Abstract
EZH2, acting as a catalytic subunit of PRC2 to catalyze lysine 27 in histone H3, induces the suppression of gene expression. EZH2 can regulate cell proliferation and differentiation of retinal progenitors, which are required for physiological retinal development. Meanwhile, an abnormal level of EZH2 has been observed in ocular tumors and other pathological tissues. This review summarizes the current knowledge on EZH2 in retinal development and ocular diseases, including inherited retinal diseases, ocular tumors, corneal injury, cataract, glaucoma, diabetic retinopathy and age-related retinal degeneration. We highlight the potential of targeting EZH2 as a precision therapeutic target in ocular diseases.
Plain language summary
EZH2 is a protein that helps to regulate the activity of genes in cells. It works as a part of a complex called PRC2 to control a chemical group called lysine 27 in histone H3 and then inhibit the expression of genes. EZH2 is important for the normal development of the retina. Abnormal levels of EZH2 are associated with various eye diseases. This review summarizes the role of EZH2 in different ocular diseases and the potential mechanisms. Targeting EZH2 may be a novel way to treat or prevent ocular diseases.
Tweetable abstract
Review discussing the role of EZH2 in retinal development and ocular diseases to highlight the potential of EZH2 as a precision therapeutic target for treating ocular diseases.
Ocular diseases can lead to visual impairment (VI) and greatly affect the quality of life. A survey in the USA indicated that 1.02 million adults suffered from vision loss and 3.22 million had VI in 2015, with the numbers expected to double by 2050 [Citation1]. Moreover, patients with VI showed higher mortality in a 10-year study in China [Citation2]. A deeper understanding of the pathological mechanisms and better management of eye diseases have been achieved in the last few decades. However, current therapies can seldom fully restore vision or arrest disease progression.
Epigenetics is characterized by heritable alterations of gene expression which lead to phenotype changes, without alteration in DNA sequences [Citation3]. Epigenetic modifications including DNA methylation, noncoding RNA and histone modifications are critical components in regulating various biological processes [Citation4]. EZH2, acting as a core component of PRC2, promotes transcriptional silencing through methylating lysine 27 in histone H3 (H3K27me3) [Citation5,Citation6]. As a key regulator in cell fate determination, DNA damage repair, cell proliferation and differentiation [Citation7], EZH2 has been identified to be implicated in many diseases, including ocular diseases.
Accumulating evidence shows that EZH2 is required for retinal development [Citation8]. Abnormal expression of EZH2 was observed in eye tumors [Citation9–11], ocular fibrotic tissues [Citation12,Citation13] and diabetic retinopathy (DR) [Citation14,Citation15]. Genetic or pharmacological suppression of EZH2 was shown to protect photoreceptor cells from dying and delay the pathological progression of various eye diseases [Citation16–18]. Targeting EZH2 to prevent ocular disorders or delay disease progression could be a promising therapeutic approach. In this article we review the current knowledge of EZH2 in retinal development and a variety of ocular diseases.
EZH2 structure & its functional models
Structure of EZH2
Encoded by a gene located on chromosome 7q35, EZH2 is composed of regulatory segment domains and catalytic domains. The activity of histone methyltransferase is implemented by the C-terminal SET domain and CXC domain. The SET domain is the predominant component for EZH2 methyltransferase function, where methyl groups of S-adenosyl methionine are transferred to histones [Citation19]. N-terminal domains, including the EID domain, domain I and domain II, act as binding sites to promote assembly with other PRC2 subunits, including EED and SUZ12, to ensure the proper functioning of PRC2 [Citation6].
EZH2 functional models
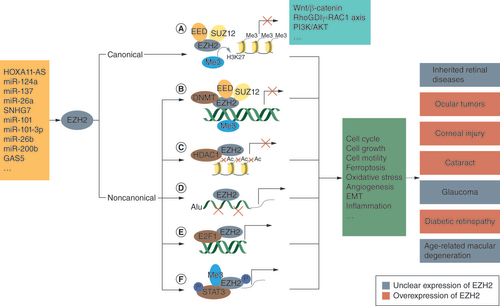
As shown in , upstream mediators including noncoding RNAs can regulate EZH2 expression. Canonically, EZH2, in the form of a catalytic subunit, interacts with two other PRC2 subunits, EED and SUZ12, to catalyze H3K27me3 and thus suppresses gene expression and regulates biological processes in several signaling pathways, including the Wnt/β-catenin pathway and PI3K/AKT pathway; however, mounting evidence suggests that EZH2 also has noncanonical epigenetic functions [Citation20,Citation21]. EZH2, in a PRC2-dependent or -independent manner, can methylate nonhistone molecules such as DNA [Citation22] and STAT3 [Citation23] to inhibit or activate gene transcription. In melanoma cells, EZH2 was involved in histone H3 deacetylation by maintaining HDAC1 at the CDKN1A site, thus repressing p21 expression during tumor progression [Citation24]. EZH2 can also function as a coactivator with transcription factors such as E2F1 or as a chaperone to promote the cleavage of the transposable element Alu RNA for transactivation [Citation25,Citation26].
Upstream mediators including noncoding RNAs and other factors (orange) can regulate EZH2 expression. (A) Canonically, EZH2, in the form of a catalytic subunit, interacts with two other PRC2 subunits (EED and SUZ12) to catalyze lysine 27 in histone H3 (H3K27me3) and thus suppresses gene expression. EZH2 regulates biological processes (green) in several signaling pathways (blue), including the Wnt/β-catenin pathway and PI3K/AKT pathway. (B) Noncanonically, EZH2, in the context of the PRC2 complex, methylates DNA through recruiting DNA methyltransferase (DNMT). (C) EZH2 is involved in histone 3 deacetylation by maintaining HDAC1, thus repressing p21 expression during tumor progression. (D) EZH2 acts as a chaperone to promote the cleavage of Alu RNA. (E) EZH2 functions as a coactivator with transcription factors such as E2F1. (F) EZH2 methylates nonhistone molecules including STAT3 to regulate the expression of downstream genes. EZH2 is overexpressed in pathological tissues in ocular tumors, corneal injury, cataract and diabetic retinopathy. However, the significance of the EZH2 level in inherited retinal diseases, glaucoma and age-related macular disease is unclear.
EZH2&retinal development
During retinal development, the responses of progenitor cells to extrinsic and intrinsic stimuli are critical to regulate cell proliferation and differentiation. Genetic and epigenetic regulation enables accurate and robust developmental transitions. Mounting evidence suggests that EZH2, as a methyltransferase for H3K27me3, is a key mediator in retinal development ().
Table 1. Summary of EZH2 inhibitors in retinal development.
EZH2 has been reported to be dynamically expressed in different stages of retinal development, with high levels in retinal progenitors and weak expression in adult retinas in mice [Citation27]. At embryonic stages E14 and E17, EZH2 was evident in all layers in murine retinas, especially in the peripheral retina. After birth, EZH2 expression was restricted to the inner nuclear layer and ganglion cell layer, and negligible in adult retinas [Citation31]. This pattern may suggest that EZH2 actively participates in retinogenesis. Moreover, EZH2 also has an impact on cell differentiation. Rhodopsin was first detected at P2 and Müller glia started to differentiate at P8 in Ezh2 knockout mice, which was not observed in the control group [Citation31]. This shows the accelerated differentiation of Müller glia and rod photoreceptors after EZH2 inhibition. In addition, the number of retinal ganglion cells (RGCs) was significantly reduced at P3 and amacrine cells were damaged in Ezh2 knockout mice [Citation8]. Therefore, EZH2 may act as a critical regulator in the timing of the differentiation of retinal progenitors.
The proliferation of progenitors is also critical for the development of the retina. Suppression of EZH2 could reduce the proportion of cycling cells and growth fraction in the optic vesicle, and contribute to a 15% reduction in eye diameter in embryos [Citation29]. However, no difference in the proportion of S-phase cells was observed, and there was no change in the proportion of cycling cells, in E15 Ezh2 knockout mice [Citation8]. However, reduction in the proliferation of retinal progenitors was observed since P0 [Citation8], revealing that EZH2 regulates the proliferation of progenitors after birth, but not in embryonic stages. The mechanism of the regulation of EZH2 in retinal cell proliferation may be associated with upregulation of cycle-related genes, including CDKN2A and CDKN2B [Citation8], although the underlying mechanism remains to be elucidated.
EZH2&ocular diseases
EZH2&inherited retinal diseases
EZH2 is involved in controlling cell fate during retinal development, especially in photoreceptors, and thus may provide a novel target for inherited retinal diseases (IRDs) (). Retinitis pigmentosa is the most common form of IRD, characterized by the loss of photoreceptors [Citation35]. In the Rd1 mouse, which is a well-known animal model of retinitis pigmentosa, the thickness of the outer nuclear layer increased from 25.38 ± 6.48 μm to 43.21 ± 3.78 μm after injection of the EZH2 inhibitor DZNep [Citation36]. At P21, when nearly all rods die, immunostaining of retinas showed that there were still at least three layers of photoreceptors with DZNep treatment, while only one layer of photoreceptors was observed in the control group. Additionally, the electroretinogram results showed the visual functions were significantly preserved in the Rd1 eyes with DZNep treatment, indicating that EZH2 inhibition can suppress the death of rods and partially restore visual functions. In agreement with this finding, a reduction of about 70% in apoptotic photoreceptor cells was observed in Rd1 retinas when treated with an EZH2 inhibitor [Citation16].
Table 2. Summary of EZH2 inhibitors in inherited retinal diseases.
However, Yan et al. reported that embryonic knockdown of Ezh2 induced progressive photoreceptor degeneration and decreased the thickness of the outer nuclear layer by regulating photoreceptor-related genes in postnatal eyes [Citation38], which is in disagreement with the aforementioned studies. Given the complex mechanism of disease development, further studies of the function of EZH2 in photoreceptors are needed. Besides, the role of EZH2 in other forms of IRD is also worthy of investigation.
EZH2&ocular tumors
The importance of EZH2 in the pathophysiology of cancer is now widely recognized, and EZH2 has been a promising target. Inhibitors of EZH2 have been investigated in clinical trials to evaluate their efficacies and safety in malignant pleural mesothelioma, follicular lymphoma, advanced epithelioid sarcoma, B-cell non-Hodgkin lymphomas and solid tumors [Citation39–47]. The study of EZH2 in ocular tumors is still preliminary and there has been no clinical trial for EZH2 inhibitors yet. comprises a list of the related studies.
Table 3. Summary of EZH2 inhibitors in ocular tumors.
Uveal melanoma
Uveal melanoma (UM) is a malignant neoplasm that originates from melanocytes from the choroid, ciliary body and iris [Citation53]. Even if UM patients receive radiation or surgery to remove the primary tumor, almost half of them will develop metastatic cancer eventually [Citation54]. Therefore, better therapies for UM are needed. In 89 primary UM patients, upregulated expression of EZH2 in UM tissues was noted, and the higher EZH2 level was related to an increased risk of metastases and a worse prognosis [Citation55]. Similar results were also observed in studies by Zhang et al. and Wu et al. [Citation50,Citation56]. Additionally, pharmacological suppression of EZH2 induced apoptosis and inhibited the proliferation of UM cells, but did not affect the noncancerous cells [Citation9,Citation57]. The results were further confirmed in vivo. After treatment with the EZH2 inhibitor GSK126, a significant decrease in the volumes and weights of tumors was noted in the subcutaneous xenografted mouse model [Citation9]. EZH2 may act as a target of epigenetic mediators such as miR-124a, miR-26a and miR-137 and therefore epigenetically inhibit the development of UM [Citation49,Citation50,Citation57]. However, the association between EZH2 and UM progression is still unclear.
Conjunctival melanoma
Conjunctival melanoma (CM) is an uncommon aggressive tumor, accounting for 5% of all ocular melanomas [Citation58]. EZH2 mutations are abundant in this invasive melanoma [Citation59]. Treatment with EZH2 inhibitors inhibited cell proliferation and colony formation in CM cell lines [Citation10]. EZH2 ablation mainly increased the proportion of cells in the G2/M phase and promoted cell death by activating the transcription of tumor suppressor gene p21/CDKN1A. The same results were also shown in a zebrafish xenograft model. Inhibition of EZH2 suppressed the proliferation and migration of tumor cells [Citation10]. Given the tumor suppressive effects of EZH2 inhibitors, EZH2 may offer a novel therapeutic target for CM patients. However, reports of EZH2 in CM are limited, and more studies are needed.
Retinoblastoma
Retinoblastoma (Rb), with an incidence rate of 1:16,000, is the most common intraocular malignant tumor in children [Citation60,Citation61]. Biallelic loss of the tumor suppressor gene RB1 causes over 95% of all Rb cases [Citation62]. After RB1 inactivation, tumors develop rapidly. EZH2 has been reported to be downstream of RB1, producing repetitive DNA sequences to promote cancer development [Citation63]. Therefore, EZH2 may be relevant to Rb tumorigenesis. Increased expression of EZH2 was observed in Rb tissues, especially in patients with orbital and choroidal invasion and subretinal and vitreous seeding [Citation64]. Pharmacological suppression of EZH2 inhibited tumor proliferation and colony formation, and also reactivated RB1 expression to regulate the tumorigenesis [Citation17]. Importantly, the effects of EZH2 inhibitors were specific to Rb cells, as there were no signs of suppression on the viability of nontumor primary retinal pigment epithelium (RPE) cells [Citation11].
Intraocular medulloepithelioma
Intraocular medulloepithelioma (IM) is a congenital tumor originating from the neuroepithelial progenitor cells, mostly arising from the nonpigmented ciliary epithelial body [Citation65]. Current therapy includes brachytherapy, tumor resection, enucleation and exenteration, depending on the cancer stages and clinical manifestations [Citation66]. Recently, precision medicine based on epigenetic regulation has been explored. Studies showed that EZH2 staining was exclusively positive in IM tumor cells, especially in poorly differentiated cells [Citation5], suggesting EZH2 may be a potential diagnostic biomarker to evaluate tumor invasion. Besides, given that EZH2 was exclusively expressed in IM tumor cells, targeting EZH2 seems an attractive approach to treat this intraocular neoplasm.
EZH2&corneal injury
Corneal transparency is important to maintain clear vision. When the cornea is damaged by external stimuli, such as inflammation, infection and traumatic injury, corneal wound healing is initiated [Citation67,Citation68]. Unlike the repair processes in other tissues, wound healing of the cornea is a complex procedure involving migration and differentiation of limbal stem cells, cross-talk among different corneal cell types and remodeling of extracellular matrix [Citation69,Citation70]. After corneal scarring is formed, it largely decreases corneal transparency and the reduction cannot be reversed [Citation71]. During this process, myofibroblast transdifferentiation induced by TGF-β plays an important role [Citation72–74]. Inhibiting the dysfunction of transdifferentiation to corneal myofibroblasts is a promising approach to prevent corneal opacity. An increased expression of EZH2 was observed in a corneal injury mouse model [Citation12]. Genetic or pharmacological suppression of EZH2 caused a reduction of COL1A1, FN1 and ACTA2 expressions, which are classical fibrotic markers in TGF-β1-induced corneal myofibroblasts. Meanwhile, EZH2 inhibitor EPZ-6438 alleviated cell migration and collagen contraction, the hallmarks of activated fibroblasts [Citation12]. Corneal neovascularization is another important process for wound healing. EZH2 expression was upregulated in a corneal neovascularization mouse model, and the release of proangiogenesis factors, including VEGF, was reduced following treatment with EZH2 inhibitors [Citation75]. These studies suggested EZH2 actively participated in the process of corneal injury repair and it may serve as a new avenue for preventing corneal scarring ().
Table 4. Summary of EZH2 inhibitors in corneal injury.
EZH2&cataract
Cataract is a globally widespread, vision-threatening disorder of which aging is the primary cause [Citation76]. During aging, the anterior epithelial cells lose their functions and undergo degeneration, finally leading to cataract [Citation77]. Anti-aging therapy may help to prevent the development of cataract. It has been reported that the suppression of HSF4 could impair lens differentiation and cause cataracts to develop [Citation78,Citation79]. In lens epithelial cells (LECs), HSF4 colocalized with EZH2 in the nucleus. Recruitment of EZH2 by HSF4 downregulated p21 expression and prevented senescence. Conversely, knocking out EZH2 increased the level of p21 and promoted senescence [Citation80].
Till now, opaque lens removal followed by artificial intraocular lens implantation has been the primary treatment modality for cataract [Citation81]. Nevertheless, a wound healing response is initiated by residual LECs after surgery and therefore leads to secondary vision loss, which is known as posterior capsule opacification (PCO) [Citation82–84]. Inhibiting fibrotic responses of LECs is worth investigating to prevent or suppress VI resulting from PCO. Overexpression of EZH2 was observed in human PCO-attached LECs, indicating that EZH2 may be involved in the pathogenesis of PCO [Citation85]. Moreover, the central mediator in fibrosis, EGF, could induce expression of fibrotic markers via a miR-26b-dependent pathway and in turn upregulated EZH2 levels to promote the progression of PCO [Citation85].
Epithelial–mesenchymal transition (EMT) of LECs is an important process in anterior subcapsular cataract [Citation86]. Previous studies showed that MYPT1/PP1 specifically phosphorylated EZH2 at S21, and then enhanced H3K27Me3 expression via the AKT–EZH2 axis and contributed to the prevention of LEC fibrosis [Citation13]. These results provide important insights into the functions and regulation of EZH2 in aging and the fibrotic process of LECs, not only as an enzymatic subunit of the PRC2 complex but also as an independent nonhistone methyltransferase to regulate other transcription factors ().
Table 5. Summary of EZH2 inhibitors in cataract.
EZH2&glaucoma
Loss of RGCs is an important pathology in glaucoma, and preventing RGC death has been an intense research direction [Citation87,Citation88]. The roles of EZH2 in RGC protection are still not fully understood (). In the N-methyl-d-aspartate-induced acute RGC death model, treatment with the EZH2 inhibitor DZNep prevented the reduction of RGCs and inner nuclear layer thickness after N-methyl-d-aspartate-induced damage [Citation89]. However, another study showed EZH2 silencing had little impact on the maturation and functions of RGCs, nor did it have an influence on injury reaction induced by elevated intraocular pressure or optic nerve damage [Citation90]. In a glaucoma animal model established by translimbal laser photocoagulation, inhibition of EZH2 even promoted the apoptosis of RGCs, while upregulation of EZH2 reduced the RGC degeneration rate [Citation91,Citation92]. There are thus substantial variations about the roles of EZH2 in RGCs. More investigations are needed.
Table 6. Summary of EZH2 inhibitors in glaucoma.
EZH2&DR
Activation of MMP9 is a trigger factor in DR, causing angiogenesis, mitochondrial injury and the apoptosis of capillary cells [Citation93]. MMP9 has been proven to be the downstream gene of EZH2 [Citation94,Citation95]. Targeting EZH2 may epigenetically regulate MMP9 and therefore alleviate the progression of DR. High expression of H3K27me3 in the MMP9 promoter region and an approximately twofold increase in EZH2 levels were detected after high glucose stimulation in human retinal endothelial cells (HRECs). EZH2 inhibition reduced the levels of H3K27me3, alleviated the activity of MMP9 and prevented cell apoptosis [Citation18]. Moreover, EZH2 could regulate DNA methylation through the recruitment of DNMT1 and TET2 to control the transcription of MMP9: similar results were observed in the animal model and DR patient retinas, where a significant increase in H3K27me3 and EZH2 levels at the MMP9 promoter and higher expression of DNMT1 and TET2 were noted [Citation18], supporting the fact that EZH2 regulates MMP9 by different epigenetic mechanisms to maintain the cellular integrity in DR.
As well as its regulation of MMP9, EZH2 can also interact with lncRNAs to actively participate in DR. A lncRNA microarray analysis showed that the lncRNA ANRIL was one of the most differentially expressed genes in a normal-glucose-treated group versus a high-glucose-treated group in human retinal endothelial cells [Citation14]. Cooperation between ANRIL and EZH2 in regulating disease development has been reported previously [Citation96,Citation97]. In the diabetic mice, significant elevation of EZH2 and VEGF was detected, while no changes to the levels of EZH2 and VEGF were found in ANRIL knockout diabetic mice. In addition, pharmacological suppression of EZH2 resulted in significant reductions in VEGF and ANRIL levels [Citation14], indicating the interaction between ANRIL and EZH2 in the VEGF signaling axis. Given that VEGF is a key player in angiogenesis in DR [Citation98–101], the role of EZH2 in regulating VEGF and angiogenesis-related diseases may be worth further investigating ().
Table 7. Summary of EZH2 inhibitors in diabetic retinopathy.
EZH2&age-related macular degeneration
Epigenetic regulation, which is potentially conducive to the aging process, has been widely recognized to be involved in the pathology of age-related macular degeneration (AMD).
As shown in , EZH2, along with miRNAs, co-regulates AMD-associated genes such as ELL2 and ENTPD1 [Citation105]. Dysfunction of RPE cells is the primary feature of AMD, and EMT of RPE cells is implicated in disease development. In TGF-β1-induced EMT in RPE cells, EZH2 inhibition caused a drastic decrease in the expression of EMT markers, including α-smooth muscle actin, fibronectin and collagen-1 [Citation106]. The level of the tight junction protein ZO-1 was upregulated. Suppression of EZH2 also inhibited the proliferative and migratory abilities of RPE cells, enhancing transepithelial electrical resistance and barrier functions [Citation106]. However, the effect of EZH2 inhibitors in vivo is still unclear.
Table 8. Summary of EZH2 inhibitors in age-related macular degeneration.
The importance of Alu RNA accumulation in AMD has been widely recognized in recent years. As a transposable element, Alu RNAs actively participate in gene translation and modify human genomes [Citation107]. An abundance of Alu elements was found to be present in RPE cells from patients with atrophic AMD; Alu inhibition increased cell viability and the barrier function of RPE cells in vitro and invivo [Citation108]. Alu RNA could also promote retinal degeneration through the activation of the NLRP3 inflammasome and therefore facilitate inflammatory responses [Citation109,Citation110]. Interestingly, studies showed that EZH2 could enhance the activity of Alu RNA by accelerating its cleavage [Citation25], suggesting a nonmethylase activity of EZH2. Nevertheless, whether EZH2 interacts with Alu RNAs in AMD pathogenesis remains to be elucidated.
EZH2&other ocular diseases
Studies about EZH2 and its relevance to uveitis and dry eye diseases are lacking. Limited data in one study showed that EZH2 expression was higher in samples from patients with Sjögren’s syndrome and its level paralleled the pathological damage in salivary glands [Citation111]. In addition, EZH2 is closely related to the activity of CD4+ T cells in Sjögren’s syndrome. A significant reduction of cell activation and the release of proinflammatory cytokines from CD4+ T cells was noted after EZH2 inhibition [Citation111,Citation112]. Sjögren’s syndrome is an important cause of dry eye diseases; thus EZH2 inhibitors may be a potential avenue for Sjögren’s syndrome-related dry eye. Meanwhile, because of the vital role of CD4+ T cells in inflammatory reactions, the regulation of EZH2 in uveitis is worth investigating.
Conclusion & future perspective
Previous studies suggested that EZH2 was highly elevated in ocular lesion tissues and participated in many types of eye diseases. However, the findings were mainly from experimental models. Moreover, although current studies demonstrate the benefits of EZH2 inhibitors in different eye diseases, their potential risks – such as damage to cognitive ability, autoimmunity and heart muscles – should be noted [Citation113], and there has been no clinical trial yet. Due to the complex function of EZH2, there remains much that we still do not fully understand. Further investigations to fully explain the EZH2 network, including the PRC2-dependent and -independent effects, will provide insights into a variety of ocular diseases. Meanwhile, the development of efficient, selective and safe EZH2 inhibitors and the realization of translational preclinical pharmacology should be an important goal for its clinical application in ophthalmology.
EZH2&retinal development
EZH2 is suggested to actively regulate retinal proliferation and differentiation.
EZH2 is dynamically expressed in different stages of retinal development, with high levels in embryonic retinas but weak expression after birth.
EZH2&inherited retinal diseases
The effect of EZH2 inhibitors on photoreceptor degeneration in inherited retinal diseases is still controversial, with some studies showing a delay in photoreceptor loss but one other study showing an induced progression.
EZH2&ocular tumors
EZH2 was shown to be overexpressed in ocular tumors and related to a worse prognosis.
EZH2 suppression reduced the growth and invasion of tumors through interactions with noncoding RNAs and tumor suppressor genes.
EZH2&corneal injury
EZH2 inhibition prevented the formation of corneal scarring in a corneal injury animal model by suppressing extracellular matrix synthesis and corneal myofibroblast activation.
EZH2&cataract
EZH2 is suggested to be related to the aging process and involved in the development of cataract.
EZH2 inhibition halted the epithelial–mesenchymal transition process and interacted with other mediators, such as miRNAs and transposable elements, to co-regulate disease progression.
EZH2&glaucoma
The effect of EZH2 inhibitors on retinal ganglion cells is still unclear, with substantial variations in research on the roles of EZH2 in prevention and promotion of retinal ganglion cell loss.
EZH2&diabetic retinopathy
Upregulated expression of EZH2 was detected in high-glucose-induced human retinal endothelial cells and diabetic animal models.
Blockade of EZH2 activity reduced MMP9 and VEGF levels in both in vivo and in vitro studies.
EZH2&age-related macular degeneration
EZH2 inhibition suppressed epithelial–mesenchymal transition and enhanced the barrier functions of retinal pigment epithelium cells.
The potential interactions between EZH2 and Alu RNA in the pathogenesis of age-related macular degeneration require investigation.
Conclusion & future perspective
EZH2, as an important epigenetic regulator, acts in both PRC2-dependent and -independent ways to participate in various ocular diseases.
Despite the benefits of EZH2 inhibitors in different eye diseases, their potential risks, including damage to cognitive ability, autoimmunity and heart muscles, should be noted.
The development of efficient, selective and safe EZH2 inhibitors and the realization of translational preclinical pharmacology should be an important goal for its clinical application in ophthalmology.
Conceptualization: J Yam. Writing (original draft preparation): Y Peng, C Bui and X Zhang. Writing (review and editing): C Bui, J Chen, C Tham, W Chu, L Chen, C Pang and J Yam. All the authors read and approved the final manuscript.
Financial & competing interests disclosure
This study was supported in part by the CUHK Jockey Club Children’s Eye Care Programme; the CUHK Jockey Club Myopia Prevention Programme; and Health and Medical Research Fund (HMRF), Hong Kong (07180306 and PR-HKCH-8). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Varma R , VajaranantTS , BurkemperBet al. Visual impairment and blindness in adults in the United States: demographic and geographic variations from 2015 to 2050. JAMA Ophthalmol.134(7), 802–809 (2016).
- Wang L , ZhuZ , ScheetzJ , HeM. Visual impairment and ten-year mortality: the Liwan Eye Study. Eye35(8), 2173–2179 (2021).
- Berger SL , KouzaridesT , ShiekhattarR , ShilatifardA. An operational definition of epigenetics. Genes Dev.23(7), 781–783 (2009).
- Rodenhiser D , MannM. Epigenetics and human disease: translating basic biology into clinical applications. CMAJ174(3), 341–348 (2006).
- Vedschmidt SE , StagnerAM , EagleRCJr , HarocoposGJ , DouY , RaoRC. The targetable epigenetic tumor protein EZH2 is enriched in intraocular medulloepithelioma. Invest. Ophthalmol. Vis. Sci.57(14), 6242–6246 (2016).
- Simon JA , LangeCA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat. Res.647(1–2), 21–29 (2008).
- Duan R , DuW , GuoW. EZH2: a novel target for cancer treatment. J. Hematol. Oncol.13(1), 104 (2020).
- Zhang J , TaylorRJ , LaTorre Aet al. EZH2 maintains retinal progenitor proliferation, transcriptional integrity, and the timing of late differentiation. Dev. Biol.403(2), 128–138 (2015).
- Jin B , ZhangP , ZouHet al. Verification of EZH2 as a druggable target in metastatic uveal melanoma. Mol. Cancer19(1), 52 (2020).
- Cao J , PontesKC , HeijkantsRCet al. Overexpression of EZH2 in conjunctival melanoma offers a new therapeutic target. J. Pathol.245(4), 433–444 (2018).
- Khan M , WaltersLL , LiQet al. Characterization and pharmacologic targeting of EZH2, a fetal retinal protein and epigenetic regulator, in human retinoblastoma. Lab. Invest.95(11), 1278–1290 (2015).
- Liao K , CuiZ , ZengYet al. Inhibition of enhancer of zeste homolog 2 prevents corneal myofibroblast transformation in vitro. Exp. Eye Res.208, 108611 (2021).
- Zhang L , WangL , HuXBet al. MYPT1/PP1-mediated EZH2 dephosphorylation at S21 promotes epithelial-mesenchymal transition in fibrosis through control of multiple families of genes. Adv. Sci. (Weinh.)9(14), e2105539 (2022).
- Thomas AA , FengB , ChakrabartiS. ANRIL: a regulator of VEGF in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci.58(1), 470–480 (2017).
- Di S , AnX , PangBet al. Yiqi Tongluo Fang could preventive and delayed development and formation of diabetic retinopathy through antioxidant and anti-inflammatory effects. Biomed. Pharmacother.148, 112254 (2022).
- Mbefo M , BergerA , SchouweyKet al. Enhancer of zeste homolog 2 (EZH2) contributes to rod photoreceptor death process in several forms of retinal degeneration and its activity can serve as a biomarker for therapy efficacy. Int. J. Mol. Sci.22(17), 9331 (2021).
- Wen X , DingT , LiFet al. Interruption of aberrant chromatin looping is required for regenerating RB1 function and suppressing tumorigenesis. Commun. Biol.5(1), 1036 (2022).
- Duraisamy AJ , MishraM , KowluruRA. Crosstalk between histone and DNA methylation in regulation of retinal matrix metalloproteinase-9 in diabetes. Invest. Ophthalmol. Vis. Sci.58(14), 6440–6448 (2017).
- Zeng J , ZhangJ , SunYet al. Targeting EZH2 for cancer therapy: from current progress to novel strategies. Eur. J. Med. Chem.238, 114419 (2022).
- Völkel P , DupretB , LeBourhis X , AngrandPO. Diverse involvement of EZH2 in cancer epigenetics. Am. J. Transl. Res.7(2), 175–193 (2015).
- Yamaguchi H , HungMC. Regulation and role of EZH2 in cancer. Cancer Res. Treat.46(3), 209–222 (2014).
- Viré E , BrennerC , DeplusRet al. The polycomb group protein EZH2 directly controls DNA methylation. Nature439(7078), 871–874 (2006).
- Kim E , KimM , WooDHet al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell23(6), 839–852 (2013).
- Fan T , JiangS , ChungNet al. EZH2-dependent suppression of a cellular senescence phenotype in melanoma cells by inhibition of p21/CDKN1A expression. Mol. Cancer Res.9(4), 418–429 (2011).
- Hernandez AJ , ZovoilisA , Cifuentes-RojasC , HanL , BujisicB , LeeJT. B2 and ALU retrotransposons are self-cleaving ribozymes whose activity is enhanced by EZH2. Proc. Natl Acad. Sci. USA117(1), 415–425 (2020).
- Tabbal H , SeptierA , MathieuMet al. EZH2 cooperates with E2F1 to stimulate expression of genes involved in adrenocortical carcinoma aggressiveness. Br. J. Cancer121(5), 384–394 (2019).
- Rao RC , TchedreKT , MalikMTet al. Dynamic patterns of histone lysine methylation in the developing retina. Invest. Ophthalmol. Vis. Sci.51(12), 6784–6792 (2010).
- Rapicavoli NA , PothEM , ZhuH , BlackshawS. The long noncoding RNA Six3OS acts in trans to regulate retinal development by modulating Six3 activity. Neural Dev.6, 32 (2011).
- Aldiri I , MooreKB , HutchesonDA , ZhangJ , VetterML. Polycomb repressive complex PRC2 regulates Xenopus retina development downstream of Wnt/β-catenin signaling. Development140(14), 2867–2878 (2013).
- Iida A , IwagawaT , KuribayashiHet al. Histone demethylase JMJD3 is required for the development of subsets of retinal bipolar cells. Proc. Natl Acad. Sci. USA111(10), 3751–3756 (2014).
- Iida A , IwagawaT , BabaYet al. Roles of histone H3K27 trimethylase Ezh2 in retinal proliferation and differentiation. Dev. Neurobiol.75(9), 947–960 (2015).
- Watanabe S , MurakamiA. Regulation of retinal development via the epigenetic modification of histone H3. Adv. Exp. Med. Biol.854, 635–641 (2016).
- Fujimura N , KuzelovaA , EbertAet al. Polycomb repression complex 2 is required for the maintenance of retinal progenitor cells and balanced retinal differentiation. Dev. Biol.433(1), 47–60 (2018).
- Andrews D , OlivieroG , DeChiara Let al. Unravelling the transcriptional responses of TGF-β: Smad3 and EZH2 constitute a regulatory switch that controls neuroretinal epithelial cell fate specification. FASEB J.33(5), 6667–6681 (2019).
- Ben-Yosef T . Inherited retinal diseases. Int. J. Mol. Sci.23(21), 13467 (2022).
- Zheng S , XiaoL , LiuYet al. DZNep inhibits H3K27me3 deposition and delays retinal degeneration in the rd1 mice. Cell Death Dis.9(3), 310 (2018).
- Ueno K , IwagawaT , KuribayashiHet al. Transition of differential histone H3 methylation in photoreceptors and other retinal cells during retinal differentiation. Sci. Rep.6, 29264 (2016).
- Yan N , ChengL , ChoKet al. Postnatal onset of retinal degeneration by loss of embryonic Ezh2 repression of Six1. Sci. Rep.6, 33887 (2016).
- Italiano A , SoriaJC , ToulmondeMet al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol.19(5), 649–659 (2018).
- Morschhauser F , TillyH , ChaidosAet al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol.21(11), 1433–1442 (2020).
- Gounder M , SchöffskiP , JonesRLet al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open-label, phase 2 basket study. Lancet Oncol.21(11), 1423–1432 (2020).
- Song Y , LiuY , LiZMet al. SHR2554, an EZH2 inhibitor, in relapsed or refractory mature lymphoid neoplasms: a first-in-human, dose-escalation, dose-expansion, and clinical expansion phase 1 trial. Lancet Haematol.9(7), e493–e503 (2022).
- Izutsu K , AndoK , NishikoriMet al. Phase II study of tazemetostat for relapsed or refractory B-cell non-Hodgkin lymphoma with EZH2 mutation in Japan. Cancer Sci.112(9), 3627–3635 (2021).
- Vaswani RG , GehlingVS , DakinLAet al. Identification of (R)-N-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1-(1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)ethyl)-1H-indole-3-carboxamide (CPI-1205), a potent and selective inhibitor of histone methyltransferase EZH2, suitable for phase I clinical trials for B-cell lymphomas. J. Med. Chem.59(21), 9928–9941 (2016).
- Yap TA , WinterJN , Giulino-RothLet al. Phase I study of the novel enhancer of zeste homolog 2 (EZH2) inhibitor GSK2816126 in patients with advanced hematologic and solid tumors. Clin. Cancer Res.25(24), 7331–7339 (2019).
- Munakata W , ShirasugiY , TobinaiKet al. Phase 1 study of tazemetostat in Japanese patients with relapsed or refractory B-cell lymphoma. Cancer Sci.112(3), 1123–1131 (2021).
- Zauderer MG , SzlosarekPW , LeMoulec Set al. EZH2 inhibitor tazemetostat in patients with relapsed or refractory, BAP1-inactivated malignant pleural mesothelioma: a multicentre, open-label, phase 2 study. Lancet Oncol.23(6), 758–767 (2022).
- Lu Q , ZhaoN , ZhaG , WangH , TongQ , XinS. LncRNA HOXA11-AS exerts oncogenic functions by repressing p21 and miR-124 in uveal melanoma. DNA Cell Biol.36(10), 837–844 (2017).
- Li Y , ZhangM , FengH , MahatiS. The tumorigenic properties of EZH2 are mediated by miR-26a in uveal melanoma. Front. Mol. Biosci.8, 713542 (2021).
- Zhang J , LiuG , JinHet al. MicroRNA-137 targets EZH2 to exert suppressive functions in uveal melanoma via regulation of Wnt/β-catenin signaling and epithelial-to-mesenchymal transition. J. BUON26(1), 173–181 (2021).
- Hou C , XiaoL , RenXet al. EZH2-mediated H3K27me3 is a predictive biomarker and therapeutic target in uveal melanoma. Front. Genet.13, 1013475 (2022).
- Zhao Y , ChengY , QuY. The role of EZH2 as a potential therapeutic target in retinoblastoma. Exp. Eye Res.227, 109389 (2023).
- Singh AD , TurellME , TophamAK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology118(9), 1881–1885 (2011).
- Smit KN , JagerMJ , DeKlein A , KiliçE. Uveal melanoma: towards a molecular understanding. Prog. Retin. Eye Res.75, 100800 (2020).
- Cheng Y , LiY , HuangX , WeiW , QuY. Expression of EZH2 in uveal melanomas patients and associations with prognosis. Oncotarget8(44), 76423–76431 (2017).
- Wu X , YuanY , MaR , XuB , ZhangR. lncRNA SNHG7 affects malignant tumor behaviors through downregulation of EZH2 in uveal melanoma cell lines. Oncol. Lett.19(2), 1505–1515 (2020).
- Chen X , HeD , DongXDet al. MicroRNA-124a is epigenetically regulated and acts as a tumor suppressor by controlling multiple targets in uveal melanoma. Invest. Ophthalmol. Vis. Sci.54(3), 2248–2256 (2013).
- Rossi E , SchinzariG , MaioranoBAet al. Conjunctival melanoma: genetic and epigenetic insights of a distinct type of melanoma. Int J Mol Sci20(21), 5447 (2019).
- Mudhar HS , SalviSS , PissalouxD , DeLa Fouchardiere A. Single time frame overview of the genetic changes in conjunctival melanoma from intraepithelial disease to invasive melanoma: a study of 4 exenteration specimens illustrating the potential role of cyclin D1. Ocul. Oncol. Pathol.8(1), 52–63 (2022).
- Kaewkhaw R , RojanapornD. Retinoblastoma: etiology, modeling, and treatment. Cancers (Basel)12(8), 2304 (2020).
- Fabian ID , OnadimZ , KaraaEet al. The management of retinoblastoma. Oncogene37(12), 1551–1560 (2018).
- Dimaras H , CorsonTW , CobrinikDet al. Retinoblastoma. Nat. Rev. Dis. Primers1, 15021 (2015).
- Ishak CA , MarshallAE , PassosDTet al. An RB–EZH2 complex mediates silencing of repetitive DNA sequences. Mol. Cell64(6), 1074–1087 (2016).
- Lei Q , ShenF , WuJ , ZhangW , WangJ , ZhangL. MiR-101, downregulated in retinoblastoma, functions as a tumor suppressor in human retinoblastoma cells by targeting EZH2. Oncol. Rep.32(1), 261–269 (2014).
- Saunders T , MargoCE. Intraocular medulloepithelioma. Arch. Pathol. Lab. Med.136(2), 212–216 (2012).
- Edward DP , AlkatanH , RafiqQet al. MicroRNA profiling in intraocular medulloepitheliomas. PLOS ONE10(3), e0121706 (2015).
- Fini ME . Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res.18(4), 529–551 (1999).
- Wilson SE . Corneal wound healing. Exp. Eye Res.197, 108089 (2020).
- Ljubimov AV , SaghizadehM. Progress in corneal wound healing. Prog. Retin. Eye Res.49, 17–45 (2015).
- Kamil S , MohanRR. Corneal stromal wound healing: major regulators and therapeutic targets. Ocul. Surf.19, 290–306 (2021).
- Zhao X , SongW , ChenY , LiuS , RenL. Collagen-based materials combined with microRNA for repairing cornea wounds and inhibiting scar formation. Biomater. Sci.7(1), 51–62 (2018).
- Shu DY , LovicuFJ. Myofibroblast transdifferentiation: the dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res.60, 44–65 (2017).
- Myrna KE , PotSA , MurphyCJ. Meet the corneal myofibroblast: the role of myofibroblast transformation in corneal wound healing and pathology. Vet. Ophthalmol.12(Suppl. 1), 25–27 (2009).
- Wilson SE . Corneal myofibroblasts and fibrosis. Exp. Eye Res.201, 108272 (2020).
- Wan S-S , PanY-M , YangW-J , RaoZ-Q , YangY-N. Inhibition of EZH2 alleviates angiogenesis in a model of corneal neovascularization by blocking FoxO3a-mediated oxidative stress. FASEB J.34(8), 10168–10181 (2020).
- Thompson J , LakhaniN. Cataracts. Prim. Care42(3), 409–423 (2015).
- Liu YC , WilkinsM , KimT , MalyuginB , MehtaJS. Cataracts. Lancet390(10094), 600–612 (2017).
- Gao M , HuangY , WangLet al. HSF4 regulates lens fiber cell differentiation by activating p53 and its downstream regulators. Cell Death Dis.8(10), e3082 (2017).
- Mou L , XuJY , LiWet al. Identification of vimentin as a novel target of HSF4 in lens development and cataract by proteomic analysis. Invest. Ophthalmol. Vis. Sci.51(1), 396–404 (2010).
- Cui X , DuC , WanSet al. Deficiency of heat shock factor 4 promotes lens epithelial cell senescence through upregulating p21cip1 expression. Biochim. Biophys. Acta Mol. Basis Dis.1867(11), 166233 (2021).
- Lee CM , AfshariNA. The global state of cataract blindness. Curr. Opin. Ophthalmol.28(1), 98–103 (2017).
- Wormstone IM , WangL , LiuCS. Posterior capsule opacification. Exp. Eye Res.88(2), 257–269 (2009).
- Wormstone IM , EldredJA. Experimental models for posterior capsule opacification research. Exp. Eye Res.142, 2–12 (2016).
- Wormstone IM , WormstoneYM , SmithAJO , EldredJA. Posterior capsule opacification: what’s in the bag?Prog. Retin. Eye Res.82, 100905 (2021).
- Dong N , XuB , XuJ. EGF-Mediated overexpression of Myc attenuates miR-26b by recruiting HDAC3 to induce epithelial–mesenchymal transition of lens epithelial cells. Biomed. Res. Int.2018, 7148023 (2018).
- Imaizumi T , KurosakaD , TanakaU , SakaiD , FukudaK , SanbeA. Topical administration of a ROCK inhibitor prevents anterior subcapsular cataract induced by UV-B irradiation. Exp. Eye Res.181, 145–149 (2019).
- Almasieh M , WilsonAM , MorquetteB , CuevaVargas JL , DiPolo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res.31(2), 152–181 (2012).
- Fry LE , FahyE , ChrysostomouVet al. The coma in glaucoma: retinal ganglion cell dysfunction and recovery. Prog. Retin. Eye Res.65, 77–92 (2018).
- Xiao L , HouC , ChengL , ZhengS , ZhaoL , YanN. DZNep protects against retinal ganglion cell death in an NMDA-induced mouse model of retinal degeneration. Exp. Eye Res.212, 108785 (2021).
- Cheng L , WongLJ , YanNet al. Ezh2 does not mediate retinal ganglion cell homeostasis or their susceptibility to injury. PLOS ONE13(2), e0191853 (2018).
- Zhou RR , LiHB , YouQSet al. Silencing of GAS5 alleviates glaucoma in rat models by reducing retinal ganglion cell apoptosis. Hum. Gene Ther.30(12), 1505–1519 (2019).
- Zhang N , CaoW , HeX , XingY , YangN. Long non-coding RNAs in retinal ganglion cell apoptosis. Cell. Mol. Neurobiol.43(2), 561–574 (2023).
- Kowluru RA , MohammadG , DosSantos JM , ZhongQ. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes60(11), 3023–3033 (2011).
- Huang Y , YuSH , ZhenWXet al. Tanshinone I, a new EZH2 inhibitor restricts normal and malignant hematopoiesis through upregulation of MMP9 and ABCG2. Theranostics11(14), 6891–6904 (2021).
- Delgado-Olguín P , DangLT , HeDet al. Ezh2-mediated repression of a transcriptional pathway upstream of Mmp9 maintains integrity of the developing vasculature. Development141(23), 4610–4617 (2014).
- Song Z , WuW , ChenMet al. Long noncoding RNA ANRIL supports proliferation of adult T-cell leukemia cells through cooperation with EZH2. J. Virol.92(24), e00909–18 (2018).
- Yang LH , DuP , LiuWet al. LncRNA ANRIL promotes multiple myeloma progression and bortezomib resistance by EZH2-mediated epigenetically silencing of PTEN. Neoplasma68(4), 788–797 (2021).
- Witmer AN , VrensenGF , Van NoordenCJ , SchlingemannRO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog. Retin. Eye Res.22(1), 1–29 (2003).
- Osaadon P , FaganXJ , LifshitzT , LevyJ. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye28(5), 510–520 (2014).
- Yang Y , LiuY , LiYet al. MicroRNA-15b targets VEGF and inhibits angiogenesis in proliferative diabetic retinopathy. J. Clin. Endocrinol. Metab.105(11), 3404–3415 (2020).
- Wong TY , CheungCM , LarsenM , SharmaS , SimóR. Diabetic retinopathy. Nat. Rev. Dis. Primers2, 16012 (2016).
- Ruiz MA , FengB , ChakrabartiS. Polycomb repressive complex 2 regulates miR-200b in retinal endothelial cells: potential relevance in diabetic retinopathy. PLOS ONE10(4), e0123987 (2015).
- Biswas S , ThomasAA , FengBet al. MALAT1 and HOTAIR – key epigenetic regulators in diabetic retinopathy. Diabetes67(Suppl. 1), 240-OR (2018).
- Di S , AnX , PangBet al. Yiqi Tongluo Fang could preventive and delayed development and formation of diabetic retinopathy through antioxidant and anti-inflammatory effects. Biomed. Pharmacother.148, 112254 (2022).
- Olivares AM , JelcickAS , ReineckeJet al. Multimodal regulation orchestrates normal and complex disease states in the retina. Sci. Rep.7(1), 690 (2017).
- Peng Y , LiaoK , TanFet al. Suppression of EZH2 inhibits TGF-β1-induced EMT in human retinal pigment epithelial cells. Exp. Eye Res.222, 109158 (2022).
- Lee HE , AyarpadikannanS , KimHS. Role of transposable elements in genomic rearrangement, evolution, gene regulation and epigenetics in primates. Genes Genet. Syst.90(5), 245–257 (2015).
- Kaneko H , DridiS , TaralloVet al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature471(7338), 325–330 (2011).
- Tarallo V , HiranoY , GelfandBDet al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell149(4), 847–859 (2012).
- Gelfand BD , WrightCB , KimYet al. Iron toxicity in the retina requires Alu RNA and the NLRP3 inflammasome. Cell Rep.11(11), 1686–1693 (2015).
- Zhu S , LiuM , ZhuF , YuX , WenJ , LiC. Targeting EZH2 prevents the occurrence and mitigates the development of Sjögren’s syndrome in mice. Int. Immunopharmacol.110, 109073 (2022).
- He C , YangY , ChenZet al. EZH2 promotes T follicular helper cell differentiation through enhancing STAT3 phosphorylation in patients with primary Sjögren’s syndrome. Front. Immunol.13, 922871 (2022).
- Katoh M . Mutation spectra of histone methyltransferases with canonical SET domains and EZH2-targeted therapy. Epigenomics8(2), 285–305 (2016).
