for 5 min before seeding SVF in a 55-cm2 petri dish. After 3 days, the inflammatory cells (CD14+ and CD45+ cells) and endothelial cells (CD31+ cells) were removed from the adipocyte precursor cell fraction by immune magnetic separation (Miltenyi Biotech, Bergisch Gladbach, Germany). The adipocyte precursor cells were then cultured with DMEM:F12 medium (LONZA, Basel, Switzerland) supplemented with 10% fetal bovine serum (ThermoFisher Scientific, MA, USA), 2 mmol/l glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (LONZA). After 2 weeks, any remaining inflammatory cells were removed by immune magnetic separation as described in [Citation4,Citation19].
Replication cohort
20 caucasic individuals from an independent cohort recruited at the Federico II University of Naples were included in the replication analysis. The cohort included ten obese patients and ten lean individuals (Supplementary Table 1). Recruitment and clinical phenotyping of these subjects have been previously described [Citation20]. The study was approved by the Ethics Committee of the Federico II University of Naples (Ethics Approval Number: no. 254/17) and all subjects provided written informed consent for sampling.
MeDIP-Seq
Genomic DNA (gDNA), obtained from isolated SVF cells, was extracted using the AllPrep DNA/RNA Universal Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. All gDNA samples were tested for integrity and quantified by using a Qubit 4 Fluorometer (ThermoFisher Scientific). Equimolar amounts of gDNA for each subject were pooled (two pools for each condition). Methylated DNA Immunoprecipitation followed by massively parallel sequencing (MeDIP-Seq) was performed by Beijing Genomics Institute (https://www.bgi.com/global/). Sequencing was performed on a HiSeq 2000 system (Illumina Inc., CA, USA). Reads were mapped to the reference human genome (hg19 release) using SOAP2.21 aligner (http://soap.genomics.org.cn). Read alignments in sequence alignment map format were converted to binary alignment map for further analyses using SAMtools [Citation21] and browser extensible data (BED)tools were used to convert mapped reads in BED format and to generate Bedgraph files uploaded in UCSC Genome Browser (http://genome.ucsc.edu). MeDIP-Seq datasets obtained were independently analyzed for whole genome peak scanning by MACS2 (http://liulab.dfci.harvard.edu/MACS/) [Citation22]. By using BEDtools, we defined a set of common peaks within each experimental condition (both in CTRL and FDR individuals). ≥50% overlap across the entire peak length was used as arbitrary threshold for the inclusion in this common set of peaks. The genomic coordinates of these peaks were extracted and unique reads mapping within these regions were used to compute differential methylation between CTRL and FDR individuals. Then, differentially methylated regions (DMR) in the comparison FDR versus CTRL subjects were computed using the R/Bioconductor package edgeR [Citation23] and Benjamini“Hochberg approach was used for multiple testing correction [Citation24]. For further analyses, only regions with an arbitrary threshold of 5% false discovery rate (fdr) were selected. The relative position of the peaks and proximity to genes were assessed using the online tool PAVIS2 [Citation24]. The functional annotation tool DAVID (https://david.ncifcrf.gov/home.jsp) was used to classify genes with methylation peaks according to their biological function. Also see Supplementary methods for details.
RNA-Seq
Total RNA, obtained from isolated SVF cells, was extracted using the AllPrep DNA/RNA Universal Kit. RNA samples were tested for integrity and quantified by using a Qubit 4 Fluorometer. Equimolar amounts of total RNA for each subject were pooled (two pools for each condition). RNA-Seq was carried out by Beijing Genomics Institute (https://www.bgi.com/global/). Trimmed reads were aligned to Gencode Human release v19 transcripts by running RSEM version 1.2.19 with standard parameters [Citation25]. Aligned reads (about 47 million per sample) were then used to estimate the expression level of each Gencode gene. Only genes with at least one count per million reads (CPM) in at least two samples were considered as expressed. Differential expression analysis between CTRL and FDR individuals was performed on the read counts of the expressed genes. The Generalized Linear Model approach implemented in the R/Bioconductor package edgeR was used to correct for possible bias [Citation23]. The Benjamini“Hochberg approach was used for multiple testing correction [Citation24]. Genes with log2 Fold Change (log FC) ≥1 and an fdr ≤0.05 were considered as differentially expressed genes (DEG). To assess the robustness of our pooling design, we performed further RNA-Seq analyses on individual samples of total RNA from SAT pre-adipocytes of FDR and CTRL subjects. Also see Supplementary Methods for details.
Bisulphite sequencing
Bisulphite treatment of gDNA extracted from SVF and peripheral blood leukocytes (PBL) by the AllPrep DNA/RNA Mini Kit (Qiagen, Hilden, Germany), was performed using the EZ DNA Methylation Kit (Zymo Research, CA, USA). Converted gDNA was amplified by PCR using specific primers for PTPRD-DMR. Bisulphite sequencing (BS) was performed as reported in [Citation17]. Primers are shown in Supplementary Table 2>. See Supplementary Methods for details.
Total RNA isolation &quantitative real-time PCR
Total RNA was extracted from SVF and 3T3-L1 cells using the AllPrep DNA/RNA Mini Kit (Qiagen). Real-time PCR (RT-PCR) was performed as described in [Citation26]. Primers are shown in Supplementary Table 2. See Supplementary methods for details.
Western blots
Western blots were carried out as described in [Citation26]. Immunoblots were performed with antibodies against DNMT3A (ab2850, Abcam, Cambridge, UK) and β-ACTIN (sc-47778, Santa Cruz Biotechnology, TX, USA).
Human SVF cell culture &treatment
SVF cells, isolated from control donors, at passages 3“5 were treated with 5-azacytidine (5²AZA; Sigma-Aldrich, MO, USA) for 2Â days. See Supplementary methods for details.
Luciferase assay
The PTPRD-associated DMR chr9:10448607-10450000 was amplified by PCR and cloned into a CpG-free promoter firefly luciferase reporter vector (pCpGL-promoter; InvivoGen, CA, USA). Luciferase assays were performed as reported [Citation27]. Constructs were transfected into ChubS7 pre-adipose cell line by lipofectamine (ThermoFisher Scientific), following manufacturer’s instructions. Firefly luciferase activity was normalized against renilla luciferase activity (Promega, WI, USA). See Supplementary methods for details.
Overexpression of Ptprd in 3T3-L1 pre-adipocytes
3T3-L1 cells were grown and allowed to differentiate as described in [Citation28,Citation29]. Six hours before adipogenesis induction, 3T3-L1 pre-adipocytes were transfected with Ptprd-overexpressing vector (pCMV-Ptprd) or control vector (pCMV) using lipofectamine (ThermoFisher Scientific), following manufacturer’s instructions. See Supplementary methods for details.
Statistical analysis
Data are expressed as mean ± SD. Mann“Whitney U-test was used to compare anthropometric and biochemical variables, gene expression and DNA methylation data between groups. The correlation between quantitative variables was tested using Spearman’s rank correlation test. Two-tailed Student’s t-test or the one-way analysis of variance (ANOVA) followed by Tukey’s multi-comparison test was used, as appropriate, for the in vitro data. The group size (n) for each experimental group/condition is reported in the figure legends. The value of n refers to either the number of biological independent samples/individuals per group (in vivo analyses) or the number of independently repeated experiments, each performed at least in triplicate (in vitro studies). Differences were considered statistically significant at a p <0.05. Statistical analyses were performed using the GraphPad Software (version 6.01, CA, USA).
Results
Differential DNA methylation in pre-adipocytes from first-degree relatives of individuals with T2D
To explore the molecular bases of the increased risk of T2D in FDR individuals, we have first selected a suitable study group from the EUGENE2 population [Citation18]. As shown in , these subjects were healthy and nonobese individuals with (n = 9; FDR) or without (n = 11; CTRL) one first-degree relative with T2D and matched for age, BMI, body fat percentage and distribution. As described in [Citation4], individuals who were FDR featured significantly reduced insulin sensitivity, increased HOMA-IR values, higher fasting plasma insulin and glucose levels and higher glucose levels following 75 g glucose loading. Also, the FDR had higher triglyceride levels and a reduced amount of high-density lipoproteins cholesterol compared with the CTRL individuals. Importantly, FDR also featured larger subcutaneous adipose cells when compared with control subjects, consistent with the finding that FDR individuals have subcutaneous adipose cell hypertrophy [Citation4].
Table 1. Clinical characteristics of first-degree relative and CTRL subjects.
We then hypothesized that increased risk of T2D in FDR is contributed by epigenetic changes impairing pre-adipocyte differentiation. To explore this possibility, we have used SVF adipocyte precursor cells from the abdominal SAT of the FDR and control individuals shown in . Genomic DNA from these individuals was subjected to MeDIP-Seq. We have generated about 180 million reads per sample, 70% of which could be uniquely aligned to the human genome. These reads were uniformly distributed through the entire SVF genome, as indicated by detection of methylation events in most chromosomal regions (data not shown). Subsequent analyses were exclusively based on these sequences.
To identify DMR associated with T2D family history, MeDIP-Seq profiles from the FDR and control individuals were compared. This analysis led us to identify 2841 unique DMR, 2628 of which were hypomethylated with further 213 resulting hypermethylated in FDR. The highest frequency of DMR was observed in the gene bodies, particularly in the intronic (2143) and the exonic (346) regions, where hypomethylation events were more common ().
Table 2. DMR distribution in first-degree relative subjects.
DNA methyltransferases in FDR pre-adipocytes
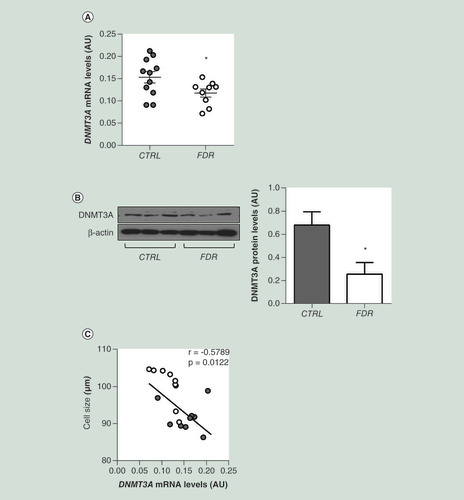
We then sought to explore whether differences in the DNA methylation profile observed in the FDR subjects can be attributed to changes in the expression of DNA-methylating enzymes and therefore we focused our attention on the three catalytically active DNA methyltransferases (DNMTs) [Citation17,Citation30]. In SVF isolated from the FDR group, we observed a significant reduction in the mRNA expression of DNMT3A, which is responsible for de novo methylation events (A). Furthermore, the amounts of DNMT3A protein in the SVF were decreased in the FDR compared with the control subjects (B). No significant difference was found in the expression of DNMT1 and DNMT3B between FDR and control subjects (Supplementary Figure 1), suggesting that the global hypomethylation observed in the SVF of the FDR individuals is contributed by reduced expression of DNMT3A. Interestingly, DNMT3A mRNA levels inversely correlated to the adipose cell size in the study group (C).
(A) DNMT3A mRNA expression was measured by qPCR in SVF from FDR (n = 9) and control (CTRL; n = 11) subjects. Data points represent AU from each individual subject. Mean values ± SD are also shown. *p<0.05 in a two-tailed Mann“Whitney U-test. (B) Representative immunoblot analysis of DNMT3A and β-ACTIN protein levels in SVF from FDR (n = 6) and control (CTRL; n = 6) subjects. Data are expressed as arbitrary units and shown as mean values ± SD. *p<0.05 in a two-tailed Mann-Whitney U-test. (C) Correlation between SVF DNMT3A mRNA levels and adipose cell size in FDR (n = 9) and CTRL (n = 11) subjects. r correlation coefficient and p-values are indicated on the graph.
AU: Absolute units; CTRL: Control; FDR: First-degree relatives; SD: Standard deviation; SVF: Stromal vascular fraction cells.
DMR-associated genes feature functions related to adipocyte biology &T2D
Further analysis revealed that, in the FDR, 2392 genes overlapped the identified DMR with their promoter and/or gene body regions. These were termed differentially methylated genes (DMG), >90% of which featured decreased methylation signals. We then adopted the DAVID annotation tools (http://david.ncifcrf.gov/home.jsp) to analyze the identified DMG for over-representation of specific pathways. Interestingly, over-represented pathways were found to include the Protein Kinase A, Insulin/IGF pathway-Protein Kinase B signalling, Inflammation by chemokine and cytokine pathway, Fibroblast Growth Factor signalling pathway and the WNT/β-catenin signaling pathway, all of which are linked to adipose cell function and T2D development (Supplementary Table 3).
Transcriptional effect of differential methylation
To explore the functional relevance of the FDR differential methylation, we complemented the Methylome dataset with Transcriptome analysis of SVF from FDR and control individuals. RNA-Seq analysis revealed 31 genes which were differentially expressed between FDR and control subjects. Out of these genes, 12 appeared to be downregulated while 19 were upregulated in FDR. We then merged the Transcriptome data with the identified intragenic DMR aiming at sorting differentially methylated sequences associated to functional effects on gene transcription. Three SVF genes exhibited significant expression changes associated to intragenic DMR in the FDR (). Interestingly, the expression-associated differential methylation changes occurred in gene body sequences rather than in promoter regions, underlining the significance of gene body methylation to transcriptional regulation.
Table 3. List of the genes differentially methylated and expressed between first-degree relative and CTRL subjects.
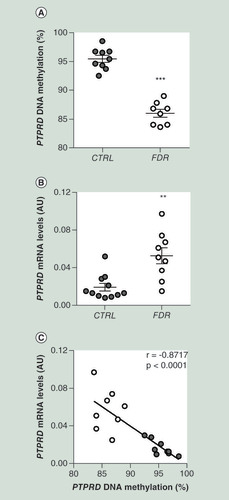
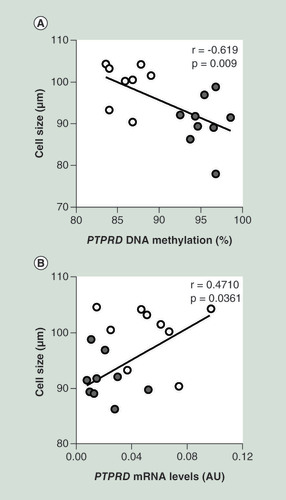
To validate these findings, we focused on the PTPRD gene, previously associated to risk of T2D [Citation31]. This gene was also found to be upregulated in FDR subjects by additional sequencing of individual samples from our study group (Supplementary Table 4). MeDIP-Seq analysis identified six hypomethylated DMR in PTPRD gene body spanning a region encompassing gene intron 1 to exon 12 (). DMR exhibiting the higher significance was subjected to bisulphite sequencing, which provided direct confirmation of DNA hypomethylation in the FDR (A). Importantly, as shown in B, PTPRD gene exhibited significant overexpression in the FDR compared with the control group that was inversely correlated to its hypomethylation (C). Of note, PTPRD DNA methylation (A) and its mRNA levels (B) significantly correlated with adipose cell size in the same FDR and control individuals.
Table 4. Characteristics of hypomethylated DMR associated with PTPRD in first-degree relative subjects.
(A) BS analysis of PTPRD DMR in SVF from FDR (n = 8) and CTRL (n = 9) subjects available from the study group. Data points represent DNA methylation percentage at the PTPRD-associated DMR. Mean values ± SD are also shown. ***p<0.001 in a two-tailed Mann“Whitney U-test. (B)PTPRD mRNA expression was measured by qPCR in SVF from FDR (n = 9) and control (CTRL; n = 11) subjects. Data points represent AU from each individual subject. Mean values ± SD are also shown. **p<0.01 in a two-tailed Mann“Whitney U-test. (C) Correlation between PTPRD DNA methylation and PTPRD mRNA levels in SVF from FDR (n = 8) and CTRL (n = 9) subjects. r correlation coefficient and p-values are indicated on the graph.
AU: Absolute units; BS: Bisulphite sequencing; CTRL: Control; DMR: Differentially methylated region; FDR: First-degree relatives; SD: Standard deviation; SVF: Stromal vascular fraction cells.
(A) Correlation between SVF PTPRD DNA methylation and adipose cell size in FDR (n = 8) and CTRL (n = 9) subjects. (B) Correlation between SVF PTPRD mRNA levels and adipose cell size in FDR (n = 9) and CTRL (n = 11) subjects. r correlation coefficient and p-values are indicated on the graph.
AU: Absolute units; CTRL: Control; FDR: First-degree relatives; SVF: Stromal vascular fraction cells.
Functional analysis of PTPRD intronic DMR
To further explore the transcriptional consequence of the hypomethylation occurring at the PTPRD intronic DMR in FDR individuals, we exposed SVF cells to the demethylating agent 5-azacytidine (5²AZA). As shown in Supplementary Figure 2, we observed that, in parallel with reducing DNA methylation, 5²AZA treatment significantly upregulated PTPRD transcription.
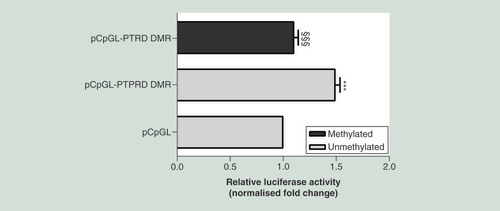
To assess this issue mechanistically, we have used a CpG-free promoter plasmid with luciferase reporter gene (pCpGL) where the PTPRD-associated DMR chr9:10448607-10450000 was cloned upstream the luciferase gene. This construct was then methylated in vitro or mock-treated and transfected in ChubS7 cells. In luciferase assays, the unmethylated PTPRD-associated DMR construct (pCpGL-PTPRD DMR) showed higher luciferase activity compared with the mock-treated empty vector (pCpGL; ). In contrast, the methylated PTPRD-associated DMR construct exhibited a significantly decreased transcriptional activity compared with the unmethylated pCpGL-PTPRD DMR (), indicating that the intronic DMR functions as a DNA methylation-sensitive region regulating PTPRD expression.
The 590 bp sequence corresponding to the intronic PTPRD-associated DMR chr9:10448607-10450000, was cloned into a pCpGL-promoter vector (pCpGL-PTPRD DMR), upstream the luciferase gene. The construct was either methylated by M.SssI (methylated pCpGL-PTPRD DMR) or mock-treated (unmethylated pCpGL-PTPRD DMR) and then transfected in ChubS7 cells. The data were normalized using a co-transfected renilla luciferase vector and are presented as fold change relative to the mock-treated empty vector (pCpGL). Data are shown as means ± SD of n = three independent experiments. Statistical significance was tested by one-way ANOVA followed by Tukey’s multi-comparison test: ***p<0.001 for comparison versus pCpGL; §§§p<0.001 for comparison versus unmethylated pCpGL-PTPRD DMR.
ANOVA: Analysis of variance;Â DMR: Differentially methylated region; SD: Standard deviation.
Ptprd restrains adipogenic differentiation in 3T3-L1 pre-adipocytes
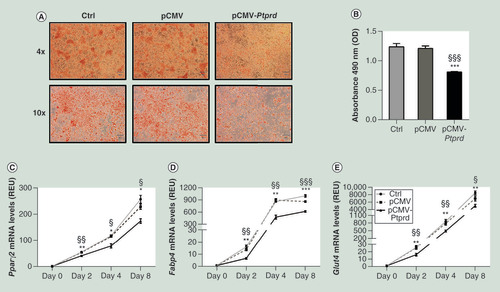
We have also examined whether PTPRD has a functional role in adipogenesis in vitro. To explore this possibility, 3T3-L1 pre-adipocytes were transfected with a pCMV vector expressing Ptprd. Eight days after the exposure to the differentiation cocktail, the cells overexpressing Ptprd accumulated significantly fewer lipids than either un-transfected or empty vector transfected cells, as revealed by Oil Red O staining (A &B). In parallel, expression of adipogenic markers was reduced in 3T3-L1 overexpressing Ptprd (C“E), indicating that, in vitro, adipogenesis is impaired by Ptprd overexpression.
Un-transfected cells (Ctrl) and cells transfected with either the empty vector (pCMV) or the PTPRD expressing vector (pCMV-PTPRD), at differentiation day eight, were stained with Oil Red O and then subjected to phase contrast microscopy. Representative fields are shown in figure A. Lipid quantification (B) was assessed at 490 nm as described under Materials and methods. Expression levels of Pparγ2(C), Fabp4(D), and Glut4(E) were assessed by qPCR at the indicated time points. All data are presented as means ± SD of n = three independent experiments. Statistical significance was tested by one-way ANOVA followed by Tukey’s multi-comparison test at each indicated time point: *p<0.05; **p<0.01; ***p<0.001 for comparison versus Ctrl cells; §p<0.05, §§p<0.01, §§§p<0.001 for comparison versus pCMV transfected cells.
ANOVA: Analysis of variance;Â Ctrl: Control;Â OD: Optical density; pCMV: Cytomegalovirus promoter; qPCR: Quantitative PCR;Â REU: Relative expression units;Â SD: Standard deviation.
PTPRD epigenetic signature in peripheral blood leukocytes
Blood cells can be more easily accessed than the adipose tissue. Thus, search for epigenetic marks associated to disease traits has been so far largely conducted in blood [Citation32]. Accordingly, we have further investigated whether the PTPRD hypomethylation observed in the SVF cells obtained from FDR individuals is accompanied by similar changes also in peripheral blood leukocytes.
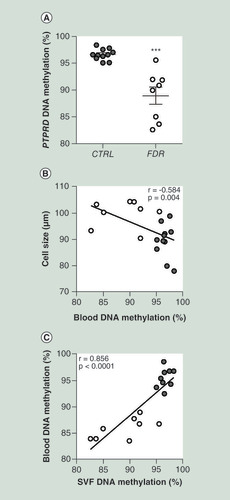
Upon bisulphite treatment, we have sequenced the PTPRD-associated DMR in PBL from the same FDR and control subjects whose SVF cells have been previously assessed. Interestingly, similar as with the SVF cells, PBL methylation levels at PTPRD were also significantly decreased in the FDR subjects (A). PBL DNA methylation at this locus also inversely correlated with subcutaneous adipose cell size (B) in the study group. In addition, a positive correlation was established between PTPRD DNA methylation levels in PBL and SVF cells from each individual subject (C), indicating that PBL represent a convenient proxy of the SVF epigenetic signature at this gene.
(A) BS analysis of PTPRD DMR in PBL from FDR (n = 8) and CTRL (n = 11) subjects available from the study group. Data points represent DNA methylation percentage at the PTPRD-associated DMR. Mean values ± SD are also shown. ***p<0.001 in a two-tailed Mann“Whitney U-test. Correlation between PBL PTPRD DNA methylation and adipose cell size in FDR (n = 8) and CTRL (n = 11) (B) or SVF PTPRD. DNA methylation in FDR (n = 8) and CTRL (n = 9) subjects (C)r correlation coefficient and p values are indicated on the graph.
BS: Bisulphite sequencing; CTRL: Control; DMR: Differentially methylated region; FDR: First-degree relatives; PBL: Peripheral blood leukocytes; SD: Standard deviation; SVF: Stromal vascular fraction cells.
Replication of the association to increased risk of T2D for the PTPRD DMR
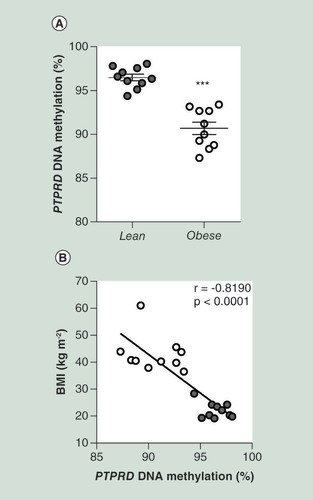
Obesity, estimated by BMI, is one of the well-established risk factors for T2D [Citation33]. Therefore, we sought to replicate the hypomethylation signal at the PTPRD gene in the PBL from an independent cohort of obese subjects. Similar to FDR, obese subjects have been reported to feature a higher risk of developing T2D (Supplementary Table 1). Interestingly, as evidenced in the FDR subjects, we observed that the PBL DNA methylation levels at PTPRD DMR were also significantly decreased in the obese compared with the lean individuals (A). Of note, the PTPRD DNA methylation levels in PBL were found to negatively correlate with the BMI in these subjects (B).
(A) BS analysis of PTPRD DMR in PBL from obese (n = 10) and lean (n = 10) subjects from the replication group. Data points represent DNA methylation percentage at the PTPRD-associated DMR. Mean values ± SD are also shown. ***p < 0.001 in a two-tailed Mann“Whitney U-test. (B) Correlation between PBL PTPRD DNA methylation and BMI in obese (n = 10) and lean (n = 10) subjects. r correlation coefficient and p-values are indicated on the graph.
BS: Bisulphite sequencing; DMR: Differentially methylated region; PBL: Peripheral blood leukocytes; SD: Standard deviation.
Discussion
In the present manuscript, we report the first genome-wide DNA methylation profile in human pre-adipocytes from healthy nonobese FDR. As previously described [Citation4], these subjects are characterized by an ˜obese™ phenotype in the SAT even if they are normal weight, leading to a reduced insulin sensitivity and a more proatherogenic lipid profile with an increased risk of developing T2D. We found that, in SAT pre-adipocytes from the FDR individuals, a number of hypomethylation events associate to their Methylome profile and family history of T2D. Consistent with these novel findings and the high risk of T2D in FDR, T2D is also accompanied by whole genome hypomethylation events occurring both in the liver and in pancreatic islets [Citation34,Citation35]. Crujeiras and co-workers [Citation36] have further reported the presence of hypomethylation in SAT and PBL genome from obese patients, who also feature excess risk of T2D [Citation14]. Thus, the occurrence of hypomethylation events at specific genomic site in tissues representing major determinants of glucose homeostasis might contribute to increasing T2D risk. Indeed, our results in SAT from FDR are consistent with a previous study showing that the majority of the methylation changes associated with a family history of T2D in human skeletal muscle are hypomethylation events [Citation37].
DNA methylation profiles are shaped by DNMTs [Citation16]. In the present study, we report that the hypomethylation occurring at several genomic loci in the pre-adipocytes from FDR is accompanied by DNMT3A mRNA and protein downregulation, suggesting a causal relationship with at least some of the identified hypomethylation events. Indeed, previous studies document that DNMT3A inactivation decreases methylation at the genome-wide level in both human and mouse embryonic stem cells [Citation38,Citation39]. Evidence has also been reported that DNMT3A deficiency restricts adipogenesis [Citation40,Citation41]. We now find that the reduced expression of DNMT3A negatively correlates with SAT adipose cell hypertrophy in FDR, where the increased adipose cell size is a marker of impaired adipogenesis [Citation2]. It is possible therefore that the downregulation of DNMT3A occurring in these subjects impacts on the methylation of distinct genomic regions which are relevant to adipogenesis, contributing to impair this function.
Functional analysis of the differential methylation profile identified in FDR led us to recognize that most DMG are related to biological pathways which are relevant to adipocyte biology or adipogenesis and T2D pathogenesis. These include the Insulin and IGF, PKA, FGF and WNT signalling cascades. Interestingly, methylation changes were more prevalent in gene body and intragenic regions as compared with gene promoter regions. This finding is consistent with previous studies by our own as well as other groups of investigators showing that DNA methylation events often involve regions other than promoters [Citation15,Citation42,Citation43]. How gene body methylation may affect gene expression remains unclear [Citation44“46]. However, our Methylome and Transcriptome findings in human FDR pre-adipocytes suggest that hypomethylation occurring at the gene body level may enhance gene expression; indeed, some genes in which both DNA methylation and mRNA expression were found simultaneously altered in the FDR pre-adipocytes show reduced methylation and increased expression.
In this study, PTPRD has been identified as the most significantly hypomethylated gene in FDR individuals. PTPRD encodes a transmembrane PTP termed PTPδ, which is highly homologous to the LAR protein and PTPσ tyrosine phosphatases [Citation47]. All of them have been previously shown to modulate insulin signalling and may affect glucose tolerance in vivo [Citation48“50]. Moreover, overexpression of LAR leads to suppression of adipocyte differentiation in both 3T3-L1 pre-adipocytes and human mesenchymal stem cells [Citation51]. Whether these phosphatases also play a role in the evolution toward T2D and how, at the mechanistic level, these deranging functions occur has not yet been identified. Previous GWAS for T2D in a Han Chinese population have identified PTPRD as a T2D susceptibility gene [Citation31]. Thus, the functional consequences of the PTPRD hypomethylation we have found in SVF from healthy FDR individuals deserve to be addressed. In this work, we have firstly shown that, in the FDR pre-adipocytes, decreased methylation at the PTPRD-associated intronic DMR chr9:10448607-10450000 is accompanied by PTPRD upregulation. Importantly, we demonstrated that methylation at this locus directly represses transcriptional activity of PTPRD in vitro, which may occur in vivo as well. This novel finding prompted us to hypothesize that methylation changes causing PTPRD upregulation contribute to the abnormalities in SAT function occurring in FDR and to the consequences of these abnormalities. Consistent with this hypothesis, we have found that methylation at the PTPRD-DMR negatively correlates with subcutaneous adipose cell size, while the PTPRD mRNA levels were positively correlated. In addition, we have further demonstrated that overexpression of Ptprd in cultured 3T3-L1 pre-adipocytes impairs their differentiation into mature adipocytes, as shown by reduced adipogenic gene expression and decreased lipid droplet accumulation. Collectively, these data suggest that, by impairing adipocyte precursor cell differentiation, methylation changes at PTPRD gene associate to increased risk of T2D in FDR.
The PTPRD methylation profile distinguishing SVF cells from FDR and from individuals who have no familiarity for T2D closely reflects that in blood-borne DNA obtained from PBL of the same study group subjects. Similar to the SVF adipocyte precursor cells, PBL methylation at the PTPRD locus negatively correlated with the subcutaneous adipose cell size in FDR where adipocyte hypertrophy is an independent predictor of T2D [Citation4,Citation5]. Thus, PBL seem to represent a convenient and easily accessible proxy of the SVF epigenetic profile at this gene in FDR. Our study further suggests the potential use of the PTPRD DNA methylation pattern in PBL as a promising biomarker of T2D risk since we have been able to replicate the FDR-associated PTPRD epigenetic profile in an independent cohort of severely obese patients, who feature “ similar to the FDR individuals “ an increased risk of T2D [Citation33,Citation52]. Of note, the risk of T2D increases as BMI increases, from three-times in overweight people to more than 15-times in severely obese patients compared with individuals with a healthy BMI [Citation53]. In our study, PBL DNA methylation at the PTPRD locus negatively correlated with BMI in individuals with severe obesity, strongly supporting a role for PTPRD methylation pattern in identifying individuals at high risk of developing T2D.
Previous investigations have shown that hypermethylation occurs at the PTPRD locus in PBL from Han Chinese individuals diagnosed to have T2D [Citation54]. Interestingly, these methylation events were reported to induce downregulation of PTPRD with progressive repression of its transcription with increasing duration of diabetes in the patients. These methylation changes may therefore be secondary to diabetes itself unlike those occurring in our FDR subjects who were not diabetic at the time they were recruited in the study. Importantly, these methylation events were caused by increased expression of DNMT1, which does not occur in our population, and involved the promoter rather than the intronic chr9:10448607-10450000 region at PTPRD. The genetic background of the Han Chinese population and the subjects in our study group is likely different as PTPRD and DNMT1 diabetes risk polymorphisms occurring in the former were not detected in the individuals we have studied (data not shown). This diversity might have contributed to the occurrence of different epigenetic profile in the two groups.
Although we have included two independent cohorts in our analyses and different types of tissues (i.e., PBL and SVF samples), we are aware that the small sample size of the recruited individuals could represent a limitation of our study which, however, deserves to be replicated in larger populations and analysed in further prospective studies.
Conclusion
This work provides the first genome-wide DNA methylation analysis in human adipocyte precursor cells from SAT of healthy non-obese individuals who are FDR of diabetic patients. It is shown that the Methylome profile of these subjects features significant differences at genes known to be implicated in both adipose cell function and T2D development. In addition, these studies have revealed a previously unrecognized function of PTPRD in restraining adipogenesis and found evidence that PTPRD hypomethylation, which is also reflected in blood-borne DNA, may contribute to adipose cell hypertrophy in FDR of T2D patients.
Future perspective
Use of data from the present work may, in the future, reveal epigenetic changes contributing to T2D risk. Some of these changes may further represent marks of disease applicable to prediction and treatment, particularly lifestyle-based treatment.
Epigenetic signature of first-degree relatives (FDR) subcutaneous adipose tissue (SAT) pre-adipocytes is characterized by global DNA hypomethylation.
DNA methylation changes in FDR SAT pre-adipocytes occur in key genes implicated in adipocyte functions and Type 2 diabetes.
Hypomethylation at the PTPRD risk gene strongly associates with SAT hypertrophy in FDR subjects.
Adipogenesis is impaired by Ptprd overexpression in cultured 3T3-L1 pre-adipocytes.
Pre-adipocyte PTPRD hypomethylation reflects in blood-borne DNA, indicating that peripheral blood leukocytes represent a proxy of epigenetic signature at this locus.
PTPRD hypomethylation could identify individuals with a high risk of developing Type 2 diabetes.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval for all human experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Supplemental Information 1
Download MS Word (14.3 KB)Supplemental Information 2
Download MS Word (14.4 KB)Supplemental Information 3
Download MS Word (86.4 KB)Supplemental Information 4
Download MS Word (132.9 KB)Supplemental Information 5
Download MS Word (21.5 KB)Supplemental Information 6
Download MS Word (16.2 KB)Supplemental Information 7
Download MS Word (20.1 KB)Acknowledgments
The authors thank M Ceccarelli for his valuable support and comments.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.comhttp://doi/suppl/10.2217/epi-2019-0267
Financial &competing interest disclosure
L Parrillo is the recipient of the SID-FO.DI.RI/MSD ITALIA 2017 Research Fellowship and the EFSD/Lilly Research Fellowship 2015. This study was funded, in part, by the European Foundation for the Study of Diabetes (EFSD), by the Ministero dell’Istruzione, Università e della Ricerca Scientifica, by Regione Campania POR FESR 2014–2020 (Obiettivo specific 1.2.) Manifestazione di Interesse per la Realizzazione di Technology Platform nell’ambito della Lotta alle Patologie Oncologiche” Projects: RARE PLAT NET, SATIN, and COEPICA, by the Società Italiana di Diabetologia (SID-FO.DI.RI), by the Swedish Research Council, Torsten Söderberg and by the Novo Nordisk Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Lönn M , MehligK , BengtssonC , LissnerL. Adipocyte size predicts incidence of Type 2 diabetes in women. FASEB J.24, 326“331 (2010).
- Longo M , RacitiGA , ZatteraleFet al. Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity. Diabetologia61, 369“380 (2018).
- Longo M , ZatteraleF , NaderiJet al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci.20(9), 2358 (2019).
- Arner P , ArnerE , HammarstedtA , SmithU. Genetic predisposition for Type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS ONE6, e18284 (2011).
- InterAct Consortium , ScottRA , LangenbergCet al. The link between family history and risk of Type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia56, 60“69 (2013).
- Henninger AM , EliassonB , JenndahlLE , HammarstedtA. Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to Type 2 diabetes. PLoS ONE9, e105262 (2014).
- Lotta LA , GulatiP , DayFRet al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet.49, 17“26 (2014).
- Cederberg H , Stancˇakov ´A , KuusistoJ , LaaksoM , SmithU. Family history of Type 2 diabetes increases the risk of both obesity and its complications: is Type 2 diabetes a disease of inappropriate lipid storage?J. Intern. Med.277, 540“551 (2015).
- Smith U , KahnBB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med.280, 465“475 (2016).
- Acosta JR , DouagiI , AnderssonDPet al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with Type 2 diabetes. Diabetologia59, 560“570 (2016).
- Multhaup ML , SeldinMM , JaffeAEet al. Mouse-human experimental epigenetic analysis unmasks dietary targets and genetic liability for diabetic phenotypes. Cell Metab.21, 138“149 (2015).
- Keller M , HoppL , LiuXet al. Genome-wide DNA promoter methylation and transcriptome analysis in human adipose tissue unravels novel candidate genes for obesity. Mol. Metab.6, 86“100 (2016).
- Rönn T , VolkovP , DavegårdhCet al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet.9, e1003572 (2013).
- Vazquez G , DuvalS , JacobsDRJr , SilventoinenK. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol. Rev.29, 115“128 (2007).
- Broholm C , OlssonAH , PerfilyevAet al. Human adipogenesis is associated with genome-wide DNA methylation and gene-expression changes. Epigenomics8, 1601“1617 (2016).
- Parrillo L , SpinelliR , NicolòAet al. Nutritional factors, DNA methylation, and risk of Type 2 diabetes and obesity: perspectives and challenges. Int. J. Mol. Sci.20(12), 2983 (2019).
- Parrillo L , CostaV , RacitiGAet al. Hoxa5 undergoes dynamic DNA methylation and transcriptional repression in the adipose tissue of mice exposed to high-fat diet. Int. J. Obes.40, 929“937 (2016).
- Laakso M , ZilinskaiteJ , HansenTet al. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia51, 502“511 (2008).
- Isakson P , HammarstedtA , GustafsonB , SmithU. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes58, 1550“1557 (2009).
- Desiderio A , LongoM , ParrilloLet al. Epigenetic silencing of the ANKRD26 gene correlates to the pro-inflammatory profile and increased cardio-metabolic risk factors in human obesity. Clin. Epigenetics11, 181 (2019).
- Li H , HandsakerB , WysokerAet al. The Sequence Alignment/Map format and SAMtools. Bioinformatics25, 2078“2079 (2009).
- Zhang Y , LiuT , MeyerCAet al. Model-based analysis of ChIP-Seq (MACS). Genome Biol.9, R137 (2008).
- Robinson MD , McCarthyDJ , SmythGK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics26, 139“140 (2010).
- Benjamini Y , HochbergY. Controlling the false discovery rate “ a practical and powerful approach to multiple testing. J. Royal Statist. Soc., Series B.57, 289“300 (1995).
- Li B , DeweyCN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics12, 323 (2011).
- de Cristofaro T , DiPalma T , FicheraIet al. An essential role for Pax8 in the transcriptional regulation of cadherin-16 in thyroid cells. Mol. Endocrinol.26, 67“78 (2012).
- Raciti GA , SpinelliR , DesiderioAet al. Specific CpG hyper-methylation leads to Ankrd26 gene down-regulation in white adipose tissue of a mouse model of diet-induced obesity. Sci. Rep.7, 43526 (2017).
- Liotti A , CabaroS , CimminoIet al. Prep1 deficiency improves metabolic response in white adipose tissue. Biochim. Biophys. Acta1863(5), 515“525 (2018).
- Longo M , SpinelliR , D’EspositoVet al. Pathologic endoplasmic reticulum stress induced by glucotoxic insults inhibits adipocyte differentiation and induces an inflammatory phenotype. Biochim. Biophys. Acta1863, 1146“1156 (2016).
- Lyko F . The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat. Rev. Genet.19, 81“92 (2018).
- Tsai FJ , YangCF , ChenCCet al. A genome-wide association study identifies susceptibility variants for Type 2 diabetes in Han Chinese. PLoS Genet.6, e1000847 (2010).
- Barciszewska AM , NowakS , Naskręt-BarciszewskaMZ. The degree of global DNA hypomethylation in peripheral blood correlates with that in matched tumor tissues in several neoplasia. PLoS ONE9, e92599 (2014).
- Laakso M . Biomarkers for Type 2 diabetes. Mol. Metab.27S, S139“S146 (2019).
- Nilsson E , MatteA , PerfilyevAet al. Epigenetic alterations in human liver from subjects with Type 2 diabetes in parallel with reduced folate levels. J. Clin. Endocrinol. Metab.100, e1491“e1501 (2015).
- Dayeh T , VolkovP , SalöSet al. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and nondiabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet.10(3), e1004160 (2014).
- Crujeiras AB , Diaz-LagaresA , SandovalJet al. DNA methylation map in circulating leukocytes mirrors subcutaneous adipose tissue methylation pattern: a genome-wide analysis from non-obese and obese patients. Sci. Rep.7, 41903 (2017).
- Nitert MD , DayehT , VolkovPet al. Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with Type 2 diabetes. Diabetes61, 3322“3332 (2012).
- Chen T , UedaY , DodgeJE , WangZ , LiE. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol. Cell. Biol.23, 5594“5605 (2003).
- Liao J , KarnikR , GuHet al. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat. Genet.47, 469“478 (2015).
- Yang X , WuR , ShanW , YuL , XueB , ShiH. DNA methylation biphasically regulates 3T3-L1 preadipocyte differentiation. Mol. Endocrinol.30, 677“687 (2016).
- Guo W , ChenJ , YangY , ZhuJ , WuJ. Epigenetic programming of Dnmt3a mediated by AP2α is required for granting preadipocyte the ability to differentiate. Cell Death Dis.7, e2496 (2016).
- Zhang P , ZhaoM , LiangGet al. Whole-genome DNA methylation in skin lesions from patients with psoriasis vulgaris. J. Autoimmun.41, 17“24 (2013).
- Maunakea AK , NagarajanRP , BilenkyMet al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature466, 253“257 (2010).
- Jones PA . Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet.13, 484“492 (2012).
- Blattler A , YaoL , WittHet al. Global loss of DNA methylation uncovers intronic enhancers in genes showing expression changes. Genome Biol.15, 469 (2014).
- Bakshi A , BretzCL , CainTL , KimJ. Intergenic and intronic DNA hypomethylated regions as putative regulators of imprinted domains. Epigenomics10, 445“461 (2018).
- Toker AW . Protein tyrosine phosphatases and signalling. J. Endocrinol.185, 19“33 (2005).
- Ren JM , LiPM , ZhangWRet al. Transgenic mice deficient in the LAR protein-tyrosine phosphatase exhibit profound defects in glucose homeostasis. Diabetes47, 493“497 (1998).
- Chagnon MJ , ElcheblyM , UetaniNet al. Altered glucose homeostasis in mice lacking the receptor protein tyrosine phosphatase sigma. Can. J. Physiol. Pharmacol.84, 755“763 (2006).
- Batt J , AsaS , FladdC , RotinD. Pituitary, pancreatic and gut neuroendocrine defects in protein tyrosine phosphatase-sigma-deficient mice. Mol. Endocrinol.16, 155“169 (2002).
- Kim WK , JungH , KimDHet al. Regulation of adipogenic differentiation by LAR tyrosine phosphatase in human mesenchymal stem cells and 3T3-L1 preadipocytes. J. Cell Sci.122, 4160“4167 (2009).
- Corbin LJ , RichmondRC , WadeKHet al. BMI as a modifiable risk factor for Type 2 diabetes: refining and understanding causal estimates using mendelian randomization. Diabetes65, 3002“3007 (2016).
- Kivimäki M , KuosmaE , FerrieJEet al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health2, e277“e285 (2016).
- Chen YT , LinWD , LiaoWL , LinYJ , ChangJG , TsaiFJ. PTPRD silencing by DNA hypermethylation decreases insulin receptor signaling and leads to Type 2 diabetes. Oncotarget6, 12997“13005 (2015).
