Abstract
Aim: We conducted this study to identify gene promoter methylation status and clinical predictors for upper tract urothelial carcinoma (UTUC) patients. Materials & methods: Using methylation-sensitive PCR, we examined ten genes promoter methylation status in 687 UTUC patients. Results: A methylated promoter of three genes to predict higher tumor stage (T3 and T4), five genes to predict higher tumor grade (G3) and one gene to predict pN+ were certified in this study. Nine factors were significantly associated with poor cancer-specific survival. Six factors were considered as predictors to develop bladder recurrence after surgery. Conclusion: Methylation occurs commonly in UTUCs, may affect carcinogenic mechanisms, and is a well predictive factor for cancer-specific survival and bladder recurrence in UTUCs.
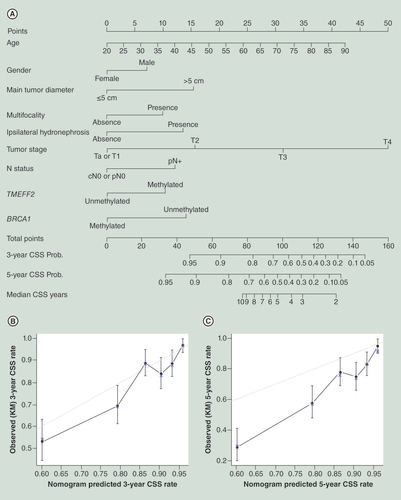
(A) Calibration plot for predicted (gray straight line) and observed (patients were divided into six equal groups, vertical lines represent the 95% CI) (B) 3-year CSS probability and (C) 5-year CSS probability.
CSS: Cancer-specific survival.
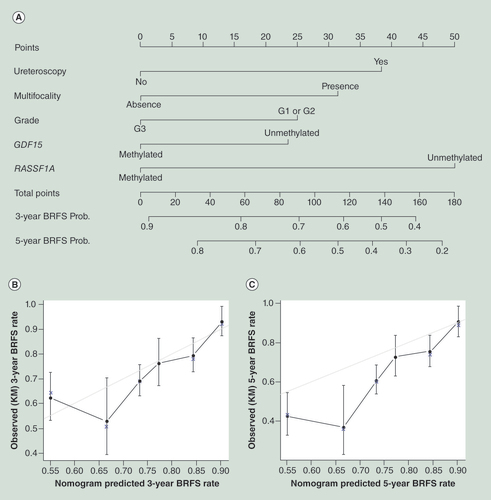
(A) Calibration plot for predicted (gray straight line) and observed (patients were divided into six equal groups, vertical lines represent the 95% confidence interval) (B) 3-year BRFS probability and (C) and 5-year BRFS probability.
BRFS: Bladder recurrence free survival; Prob: Probability.
Upper tract urothelial carcinoma is a rare disease and accounts for approximately 5–10% of all urothelial carcinomas and 10% of renal tumors [Citation1,Citation2]. It is estimated that new upper tract urothelial carcinoma (UTUC) patients amounted to more than 2800 in the USA in 2012, with a male to female ratio of approximately 2:1 [Citation2]. Although the oncological characteristics of UTUC are well defined in guidelines, few studies have systematically analyzed gene promoter methylation and oncological outcomes for this rare carcinoma [Citation1]. In addition, there was no high credible model to predict bladder recurrence and cancer-specific survival (CSS) after surgery for UTUCs. However, accurate outcome projections made based on pathological diagnosis, can provide significant benefits and convenience to clinicians and patients.
The alteration of DNA methylation is considered a key event in transcriptionally repressed regions of the genome. Aberrant promoter methylation at several gene loci has been reported to be associated with cancer stage, progression and survival in bladder urothelial carcinoma [Citation3–8]. Because bladder urothelial carcinoma and UTUC display genomic and clinical similarities, we hypothesized they might share some common epigenetic characteristics. Therefore, we chose ten genes (ABCC6, BRCA1, CDH1, GDF15, HSPA2, RASSF1A, SALL3, THBS1, TMEFF2 and VIM) with a high frequency of methylation in bladder UC and assessed their epigenetic status in UTUC and relationships with clinical outcomes.
As one of the largest urological centers in China, we conducted this study to identify the gene promoter methylation status and prognostic factors in UTUC patients. We also constructed prognostic models for UTUC patients treated with surgery with the purpose of predicting CSS and bladder recurrence-free survival (BRFS).
Materials & methods
Patient population
In this retrospective analysis, we obtained data from Peking University First Hospital (Beijing, China) for the period from 1 August 1999, to 31 December 2011. Among the 820 pathologically diagnosed UTUC patients with complete follow-up, 133 were excluded from the study: 76 had concomitant/previous bladder tumor, 30 had bilateral UTUCs (the bilateral UTUCs presents some distinctive clinical characteristics compared with normal UTUC [Citation9], and there might be some different epigenetics mechanism to bilateral UTUCs), and 27 did not obtain a permission to extract DNA from pathological department, as there was only one paraffin specimen stored in our bank. The remaining 687 consecutive patients constituted the basis of this large-scale study. The ethics committee of Peking University First Hospital approved the study.
Diagnosis & treatment
Radical nephroureterectomy or partial ureterectomy (in solitary kidney patients or chronic kidney disease [CKD] IV–V patients with evidence of ipsilateral functional kidney) procedures were performed on all members of the study population. Ureteroscopy with tumor biopsy was performed to determine treatment strategy when radiography was atypical. Patients with positive pathological evidence received surgical treatment, and patients with negative evidence received re-ureteroscopy or were closely followed. Ipsilateral hydronephrosis was determined by urinary system ultrasound, MRI or CT prior to the operation. All surgical specimens were processed according to standard pathological procedures, and all slides were reevaluated by another senior pathologist. All pathologists were blinded to the clinical outcomes. Tumor stage was assessed according to the UICC TNM classification of malignant tumors of 2002 [Citation10]. Tumor grade was assessed according to the WHO classification of 1973 [Citation11]. Tumor architecture was defined as papillary or sessile. Tumor multifocality was defined as the simultaneous presence of two or more pathologically confirmed macroscopic tumors in any location(s) (renal, pelvis or ureter).
DNA extraction & methylation-specific PCR
All of the DNA samples were extracted from formalin-fixed paraffin-embedded tumor (>70% tumor) samples using the QIAamp DNA formalin-fixed paraffin-embedded Tissue Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. For bisulfite transformation, approximately 1.5 μg tumor DNA was treated with sodium bisulfite using EpiTect Fast Bisulfite Conversion Kits (Qiagen, Hilden, Germany). The methylation status of the ten gene promoters was analyzed by methylation-sensitive PCR (MSP), as previously described by Herman et al. [Citation12]. Thirty-eight cycles of PCR were carried out for every reaction, and primers and reaction conditions were described in . Commercially available methylated human genomic DNA (Qiagen, Hilden, Germany) was used as positive control. Water blanks and PCR mixtures were used as negative control. The MSP results were analyzed on 3% agarose gels stained with ethidium bromide at UV light. Because all of the CpG islands we investigated have been previously certified with a low prevalence of hypermethylation in normal tissue [Citation3–8,Citation13], we did not compare the epigenetic status at each promoter region between UTUC and cancer-free subjects any further in our study.
Follow up
Routine follow-up consisted of physical examination, routine urinalysis, cystoscopy, ultrasound, MRI/CT and urine cytology (or urine fluorescence in situ hybridization) every 3 months in the first 2 years and once a year thereafter. However, approximately 40% of the patients lived far from Beijing, and their postoperative examinations were performed at local clinical centers. We obtained follow-up data via telephone or letter for this cohort of patients.
Statistical analysis
Univariate log–rank tests and multivariate Cox regression were performed to assess the prognostic significance of each variable with regard to CSS and BRFS. To predict CSS and BRFS probability, the construction of two nomograms was carried out. Two Cox regression model were built, each containing all of predictors in . The calculation of c-index, generation of the nomogram and calibration plots was performed with the open-source statistical software R (The R Foundation for Statistical Computing) and rms package [Citation14], and other statistical tests were performed with SPSS 21.0 software (IBM Corp, NY, USA). A p-value of less than 0.05 was considered significant.
Results
A total of 687 pathologically diagnosed UTUC patients were enrolled in our study. Two hundred and twenty five patients (32.8%) died as a consequence of UTUC during the follow-up period (median: 65 months, range: 3–144); the median survival time was 115 months (95% CI: 99–131 months). Two hundred and twenty eight patients (33.2%) developed bladder recurrence after surgery during the follow-up period (median: 49 months, 2–144).
Epigenetic biomarkers & tumor malignancy
We determined the prevalence of the methylation status of the ABCC6, BRCA1, CDH1, GDF15, HSPA2, RASSF1A, SALL3, THBS1, TMEFF2 and VIM genes in the 687 UTUC patients by MSP. The patient characteristics and promoter methylation status are summarized in . The median age was 68 years (range: 20–90), and the ratio of male to female patients was 1: 1.2. In total, 88.9% tumor DNA samples presented hypermethylation for at least one gene promoter locus. We verified the association between epigenetic biomarkers and tumor malignancy (pT3/T4, tumor grade 3 and pN+) using univariate and multivariate logistic regressions (). A methylated promoter of CDH1, HSPA2 and RASSF1A was significantly associated with a higher tumor stage (T3 and T4). A methylated promoter of BRCA1, HSPA2, RASSF1A and THBS1 and an unmethylated promoter of GDF15 were significantly associated with grade 3. A methylated promoter of RASSF1A was associated with pN+.
Prognostic factors for UTUCs
The univariate and multivariate analyses for prognostic significance are summarized in . Older age, male sex, tumor multifocality, ipsilateral hydronephrosis, larger main tumor diameter (>5 cm), higher tumor stage, positive N status, methylated TMEFF2 promoter and methylated BRCA1 promoter were significantly associated with CSS. Tumor multifocality, ureteroscopy history (51/86 for ureteroscopy group vs 177/601 for nonureteroscopy group), lower tumor grade, unmethylated promoter of GDF15 and RASSF1A promoters were significantly associated with bladder recurrence after surgery for UTUC patients. Considering the significantly negative effect on CSS, we eliminated ‘N status’ in the construction of the BRFS nomogram because there might be a statistic bias that some pN+ patients would not be live long enough to develop BR.
Predictive model for CSS & BRFS
The nomogram for predicting CSS probability and median survival time is illustrated in . The calibration plots were separately demonstrated for a 3-year CSS probability and 5-year CSS probability ( & C), and the bootstrap-corrected Harrell C statistic (c-index) for CSS was 0.75 (95% CI: 0.73–0.77). The nomogram for predicting BRFS probability and the calibration plots are illustrated in –C; the c-index for BRFS model was 0.71 (95% CI: 0.69–0.73).
Discussion
Aberrant methylation was detected in 88.9% of UTUC patients in our study, suggesting that epigenetic gene silencing is typical in this tumor type. In our study, the most frequent hypermethylation was detected in the VIM (63.2%) gene. Through univariate and multivariate logistic regressions, we confirmed that hypermethylation of the CDH1, HSPA2 and RASSF1A promoters was significantly associated with a higher tumor stage, that a methylated BRCA1, HSPA2, RASSF1A and THBS1 and an unmethylated GDF15 promoter were predictors for grade 3 and that hypermethylation of the RASSF1A promoter was associated with pN+. Our findings suggested that different classes and degrees of methylation were significantly associated with tumor malignancy (tumor stage, tumor grade and pN+ status).
The oncological outcomes of UTUC after treatment were associated with the methylation status of individual CpG islands. For example, tumors with TMEFF2 methylation had higher mortality rates (HR = 1.53, p = 0.002) than those without it, whereas tumors with BRCA1 methylation presented better CSS rates (HR = 0.56, p = 0.004) than those without it. More surprisingly, in our large-scale study, UTUC patients with RASSF1A (HR = 0.50, p = 0.001) and GDF15 (HR = 0.74, p = 0.030) hypermethylation presented less bladder recurrence after surgery. In previous study, methylated GDF15 in UTUC [Citation15], methylated BRCA1 in breast cancer [Citation16] and methylated RASSF1A in bladder cancer [Citation3] was associated with higher tumor stage, worse molecular pathology type and poorer survival. However, in our study group, the methylated BRCA1 patients presented better survival, and more bladder recurrence developed in the patients with unmethylated RASSF1A and GDF15 promoters. It is difficult to explain this interesting phenomenon with existing theories; however, new insight was appeared when we investigated the relationship between the epigenetic and patient population characteristics. Through logistic regression using ten epigenetic biomarkers as a variable, we demonstrated that female patients showed a higher proportion of methylated BRCA1 promoters (p = 0.012) and unmethylated GDF15 promoters (p = 0.042) than males.
In the Chinese population, there is a very different gender-related incidence of UTUC compared with Western populations [Citation17–19]. However, the gender-related oncological outcomes of this disease from different studies are controversial [Citation20–23]. Based on our study, we consider the male sex (p = 0.033) as a significant predictor of a poor CSS in UTUC in the Chinese population. The most plausible explanation for this could be the use of traditional Chinese medicine (TCM) containing aristolochic acid (AA). AA-containing preparations are mostly used by women for weight loss [Citation24]. Moreover, according to TCM theory, Caulis aristolochiae manshuriensis (containing AA) can regulate the menstrual cycle, increase breast milk production and reduce the secretion of leucorrhea. Consequently, it was widely used by Chinese women until the State Drug Administration of PR China forbade its use in April of 2003. The carcinogenicity of AA has been clearly demonstrated [Citation25], and there were some distinctive characteristics for this cohort of UTUCs. In one retrospective study of UTUC in Balkan endemic nephropathy patients, a lower tumor stage predominated among UTUC patients from the endemic settlements from 1957 to 1986 [Citation26]. This phenomenon led to the prediction that UTUCs caused by AA might have lower malignancy and better CSS rates. Based on the aforementioned hypothesis (AA-related UTUC might have a lower tumor stage, and TCM containing aristolochic is widely used in Chinese women), we further verified that the female group had a lower tumor stage than males (p = 0.006) in our study cohort. Furthermore, as a multifunctional tumor suppressor gene, BRCA1 plays an important role in DNA damage repair, genomic stability maintenance, transcriptional and cell apoptosis regulation [Citation27]. In the previous study, researchers found that breast cancer patients with BRCA1 mutation and decreased expression of BRCA1 protein were more sensitive to chemotherapy and inclined to have better survival [Citation28]. In the UTUC patients, hypermethylated BRCA1 might downregulate its expression and then increased the sensitivity of chemotherapy, and consequently improved the survival time. This may in part explain why female sex and an unmethylated BRCA1 promoter result in better survival in UTUC.
The classic mechanisms for bladder recurrence are the tumor intraluminal seeding theory and field cancerization theory. The former considers that recurrence is caused by implanted cancer cells from primary tumor. In contrast, the latter indicates that in addition to the primary carcinoma, there might be the activation of oncogenes or inactivation of suppressor genes in any location of the urothelium caused by exposure to carcinogenic factors (e.g., benzidine, tobacco and AA), which could be the origin of the new carcinoma. In our study, diagnostic ureteroscopy (HR = 1.68, p = 0.001) was associated with bladder recurrence after resection surgery, and there was another study reached a similar conclusion [Citation29]. As an effective tool to evaluate UTUCs visually or by biopsy, ureteroscopy was selectively used in early UTUC patients without atypical radiography. However, retrograde flow, increased urine flow rate and intraluminal pressure might lead to the shedding of tumor cells (intraluminal tumor seeding is thought to contribute to intravesical recurrence after nephroureterectomy [Citation30]), which implant in the bladder to develop recurrences. The other four predictors for BRFS were tumor multifocality (HR = 1.54, p = 0.002), lower tumor grade (G1 or G2 vs G3, HR = 1.34, p = 0.046) and unmethylated GDF15 (HR = 1.35, p = 0.030) and RASSF1A (HR = 1.98, p = 0.001) promoters. Multifocality was confirmed to be related to BRFS in several studies and could be caused by seeding tumor cells or original tumor cells developed due to carcinogens [Citation31–33]. The co-existence mechanism could lead to the further recurrence of bladder tumor. As a member of the TGF-β family, GDF15 might act as a tumor suppressor in early cancer stages and a protumorigenic factor at later stages of tumor progression [Citation34]. GDF15 exerts pleiotropic roles in cancer development and seems to contribute to proliferation, invasion, migration and metastases in cancer [Citation35]. According to this theory, unmethylation of the GDF15 promoter in UTUC could positively regulate gene expression, and finally resulted in bladder recurrence.
It is difficult to explain the abnormal phenomenon that unmethylated RASSF1A and lower grade resulted in a higher probability of developing bladder recurrence. In general, a methylated RASSF1A promoter was considered to be an independent risk factor for higher malignancy and progression, which is further supported by our study: hypermethylation of RASSF1A was associated with a higher tumor stage (p = 0.037), higher grade (p = 0.031) and pN+ (p = 0.005) in our UTUC cohort. Tumors with higher malignancy should present worse biological behavior; however, we observed the opposite results for bladder recurrence in UTUC at our center. In our previous study, we hypothesized that there might be a possible correlation between a lower grade and the molecular recurrent mechanism [Citation36]. Combined with the results of the current study, we identified that the methylation rate was statistically higher in the unmethylated RASSF1A (p = 0.007) promoter in lower grade (G1 + G2) patients. Coincidentally, these variables were two independent predictors for BRFS. Because there was no evidence that an unmethylated RASSF1A promoter and a lower grade could increase tumor seeding, we attempted to explain the phenomenon based on the field cancerization theory. Because AA-related UTUCs present lower grade tumors [Citation26], we hypothesized that the lower grade and unmethylated RASSF1A patient cohort had more AA-related UTUCs than the higher grade and methylated RASSF1A cohort in our study. According to the field cancerization theory, AA could influence all urinary urothelium and lead to more BRFS, which may in part explain this interesting phenomenon in UTUC in the Chinese population.
In the final points-based CSS nomogram (), nine predictors are located on the left side, tailing with their respective scales on the right. Each scale position has corresponding points located on the ‘point’ scale. The sum of all points for each variable was used to calculate the ‘Total Points.’ Each ‘Total Points’ represents the different 3- and 5-year probabilities of being alive, and the corresponding position on the ‘Median Survival Years’ represents median life expectancy for patients with identical characteristics. For example, a 58-year-old female patient was treated with surgery, the postoperative pathological diagnosis was UTUC, single tumor, diameter = 5.5 cm, pT3, N0, G3, methylated TMEFF2, methylated BRCA1 and there was no evidence of hydronephrosis in preoperative examinations. For this patient, the ‘Total Points’ = 22 (58 years old) + 0 (female) + 15 (tumor diameter = 5.5 cm) + 0 (single tumor) + 0 (without hydronephrosis) + 31 (pT3) + 0 (N0) + 11 (methylated TMEFF2) + 0 (methylated BRCA1) = 79. The 3- and 5-year probabilities of survival for this patient were approximately 83 and 72%, and the median survival time after operation was approximately 9 years for patients with identical characteristics. The calibration plots (–C) suggested that the nomogram was well calibrated for all predictions in ‘3-year Survival Pro’ and in ‘5-year Survival Pro.’ Furthermore, we can access bladder recurrence probability using the BRFS nomogram showing in .
This study has certain limits and constraints. First, the research data represent a retrospective review of findings at a single center. Second, the accuracy of the study was affected by the single methods to detect methylation status (MSP). Third, the lack of subsequent gene methylation function analysis limits explaining the detailed mechanism of methylation status (TMEFF2, RASSF1A, GDF15 and BRCA1) and oncological outcomes. Fourth, the lack of accurate information on AA medication history, surgical margin status, lymphovascular invasion status and new tumor grading (WHO 2004) results may reduce the strength of this study.
Conclusion & future perspective
In conclusion, our results suggest that different classes and degrees of gene methylation could regulate tumor malignancy and progression. We have constructed gene methylation related prognostic models for the UTUC population. In the future, the methylation status of GDF15, BRCA1, RASSF1A and TMEFF2 could be detected after radical surgery, survival probability, median survival time and bladder recurrence-free time can be valuable measures that permit more accurate prognoses for UTUC patients and clinicians as they formulate subsequent treatment plans. Our findings suggest that future research is required to describe the mechanisms leading to altered DNA methylation including environment factors such as AA. Future research also have to describe the relationship between DNA methylation and tumor malignancy, progression and prognosis.
Table 1. Primer sequences for methylation-sensitive PCR.
Table 2. Patients characteristics and methylation status.
Table 3. Predictive effect of epigenetic biomarkers in upper tract urothelial carcinomas for high tumor stage (T3 and T4), grade 3 and pN+ using univariable and multivariable logistic regression.
Table 4. Prognostic factors for cancer-specific survival and bladder recurrence after surgery.
Prognostic epigenetic biomarkers and clinical factors in upper tract urothelial carcinoma (UTUC) are inconclusive.
We conducted this study to identify gene promoter (ABCC6, BRCA1, CDH1, GDF15, HSPA2, RASSF1A, SALL3, THBS1, TMEFF2 and VIM) methylation status and clinical predictors for UTUC patients (n = 687).
The log–rank test and Cox regression were applied, and two nomograms were utilized to construct the prognostic model for cancer-specific survival (CSS) and bladder recurrence-free survival, respectively.
Methylation in tumor tissue was detected in 14.3% for ABCC6, 17.3% for BRCA1, 14.3% for CDH1, 49.9% for GDF15, 41.2% for HSPA2, 26.6% for RASSF1A, 34.4% for SALL3, 25.2% for THBS1, 43.2% for TMEFF2 and 63.2% for VIM.
A methylated promoter of CDH1 (p = 0.045), HSPA2 (p < 0.001) and RASSF1A (p = 0.037) was significantly associated with a higher tumor stage (T3 and T4). A methylated promoter of BRCA1 (p = 0.013), HSPA2 (p = 0.030), RASSF1A (p = 0.031) and THBS1 (p = 0.001), and an unmethylated promoter of GDF15 (p < 0.001) were significantly associated with tumor grade 3. A methylated promoter of RASSF1A (p = 0.005) was associated with pN+.
Older age (p < 0.001), male sex (p = 0.033), tumor multifocality (p = 0.008), ipsilateral hydronephrosis (p < 0.001), larger main tumor diameter (>5 cm, p < 0.001), higher tumor stage (p < 0.001), positive N status (p = 0.018), methylated TMEFF2 promoter (p = 0.002) and unmethylated BRCA1 promoter (p = 0.004) were significantly associated with poor CSS.
Tumor multifocality (p = 0.002), ureteroscopy history (p = 0.001), lower tumor grade (p = 0.046), unmethylated promoter of GDF15 (p = 0.030) and RASSF1A (p = 0.001) were considered as predictors to develop bladder recurrence after surgery.
Methylation occurs commonly in UTUCs, may affect carcinogenic mechanisms, and is a well predictive factor for CSS and bladder recurrence-free survival in UTUCs.
Ethical conduct
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Acknowledgements
We thank L Zhang, Q Liu, Y Cui and J Gai for their excellent experimental techniques.
Financial & competing interests disclosure
The authors gratefully acknowledge financial support from the Collaborative Research Foundation of Peking University Health Science Center and National Taiwan University, College of Medicine (BMU20120318), Natural Science Foundation of Beijing (7122183, 7152146), Natural Science Foundation of China (81172419, 81372746) and The Clinical Features Research of Capital (No. Z151100004015173). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Rouprêt M1 , BabjukM , CompératEet al. European guidelines on upper tract urothelial carcinomas: 2013 update . Eur. Urol.63 ( 6 ), 1059 – 1071 ( 2013 ).
- Siegel R , MaJ , ZouZ , JemalA . Cancer statistics, 2014 . CA Cancer J. Clin.64 ( 1 ), 9 – 29 ( 2014 ).
- Catto JW , AzzouziAR , RehmanIet al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma . J. Clin. Oncol.23 ( 13 ), 2903 – 2910 ( 2005 ).
- Yu J , ZhuT , WangZet al. A novel set of DNA methylation markers in urine sediments for sensitive/specific detection of bladder cancer . Clin. Cancer Res.13 ( 24 ), 7296 – 7304 ( 2007 ).
- Maruyama R , ToyookaS , ToyookaKOet al. Aberrant promoter methylation profile of bladder cancer and its relationship to clinicopathological features . Cancer Res.61 ( 24 ), 8659 – 8663 ( 2001 ).
- Costa VL1 , HenriqueR , DanielsenSAet al. Three epigenetic biomarkers, GDF15, TMEFF2, and VIM, accurately predict bladder cancer from DNA-based analyses of urine samples . Clin. Cancer Res.16 ( 23 ), 5842 – 5851 ( 2010 ).
- Lee MG , KimHY , ByunDSet al. Frequent epigenetic inactivation of RASSF1A in human bladder carcinoma . Cancer Res.61 ( 18 ), 6688 – 6692 ( 2001 ).
- Casadio V1 , MolinariC , CalistriDet al. DNA Methylation profiles as predictors of recurrence in non-muscle invasive bladder cancer: an MS-MLPA approach . J. Exp. Clin. Cancer Res.32 , 94 ( 2013 ).
- Fang D , XiongG , LiXet al. Incidence, characteristics, treatment strategies, and oncologic outcomes of synchronous bilateral upper tract urothelial carcinoma in the Chinese population . Urol. Oncol.33 ( 2 ), 66.e1 – 66.e11 ( 2015 ).
- TNM Classification Of Malignant Tumours. Urological tumours, renal pelvis and ureter. Sobin LH , GospodarowiczMK , WittekindC ( Eds ). Wiley-Blackwell, NY USA , 258 – 261 ( 2009 ).
- Mostofi FK , SobinLH , TorloniH . Histological typing of urinary bladder tumours . WHO ( 1973 ). http://whqlibdoc.who.int .
- Herman JG , GraffJR , MyöhänenS , NelkinBD , BaylinSB . Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands . Proc. Natl Acad. Sci. USA93 ( 18 ), 9821 – 9826 ( 1996 ).
- Kang GH , ShimYH , JungHY , KimWH , RoJY , RhyuMG . CpG island methylation in premalignant stages of gastric carcinoma . Cancer Res.61 ( 7 ), 2847 – 2851 ( 2001 ).
- Frank E Harrell Jr . Rms Package. R package version 4.2-1 . http://cran.r-project.org
- Monteiro-Reis S , LeçaL , AlmeidaMet al. Accurate detection of upper tract urothelial carcinoma in tissue and urine by means of quantitative GDF15, TMEFF2 and VIM promoter methylation . Eur. J. Cancer50 ( 1 ), 226 – 233 ( 2014 ).
- Hsu NC , HuangYF , YokoyamaKK , ChuPY , ChenFM , HouMF . Methylation of BRCA1 promoter region is associated with unfavorable prognosis in women with early-stage breast cancer . PLoS ONE8 ( 2 ), e56256 ( 2013 ).
- Chen XP , XiongGY , LiXSet al. Predictive factors for worse pathological outcomes of upper tract urothelial carcinoma: experience from a nationwide high-volume centre in China . BJU Int.112 ( 7 ), 917 – 924 ( 2013 ).
- Chou YH , HuangCH . Unusual clinical presentation of upper urothelial carcinoma in Taiwan . Cancer85 , 1342 – 1344 ( 1999 ).
- Yang MH , ChenKK , YenCCet al. Unusually high incidence of upper tract urothelial carcinoma in Taiwan . Urology59 , 681 – 687 ( 2002 ).
- Lughezzani G , JeldresC , IsbarnHet al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: a population-based study of 2299 patients . Eur. J. Cancer45 , 3291 – 3297 ( 2009 ).
- Rouprêt M , TraxerO , TliguiMet al. Upper urinary tract urothelial carcinoma: recurrence rate after percutaneous endoscopic resection . Eur. Urol.51 , 709 ( 2007 ).
- Fernández MI , ShariatSF , MargulisVet al. Evidence-based sex related outcomes after radical nephroureterectomy for upper tract urothelial carcinoma: results of large multicenter study . Urology73 , 142 – 146 ( 2009 ).
- Munoz JJ , EllisonLM . Upper tract urothelial neoplasms: incidence and survival during the last 2 decades . J. Urol.164 , 1525 – 1532 ( 2000 ).
- Chen CH , DickmanKG , HuangCYet al. Aristolochic acid-induced upper tract urothelial carcinoma in Taiwan: clinical characteristics and outcomes . Int. J. Cancer133 ( 1 ), 14 – 20 ( 2013 ).
- Nortier JL , MartinezMC , SchmeiserHHet al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) . N. Engl. J. Med.342 ( 23 ), 1686 – 1692 ( 2000 ).
- Cukuranovic R , IgnjatovicI , VisnjicMet al. Characteristics of upper urothelial carcinoma in an area of Balkan endemic nephropathy in South Serbia. A fifty-year retrospective study . Tumori96 , 674 – 679 ( 2010 ).
- Paul A , PaulS . The breast cancer susceptibility genes (BRCA) in breast and ovarian cancers . Front. Biosci. (Landmark Ed).19 , 605 – 618 ( 2014 ).
- Byrski T , DentR , BlecharzPet al. Results of a Phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer . Breast Cancer Res.14 ( 4 ), R110 ( 2012 ).
- Luo HL , KangCH , ChenYTet al. Diagnostic ureteroscopy independently correlates with intravesical recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma . Ann. Surg. Oncol.20 ( 9 ), 3121 – 3126 ( 2013 ).
- Hafner C , KnuechelR , ZanardoLet al. Evidence for oligoclonality and tumor spread by intraluminal seeding in multifocal urothelial carcinomas of the upper and lower urinary tract . Oncogene20 , 4910 – 4915 ( 2001 ).
- Matsui Y , UtsunomiyaN , IchiokaKet al. Risk factors for subsequent development of bladder cancer after primary transitional cell carcinoma of the upper urinary tract . Urology65 ( 2 ), 279 – 283 ( 2005 ).
- Terakawa T , MiyakeH , MuramakiM , TakenakaA , HaraI , FujisawaM . Risk factors for intravesical recurrence after surgical management of transitional cell carcinoma of the upper urinary tract . Urology71 ( 1 ), 123 – 127 ( 2008 ).
- Hisataki T , MiyaoN , MasumoriNet al. Risk factors for the development of bladder cancer after upper tract urothelial cancer . Urology55 ( 5 ), 663 – 667 ( 2000 ).
- Eling TE , BaekSJ , ShimM , LeeCH . NSAID activated gene (NAG-1), a modulator of tumorigenesis . J. Biochem. Mol. Biol.39 ( 6 ), 649 – 655 ( 2006 ).
- Mimeault M , BatraSK . Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer . J. Cell. Physiol.3 , 626 – 635 ( 2010 ).
- Fang D , XiongGY , LiXSet al. Pattern and risk factors of intravesical recurrence after nephroureterectomy for upper tract urothelial carcinoma: a large Chinese center experience . J. Formos. Med. Assoc.113 ( 11 ), 820 – 827 ( 2014 ).
