Abstract
Background: Little is known about the determinants of epigenetic aging in pediatric populations. Methods: Epigenetic age was estimated from 258 1-year-olds, using pediatric buccal epigenetic and Horvath clocks. We explored associations between epigenetic age and maternal indicators of mental and relational health, substance use and general physical health assessed during trimester three. Results: Higher anxiety and stress, BMI and higher parent–parent relationship quality were associated with pediatric buccal epigenetic clock differences. High blood pressure during pregnancy was associated with Horvath age acceleration. Third-trimester smoking and pre-pregnancy weight were associated with acceleration and deceleration respectively, and concordant across clocks. Conclusion: A broad range of maternal factors may shape epigenetic age in infancy; further research is needed to explore the possible effects on health and development.
Plain language summary
Molecules on our DNA, called DNA methylation, can be used in a laboratory test to estimate how old we are – also known as epigenetic age. In adults, a higher risk of age-related disease has been attributed to older epigenetic age. However, we know very little about epigenetic age in children. In this study, we look at the how measures of a mother’s health during pregnancy – such as using alcohol or tobacco, mental health (stress, anxiety and depression), or general health such as weight or high blood pressure – affect epigenetic age in children.
The ‘developmental origins of health and disease’ (DOHaD) hypothesis has long maintained that social and environmental exposures prior to conception, in utero and in early life can be causally related to later life health outcomes [Citation1]. One mechanism driving risk is epigenetic programming of gene expression. Epigenetics is the study of structural and molecular mechanisms – primarily DNA methylation, histone modification and noncoding RNAs – that do not alter the DNA code but are involved in gene regulation [Citation2]. Because epigenetic mechanisms are dynamic and respond to the environment, they have been of particular interest within the DOHaD framework [Citation3,Citation4].
A wide range of environmental exposures during pregnancy have now been associated with DNA methylation profiles in offspring. One of the most replicated examples of this is exposure to maternal smoking [Citation5]. Current research also shows that DNA methylation differences, at least in part, may mediate smoking exposure-related outcomes such as low birthweight [Citation6] and childhood asthma [Citation7]. Maternal exposure to second-hand smoke can also affect the fetus’s epigenome, altering DNA methylation in genes associated with inflammation, carcinogens and neuronal functioning [Citation8]. Further, exposure to less obvious environmental factors, such as maternal adverse childhood experiences years before conception [Citation9] and poorer maternal mental health during pregnancy [Citation10] has been shown to affect the offspring’s epigenome. Thus exploring the effects of factors such as maternal mental, social and general health on the offspring epigenetic profile is of great importance, given that they may be linked to negative health and developmental outcomes.
More recently, it has become clear that there are stable DNA methylation changes over a lifespan which track with age [Citation11]. This gradual change, which reflects biological aging, regardless of chronological age, is known as epigenetic aging and is measured using epigenetic/DNA methylation ‘clocks’. There are now several clocks available, each measuring different aspects of aging [Citation12]. The most popular clock, Horvath’s clock, was built by using methylation profiles of multiple tissues and finding methylation sites across the epigenome that correlate highly with age [Citation13]. Another clock, by Hannum et al., was built similarly, but by using DNA methylation signatures from blood samples only [Citation14]. There are also other clocks that measure ‘aging’ indirectly; for example, GrimAge is a measure of age reflected by time to death, derived using measures of morbidity and mortality that are associated with plasma-based proteins, as well as DNA methylation estimations of smoking pack-years [Citation15]. A newly developed clock, DunedinPACE, estimates age based on methylation values associated with several other age-related biomarkers [Citation16] rather than age itself, as Horvath’s clock does.
While measures such as these may be useful in predicting age-related disease and mortality risk [Citation17], the majority of studies that develop or use these clocks focus on middle-aged to older adults. This has left a significant gap in pediatric populations and limited understanding of the developmental origins of biological aging. Most clocks are developed using blood, as well as using age ranges that are not particularly appropriate for the pediatric population. Indeed, a recently published review of aging clocks in pediatric populations has shown that few studies currently exist, and even then, seldom in the context of early development [Citation18]. However, the recent development of the pediatric buccal epigenetic (PedBE) clock, which was built using similar methods to Horvath’s clock, now provides unique opportunities to extend work on adult biological aging to biological aging at the beginning of the life course. The PedBE clock has the added advantage of being derived from methylation signatures generated from easily collected buccal swabs, and only using pre-adulthood years (0–20), capturing pertinent developmental periods [Citation19]. Indeed, a recent review of the use of epigenetic clocks in pediatric populations has highlighted that the PedBE clock is the best performer of age estimation when using DNA methylation generated from pediatric buccal samples [Citation20].
The aim of this study was to explore and identify perinatal exposures that may be associated with epigenetic age acceleration at 1 year of age, estimated with the PedBE and Horvath clocks. Specifically, the aims of the study were to examine the extent to which epigenetic aging in 12-month-old infants is associated with perinatal measures of: maternal mental and relational health status, including anxiety, stress, depression and intimate partner relationship quality; maternal substance use, including alcohol, tobacco and cannabis; and maternal physical health, including weight, blood pressure and BMI.
Materials & methods
Study participants
The Australian Temperament Project (ATP) is a multigenerational, longitudinal cohort, initially started as a population representative survey of social and emotional health [Citation21]. For this study we used child participants from ATP generation 3 (G3), as well as data collected from their mothers from ATP generation 2 (97% born in Australia). Details of the cohort, informed consent and ethics have been described previously [Citation22]. ATP G3 study protocols have been approved by the Royal Children’s Hospital Human Research Ethics Committee.
Biological samples, methylation data & epigenetic clock generation
Buccal swabs were collected from n = 281 G3 children, at approximately 1 year of age. DNA was extracted from buccal swabs using the salting-out extraction method. A subsample of 270 participants had an adequate amount of DNA for generation of methylation data and were subsequently used for this study. DNA methylation data were generated by the Australian Genome Research Facility, Melbourne, Australia (www.agrf.org.au/) using the Illumina Infinium HumanMethylationEPIC BeadChip. R version 3.6.1 was used to process all data [Citation23]. Technical or biological replicates were not used in this study due to this being an exploratory study and the restrictive cost of generating epigenome-wide data. The minfi package [Citation24] was used to carry out preprocessing and quality control. Methylation at one probe was missing for one participant and imputed using impute [Citation25]. Methylation data were normalized using the β-mixture quantile method [Citation26]. Data from 12 participants were removed due to poor quality of DNA samples, resulting in methylation data not passing quality control. This left 258 children (54.7% female; mean age = 62.77 weeks, standard deviation = 7.7; 48% first child) from 213 mothers (mean age = 32.5 years, standard deviation = 1.4) in the dataset.
The PedBE clock was generated following publicly available code from https://github.com/kobor-lab/Public-Scripts/blob/master/PedBE.Md. The Horvath clock was generated using the online portal https://dnamage.genetics.ucla.edu/new. Estimated biological ages from PedBE and Horvath clocks are reported in weeks rather than years. Measures of age acceleration residuals were derived from regressing estimated biological age onto chronological age (i.e., biological age adjusted for chronological age). As both clocks adjust for cell type, they may be considered measures of ‘intrinsic’ epigenetic age acceleration [Citation27].
Maternal perinatal measures
Mothers responded to questionnaires during pregnancy (89.19% during the third trimester and 10.81% retrospectively about the third trimester) which covered a wide range of biopsychosocial factors [Citation22]. Maternal anxiety and stress were measured using the 21-question short report Depression Anxiety Stress Scale (note that scores were not multiplied by two) [Citation28]. Maternal depression was measured using the Edinburgh Postnatal Depression Scale [Citation29]. Higher scores for anxiety, stress and depression indicate worse mental health. Relationship quality included in this study was measured using the dyadic adjustment scale adapted for the ATP study, which averages scores from seven items, ranked from 1 to 6 [Citation30]. Social support was measured using the Maternity Social Support Scale [Citation31]. Maternal–fetal attachment was measured using a maternal–fetal attachment scale adapted from the work of Cranley [Citation32], using an average score from six items, ranked from 1 to 4, with each item multiplied by six. Higher scores represent higher relational quality.
Alcohol, tobacco and cannabis use were assessed at three time points: before pregnancy awareness (retrospectively), after pregnancy awareness (retrospectively) and during the third trimester (prospectively). For each time point and substance type, responses were recoded to indicate any use (none or any). For cannabis, due to the small number of mothers reporting use, responses for ‘during pregnancy’ were collapsed across ‘after pregnancy awareness’ and ‘during third trimester’.
Self-reported physical health measures used in this study included weight (kg), height (cm) and BMI leading up to pregnancy; a five-point scale of general physical health (poor, average, good, very good and excellent); gestational diabetes (no or yes); high blood pressure or preeclampsia (no or yes); and infections or fever (no or yes) during pregnancy.
Potential confounding factors
Common factors that confound epigenetic data were considered in statistical models. Proportions of epithelial and immune cells were estimated using epidish [Citation33], and epithelial cell proportions were included as an adjustment in all models, as suggested by both Horvath and McEwen et al. [Citation13,Citation19]. Other adjustments included maternal age [Citation34], parity (mother’s first pregnancy: no or yes), financial strain (scored from 0 [living comfortably] to 4 [finding it very difficult]), maternal smoking and alcohol use during any of the aforementioned time points [Citation35,Citation36], child sex and gestational age [Citation37,Citation38], child chronological age [Citation39] and experimental batch (EPIC array data were collected from three separate experimental batches) [Citation40].
Statistical analysis
Stata v. 17 (StataCorp, LLC, TX, USA) [Citation41] was used to carry out all statistical models. Correlations between age estimated by PedBE and Horvath clocks and chronological age of children were examined. Age acceleration residuals were used to explore relationships between biological age and maternal biopsychosocial factors. Specifically, linear regression models regressed age acceleration residuals onto maternal perinatal measures, separately, using a robust standard error estimator to account for within-family clustering. Models were run adjusting for only cell proportions (model 1) and then additionally adjusting for all other potential confounding factors, specifically maternal age, parity, financial strain, maternal alcohol use and smoking during pregnancy, child sex, chronological age and gestational age, epithelial cell proportion and experimental batch (model 2), unless the covariate was the exposure of interest (i.e., we did not adjust for smoking if smoking was the primary exposure in the model). For inferential analyses, continuous exposure variables are standardized (z-score). Results are reported as regression coefficients representing divergence from chronological age in weeks (β), with 95% CIs and findings considered significant using a two-sided test p-value of < 0.05. Where data were missing on any maternal participant from the 258 mother–child dyads, they were excluded from the specific analytical test, and the number within each test was tabulated in the results.
Results
Participant characteristics
Child and maternal characteristics can be seen in . On average, children were 1.2 years old when swabs were collected, with slightly more female participants (54.7%) than males. Epithelial cells contributed to the majority of estimated cell types within buccal swabs collected (mean = 80% of all cells across samples collected). Mothers were on average 32.1 years old, and a minority experienced greater than mild depression (15% ≥10), stress (8% ≥8) or anxiety (6% ≥4). Most women did not smoke during pregnancy; however, most women did consume alcohol before awareness of pregnancy (84%). Cannabis was only used during pregnancy by a very small proportion of women (1%).
Table 1. Participant characteristics.
Clock estimate correlations
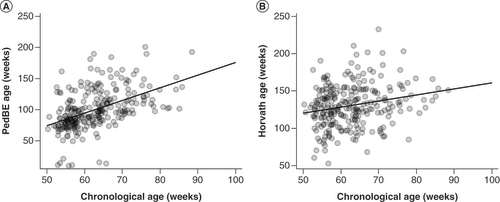
PedBE clock age estimates were more highly correlated with child chronological age compared with Horvath age estimates (r = 0.49; p < 0.001; and r = 0.22; p < 0.001, respectively) (A & B).
Age acceleration
Associations between age acceleration residuals and maternal perinatal biopsychosocial factors are shown in . Supplementary analyses between age acceleration residuals and any potential confounding birth-related factors (adjusted for epithelial cell proportion, batch and child age) can be seen in Supplementary Table 1.
Table 2. Associations between infant epigenetic age acceleration residuals and perinatal maternal characteristics.
Maternal perinatal mental & relational health
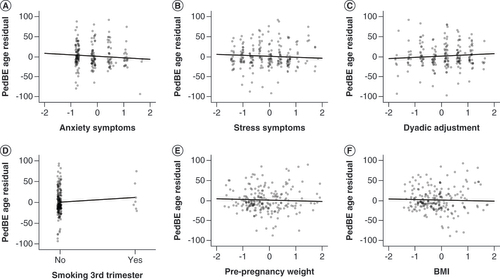
When adjusting for only epithelial cell proportions (model 1), higher maternal depression and anxiety were associated with decelerated PedBE clock age (β = -2.30; 95% CI: -4.54 to -0.05; p = 0.045; and β = -3.79; 95% CI: -6.34 to -1.23; p = 0.004, respectively). In contrast, higher parent–parent relational quality was associated with accelerated PedBE clock age (β = 2.80; 95% CI: 0.64 to 4.96; p = 0.011). When further accounting for potential confounding factors (model 2), the associations with maternal anxiety and decelerated PedBE clock age strengthened (β = -3.60; 95% CI: -5.92 to -1.28; p = 0.003; A) and there was further indication that maternal stress may also be associated with decelerated PedBE clock age (β = -2.32; 95% CI: -4.49 to -0.16; p = 0.036; B). However, the association with depression was attenuated (β = -1.91; 95% CI: -4.27 to 0.44; p = 0.111). The association between higher parent–parent relationship quality and accelerated PedBE clock age remained significant (β = 2.24; 95% CI: 0.24 to 4.26; p = 0.03; C). No measures of maternal mental health were associated with measures of Horvath clock age acceleration or deceleration.
Continuous measures are displayed as standardized z-scores, regression line shown. (A) Prenatal anxiety: β = -3.60; 95% CI: -5.92 to -1.28; p = 0.003. (B) Prenatal stress: β = -2.32; 95% CI: -4.49 to -0.16; p = 0.036. (C) Dyadic adjustment: β = 2.24; 95% CI: 0.24 to 4.26; p = 0.03. (D) Maternal smoking during the third trimester: β = 10.52; 95% CI: 2.07 to 18.98; p = 0.015. (E) Pre-pregnancy weight: β = -2.20; 95% CI: -4.33 to -0.08; p = 0.042. (F) Pre-pregnancy BMI: β = -1.87; 95% CI: -3.70 to -0.04; p = 0.045.
Maternal perinatal substance use
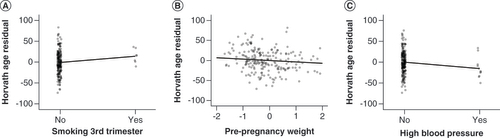
Maternal smoking during the third trimester (β = 9.00; 95% CI: 0.35 to 17.64; p = 0.041) and cannabis use during pregnancy (β = 3.58; 95% CI: 1.17 to 5.98; p = 0.004) were associated with accelerated PedBE clock age when adjusting for cell proportions (model 1). Associations remained for smoking exposure after adjusting for other potential confounding factors (β = 10.52; 95% CI: 2.07 to 18.98; p = 0.015) (D), but not cannabis (β = 11.48; 95% CI: -3.74 to 26.71; p = 0.138). Tobacco smoking during the third trimester and alcohol use shortly after pregnancy knowledge were both associated with increased Horvath clock age acceleration (β = 15.84; 95% CI: 5.47 to 26.20; p = 0.003; and β = 11.68; 95% CI: 0.98 to 22.38; p = 0.033, respectively), of which smoking exposure remained significant after full adjustment (β = 14.94; 95% CI: 3.56 to 26.32; p = 0.01; A), but the trend was not as strong with exposure to alcohol (β = 10.64; 95% CI: -0.23 to 21.51; p = 0.055).
Continuous measures are displayed as standardized z-scores, regression line shown. (A) Maternal smoking during third trimester: β = 14.94; 95% CI: 3.56 to 26.32; p = 0.01. (B) Pre-pregnancy weight: β = -3.49; 95% CI: -6.48 to -0.51; p = 0.022. (C) Reported high blood pressure, including preeclampsia, during pregnancy: β = -15.50; 95% CI: -27.95 to -3.05; p = 0.015.
Maternal perinatal physical health measures
PedBE age acceleration was not associated with any self-reported perinatal health measures before adjustments. However, after adjustment, both weight (β = -2.20; 95% CI: -4.33 to -0.08; p = 0.042; E) and BMI (β = -1.87; 95% CI: -3.70 to -0.04; p = 0.045; F) were separately associated with a deceleration of the PedBE clock age. As well, higher reported weight (but not BMI) before pregnancy was associated with decelerated Horvath age (β = -3.38; 95% CI: -6.20 to -0.56; p = 0.019), as was having reported issues with high blood pressure during pregnancy (β = -13.68; 95% CI: -26.32 to -1.03; p = 0.034). Both of these associations remained after full adjustment models (β = -3.49; 95% CI: -6.48 to -0.51; p = 0.022; and β = -15.50; 95% CI: -27.95 to -3.05; p = 0.015) (B & C).
Discussion
Here we report findings from an exploratory study, using a nested epigenetic sample from infants paired with maternal biopsychosocial data, drawn from a larger population-based cohort study. These findings suggest that offspring epigenetic aging may be shaped by a range of maternal biopsychosocial perinatal factors. In studies of older adults, exposures that negatively impact health are generally associated with an acceleration of epigenetic aging [Citation17,Citation42]. In our early-life study, findings were more variable, potentially reflecting the more dynamic nature of methylation events in early than later life. Specifically, while maternal substance use was associated with accelerated epigenetic age, maternal anxiety, stress and high blood pressure were associated with the opposite; that is, decelerated epigenetic age. Furthermore, dyadic adjustment representing low-conflict parent–parent relationships was associated with accelerated epigenetic aging. Small sample sizes necessitate cautious interpretation of the findings; however, if replicated, the results may demarcate early childhood as a unique developmental period within which the nature of biological aging follows a more variable, and not necessarily contiguous, course than biological aging later in the life course.
Increased maternal smoking, alcohol and cannabis use showed an association with accelerated infant age. Specifically, maternal smoking during the third trimester had the strongest association with accelerated biological age in infant offspring (PedBE age acceleration: +10.5 weeks; Horvath acceleration: +14.9 weeks). These findings are consistent with previous work using Horvath’s ‘skin and blood’ epigenetic clock [Citation43] measured in blood samples of 1173 6- to 11-year-olds (average age: 7.2 years) [Citation44]. Our findings should, however, be interpreted with caution due to the particularly low level of individuals reporting substance use. Of note, concordant findings across PedBE and Horvath clocks (in this study, smoking in the third trimester and pre-pregnancy weight) are of particular interest, as the two clocks are based on different methylation sites, with none overlapping (PedBE: 94 methylation sites; Horvath: 353 methylation sites). Together, these findings imply that substance use exposure in utero puts environmental pressure on the developing offspring to age.
In contrast, higher maternal stress and anxiety were associated with decelerated epigenetic age in infant offspring. These findings were unexpected and not concordant with the few prior studies in the field. For example, one recent study measured both Horvath and PedBE epigenetic age acceleration from buccal swabs in two cohorts, collected at different time points (n = 165 at 6 and 10 years; n = 340 at 3, 9 and 48 months) [Citation45]. After adjusting for genetic principal component scores, sex, time point, gestational age, maternal education and buccal cell heterogeneity, the authors found, in contrast to our observations, that prenatal anxiety was associated with a small but significant PedBE clock age acceleration between time points in both cohorts. Similarly, previous work using the Knight et al. gestational epigenetic age clock [Citation46] from the cord blood of 303 neonates found a weak positive correlation between prenatal maternal depression and gestational epigenetic age (although the association did not withstand adjustment for selective serotonin-reuptake inhibitor use), but no similar association for stress [Citation47]. Similar to maternal anxiety and stress, maternal high blood pressure (which is also associated with infant offspring developmental problems [Citation48,Citation49]) was associated with decelerated aging, an unexpected direction, and the largest decelerated aging effect (Horvath age deceleration: -15.5 weeks). Yet another study of 1801 neonates, which used the Knight et al. gestational epigenetic age clock, found an association in the same direction as in this study, in which preeclampsia (but not gestational hypertension) was associated with decelerated gestational epigenetic age [Citation50]. This association remained after adjusting for maternal ethnicity, race, pre-pregnancy BMI, age, prenatal smoking, educational level, child sex and cord blood cell composition estimates.
One explanation of this finding is that high blood pressure may be an indicator of factors involved with disrupted growth in the womb (e.g., intrauterine growth restriction, preterm birth, lower birth weight and size), with age deceleration simply marking ‘lagging age’. Indeed, prenatal anxiety, stress and hypertension are associated with intrauterine growth restriction [Citation51,Citation52]. It is important to note, however, that substance use is also well documented to cause intrauterine growth restriction [Citation53–55] and did not exhibit the same pattern of findings.
Taken together, epigenetic age in infants appears dynamic in association with different exposures, and further research in early life is needed to resolve the nature of relationships between maternal perinatal exposures (risk and protective) and infant offspring biological aging. Additionally, the stability and continuity of patterns of infant epigenetic aging into later life ages and stages remains unknown. For example, it could be that any divergence (either ‘older’ or ‘younger’) from the linear relationship between biological and chronological age before development is completed (i.e., before adulthood) may result in possible downstream health or developmental effects. Even so, there must be a transition point in development where accelerated aging becomes more consistently associated with adverse health and developmental exposures and outcomes. For example, when examining environmental exposures during childhood, rather than perinatal factors mediated through the mother, most studies show results that are more in line with adult aging studies, in that risk exposures associate with accelerated aging across domains such as maltreatment and internalizing [Citation56,Citation57]. Longitudinal studies that assess biological aging during development, as well as following up in early, middle and late adulthood, are needed to understand whether changes to aging trajectories in early life affect trajectories thereafter, and where these divergences affect health or development.
Strengths, limitations & future directions
One of the primary strengths of this study is the broad range of biopsychosocial factors that were able to be explored in association with age acceleration. This also gave us the ability to adjust for a wide range of possible confounding factors, bringing robustness to our statistical models. Further, our epigenetic clock estimates for PedBE are more highly correlated (r = 0.49) compared with Horvath’s estimate (r = 0.22). This is most likely due to the fact that we used the same specific tissue (buccal) and were within a closer range of ages (0–20 years, vs Horvath’s 0–100 years) that were used to build the PedBE clock. This increased strength of association may also reflect an increased sensitivity in PedBE versus Horvath, in finding associations between maternal exposures and differences in aging in the offspring. This may explain why associations with maternal mental health were found in association with PedBE but not Horvath clock estimates.
A limitation of this study is that many of our groups – for example, cannabis use (n = 3) versus no cannabis use (n = 218) during pregnancy – are very small, which results in imprecise estimates (wide confidence intervals) of effects due to low power. These types of findings need to be interpreted with caution, with replication sought in other studies in the future. A further limitation of this study is that all maternal data collected were self-reported, and thus social desirability bias may be an issue. While 97% of the study population were born in Australia, we were unable to adjust for specific ethnicities, which is an important consideration in DNA methylation studies [Citation58]. The two clock algorithms used in this study were selected because of their suitability for use with DNA methylation data extracted from buccal cells. We did not apply clocks developed on DNA methylation data extracted from blood cells (e.g., the Wu et al. epigenetic clock [Citation59]). Future studies with access to both blood and buccal DNA will be needed to compare the performance of clocks based on different tissue types. Another limitation is that we were not able to discern whether the differences found in aging were apparent at birth, or whether they persisted into early childhood. This of course is an important consideration, due to the fact that specific methylation differences track steadily with age [Citation13]. However, this will be a major point of interest in future longitudinal epigenetic studies of the ATP cohort, given that 8-week and 4-year buccal samples are available. Further, epigenetic aging at these time points will also be examined in the context of anthropometric (height, weight, BMI), socio-emotional and developmental outcomes of the offspring. This will be a major step in determining whether biological age divergences in the early years have positive or negative impacts when associated with outcomes.
Conclusion
Findings from this study suggest that several maternal perinatal factors may impact the overall rate of biological aging measured in 1-year-old infant offspring. PedBE clock measures are likely more appropriate than Horvath clock measures in a pediatric setting, given that the clock is more highly specific to younger ages. This potentially highlights the need to split up aging clocks before adulthood for use in specific age ranges (neonatal, early childhood, puberty etc.), as ‘aging’ during development may have a different, more variable, natural history to the more linear aging in later years. Our findings emphasize the importance of studies of epigenetic aging in pediatric populations, with future studies needing to include different time points and to explore the relationship between age acceleration and developmental outcomes. Finally, due to the varying results of acceleration and deceleration of biological age, studies in this field should consider that it may not be that aging faster is bad, or slower is good, but that a divergence from the biological aging norms may be negatively affecting a foundation of age-related epigenetic integrity established during development, and that may lead to an instability which increases the risk of later-life accelerated aging and disease.
Maternal perinatal factors may influence the offspring epigenetic profile, but less is known about how epigenetic aging is influenced.
The pediatric buccal epigenetic (PedBE) clock and Horvath’s multi-tissue clock were generated from buccal swabs from 258 1-year-olds.
A series of maternal perinatal biopsychosocial factors were used in covariate adjusted multivariate models to assess their associations with offspring epigenetic age.
Higher maternal anxiety and stress, as well as maternal pre-pregnancy BMI, were associated with a slight deceleration of PedBE clock aging, while higher parent–parent relationship quality was associated with accelerated PedBE clock aging.
High maternal blood pressure was associated with a large deceleration of Horvath epigenetic age (β = -15.50 weeks).
Concordant findings between the PedBE and Horvath clocks include acceleration in epigenetic age associated with smoking in the third trimester and deceleration in association with higher pre-pregnancy weight.
Further research is needed to determine whether the findings can be replicated and to ascertain whether early differences in biological age remain over time and to what extent early differences in biological age are predictive of health and development.
Author contributions
P Fransquet, C Greenwood and C Olsson contributed to the concept of this work. P Fransquet oversaw bioinformatics, epigenetic age generation and primary authorship of the article. C Greenwood oversaw statistical analysis. P Fransquet, C Greenwood and C Olsson interpreted the data for discussion. P Fransquet, J Macdonald, J Ryan, C Greenwood and C Olsson all contributed to drafting and revising the manuscript.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study involves human participants, and the main ATP study was approved by the Human Research Ethics Committee (HREC) at the Royal Children’s Hospital (RCH) from 1983 to 1993; La Trobe University HREC from 1994 to 1995; RCH HREC from 1996 to 1997; University of Melbourne HREC from 1998 to 2000; and the Australian Institute of Family Studies Ethics Committee from 2000. ATPG3 protocols have been approved by RCH HREC and have been ratified by Deakin University and The University of Melbourne. Parents of the ATP G3 sample have given written informed consent.
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Supplemental Table S1
Download MS Word (14.9 KB)Acknowledgments
The Australian Temperament Project (ATP) and Generation 3 (ATPG3) studies are located at The Royal Children’s Hospital Melbourne and are a collaboration between Deakin University, The University of Melbourne, La Trobe University, The Australian Institute of Family Studies, The University of New South Wales, The University of Otago (New Zealand) and the Royal Children’s Hospital; further information is available at https://www.melbournechildrens.com/atp/. The views expressed in this paper are those of the authors and may not reflect those of their organizational affiliations, nor of other collaborating individuals or organizations. The authors acknowledge all collaborators who have contributed to the ATP, especially A Sanson, M Prior, F Oberklaid and D Smart. They are grateful to all study research team members involved in data collection and management. They would also like to sincerely thank the participating families for their time and invaluable contribution to the study.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/epi-2023-0284
Financial disclosure
Funding for this work was supported by grants from the Australian Research Council (DP130101459; DP160103160; DP180102447), the National Health and Medical Research Council of Australia (APP1082406; APP1175086) and Financial Markets Foundation for Children. Data collection for the ATP study was supported primarily through Australian grants from the Melbourne Royal Children’s Hospital Research Foundation, National Health and Medical Research Council, Australian Research Council and the Australian Institute of Family Studies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Data sharing statement
Inquires about collaboration are possible through our institutional data access protocol: https://lifecourse.melbournechildrens.com/data-access/. Data from this study are available upon reasonable request.
References
- Lacagnina S . The developmental origins of health and disease (DOHaD). Am. J. Lifestyle Med.14(1), 47–50 (2020).
- Li Y . Modern epigenetics methods in biological research. Methods187, 104–113 (2021).
- Lynch F , LewisS , MaccioccaI , CraigJM. Epigenetics and DOHaD: how translation to predictive testing will require a better public understanding. J. Dev. Orig. Health Dis.13(4), 424–430 (2022).
- Felix JF , CecilCaM. Population DNA methylation studies in the Developmental Origins of Health and Disease (DOHaD) framework. J. Dev. Orig. Health Dis.10(3), 306–313 (2019).
- Joubert BR , FelixJF , YousefiPet al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am. J. Hum. Genet.98(4), 680–696 (2016).
- Xu R , HongX , ZhangBet al. DNA methylation mediates the effect of maternal smoking on offspring birthweight: a birth cohort study of multi-ethnic US mother–newborn pairs. Clin. Epigenetics13(1), 47 (2021).
- Neophytou AM , OhSS , HuDet al. In utero tobacco smoke exposure, DNA methylation, and asthma in Latino children. Environ. Epidemiol.3(3), e048 (2019).
- Fuemmeler BF , DozmorovMG , DoEKet al. DNA methylation in babies born to nonsmoking mothers exposed to secondhand smoke during pregnancy: an epigenome-wide association study. Environ. Health Perspect.129(5), 57010 (2021).
- Folger AT , NideyN , DingLet al. Association between maternal adverse childhood experiences and neonatal SCG5 DNA methylation – effect modification by prenatal home visiting. Am. J. Epidemiol.191(4), 636–645 (2021).
- Cao-Lei L , VanDen Heuvel MI , HuseKet al. Epigenetic modifications associated with maternal anxiety during pregnancy and children’s behavioral measures. Cells10(9), 2421 (2021).
- Li A , KochZ , IdekerT. Epigenetic aging: biological age prediction and informing a mechanistic theory of aging. J. Intern. Med.292(5), 733–744 (2022).
- Bell CG , LoweR , AdamsPDet al. DNA methylation aging clocks: challenges and recommendations. Genome Biol.20(1), 249 (2019).
- Horvath S . DNA methylation age of human tissues and cell types. Genome Biol.14(10), R115 (2013).
- Hannum G , GuinneyJ , ZhaoLet al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell49(2), 359–367 (2013).
- Lu AT , QuachA , WilsonJGet al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY)11(2), 303–327 (2019).
- Belsky DW , CaspiA , CorcoranDLet al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife11, e73420 (2022).
- Fransquet PD , WrigglesworthJ , WoodsRL , ErnstME , RyanJ. The epigenetic clock as a predictor of disease and mortality risk: a systematic review and meta-analysis. Clin. Epigenetics11(1), 62 (2019).
- Wang J , ZhouWH. Epigenetic clocks in the pediatric population: when and why they tick?Chin. Med. J. (Engl.)134(24), 2901–2910 (2021).
- Mcewen LM , O’donnellKJ , McgillMGet al. The PedBE clock accurately estimates DNA methylation age in pediatric buccal cells. Proc. Natl Acad. Sci. USA117(38), 23329–23335 (2020).
- Fang F , ZhouL , PerngWet al. Evaluation of pediatric epigenetic clocks across multiple tissues. Clin. Epigenetics15(1), 142 (2023).
- Vassallo S , SansonA. The Australian Temperament Project: The first 30 years.Australian Institute of Family Studies (2013).
- Olsson CA , LetcherP , GreenwoodCJet al. The Australian Temperament Project Generation 3 study: a population-based multigenerational prospective cohort study of socioemotional health and development. BMJ Open12(9), e061854 (2022).
- R Core Team . R: a language and environment for statistical computingR Foundation for Statistical Computing, Vienna, Austria (2018).
- Aryee MJ , JaffeAE , Corrada-BravoHet al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics30(10), 1363–1369 (2014).
- Hastie T , TibshiraniR , NarasimhanB , ChuG. impute: imputation for microarray data. R package version1.72.1https://bioconductor.org/packages/release/bioc/html/impute.html(2022).
- Teschendorff A , MarabitaF , LechnerMet al. A beta-mixture quantile normalisation method for correcting probe design bias in Illumina Infinium 450k DNA methylation data. Bioinformatics29(2), 189–196 (2012).
- Gibson J , RussTC , ClarkeT-Ket al. A meta-analysis of genome-wide association studies of epigenetic age acceleration. PLOS Genet.15(11), e1008104 (2019).
- Lovibond PF , LovibondSH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther.33(3), 335–343 (1995).
- Cox JL , HoldenJM , SagovskyR. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry150(6), 782–786 (1987).
- Spanier GB . Measuring dyadic adjustment: new scales for assessing the quality of marriage and similar dyads. J. Marriage Fam.38(1), 15–28 (1976).
- Webster J , LinnaneJW , DibleyLM , HinsonJK , StarrenburgSE , RobertsJA. Measuring social support in pregnancy: can it be simple and meaningful?Birth27(2), 97–101 (2000).
- Cranley MS . Development of a tool for the measurement of maternal attachment during pregnancy. Nurs. Res.30(5), 281–284 (1981).
- Teschendorff AE , ZhengSC. Epigenetic Dissection of Intra-Sample-Heterogeneity (EPIDISH). https://bioconductor.org/packages/release/bioc/html/EpiDISH.html (2022).
- Markunas CA , WilcoxAJ , XuZet al. Maternal age at delivery is associated with an epigenetic signature in both newborns and adults. PLOS ONE11(7), e0156361 (2016).
- Rauschert S , MeltonPE , BurdgeGet al. Maternal smoking during pregnancy induces persistent epigenetic changes into adolescence, independent of postnatal smoke exposure and is associated with cardiometabolic risk. Front. Genet.10, 770 (2019).
- Bestry M , SymonsM , LarcombeAet al. Association of prenatal alcohol exposure with offspring DNA methylation in mammals: a systematic review of the evidence. Clin. Epigenetics14(1), 12 (2022).
- Solomon O , HuenK , YousefiPet al. Meta-analysis of epigenome-wide association studies in newborns and children show widespread sex differences in blood DNA methylation. Mutat. Res. Rev. Mutat. Res.789, 108415 (2022).
- Wheater ENW , GaldiP , McCartneyDLet al. DNA methylation in relation to gestational age and brain dysmaturation in preterm infants. Brain Commun.4(2), fcac056 (2022).
- Krieger N , ChenJT , TestaCet al. Use of correct and incorrect methods of accounting for age in studies of epigenetic accelerated aging: implications and recommendations for best practices. Am. J. Epidemiol.192(5), 800–811 (2023).
- Price EM , RobinsonWP. Adjusting for batch effects in DNA methylation microarray data, a lesson learned. Front Genet9, 83 (2018).
- StataCorp . Stata Statistical Software: release 17. www.stata.com/ (2021).
- Oblak L , VanDer Zaag J , Higgins-ChenAT , LevineME , BoksMP. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res. Rev.69, 101348 (2021).
- Horvath S , OshimaJ , MartinGMet al. Epigenetic clock for skin and blood cells applied to Hutchinson Gilford progeria syndrome and ex vivo studies. Aging (Albany NY)10(7), 1758–1775 (2018).
- De Prado-Bert P , Ruiz-ArenasC , Vives-UsanoMet al. The early-life exposome and epigenetic age acceleration in children. Environ. Int.155, 106683 (2021).
- Mcgill MG , PokhvisnevaI , ClappisonASet al. Maternal prenatal anxiety and the fetal origins of epigenetic aging. Biol. Psychiatry91(3), 303–312 (2022).
- Knight AK , CraigJM , ThedaCet al. An epigenetic clock for gestational age at birth based on blood methylation data. Genome Biol.17(1), 206 (2016).
- Mckenna BG , HendrixCL , BrennanPAet al. Maternal prenatal depression and epigenetic age deceleration: testing potentially confounding effects of prenatal stress and SSRI use. Epigenetics16(3), 327–337 (2021).
- Sun BZ , MosterD , HarmonQE , WilcoxAJ. Association of preeclampsia in term births with neurodevelopmental disorders in offspring. JAMA Psychiatry77(8), 823–829 (2020).
- Graham AM , DoyleO , TildenELet al. Effects of maternal psychological stress during pregnancy on offspring brain development: considering the role of inflammation and potential for preventive intervention. Biol. Psychiatry Cogn. Neurosci. Neuroimaging7(5), 461–470 (2022).
- Ladd-Acosta C , VangE , BarrettESet al. Analysis of pregnancy complications and epigenetic gestational age of newborns. JAMA Netw. Open6(2), e230672 (2023).
- Lewis AJ , AustinE , GalballyM. Prenatal maternal mental health and fetal growth restriction: a systematic review. J. Dev. Orig. Health Dis.7(4), 416–428 (2016).
- Monteiro LJ , PeñaililloR , SánchezMet al. The role of long non-coding RNAs in trophoblast regulation in preeclampsia and intrauterine growth restriction. Genes12(7), 970 (2021).
- Tarasi B , CornuzJ , ClairC , BaudD. Cigarette smoking during pregnancy and adverse perinatal outcomes: a cross-sectional study over 10 years. BMC Public Health22(1), 2403 (2022).
- Bouquet E , EidenC , FauconneauBet al. Adverse events of recreational cannabis use during pregnancy reported to the French Addictovigilance Network between 2011 and 2020. Sci. Rep.12(1), 16509 (2022).
- Naik VD , LeeJ , WuG , WashburnS , RamadossJ. Effects of nutrition and gestational alcohol consumption on fetal growth and development. Nutr. Rev.80(6), 1568–1579 (2022).
- Nishitani S , SuzukiS , OchiaiKet al. Altered epigenetic clock in children exposed to maltreatment. Psychiatry Clin. Neurosci.75(3), 110–112 (2021).
- Dammering F , MartinsJ , DittrichKet al. The pediatric buccal epigenetic clock identifies significant ageing acceleration in children with internalizing disorder and maltreatment exposure. Neurobiol. Stress15, 100394 (2021).
- Chan MH-M , MerrillSM , KonwarC , KoborMS. An integrative framework and recommendations for the study of DNA methylation in the context of race and ethnicity. Discov. Soc. Sci. Health3(1), 9 (2023).
- Wu X , ChenW , LinFet al. DNA methylation profile is a quantitative measure of biological aging in children. Aging (Albany NY)11(22), 10031–10051 (2019).