Abstract
How DNA methylation is interpreted and influences genome regulation remains largely unknown. Proteins of the methyl-CpG-binding domain (MBD) family are primary candidates for the readout of DNA methylation as they recruit chromatin remodelers, histone deacetylases and methylases to methylated DNA associated with gene repression. MBD protein binding requires both functional MBD domains and methyl-CpGs; however, some MBD proteins also bind unmethylated DNA and active regulatory regions via alternative regulatory domains or interaction with the nucleosome remodeling deacetylase (NuRD/Mi-2) complex members. Mutations within MBD domains occur in many diseases, including neurological disorders and cancers, leading to loss of MBD binding specificity to methylated sites and gene deregulation. Here, we summarize the current state of knowledge about MBD proteins and their role as readers of the epigenome.
MBD family proteins function through the combined activity of their DNA binding domains and other catalytic or protein–protein association domains. All MBD family proteins contain the MBD domain. MeCP2, MBD1 and MBD2 have TRD domains that are responsible for transcriptional repression. The predominant isoform of MeCP2 has 486 residues (52 kDa). MBD1 contains 605 residues (66 kDa) and has CxxC domains that modulate DNA binding. MBD2 contains 411 residues (44 kDa) and a CC- and G/R-rich domain that allows protein interaction and posttranslational modification, respectively. MBD3 contains 291 residues (32 kDa) as well as a CC domain similar to MBD2. MBD4 contains 580 residues (66 kDa) and a catalytic glycosylase domain for DNA repair. MBD5 contains 1494 residues (159 kDa) with a PWWP domain that binds methylated histones. MBD6 contains 1003 residues (101 kDa). MBD5 and MBD6 both have P-rich domains. SETDB1 contains 1291 residues (143 kDa) and SETDB2 contains 719 residues (81 kDa). The SET domain provides methyltransferase activity to SETDB1 and 2, while the Tudor domains allow SETDB1 to bind methylated histones. TIP5, also known as BAZ2A, contains 1905 residues (211 kDa) and BAZ2B contains 2168 residues (240 kDa). They both contain bromo and DDT domains that allow acetylated histone and DNA binding capabilities. MeCP2 and MBD1–4 are sized relative to each other.
aa: Amino acid(s).
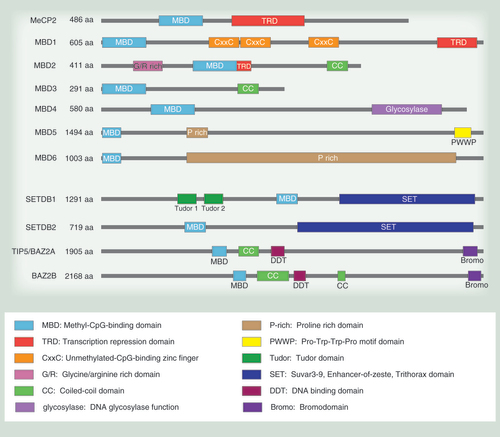
Key roles of the MBD family proteins in epigenetic remodeling and transcriptional repression. (A) MBD1 is involved in directing histone methylation to sites of DNA methylation. (B) MeCP2 has roles in heterochromatin clustering through histone methylation and nucleosome remodeling. (C) MeCP2 is also able to coordinate histone methylation and histone deacetylation at the same methylated loci via cofactor interactions such as to CoREST. (D) MBD2 directs transcriptional repression through the NuRD/Mi-2 complex binding partner that enables histone deacetylation and nucleosome remodeling. (E) MBD3 also interacts with the NuRD/Mi-2 complex and couples this with DNA methylation. MBD3′s DNA interaction at target promoters is mediated through transcription factor recruitment. (F) MBD4 maintains correct DNA methylation by repairing spontaneous 5-mC→T transitions to C and interacting with DNMTs to guide remethylation. (G) MeCP2 is able to silence select genes by creating chromatin loops via the aid of chromatin remodeler ATRX and CTCF-Cohesin. (H) MBD1 is associated with the fork via CAF1 and ensures correct histone methylation associated with DNA methylation. Red arrows indicate activity of enzymes involved. Dashed elbow arrows indicate repression or reduced expression upon MBD occupancy.
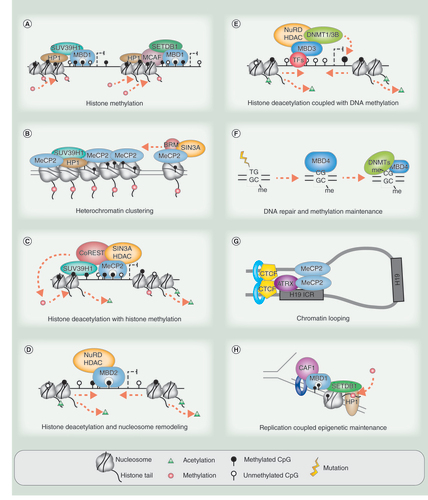
MBD proteins have a role in stem cell renewal, embryogenesis and differentiation. (A) MBD2t(/c) and MBD3 have higher expression levels in the embryo/ESC, while MBD2 has higher expression in somatic/differentiated cells. Lower levels of MBD3 persist in somatic cells; however, MBD2t expression is restricted to ESCs. (B) In ESCs or the embryonic stage, MBD2a competes for binding with MBD2t and may be prevented from repressing genes required for pluripotency. MBD3 can be ‘poised’ on active enhancers or promoters and may modulate expression and bivalency, but does not fully silence due to presence of active pluripotency transcription factors. (C) During differentiation, MBD2-NuRD/Mi-2 becomes more readily available and enacts silencing. Downregulation of pluripotency transcription factors during differentiation allow ‘poised’ MBD3 and protein partners to silence pluripotency genes and enhancers.
(A) Frequency of MBD mutations across different cancer tissue types, showing the number of donors for each type in parentheses. The results shown here are based upon data generated by the TCGA Research Network: [Citation188]. The mutational frequency of each individual MBD protein is shown in the stack graph. (B) Plots showing the location of individual nonsynonymous mutations across each MBD protein from TCGA visualized in cBioPortal for Cancer Genomics [Citation189]. Green: missense mutation; red: truncating mutation (nonsense, nonstop, frameshift deletion, frameshift insertion, splice site); purple: residues that are affected by different mutation types at the same proportion. The cBioPortal links as follows: MBD1: [Citation190]; MBD2: [Citation191]; MBD3: [Citation192], MBD4: [Citation193]; MeCP2: [Citation194]. Protein domain information is depicted and legend is included in the figure.
aa: Amino acid(s).
![Figure 4. Mutations in the methyl-binding domain family identified in cancer. (A) Frequency of MBD mutations across different cancer tissue types, showing the number of donors for each type in parentheses. The results shown here are based upon data generated by the TCGA Research Network: [Citation188]. The mutational frequency of each individual MBD protein is shown in the stack graph. (B) Plots showing the location of individual nonsynonymous mutations across each MBD protein from TCGA visualized in cBioPortal for Cancer Genomics [Citation189]. Green: missense mutation; red: truncating mutation (nonsense, nonstop, frameshift deletion, frameshift insertion, splice site); purple: residues that are affected by different mutation types at the same proportion. The cBioPortal links as follows: MBD1: [Citation190]; MBD2: [Citation191]; MBD3: [Citation192], MBD4: [Citation193]; MeCP2: [Citation194]. Protein domain information is depicted and legend is included in the figure.aa: Amino acid(s).](/cms/asset/7ced7046-42c7-484e-9ed0-7fe7fac28482/iepi_a_12325027_f0004.jpg)
The genome is epigenetically regulated at different levels of complexity, from structural compartmentalization of active and inactive chromatin, through to histone modifications, nucleosome positioning and DNA methylation of CpG dinucleotides. Epigenetic-based mechanisms do not function in isolation, but comprise extensive crosstalk and feedback regulatory loops that reinforce a cell-type-specific transcriptional program. DNA methylation is a fundamental epigenetic modification in mammalian cells and occurs at the carbon-5 position of cytosine residues at CpG nucleotides through the action of DNA methyltransferase (DNMT) enzymes, forming 5-methylcytosine (5-mC) [Citation1]. There are approximately 28 million CpG dinucleotides in the mammalian genome, though they are not evenly distributed. Typically, the genome is depleted of CpG dinucleotides except for regions with the expected frequency of CpG sites termed ‘CpG islands’ [Citation2,Citation3]. CpG islands span the promoters of house-keeping genes and commonly are unmethylated and associated with active gene expression; in contrast, methylated CpG island promoters and enhancers are associated with gene repression [Citation4–6]. The DNA methylation landscape is altered during the initiation and progression of cancer with global hypomethylation of CpG poor intergenic regions and hypermethylation of CpG islands associated with gene silencing and reduced plasticity. Through advances in genome-wide methylation sequencing technology, we can now profile the distribution of CpG methylation in normal and cancer cells; however, we still do not understand how DNA methylation patterns are interpreted and how DNA methylation plays a role in influencing genome and epigenome plasticity [Citation2].
There are several groups of proteins that can modulate and interpret DNA methylation patterns, including DNA methylation ‘writers,’ ‘readers’ and ‘editors’. DNA methylation ‘writers’ are key to establishing and maintaining CpG methylation patterns throughout development and differentiation. These proteins are members of the DNMT family: DNMT1, DNMT3A and DNMT3B [Citation1,Citation7]. DNA methylation ‘readers’ specifically bind methylated CpG dinucleotides and act as translators between DNA methylation and histone modifications to facilitate a multilayered regulatory program [Citation8,Citation9]. These include the family of methyl-CpG-binding domain (MBD) proteins, the Kaiso family proteins and the SET- and Ring finger-associated (SRA) domain family [Citation10–13]. DNA methylation ‘editors’ are the newest group to be described and comprise the ten–eleven translocation (TET) protein family, which oxidize the carbon-5 methyl group in 5-mC into a hydroxyl residue, to form 5-hydroxymethylcytosine (5-hmC) (reviewed in [Citation14]). Through a number of pathways, 5-hmC is proposed to convert to unmethylated cytosine and ultimately promote demethylation. All three groups of epigenetic modifiers play an important role in the regulation and interpretation of DNA methylation, chromatin-remodeling processes and gene expression.
Here, we review the ’readers’, the MBD protein family members, and their role in reading and regulating the epigenome and in particular, the significance of MBD-associated mutations in the etiology of disease states such as cancer.
What are MBD proteins?
The MBD family proteins are critical players in determining the transcriptional state of the epigenome. As transcriptional repressors, MBD proteins play a major role in coordinating crosstalk between DNA methylation, histone modifications and chromatin organization to achieve a coherent transcriptional program. Currently, the MBD protein family consists of eleven known proteins that contain a MBD domain (summarized in ). The methyl-CpG-binding protein 2 (MeCP2) was the first MBD-containing protein discovered, and subsequently MBDs 1–6 were identified through sequence homology to the MeCP2 MBD domain [Citation13,Citation15]. Four further proteins have also now been described to harbor a MBD domain (SETDB1, SEDTB2, BAZ2A and BAZ2B) [Citation16]. The 70–85-amino acid (aa) MBD domain has the ability to bind single symmetrically methylated CpG dinucleotides [Citation17,Citation18]. In addition to the MBD domain, MBD family members contain several differing domains that reflect their respective roles. Most family members contain a transcriptional repression domain (TRD) that mediates interactions with protein partners [Citation19–21]. Other family members possess unique domains such a glycosylase domain (MBD4) or unmethylated-CpG-binding zinc finger (CxxC) domain (MBD1). MBD family proteins can therefore overlap in function and work coherently to organize the epigenome, while retaining differences in DNA binding specificity and unique roles. This review will primarily focus on the well-studied ‘core’ MBD family proteins, MeCP2 and MBD1–4.
MBD domain proteins
MeCP2
MeCP2, the first MBD-containing protein to be discovered, contains the core 70-amino acid MBD and is also characterized by the presence of a TRD () [Citation22]. MeCP2 has histone deacetylation and histone methylation binding partners (); it is also considered essential for higher order/long-range chromatin remodeling and silencing with roles in heterochromatin formation and chromatin organization [Citation23–27]. Additionally, MeCP2 is reported to mediate the translation of intragenic methylation into alternative splicing [Citation28]. These functions are thought to be crucial for neural development as MeCP2 mutations are responsible for Rett syndrome, a severe neurodevelopmental disorder that specifically affects females [Citation29,Citation30]. Individuals affected by Rett syndrome typically have point mutations within the MBD domain or the TRD domain, therefore affecting the ability of MeCP2 to interact with crucial binding partners or bind to DNA. MeCP2 expression in the brain follows neuronal maturation and is most highly expressed in mature neurons, highlighting the importance of MeCP2 deregulation in neurological diseases () [Citation31].
MBD1
MBD1 is the largest member of the MBD family. MBD1 contains an N-terminal MBD domain and a C-terminal TRD domain, and two or three internal CxxC zinc finger domains that are present in MBD1 due to alternative splicing () [Citation116]. The MBD1 isoforms that contain only the first two CxxC domains preferentially bind methylated DNA, while the presence of the third CxxC domain provides MBD1 with the additional ability to bind unmethylated DNA [Citation60,Citation116]. The use of both MBD and CxxC domains may determine MBD1 binding specificity and target MBD1 to regions of both methylated and unmethylated DNA as appropriate. The main role of MBD1 is in transcriptional repression by directing histone methylation and maintaining heterochromatin () [Citation44]. Studies in MBD1 knockout (KO) mice show that MBD1 is not embryonically lethal but does appear to affect neurogenesis and neural stem cell differentiation [Citation117]. This is in line with the strictly somatic expression pattern of MBD1; however, there is no specific enrichment of MBD1 in the brain [Citation13,Citation118].
MBD2
While other MBD proteins maintain separate MBD and TRD domains, the two domains overlap in the center of the MBD2 protein sequence, suggesting tight integration of methylation binding and transcriptional repression functions of MBD2 () [Citation19]. MBD2 also contains a C-terminal coiled coil (CC) domain that mediates protein–protein interactions and an N-terminal glycine-arginine (GR) repeat region that is subject to posttranslational modifications () [Citation119,Citation120]. MBD2 has several protein isoforms; MBD2b utilizes an alternate translational start site at the N-terminal end of the MBD domain, while MBD2t (or MBD2c) loses the C-terminal TRD and CC domains due to the inclusion of an alternative 3rd exon, leading to an early stop codon [Citation13]. The canonical role of MBD2 is as a transcriptional repressor through interactions with binding partners such as the histone deacetylase complexes NuRD/Mi-2 and Sin3A () [Citation19,Citation121]. Mouse KO studies show that MBD2 is not embryonically lethal but does potentially affect maternal nurturing behavior, suggesting neurological effects similar to MBD1 and MeCP2 [Citation122]. MBD2 is mostly expressed in somatic tissue with very low expression in embryonic cells; therefore, loss of expression does not critically affect embryonic development [Citation13,Citation118].
MBD3
MBD3 is the smallest member of the MBD family. Of all the family members, MBD2 and MBD3 show the highest amino acid sequence similarity (71.1%) [Citation13]. The major difference between the two is that MBD3 lacks a N-terminal GR repeat region observed in the full-length MBD2 (MBD2a) (). Similarly to MBD2b, MBD3 is coded from the MBD domain and includes the C-terminal CC domain. Three splice isoforms of MBD3 have been reported: MBD3a, MBD3b and MBD3c [Citation13,Citation64]. MBD3 is the only family member that does not bind specifically to methylated DNA but can bind to unmethylated DNA and has been reported to bind to 5-hydroxymethylated DNA [Citation73,Citation123]. Binding to unmethylated DNA is due to differences at two key amino acid residues in the MBD domain, which have changed from LYS30 and TYR34 in MBD2 to HIS30 and PHE34 in MBD3 [Citation123]. The role of MBD3 in transcriptional repression is intimately related to its interaction with the NuRD/Mi-2 complex; moreover, MBD3 is considered essential for NuRD/Mi-2 formation and stability [Citation20,Citation64,Citation123]. MBD3-NuRD/Mi-2 plays a key role in embryonic stem cell (ESC) pluripotency and differentiation, and MBD3 KO mice are embryonically lethal [Citation64,Citation122]. Accordingly, MBD3 is expressed in ESCs as well as somatic tissue [Citation13,Citation118].
MBD4
Alongside an N-terminal MBD domain, MBD4 also contains a C-terminal glycosylase domain that is able to repair mismatches in symmetrically methylated CpG (mC→T transition) and unmethylated CpG dinucleotides (C→U transition) () [Citation76,Citation124–125]. While MBD4 can bind to methylated DNA, its more prominent role seems to be in DNA repair as opposed to transcriptional repression (). MBD4 is expressed in somatic tissues and ESC [Citation13,Citation118]. Interestingly, MBD4 KO mice are not embryonically lethal and do not appear to have developmental or adult phenotypes [Citation126]. However, they show an increase of mCpG to TpG transitions in the spleen and intestinal epithelium; these mutations only have a functional consequence when the mice also carry a germline mutation in the Apc tumor suppressor gene, resulting in increased tumor formation [Citation126].
MBD5 & MBD6
MBD5 and MBD6 are the most recently discovered and poorly characterized members of the MBD protein family [Citation118]. They contain an N-terminal MBD domain and localize to pericentric heterochromatin; however, MBD5 and MBD6 have not been found to bind methylated DNA in vitro [Citation127]. Both proteins contain proline-rich domains, while MBD5 contains an additional Pro-Try-Try-Pro (PWWP) motif domain that is known to direct protein binding of methylated histones () (reviewed in [Citation128]). Both MBD5 and MBD6 are highly expressed in the testes, while MBD5 is also highly expressed in the brain and oocytes, suggesting developmental functions for further research [Citation127]. Both MBD5 and MBD6 interact directly with the human polycomb deubiquitinase complex, PR-DUB, which catalyzes deubiquitination of H2AK119 [Citation15]. Notably, MBD6 is reported to be an OCT4 target gene in adipose tissue stem cells and is recruited to sites of laser-induced DNA damage [Citation15,Citation129]. Further comprehensive characterization of MBD5 and MBD6 will give more comprehensive insight into their functional roles.
SETDB & BAZ2
SETDB1/2 and BAZ2A/B are the only MBD domain containing proteins known to directly modify proteins/histones without relying on protein partners. SETDB1 and SETDB2 contain the SET domain that confers histone H3K9 lysine methyltransferase activity, while BAZ2A and BAZ2B contain bromodomains that confer acetylated histone binding capacity () [Citation130–132]. The MBD domains in these two groups of proteins are phylogenetically distinct from those of the core MBD family proteins [Citation16]. SETDB1 localizes to 5-mC through binding partner MBD1 and is involved in heterochromatin formation and transcriptional repression [Citation46,Citation133]. Interestingly, the MBD domain in BAZ2A (also known as TIP5) binds unmethylated, but not methylated DNA in vitro [Citation134]. BAZ2A is a component of the nucleolar remodelling complex NoRC, essential for epigenetically silencing ribosomal DNA (rDNA) by coordinating H3K9 dimethylation, H4 deacetylation and DNA methylation to create silent heterochromatin [Citation135]. While BAZ2B and SETDB2 are less studied, they are likely to have similar functions to BAZ2A and SETDB1 due to similarities in their functional domains.
Binding specificity & genomic distribution of MBD proteins
With the exception of MBD3, the ‘core’ MBD family proteins preferentially bind 5-mC in orders of magnitude over unmethylated cytosine and in some cases, 5-hmC () [Citation136–138]. In vitro studies show that 5-mC preference is affected by CpG density with increased binding at regions of higher CpG density such as CpG islands [Citation136,Citation137]. Interestingly, these studies show that MBD3 also prefers loci of high CpG density, irrespective of methylation status, although it is outcompeted by MBD2 for methylated and CpG dense DNA [Citation136]. Despite some discordance between studies, MBD2 and MeCP2 show the strongest preference for 5-mC over C [Citation60,Citation136–137].
As well as recognizing 5-mC and unmethylated DNA, 5-hmC has been suggested as an additional target for MBD3 and MeCP2; it has been reported that they can occupy 5-hmC-marked DNA in embryonic stem cells and brain cells, respectively [Citation40,Citation73]. However, most in vitro studies find that 5-hmC binding by any member of the MBD family is either completely absent or is several magnitudes less than 5-mC [Citation136,Citation138–140]. A recent study suggests that binding of 5-hmC by MeCP2 may actually be dependent on the dinucleotide context where MeCP2 is able to bind hmCA but not hmCG [Citation141]. Interpretation of in vivo experimental data has been complicated by technical difficulties in distinguishing 5-mC from 5-hmC and therefore, the true binding affinities of MBD1, MBD2, MBD4 and MeCP2 in vivo are unclear. As 5-hmC presence seems to be very tissue type specific, and as more sophisticated detection methods are currently in development, future studies will elucidate the relationship between the MBD proteins and the binding specificity of 5-hmC [Citation142].
The detailed genomic distribution of the MBD proteins is currently being explored with the aid of improved antibody production, protein tagging approaches and genome-wide sequencing techniques. In vivo studies have confirmed that methylation density is a key determinant of genome-wide MBD proteins recruitment, and MBD proteins, in general, are more frequently observed at genomic regions with higher than average DNA methylation density such as at CpG-rich promoters and exons, and less frequently at repetitive DNA sequences despite hypermethylation often observed at these regions [Citation60]. Specifically, chromatin immunoprecipitation experiments (ChIP-chip and ChIP-seq) demonstrated that MBD2 binds primarily at highly methylated CpG island-containing promoters of inactive genes and that MeCP2 binding mirrors methylation density genome wide [Citation60–61,Citation143–145].
As noted above, some MBD proteins have the biochemical capacity to bind to unmethylated DNA, and genome-wide ChIP-seq data suggest MBD proteins have the ability to bind a subset of unmethylated promoters, enhancers and gene bodies, as well as bivalent genes [Citation60–61,Citation68,Citation71,Citation144]. These observations primarily represent MBD3 binding patterns and only a subset of the binding patterns of other MBD proteins such as MBD2. The presence of MBD2 at these genomic regions appears to be influenced by MBD3 occupancy of the same sites, suggesting potential coordination [Citation60,Citation61]. These sites appear to be associated with a range of activating histone marks. For example, MBD3 shows enrichment at unmethylated active promoters marked by histone mark H3K4me3, as well as enhancer sites enriched in H3K27ac. Furthermore, MBD2 binding associated with transcriptional activation is often in methylated exons/gene bodies of active genes and is potentially related to modulating RNA Pol II pausing [Citation144]. Despite these exceptions, the currently accepted dogma is that 5-mC is the primary determinant for the genome-wide binding patterns of the MBD proteins containing a functional MBD domain and enrichment of binding is correlated with local methylation density.
MBD proteins have also been reported to bind to RNA. MBD2 and MeCP2 can form RNA–protein complexes (RNPs) in vitro and the MBD domain in BAZ2A/TIP5 is responsible for binding to noncoding RNA (ncRNA) that directs the BAZ2A-NoRC complex to rDNA loci for heterochromatin formation [Citation146,Citation147]. While it is unknown whether MBD2 and MeCP2 are also directed to chromatin by ncRNA, MeCP2′s RNA binding capacity may be related to its role in alternative splicing [Citation28]. Future studies will reveal the full extent of the functional relationship between the MBD proteins and RNA in epigenetic regulation.
Roles of MBD family proteins: facilitators of epigenetic-based repression
As readers, MBD proteins bind to methylated DNA and potentially guide or direct protein complexes with chromatin remodeling and/or histone modifying activity to specific locations in the genome (summarized in ). These protein complexes generally induce repressive changes to local chromatin such as catalyzing repressive histone marks, or creating an overall repressive chromatin environment through nucleosome remodeling and chromatin organization. A summary of the roles of MBD proteins and their functional protein partners is presented in .
Heterochromatin formation
Heterochromatin is an inaccessible chromatin state involved with transcriptional repression and chromatin stability that is characterized by compacted chromatin enriched in epigenetic marks such as H3K9 methylation and DNA methylation. It is typically bound by linker-histone HP1 that binds the linker DNA between nucleosomes to form the compacted chromatin fiber [Citation148]. Early immunofluorescence (IF) studies found that MBD proteins localized to methylated loci and constitutively silent pericentric heterochromatin (around the centromere), indicative of a role in transcriptional silencing [Citation13,Citation21]. Overexpression of MBD proteins, other than MBD3, have been found to induce heterochromatin clustering, while knockdown (KD) of MBD proteins results in loss of heterochromatin coupled with increased genomic instability [Citation46,Citation61,Citation117,Citation149–150]. MeCP2 and MBD1 are the two main MBD proteins involved in generating heterochromatic silencing through interactions with repressive histone methylation and chromatin remodeling binding partner HP1 ( & & B).
Histone modifications
Covalent modifications of key amino acid residues in histone tails affect chromatin function by affecting the structural dynamics of the DNA and binding of proteins that recognize these modifications. Histone methylation can be either active or repressive depending on the residue of interest. Histone acetylation typically translates into active transcription and open chromatin, while deacetylation translates into repression. DNA methylation, histone deacetylation and histone methylation are intimately linked in their control of gene expression [Citation151]. MBD proteins are among those that link DNA methylation to histone modification. MBD1 is well known for providing a connection between DNA methylation and histone methylation () [Citation46,Citation152]. MBD1 was initially identified as a transcriptional repressor through histone deacetylation, a role that was later attributed to its interactions with heterochromatin complex histone methyltransferase SUV39H1 and HP1 [Citation21,Citation44]. MBD1 also engages another histone methyltransferases SETDB1 and the binding partner MCAF1 (also known as ATF7IP) for heterochromatin maintenance and X chromosome inactivation [Citation46–47,Citation153]. SUV39H1-HP1 is also able to interact with the MeCP2 member of the MBD family to induce heterochromatin clustering at sites of MeCP2 occupancy, suggesting that MeCP2 may drive this process () [Citation26,Citation154]. MeCP2 binding can result in both histone methylation and/or deacetylation by interacting with the histone modifying complexes themselves or by modulating its binding partners, such as CoREST and N-CoR () [Citation23–24,Citation32,Citation35–36]. The CoREST-MeCP2 interaction is reported to promote silencing of neuronal genes by bringing together histone deacetylase protein complex Sin3A and SUV39H1, which are involved in deacetylation and methylation of histones at target genes, respectively () [Citation35]. The N-CoR-MeCP2 interaction directs HDAC3 to sites where MeCP2 binds, providing another avenue for MeCP2-directed histone deacetylation [Citation33]. Interestingly, posttranslational phosphorylation of MeCP2 can disrupt MeCP2 protein–protein interactions and alter MeCP2 occupancy and silencing, and similar defects are observed in mouse models of Rett syndrome, where mutations affecting the same phosphorylation sites cause H3 hyperacetylation in neurons due to loss of MeCP2-N-CoR interaction [Citation33,Citation155]. These data highlight key roles for MeCP2 in mediating epigenetic outcomes, particularly in the brain.
Interactions with histone deacetylation complexes are not limited to MeCP2. For example, NuRD/Mi-2 and/or Sin3A-HDAC complexes are capable of interacting with MBD2, MBD3 and MBD4 ( & & E) [Citation75,Citation121,Citation123]. Of these, MBD2-NuRD/Mi-2 was the first HDAC complex shown to bind specifically to methylated DNA [Citation121,Citation156]. Studies have since identified MBD2 as the key family member for coordinating silencing at methylated CpG island promoters in association with the NuRD/Mi-2 complex [Citation50,Citation157–158].
MBD2 has been implicated in histone arginine methylation. The protein arginine methyltransferase (PRMT) family catalyses methylation on the histone tail arginines of H3, H4 and H2A, as well as nonhistone proteins at glycine-arginine-rich motifs [Citation56,Citation159–160]. MBD2 interacts with two members of this family, PRMT1 and PRMT5, and MBD2 itself can be modified in the arginine-rich GR repeat region ( & ) [Citation50,Citation56]. MBD2 interactions with PRMT1 and/or PRMT5 can result in either activation or silencing depending on which one is present; PRMT1 creates activating asymmetrical dimethylation (H4R3me2a), whereas PRMT5 creates silencing symmetrical dimethylation (H4R3me2s) [Citation161,Citation162]. Furthermore, PRMT1/5 methylation of the MBD2 GR repeat region inhibits MBD2–HDAC interactions as well as methylated DNA binding ability, thereby resulting in activation [Citation56]. Overall, cooperation between PRMT1/5 and MBD2 may modulate MBD2 activity in transcriptional control [Citation56,Citation159].
Nucleosome remodeling
Nucleosome positioning can expose or hide regions of DNA and therefore determines accessibility of regulatory proteins that interact with DNA [Citation163]. NuRD/Mi-2 is one of the chromatin remodeling complexes with the ability to remodel nucleosomes, specifically enabled by the Mi-2 subunits (ATPase-dependent components of SWI/SNF complex) [Citation20]. This activity is critical to MBD2-mediated silencing mechanisms as it has been shown that by disrupting the interaction between MBD2 and the Mi-2 subunits, it is possible to increase chromatin accessibility and prevent the efficient silencing of the mb-1 gene in B cells [Citation51]. The links between nucleosome repositioning and MBD proteins are not limited to MBD2-NuRD/Mi-2. The MeCP2-Sin3A/HDAC2 interaction with BRM, another SWI/SNF family member, provides further evidence that gene silencing by MBD family members involves nucleosome remodeling and histone deacetylation activity () [Citation37].
DNA methylation
Interestingly, MBD3 can coordinate histone deacetylation and de novo DNA methylation in the context of the MBD3-NuRD/Mi-2 complex, by recruiting DNMT1 and DNMT3B to promoters of tumor-suppressor genes in colon cancer cells () [Citation74]. For example, the proto-oncogene transcription factor FBI-1 has been reported to silence the p21WAF/CDKN1A promoter by recruiting MBD3-NuRD/Mi-2, resulting in coordinated histone deacetylation and DNA methylation [Citation66]. Similarly, it has been observed in leukemia cell lines that the PML-RARα fusion protein recruits MBD3-NuRD/Mi-2 to target gene promoters, resulting in deacetylation that is followed by recruitment of DNMT3A and histone methyltransferase complex PRC2 to initiate DNA methylation and H3K27me3 silencing, respectively [Citation65].
MBD2-NuRD/Mi-2 interaction may also direct DNMT1/DNMT3B activity to sites of DNA methylation and allow MBD2 to maintain and potentially spread methylation [Citation74,Citation164–165]. MBD2 is responsible for establishing silent chromatin and promoter hypermethylation of the GSTP1 tumor-suppressor gene, a gene that is regularly mutated in prostate cancers [Citation164,Citation165]. The DNA repair abilities of MBD4 are also dependent on interactions with DNMT1 and DNMT3B (). MBD4 has been connected with DNMT1 at sites of oxidative stress lesions and with DNMT3B at heterochromatin [Citation77,Citation78]. As MBD4 repairs mC→T transitions at methylated CpG sites, interaction with DNMTs presumably allows remethylation of newly repaired CpG sites and maintenance of methylation state.
Chromatin organization
Chromatin organization is a higher order form of chromatin regulation controlling the 3D structure of the chromatin fiber and affects a wide variety of cellular functions including transcription and epigenome regulation [Citation166]. 3D organization includes the condensation of chromatin and chromatin looping where distant regulatory elements loci are brought together, controlling transcriptional activity. MeCP2 appears to have global chromatin organizational functions above its loci-specific role in histone modifications. As mentioned above, MeCP2 has a role in heterochromatin clustering by interacting with SUV39H1-HP1. However, MeCP2 itself can compact nucleosomes and chromatin in a linker-histone like function similar to HP1 [Citation26,Citation145,Citation167]. This primarily occurs when MeCP2 is highly abundant such as in mature neurons where MeCP2 is almost at 1:1 level to nucleosomes [Citation145]. In vitro studies show that MeCP2 at high concentrations can form and stabilize nucleosome arrays with either methylated or unmethylated DNA [Citation167,Citation168]. However, in vivo studies of MeCP2 mutants show that having a functional MBD domain increases MeCP2 residence time on chromatin and enhances stability of chromatin clustering, suggesting DNA methylation is required to stabilize MeCP2-induced large-scale chromatin clustering/organization [Citation169].
MeCP2 is also able to form chromatin loops in vitro and potentially bring distant loci together through homodimerization [Citation120,Citation168]. A role for MeCP2 in chromatin looping as a silencing mechanism has been described at H19/Igf2 and Dlx5/Dlx6 imprinted loci in mouse models () [Citation25,Citation170]. Both of these particular studies suggest the chromatin looping is accompanied by changes in histone modifications, concomitant for the dual role of MeCP2. At the methylated H19/Igf2 imprinting control region (ICR), MeCP2 recruits nucleosome remodeler ATRX which creates open DNA and binding sites for members of the cohesin complex and CTCF protein, responsible for looping [Citation25,Citation39]. Chromatin looping and silencing was lost in mutant MeCP2 cells due to a loss of interaction between ATRX and MeCP2 [Citation25,Citation171]. Similarly, MeCP2-null mouse cells showed loss of chromatin looping and silencing of the imprinted genes Dlx5 and Dlx6 leading to increased gene expression [Citation170].
Replication-dependent heterochromatin maturation
DNA replication-coupled chromatin maturation requires that the correct DNA methylation be faithfully copied, along with the correct histone modifications and chromatin conformation. Several MBD-histone modification complexes have been described during DNA and chromatin replication. DNMT1 interacts with both HDAC2 and MBD2/MBD3 at the replication fork during late S-phase when heterochromatin is typically replicated [Citation58,Citation172]. This suggests a model where DNMT1 methylates newly replicated DNA and brings in both MBD2-MBD3 and HDACs to ensure that DNA methylation and histone deacetylation are synchronized during heterochromatin maturation. The MBD1-SETDB1 complex further completes heterochromatin maturation as it interacts with CAF1, a replication-coupled chromatin remodeler that assembles newly replicating DNA into chromatin () [Citation45,Citation173]. Coordinated activity of CAF1 with MBD1-SETDB1 ensures methylation of newly incorporated histones where DNA is methylated.
Alternative MBD family functions: it is not all about the silencing
The portrait of MBD proteins as straightforward transcriptional repressors has been challenged recently with genome-wide ChIP and gene expression studies. Such studies revealed that genome regulation by MBD proteins, in particular MBD2 and MBD3, are much more complex than previously understood. Genome-wide studies suggest that MBD-NuRD/Mi-2 may also be involved in gene activation or expression modulation. MBD3-NuRD/Mi-2 KD revealed either little change in expression or an even spread of both up- and down-regulated genes in ESC and HeLa cells [Citation68,Citation73]. As mentioned above, ChIP-seq studies for both MBD3 and MBD2 detect some degree of binding at transcriptionally active and unmethylated promoters [Citation60–61,Citation71,Citation144]. The mechanisms or contextual cues that guide MBD2 and MBD3′s activating functions are less understood. MBD2′s activating roles may be mediated by protein–protein interactions other than NuRD/Mi-2 at specific loci (). MBD2 is reported to interact with TACC3 and activate methylated promoters through recruitment of histone acetyltransferase pCAF/p300 [Citation59]. This interaction is mutually exclusive with HDAC-containing complexes. At unmethylated target genes, MBD2 has been shown to interact with CEBPA transcriptional activator in hepatocellular carcinoma cell lines and CREB transcriptional coactivator complex in HEK293 cells [Citation62,Citation63]. Perhaps MBD2 may genuinely have activator roles or may be ‘poised’ and prevented from enforcing silencing by the transcriptional activators; these examples show that transcriptional repression is not necessary the complete story.
MBDs in reprogramming, pluripotency & differentiation
Developmental stage may be a key factor defining the role of MBD-NuRD/Mi-2 in DNA methylation and transcriptional activity. Full-length MBD2 is expressed at a higher level in somatic cells, whereas MBD3 has been shown to be more abundant in ESCs in mice () [Citation13,Citation140]. Splice isoform MBD2t (MBD2c) is the more abundant version of MBD2 present in human ESCs (hESC) [Citation174]. This shorter isoform is unable to interact with the NuRD complex and cannot act in a repressive fashion; therefore, with low levels of full length MBD2, MBD3-NuRD/Mi-2 may be the major NuRD species in ESCs (). This suggests that MBD2 and MBD3 have different activities throughout development.
The balance between MBD2a and MBD2t levels is important to reprogramming and differentiation ( & C) [Citation174]. Both MBD2a and MBD2t have been shown to bind and regulate expression of pluripotency genes OCT4 and NANOG. Overexpression of MBD2a in hESCs caused differentiation by silencing these pluripotency genes through NuRD recruitment, whereas overexpression of MBD2t maintained pluripotency and even enhanced reprogramming efficiency of human fibroblasts. MBD2t is unable to interact with NuRD, so does not silence bound loci and can also compete with MBD2a for binding sites during reprogramming to release MBD2a-NuRD repression of pluripotency genes ().
MBD3 KO mice are embryonically lethal, indicating that unlike other MBD family members, MBD3 is essential to embryogenesis [Citation122]. KD of MBD3 and therefore NuRD vastly improved reprogramming efficiency of human fibroblasts to induced pluripotent stem cells, indicating MBD3 is critical to differentiation [Citation175]. However, with MBD3 binding at active promoters and active enhancers in pluripotent ESCs, this suggests a mechanism of ‘poising’ the repressive complex at active elements in preparation for differentiation () [Citation60,Citation69]. In mouse ESCs, MBD3-NuRD/Mi-2 regulates expression of bivalent genes by deacetylating H3K27 to permit H3K27me3 repressive marks at promoters with H3K4me3 active marks [Citation68]. MBD3-NuRD/Mi-2 also lowers the expression of a set of genes required for pluripotency rather than fully silencing the genes when cells are in a renewal state [Citation72]. However, during differentiation MBD3-NuRD/Mi-2 complex containing histone demethylase LSD1 silences active enhancers by removing the H3K4me1 mark alongside the H3K27ac mark ( & C) [Citation69]. MBD3-mediated repression is only evident when ESCs are released from pluripotency factors and allowed to differentiate, suggesting activating factors may be present at these genes in the pluripotent state to prevent silencing by MBD3-NuRD [Citation64,Citation72]. This poised mechanism may be at many identified MBD3 ‘active’ binding sites and exemplify the highly contextual nature of MBD3-NuRD/Mi-2 activity.
MBD4 in DNA demethylation?
MBD4 is involved in DNA repair at methylated loci where 5-mC is frequently spontaneously deaminated to thymine (T) () [Citation125]. This editing ability potentially allows MBD4 to play a role in promoting DNA demethylation. DNA demethylation can occur passively through DNA replication or actively through enzymatic pathways. For example the AID/Apobec enzyme complexes are deaminases that actively create the mC→T or C→U deamination transitions. A study in zebrafish embryos reported that AID/Apobec deamination of 5-mC→T was subsequently repaired by MBD4 to unmodified cytosine, thereby effectively resulting in demethylation, although this phenomena is still subject to debate [Citation79,Citation176]. AID/Apobec can also deaminate 5-hmC into 5-hmU, another MBD4 glycosylase substrate, and become unmethylated after DNA repair [Citation80,Citation81]. This demethylation mechanism appears to be triggered in instances of breast cancer metastasis; however, the exact MBD4-mediated demethylation mechanism remains to be confirmed [Citation177].
MBD proteins in disease & cancer
The role of the MBD protein family as readers of the epigenome has important implications for their involvement in changes to both DNA methylation patterns and chromatin structure, potentially leading to disrupted transcriptional regulation that underlie many human diseases. Alterations in MBD proteins, through their expression, posttranslational modifications, disrupted interactions with protein-binding partners and localization, are all potential mechanisms contributing to human diseases. Rett syndrome is the first described and most studied disease directly linked to disruption of a MBD protein, caused by mutations in MeCP2 [Citation29,Citation30]. Individuals affected with Rett syndrome typically have point mutations in two discrete clusters within the MBD domain or the C terminus of the TRD domain, perturbing the role of MeCP2 function in transcriptional silencing of target genes. Other MBD proteins have more recently been implicated in many neurogenetic disorders, such as autism, schizophrenia and Prader–Willi and Angelman syndrome ().
MBD proteins have also been implicated in several human cancers with the specific aberrations shown in . The precise roles of the MBD proteins differ between different types of cancer. MBD2 overexpression is associated with aberrant hypermethylation of the GSTP1 tumor-suppressor gene promoter and 14–3–3σ gene in prostate cancer, and hTERT gene in HeLa cells [Citation164–165,Citation178–179]. Furthermore, MBD2 knockdown was shown to alleviate the repression of well-known tumor-suppressor genes such as p16INK4a and p14ARF [Citation50,Citation157,Citation180]. In addition, when a colorectal carcinogenesis mouse model with ApcMin/+ mutations was crossed to MBD2-deficient mice, the resulting offspring developed significantly smaller and fewer tumors [Citation181]. More recently, MBD2 aberrations have been reported in other human cancers including gastrointestinal and breast cancer, and brain/glioblastoma (). Importantly, MBD1, MBD3 and MBD4 have also been associated with a number of cancers, including lung, endometrial, pancreas, prostate and colorectal cancer. While MBD5 and MBD6 have been linked to several neurodevelopmental/autism spectrum disorders (ASD), there are no reports to date of their altered activity in cancer () [Citation182–184].
With the advent of cancer genome sequencing, the mutation frequency of the MBD protein family members in different cancer types is now being better elucidated. The Cancer Genome Atlas (TCGA) data show that the mutation frequency of each MBD protein across different cancer tissue types is significantly different () [Citation185,Citation186]. Overall, stomach, colorectal, pancreatic and uterine cancer have the highest total frequency of MBD mutations with skin, bladder and lung cancers also showing a total mutation frequency above 10%. Striking is the observation that MBD5 and MBD6 have the highest mutation frequency of the MBD proteins across many cancers, with MBD5 being mutated at a frequency of 7.7, 6.5 and 7.6% in skin, colorectal and uterine cancer, respectively. MBD3 is most highly mutated in pancreatic cancer. Mutation types across the MeCP2 and MBD1–6 proteins are shown in . Nonsynonymous mutations (insertions, deletions, frameshift mutations and single mutations leading to amino acid changes) may potentially lead to disrupted or aberrant binding of the MBD protein, as well as disruption of appropriate binding partners. Interestingly, while some mutations fall within the functional domains, such as the methyl-binding domain and the transcriptional repressor domain, many mutations fall outside of these domains (). Mutations outside of known protein domains may be indicative of novel domains or functionality. For example, the cluster of mutations in MBD2 between the TRD and CC domains now overlap a newly discovered intrinsically disordered region (IDR) [Citation187]. Maintaining the integrity of the IDR increases MBD2 affinity for 5-mC as well as recruitment of the NuRD/Mi-2 complex, and therefore mutations in the IDR may disrupt crucial MBD2 functions. Some sites appear to be mutational ‘hotspots’ with MBD1, MBD2 and MBD3 showing an increase in the number of reported mutations at functional domains, in contrast to MBD4, MBD5 and MBD6 where the vast majority of mutations are outside regions with known functional roles.
In addition to mutations, disrupted gene expression of MBD proteins with tumorigenic consequences in in vivo cancer mouse models, cancer cell lines and human cancers have been reported (). For example, overexpression of MBD1 in pancreatic cancer was correlated with increased lymph node metastasis due to MBD1-mediated downregulation of E-cadherin [Citation95]. Similarly, overexpression of MBD2 has been shown to have a role in glioblastoma and breast cancer due to aberrant gene silencing () [Citation99,Citation100]. Loss of MBD expression has also been reported in a number of cancers, with MBD2 and MBD3 downregulated in gastric neoplastic tissues and MBD4 downregulated in liver cancer [Citation98,Citation112]. Interestingly, loss of MBD4 expression has been shown to increase susceptibility to cancer by decreasing the ability to recover from genetic insult. In support of this, MBD4 mutations are often found in cancers with microsatellite instability [Citation111,Citation195].
Conclusion
In this review, we have described the members of the MBD family of proteins and highlighted the diverse and complex roles that they play in gene regulation and cell biology. Recent studies have revealed new roles of MBD proteins not only as static ‘readers’ but also as dynamic facilitators of DNA methylation, in ‘editing’ and ‘writing,’ playing important roles as regulators of DNA methylation patterns, and higher-level epigenomic organization. Deregulation or mutations of MBD proteins are found in a variety of cancer and neurological diseases suggesting their functions are critical for maintaining epigenetic and cellular homeostasis. Future studies will reveal the specificity and genomic context of MBD proteins, their functional role in maintaining a normal cellular homeostasis and their role in aberrant epigenome disruptions and disease outcomes.
Future perspective
With the development of whole genome analysis, the genomic context of binding and the specificity of binding partners recruited are becoming clearer; however, genome-wide studies have also produced surprises and contradictions. For instance, MBD2 and MBD3 appear to have both active and repressive roles when complexed with NuRD/Mi-2, highlighting that the relationship of the MBD proteins with NuRD/Mi-2 is not as straight forward as first thought [Citation60,Citation61]. The dichotomy of the functional roles will be revealed by future studies, including elucidating the binding patterns with improved ChIP-seq data, the binding partners by techniques such as mass spectrometry, and understanding the associated cofactors in different genomic contexts with specific functional consequences.
The recent discovery of other DNA cytosine modifications such as 5-hmC has diversified the understanding of the role of DNA methylation. It has been suggested that introduction of 5-hmC by TETs is able to detach MBD proteins and potentially initiate a process of demethylation and activation. Interestingly, studies have shown in vitro that MBD1, MBD2 and especially MeCP2 have a specific affinity to a ‘hemimethylated’ 5-hmC/5-mC state [Citation138–139,Citation196]. Binding here could prevent full hydroxymethylation by TETs. Indeed, a study in mouse brain found that the MBD domain of MeCP2 is able to inhibit hydroxymethylation in vitro [Citation41]. As 5-mC readers, introduction or removal of 5-hmC by TET enzymes will have real consequences on the localization and function of MBD proteins. This will be a critical area of research in determining the functional impact of hydroxymethylation.
Alterations in the epigenetic regulation of genome activity are as important to tumorigenesis as alterations in the genomic code itself. A common mechanism for epigenomic disruption is methylation stochasticity mediated by genetic variants of the DNA methylation machinery of the cell [Citation197]. Uncovering gene mutations in epigenetic-modifying genes is critical to elucidating the precise role these mutations may have in disrupting the functioning of the ‘readers,’ ‘writers’ and ‘editors’ of the epigenome. While limited data are available on the roles of MBD5 and MBD6, the high rate of mutations of these two MBD family members suggests they may have significant roles in gene regulation and disease phenotypes. Despite the current level of knowledge, further research will be required to elucidate the precise roles of MBD proteins to determine if and how MBD proteins can be targeted to treat human diseases.
Table 1. Summary showing binding specificity, binding partners and functional roles of the methyl-binding domain protein family.
Table 2. Summary of published studies on methyl-binding domain protein family mutations, sequence variants and expression deregulation in cancer and other diseases.
MBD domain proteins
The family is categorized by the presence of a methyl-CpG-binding domain (MBD) that has the ability to bind to singular symmetrically methylated CpG dinucleotides.
The MBD family consists of five core proteins, MeCP2 and MBD1–4.
Other MBD containing proteins include MBD5/6, SETDB1/2 and BAZ2A/B, although their MBD domains do not seem to bind 5-mC.
Binding specificity & genomic distribution
Core MBD proteins except MBD3 preferentially bind to 5-mC at both biochemical and a genome-wide distribution level.
Recent genome-wide studies suggest that MBD3 binds at a subset of unmethylated and active promoters and enhancers. MBD2 also shows some binding to unmethylated and active promoters and enhancers, typically when MBD3 is also present. The functional consequence is not entirely clear.
MBD3 and MeCP2 have been reported to bind 5-hmC, however, that is not wholly supported and further work is required.
Functional epigenetic roles
MBD proteins typically cause transcriptional repression by histone modification, nucleosome remodeling or chromatin organisation.
MBD proteins also maintain silencing by maintaining methylation and histone marks by being coupled with DNA replication and DNMT proteins.
Alternative epigenetic roles
MBD2 and MBD3 have context-specific transcriptional activation or modulation roles that are dependent on developmental stage and protein associations.
MBD2 and MBD3 also play significant roles in defining the balance between pluripotency and differentiation during development.
MBD4 DNA mismatch repair may be used in active DNA demethylation pathways.
MBD proteins in cancer biology & disease
Mutations and expression changes of MBD proteins are found in many neurological diseases and cancers.
Author contributions
The authors thank Dr Phillippa C Taberlay and Dr Brigid O’Gorman for careful reviewing of the manuscript. The authors thank Dr Kate Patterson for background artwork.
Financial & competing interests disclosure
The authors thank our funding sources; SJ Clark is supported by NHMRC Fellowship and NHMRC project grant #1088144, C Stirzaker and P-L Luu by NHMRC project grant #1088144 and Q Du by Australian Postgraduate Award. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Bestor T , LaudanoA , MattalianoR , IngramV . Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases . J. Mol. Biol.203 ( 4 ), 971 – 983 ( 1988 ).
- Stirzaker C , TaberlayPC , StathamAL , ClarkSJ . Mining cancer methylomes: prospects and challenges . Trends Genet.30 ( 2 ), 75 – 84 ( 2014 ).
- Gardiner-Garden M , FrommerM . CpG islands in vertebrate genomes . J. Mol. Biol.196 ( 2 ), 261 – 282 ( 1987 ).
- Ogoshi K , HashimotoS , NakataniYet al. Genome-wide profiling of DNA methylation in human cancer cells . Genomics98 ( 4 ), 280 – 287 ( 2011 ).
- Ball MP , LiJB , GaoYet al. Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells . Nat. Biotechnol.27 ( 4 ), 361 – 368 ( 2009 ).
- Taberlay PC , StathamAL , KellyTK , ClarkSJ , JonesPA . Reconfiguration of nucleosome-depleted regions at distal regulatory elements accompanies DNA methylation of enhancers and insulators in cancer . Genome Res.24 ( 9 ), 1421 – 1432 ( 2014 ).
- Okano M , XieS , LiE . Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases . Nat. Genet.19 ( 3 ), 219 – 220 ( 1998 ).
- Bogdanovic O , VeenstraGJ . DNA methylation and methyl-CpG binding proteins: developmental requirements and function . Chromosoma118 ( 5 ), 549 – 565 ( 2009 ).
- Hashimoto H , VertinoPM , ChengX . Molecular coupling of DNA methylation and histone methylation . Epigenomics2 ( 5 ), 657 – 669 ( 2010 ).
- Filion GJ , ZheniloS , SalozhinS , YamadaD , ProkhortchoukE , DefossezPA . A family of human zinc finger proteins that bind methylated DNA and repress transcription . Mol. Cell. Biol.26 ( 1 ), 169 – 181 ( 2006 ).
- Unoki M , NishidateT , NakamuraY . ICBP90, an E2F-1 target, recruits HDAC1 and binds to methyl-CpG through its SRA domain . Oncogene23 ( 46 ), 7601 – 7610 ( 2004 ).
- Prokhortchouk A , HendrichB , JorgensenHet al. The p120 catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor . Genes Dev.15 ( 13 ), 1613 – 1618 ( 2001 ).
- Hendrich B , BirdA . Identification and characterization of a family of mammalian methyl-CpG binding proteins . Mol. Cell. Biol.18 ( 11 ), 6538 – 6547 ( 1998 ).
- Pastor WA , AravindL , RaoA . TETonic shift: biological roles of TET proteins in DNA demethylation and transcription . Nat. Rev. Mol. Cell Biol.14 ( 6 ), 341 – 356 ( 2013 ).
- Baymaz HI , FournierA , LagetSet al. MBD5 and MBD6 interact with the human PR-DUB complex through their methyl-CpG-binding domain . Proteomics14 ( 19 ), 2179 – 2189 ( 2014 ).
- Hendrich B , TweedieS . The methyl-CpG binding domain and the evolving role of DNA methylation in animals . Trends Genet.19 ( 5 ), 269 – 277 ( 2003 ).
- Nan X , MeehanRR , BirdA . Dissection of the methyl-CpG binding domain from the chromosomal protein MeCP2 . Nucleic Acids Res.21 ( 21 ), 4886 – 4892 ( 1993 ).
- Ohki I , ShimotakeN , FujitaNet al. Solution structure of the methyl-CpG binding domain of human MBD1 in complex with methylated DNA . Cell105 ( 4 ), 487 – 497 ( 2001 ).
- Boeke J , AmmerpohlO , KegelS , MoehrenU , RenkawitzR . The minimal repression domain of MBD2b overlaps with the methyl-CpG-binding domain and binds directly to Sin3A . J. Biol. Chem.275 ( 45 ), 34963 – 34967 ( 2000 ).
- Wade PA , GegonneA , JonesPL , BallestarE , AubryF , WolffeAP . Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation . Nat. Genet.23 ( 1 ), 62 – 66 ( 1999 ).
- Ng HH , JeppesenP , BirdA . Active repression of methylated genes by the chromosomal protein MBD1 . Mol. Cell. Biol.20 ( 4 ), 1394 – 1406 ( 2000 ).
- Meehan RR , LewisJD , BirdAP . Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA . Nucleic Acids Res.20 ( 19 ), 5085 – 5092 ( 1992 ).
- Fuks F , HurdPJ , WolfD , NanX , BirdAP , KouzaridesT . The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation . J. Biol. Chem.278 ( 6 ), 4035 – 4040 ( 2003 ).
- Nan X , NgHH , JohnsonCAet al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex . Nature393 ( 6683 ), 386 – 389 ( 1998 ).
- Kernohan KD , VernimmenD , GloorGB , BerubeNG . Analysis of neonatal brain lacking ATRX or MeCP2 reveals changes in nucleosome density, CTCF binding and chromatin looping . Nucleic Acids Res.42 ( 13 ), 8356 – 8368 ( 2014 ).
- Agarwal N , HardtT , BreroAet al. MeCP2 interacts with HP1 and modulates its heterochromatin association during myogenic differentiation . Nucleic Acids Res.35 ( 16 ), 5402 – 5408 ( 2007 ).
- Dhasarathy A , WadePA . The MBD protein family-reading an epigenetic mark?Mutat. Res.647 ( 1–2 ), 39 – 43 ( 2008 ).
- Maunakea AK , ChepelevI , CuiK , ZhaoK . Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition . Cell Res.23 ( 11 ), 1256 – 1269 ( 2013 ).
- Amir RE , Van Den VeyverIB , WanM , TranCQ , FranckeU , ZoghbiHY . Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2 . Nat. Genet.23 ( 2 ), 185 – 188 ( 1999 ).
- D’esposito M , QuaderiNA , CiccodicolaAet al. Isolation, physical mapping, and northern analysis of the X-linked human gene encoding methyl CpG-binding protein, MECP2 . Mamm. Genome7 ( 7 ), 533 – 535 ( 1996 ).
- Shahbazian MD , AntalffyB , ArmstrongDL , ZoghbiHY . Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation . Hum. Mol. Genet.11 ( 2 ), 115 – 124 ( 2002 ).
- Kokura K , KaulSC , WadhwaRet al. The Ski protein family is required for MeCP2-mediated transcriptional repression . J. Biol. Chem.276 ( 36 ), 34115 – 34121 ( 2001 ).
- Lyst MJ , EkiertR , EbertDHet al. Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor . Nat. Neurosci.16 ( 7 ), 898 – 902 ( 2013 ).
- Jones PL , VeenstraGJ , WadePAet al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription . Nat. Genet.19 ( 2 ), 187 – 191 ( 1998 ).
- Ballas N , GrunseichC , LuDD , SpehJC , MandelG . REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis . Cell121 ( 4 ), 645 – 657 ( 2005 ).
- Lunyak VV , BurgessR , PrefontaineGGet al. Corepressor-dependent silencing of chromosomal regions encoding neuronal genes . Science298 ( 5599 ), 1747 – 1752 ( 2002 ).
- Harikrishnan KN , ChowMZ , BakerEKet al. Brahma links the SWI/SNF chromatin-remodeling complex with MeCP2-dependent transcriptional silencing . Nat. Genet.37 ( 3 ), 254 – 264 ( 2005 ).
- Kimura H , ShiotaK . Methyl-CpG-binding protein, MeCP2, is a target molecule for maintenance DNA methyltransferase, DNMT1 . J. Biol. Chem.278 ( 7 ), 4806 – 4812 ( 2003 ).
- Kernohan KD , JiangY , TremblayDCet al. ATRX partners with cohesin and MeCP2 and contributes to developmental silencing of imprinted genes in the brain . Dev. Cell18 ( 2 ), 191 – 202 ( 2010 ).
- Mellen M , AyataP , DewellS , KriaucionisS , HeintzN . MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system . Cell151 ( 7 ), 1417 – 1430 ( 2012 ).
- Szulwach KE , LiX , LiYet al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging . Nat. Neurosci.14 ( 12 ), 1607 – 1616 ( 2011 ).
- Vaute O , NicolasE , VandelL , TroucheD . Functional and physical interaction between the histone methyl transferase Suv39H1 and histone deacetylases . Nucleic Acids Res.30 ( 2 ), 475 – 481 ( 2002 ).
- Villa R , MoreyL , RakerVAet al. The methyl-CpG binding protein MBD1 is required for PML-RARalpha function . Proc. Natl Acad. Sci. USA103 ( 5 ), 1400 – 1405 ( 2006 ).
- Fujita N , WatanabeS , IchimuraTet al. Methyl-CpG binding domain 1 (MBD1) interacts with the Suv39h1-HP1 heterochromatic complex for DNA methylation-based transcriptional repression . J. Biol. Chem.278 ( 26 ), 24132 – 24138 ( 2003 ).
- Sarraf SA , StanchevaI . Methyl-CpG binding protein MBD1 couples histone H3 methylation at lysine 9 by SETDB1 to DNA replication and chromatin assembly . Mol. Cell15 ( 4 ), 595 – 605 ( 2004 ).
- Ichimura T , WatanabeS , SakamotoY , AotoT , FujitaN , NakaoM . Transcriptional repression and heterochromatin formation by MBD1 and MCAF/AM family proteins . J. Biol. Chem.280 ( 14 ), 13928 – 13935 ( 2005 ).
- Fujita N , WatanabeS , IchimuraTet al. MCAF mediates MBD1-dependent transcriptional repression . Mol. Cell. Biol.23 ( 8 ), 2834 – 2843 ( 2003 ).
- Uchimura Y , IchimuraT , UwadaJet al. Involvement of SUMO modification in MBD1- and MCAF1-mediated heterochromatin formation . J. Biol. Chem.281 ( 32 ), 23180 – 23190 ( 2006 ).
- Gu P , XuX , Le MenuetD , ChungAC , CooneyAJ . Differential recruitment of methyl CpG-binding domain factors and DNA methyltransferases by the orphan receptor germ cell nuclear factor initiates the repression and silencing of Oct4 . Stem Cells29 ( 7 ), 1041 – 1051 ( 2011 ).
- Le Guezennec X , VermeulenM , BrinkmanABet al. MBD2/NuRD and MBD3/NuRD, two distinct complexes with different biochemical and functional properties . Mol. Cell. Biol.26 ( 3 ), 843 – 851 ( 2006 ).
- Ramirez J , DegeC , KutateladzeTG , HagmanJ . MBD2 and multiple domains of CHD4 are required for transcriptional repression by Mi-2/NuRD complexes . Mol. Cell. Biol.32 ( 24 ), 5078 – 5088 ( 2012 ).
- Marhold J , BrehmA , KramerK . The Drosophila methyl-DNA binding protein MBD2/3 interacts with the NuRD complex via p55 and MI-2 . BMC Mol. Biol.5 ( 1 ), 20 ( 2004 ).
- Brackertz M , BoekeJ , ZhangR , RenkawitzR . Two highly related p66 proteins comprise a new family of potent transcriptional repressors interacting with MBD2 and MBD3 . J. Biol. Chem.277 ( 43 ), 40958 – 40966 ( 2002 ).
- Brackertz M , GongZ , LeersJ , RenkawitzR . p66alpha and p66beta of the Mi-2/NuRD complex mediate MBD2 and histone interaction . Nucleic Acids Res.34 ( 2 ), 397 – 406 ( 2006 ).
- Sekimata M , TakahashiA , Murakami-SekimataA , HommaY . Involvement of a novel zinc finger protein, MIZF, in transcriptional repression by interacting with a methyl-CpG-binding protein, MBD2 . J. Biol. Chem.276 ( 46 ), 42632 – 42638 ( 2001 ).
- Tan CP , NakielnyS . Control of the DNA methylation system component MBD2 by protein arginine methylation . Mol. Cell. Biol.26 ( 19 ), 7224 – 7235 ( 2006 ).
- Feng Q , ZhangY . The MeCP1 complex represses transcription through preferential binding, remodeling, and deacetylating methylated nucleosomes . Genes Dev.15 ( 7 ), 827 – 832 ( 2001 ).
- Tatematsu KI , YamazakiT , IshikawaF . MBD2-MBD3 complex binds to hemi-methylated DNA and forms a complex containing DNMT1 at the replication foci in late S phase . Genes Cells5 ( 8 ), 677 – 688 ( 2000 ).
- Angrisano T , LemboF , PeroRet al. TACC3 mediates the association of MBD2 with histone acetyltransferases and relieves transcriptional repression of methylated promoters . Nucleic Acids Res.34 ( 1 ), 364 – 372 ( 2006 ).
- Baubec T , IvanekR , LienertF , SchubelerD . Methylation-dependent and -independent genomic targeting principles of the MBD protein family . Cell153 ( 2 ), 480 – 492 ( 2013 ).
- Gunther K , RustM , LeersJet al. Differential roles for MBD2 and MBD3 at methylated CpG islands, active promoters and binding to exon sequences . Nucleic Acids Res.41 ( 5 ), 3010 – 3021 ( 2013 ).
- Stefanska B , SudermanM , MachnesZ , BhattacharyyaB , HallettM , SzyfM . Transcription onset of genes critical in liver carcinogenesis is epigenetically regulated by methylated DNA-binding protein MBD2 . Carcinogenesis34 ( 12 ), 2738 – 2749 ( 2013 ).
- Fujita H , FujiiR , ArataniS , AmanoT , FukamizuA , NakajimaT . Antithetic effects of MBD2a on gene regulation . Mol. Cell. Biol.23 ( 8 ), 2645 – 2657 ( 2003 ).
- Kaji K , CaballeroIM , MacleodR , NicholsJ , WilsonVA , HendrichB . The NuRD component Mbd3 is required for pluripotency of embryonic stem cells . Nat. Cell Biol.8 ( 3 ), 285 – 292 ( 2006 ).
- Morey L , BrennerC , FaziFet al. MBD3, a component of the NuRD complex, facilitates chromatin alteration and deposition of epigenetic marks . Mol. Cell. Biol.28 ( 19 ), 5912 – 5923 ( 2008 ).
- Choi WI , JeonBN , YoonJHet al. The proto-oncoprotein FBI-1 interacts with MBD3 to recruit the Mi-2/NuRD-HDAC complex and BCoR and to silence p21WAF/CDKN1A by DNA methylation . Nucleic Acids Res.41 ( 13 ), 6403 – 6420 ( 2013 ).
- Aguilera C , NakagawaK , SanchoR , ChakrabortyA , HendrichB , BehrensA . c-Jun N-terminal phosphorylation antagonises recruitment of the Mbd3/NuRD repressor complex . Nature469 ( 7329 ), 231 – 235 ( 2011 ).
- Reynolds N , Salmon-DivonM , DvingeHet al. NuRD-mediated deacetylation of H3K27 facilitates recruitment of Polycomb Repressive Complex 2 to direct gene repression . EMBO J.31 ( 3 ), 593 – 605 ( 2012 ).
- Whyte WA , BilodeauS , OrlandoDAet al. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation . Nature482 ( 7384 ), 221 – 225 ( 2012 ).
- Wang Y , ZhangH , ChenYet al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer . Cell138 ( 4 ), 660 – 672 ( 2009 ).
- Shimbo T , DuY , GrimmSAet al. MBD3 localizes at promoters, gene bodies and enhancers of active genes . PLoS Genet.9 ( 12 ), e1004028 ( 2013 ).
- Reynolds N , LatosP , Hynes-AllenAet al. NuRD suppresses pluripotency gene expression to promote transcriptional heterogeneity and lineage commitment . Cell Stem Cell10 ( 5 ), 583 – 594 ( 2012 ).
- Yildirim O , LiR , HungJHet al. Mbd3/NURD complex regulates expression of 5-hydroxymethylcytosine marked genes in embryonic stem cells . Cell147 ( 7 ), 1498 – 1510 ( 2011 ).
- Cai Y , GeutjesEJ , De LintKet al. The NuRD complex cooperates with DNMTs to maintain silencing of key colorectal tumor suppressor genes . Oncogene33 ( 17 ), 2157 – 2168 ( 2014 ).
- Kondo E , GuZ , HoriiA , FukushigeS . The thymine DNA glycosylase MBD4 represses transcription and is associated with methylated p16(INK4a) and hMLH1 genes . Mol. Cell. Biol.25 ( 11 ), 4388 – 4396 ( 2005 ).
- Bellacosa A , CicchillittiL , SchepisFet al. MED1, a novel human methyl-CpG-binding endonuclease, interacts with DNA mismatch repair protein MLH1 . Proc. Natl Acad. Sci. USA96 ( 7 ), 3969 – 3974 ( 1999 ).
- Laget S , MiottoB , ChinHGet al. MBD4 cooperates with DNMT1 to mediate methyl-DNA repression and protects mammalian cells from oxidative stress . Epigenetics9 ( 4 ), 546 – 556 ( 2014 ).
- Boland MJ , ChristmanJK . Characterization of DNMT3b:thymine-DNA glycosylase interaction and stimulation of thymine glycosylase-mediated repair by DNA methyltransferase(s) and RNA . J. Mol. Biol.379 ( 3 ), 492 – 504 ( 2008 ).
- Rai K , HugginsIJ , JamesSR , KarpfAR , JonesDA , CairnsBR . DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45 . Cell135 ( 7 ), 1201 – 1212 ( 2008 ).
- Cortellino S , XuJ , SannaiMet al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair . Cell146 ( 1 ), 67 – 79 ( 2011 ).
- Hashimoto H , ZhangX , ChengX . Excision of thymine and 5-hydroxymethyluracil by the MBD4 DNA glycosylase domain: structural basis and implications for active DNA demethylation . Nucleic Acids Res.40 ( 17 ), 8276 – 8284 ( 2012 ).
- Bienvenu T , ChellyJ . Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized . Nat. Rev. Genet.7 ( 6 ), 415 – 426 ( 2006 ).
- Zhou Z , QinJ , TangJet al. Down-regulation of MeCP2 in Hirschsprung’s disease . J. Pediatr. Surg.48 ( 10 ), 2099 – 2105 ( 2013 ).
- Carney RM , WolpertCM , RavanSAet al. Identification of MeCP2 mutations in a series of females with autistic disorder . Pediatr. Neurol.28 ( 3 ), 205 – 211 ( 2003 ).
- Loat CS , CurranS , LewisCMet al. Methyl-CpG-binding protein 2 polymorphisms and vulnerability to autism . Genes Brain Behav.7 ( 7 ), 754 – 760 ( 2008 ).
- Nagarajan RP , HogartAR , GwyeY , MartinMR , LasalleJM . Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation . Epigenetics1 ( 4 ), e1 – e11 ( 2006 ).
- Shibayama A , CookEHJr , FengJet al. MECP2 structural and 3’-UTR variants in schizophrenia, autism and other psychiatric diseases: a possible association with autism . Am. J. Med. Genet. B Neuropsychiatr. Genet.128B ( 1 ), 50 – 53 ( 2004 ).
- Ramocki MB , PetersSU , TavyevYJet al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome . Ann. Neurol.66 ( 6 ), 771 – 782 ( 2009 ).
- Muller HM , FieglH , GoebelGet al. MeCP2 and MBD2 expression in human neoplastic and non-neoplastic breast tissue and its association with oestrogen receptor status . Br. J. Cancer89 ( 10 ), 1934 – 1939 ( 2003 ).
- Cukier HN , RabionetR , KonidariIet al. Novel variants identified in methyl-CpG-binding domain genes in autistic individuals . Neurogenetics11 ( 3 ), 291 – 303 ( 2010 ).
- Liu H , JinG , WangHet al. Methyl-CpG binding domain 1 gene polymorphisms and lung cancer risk in a Chinese population . Biomarkers13 ( 6 ), 607 – 617 ( 2008 ).
- Bader S , WalkerM , McqueenHAet al. MBD1, MBD2 and CGBP genes at chromosome 18q21 are infrequently mutated in human colon and lung cancers . Oncogene22 ( 22 ), 3506 – 3510 ( 2003 ).
- Jang JS , LeeSJ , ChoiJEet al. Methyl-CpG binding domain 1 gene polymorphisms and risk of primary lung cancer . Cancer Epidemiol. Biomarkers Prev.14 ( 11 Pt 1 ), 2474 – 2480 ( 2005 ).
- Ghersi D , SinghM . Interaction-based discovery of functionally important genes in cancers . Nucleic Acids Res.42 ( 3 ), e18 ( 2014 ).
- Xu J , ZhuW , XuWet al. Up-regulation of MBD1 promotes pancreatic cancer cell epithelial-mesenchymal transition and invasion by epigenetic down-regulation of E-cadherin . Curr. Mol. Med.13 ( 3 ), 387 – 400 ( 2013 ).
- Xu J , ZhuW , XuWet al. Silencing of MBD1 reverses pancreatic cancer therapy resistance through inhibition of DNA damage repair . Int. J. Oncol.42 ( 6 ), 2046 – 2052 ( 2013 ).
- Patra SK , PatraA , ZhaoH , CarrollP , DahiyaR . Methyl-CpG-DNA binding proteins in human prostate cancer: expression of CXXC sequence containing MBD1 and repression of MBD2 and MeCP2 . Biochem. Biophys. Res. Commun.302 ( 4 ), 759 – 766 ( 2003 ).
- Pontes TB , ChenES , GigekCOet al. Reduced mRNA expression levels of MBD2 and MBD3 in gastric carcinogenesis . Tumour Biol.35 ( 4 ), 3447 – 3453 ( 2014 ).
- He M , FanJ , JiangR , TangWX , WangZW . Expression of DNMTs and MBD2 in GIST . Biomed. Rep.1 ( 2 ), 223 – 227 ( 2013 ).
- Billard LM , MagdinierF , LenoirGM , FrappartL , DanteR . MeCP2 and MBD2 expression during normal and pathological growth of the human mammary gland . Oncogene21 ( 17 ), 2704 – 2712 ( 2002 ).
- Sapkota Y , RobsonP , LaiR , CassCE , MackeyJR , DamarajuS . A two-stage association study identifies methyl-CpG-binding domain protein 2 gene polymorphisms as candidates for breast cancer susceptibility . Eur. J. Hum. Genet.20 ( 6 ), 682 – 689 ( 2012 ).
- Zhu Y , BrownHN , ZhangY , HolfordTR , ZhengT . Genotypes and haplotypes of the methyl-CpG-binding domain 2 modify breast cancer risk dependent upon menopausal status . Breast Cancer Res.7 ( 5 ), R745 – R752 ( 2005 ).
- Zhu D , HunterSB , VertinoPM , Van MeirEG . Overexpression of MBD2 in glioblastoma maintains epigenetic silencing and inhibits the antiangiogenic function of the tumor suppressor gene BAI1 . Cancer Res.71 ( 17 ), 5859 – 5870 ( 2011 ).
- Balada E , Ordi-RosJ , Serrano-AcedoS , Martinez-LostaoL , Vilardell-TarresM . Transcript overexpression of the MBD2 and MBD4 genes in CD4+ T cells from systemic lupus erythematosus patients . J. Leukoc. Biol.81 ( 6 ), 1609 – 1616 ( 2007 ).
- Qin HH , ZhuXH , LiangJet al. Associations between aberrant DNA methylation and transcript levels of DNMT1 and MBD2 in CD4+ T cells from patients with systemic lupus erythematosus . Australas. J. Dermatol.54 ( 2 ), 90 – 95 ( 2013 ).
- Chen ZP , GuDS , ZhouZPet al. Decreased expression of MBD2 and MBD4 gene and genomic-wide hypomethylation in patients with primary immune thrombocytopenia . Hum. Immunol.72 ( 6 ), 486 – 491 ( 2011 ).
- Zhao S , ChoiM , OvertonJDet al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma . Proc. Natl Acad. Sci. USA110 ( 8 ), 2916 – 2921 ( 2013 ).
- Schlegel J , GuneysuS , MennelHD . Expression of the genes of methyl-binding domain proteins in human gliomas . Oncol. Rep.9 ( 2 ), 393 – 395 ( 2002 ).
- Bader S , WalkerM , HendrichBet al. Somatic frameshift mutations in the MBD4 gene of sporadic colon cancers with mismatch repair deficiency . Oncogene18 ( 56 ), 8044 – 8047 ( 1999 ).
- Riccio A , AaltonenLA , GodwinAKet al. The DNA repair gene MBD4 (MED1) is mutated in human carcinomas with microsatellite instability . Nat. Genet.23 ( 3 ), 266 – 268 ( 1999 ).
- Yamada T , KoyamaT , OhwadaSet al. Frameshift mutations in the MBD4/MED1 gene in primary gastric cancer with high-frequency microsatellite instability . Cancer Lett.181 ( 1 ), 115 – 120 ( 2002 ).
- Saito Y , KanaiY , SakamotoM , SaitoH , IshiiH , HirohashiS . Expression of mRNA for DNA methyltransferases and methyl-CpG-binding proteins and DNA methylation status on CpG islands and pericentromeric satellite regions during human hepatocarcinogenesis . Hepatology33 ( 3 ), 561 – 568 ( 2001 ).
- Cukier HN , LeeJM , MaDet al. The expanding role of MBD genes in autism: identification of a MECP2 duplication and novel alterations in MBD5, MBD6, and SETDB1 . Autism Res.5 ( 6 ), 385 – 397 ( 2012 ).
- Hamdan FF , SrourM , Capo-ChichiJMet al. De novo mutations in moderate or severe intellectual disability . PLoS Genet.10 ( 10 ), e1004772 ( 2014 ).
- Mullegama SV , RosenfeldJA , OrellanaCet al. Reciprocal deletion and duplication at 2q23.1 indicates a role for MBD5 in autism spectrum disorder . Eur. J. Hum. Genet.22 ( 1 ), 57 – 63 ( 2014 ).
- J⊘rgensen HF , Ben-PorathI , BirdAP . MBD1 is recruited to both methylated and nonmethylated CpGs via distinct DNA binding domains . Mol. Cell. Biol.24 ( 8 ), 3387 – 3395 ( 2004 ).
- Zhao X , UebaT , ChristieBRet al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function . Proc. Natl Acad. Sci. USA100 ( 11 ), 6777 – 6782 ( 2003 ).
- Roloff TC , RopersHH , NuberUA . Comparative study of methyl-CpG-binding domain proteins . BMC Genomics4 ( 1 ), 1 ( 2003 ).
- Gnanapragasam MN , ScarsdaleJN , AmayaMLet al. p66Alpha-MBD2 coiled-coil interaction and recruitment of Mi-2 are critical for globin gene silencing by the MBD2-NuRD complex . Proc. Natl Acad. Sci. USA108 ( 18 ), 7487 – 7492 ( 2011 ).
- Becker A , AllmannL , HofstatterMet al. Direct homo- and hetero-interactions of MeCP2 and MBD2 . PLoS ONE8 ( 1 ), e53730 ( 2013 ).
- Ng HH , ZhangY , HendrichBet al. MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex . Nat. Genet.23 ( 1 ), 58 – 61 ( 1999 ).
- Hendrich B , GuyJ , RamsahoyeB , WilsonVA , BirdA . Closely related proteins MBD2 and MBD3 play distinctive but interacting roles in mouse development . Genes Dev.15 ( 6 ), 710 – 723 ( 2001 ).
- Saito M , IshikawaF . The mCpG-binding domain of human MBD3 does not bind to mCpG but interacts with NuRD/Mi2 components HDAC1 and MTA2 . J. Biol. Chem.277 ( 38 ), 35434 – 35439 ( 2002 ).
- Petronzelli F , RiccioA , MarkhamGDet al. Investigation of the substrate spectrum of the human mismatch-specific DNA N-glycosylase MED1 (MBD4): fundamental role of the catalytic domain . J. Cell. Physiol.185 ( 3 ), 473 – 480 ( 2000 ).
- Hendrich B , HardelandU , NgH-H , JiricnyJ , BirdA . The thymine glycosylase MBD4 can bind to the product of deamination at methylated CpG sites . Nature401 ( 6750 ), 301 – 304 ( 1999 ).
- Wong E , YangK , KuraguchiMet al. MBD4 inactivation increases C right-arrowT transition mutations and promotes gastrointestinal tumor formation . Proc. Natl Acad. Sci. USA99 ( 23 ), 14937 – 14942 ( 2002 ).
- Laget S , JoulieM , Le MassonFet al. The human proteins MBD5 and MBD6 associate with heterochromatin but they do not bind methylated DNA . PLoS ONE5 ( 8 ), e11982 ( 2010 ).
- Qin S , MinJ . Structure and function of the nucleosome-binding PWWP domain . Trends Biochem. Sci.39 ( 11 ), 536 – 547 ( 2014 ).
- Jung JS , JeeMK , ChoHTet al. MBD6 is a direct target of Oct4 and controls the stemness and differentiation of adipose tissue-derived stem cells . Cell. Mol. Life Sci.70 ( 4 ), 711 – 728 ( 2013 ).
- Falandry C , FourelG , GalyVet al. CLLD8/KMT1F is a lysine methyltransferase that is important for chromosome segregation . J. Biol. Chem.285 ( 26 ), 20234 – 20241 ( 2010 ).
- Schultz DC , AyyanathanK , NegorevD , MaulGG , RauscherFJ , 3rd . SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins . Genes Dev.16 ( 8 ), 919 – 932 ( 2002 ).
- Jones MH , HamanaN , NezuJ , ShimaneM . A novel family of bromodomain genes . Genomics63 ( 1 ), 40 – 45 ( 2000 ).
- Loyola A , TagamiH , BonaldiTet al. The HP1alpha-CAF1-SetDB1-containing complex provides H3K9me1 for Suv39-mediated K9me3 in pericentric heterochromatin . EMBO Rep.10 ( 7 ), 769 – 775 ( 2009 ).
- Strohner R , NemethA , JansaPet al. NoRC – a novel member of mammalian ISWI-containing chromatin remodeling machines . EMBO J.20 ( 17 ), 4892 – 4900 ( 2001 ).
- Santoro R , GrummtI . Epigenetic mechanism of rRNA gene silencing: temporal order of NoRC-mediated histone modification, chromatin remodeling, and DNA methylation . Mol. Cell. Biol.25 ( 7 ), 2539 – 2546 ( 2005 ).
- Cramer JM , ScarsdaleJN , WalavalkarNM , BuchwaldWA , GinderGD , WilliamsDC . Probing the dynamic distribution of bound states for methyl-cytosine binding domains on DNA . J. Biol. Chem.289 ( 3 ), 1294 – 1302 ( 2013 ).
- Fraga MF , BallestarE , MontoyaG , TaysavangP , WadePA , EstellerM . The affinity of different MBD proteins for a specific methylated locus depends on their intrinsic binding properties . Nucleic Acids Res.31 ( 6 ), 1765 – 1774 ( 2003 ).
- Hashimoto H , LiuY , UpadhyayAKet al. Recognition and potential mechanisms for replication and erasure of cytosine hydroxymethylation . Nucleic Acids Res.40 ( 11 ), 4841 – 4849 ( 2012 ).
- Valinluck V , TsaiHH , RogstadDK , BurdzyA , BirdA , SowersLC . Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2) . Nucleic Acids Res.32 ( 14 ), 4100 – 4108 ( 2004 ).
- Spruijt CG , GnerlichF , SmitsAHet al. Dynamic readers for 5-(hydroxy) methylcytosine and its oxidized derivatives . Cell152 ( 5 ), 1146 – 1159 ( 2013 ).
- Gabel HW , KindeB , StroudHet al. Disruption of DNA-methylation-dependent long gene repression in Rett syndrome . Nature doi:10.1038/nature14319 ( 2015 ) ( Epub ahead of print ).
- Nestor CE , OttavianoR , ReddingtonJet al. Tissue type is a major modifier of the 5-hydroxymethylcytosine content of human genes . Genome Res.22 ( 3 ), 467 – 477 ( 2012 ).
- Chatagnon A , PerriaudL , NazaretNet al. Preferential binding of the methyl-CpG binding domain protein 2 at methylated transcriptional start site regions . Epigenetics6 ( 11 ), 1295 – 1307 ( 2011 ).
- Menafra R , BrinkmanAB , MatareseFet al. Genome-wide binding of MBD2 reveals strong preference for highly methylated loci . PLoS ONE9 ( 6 ), e99603 ( 2014 ).
- Skene PJ , IllingworthRS , WebbSet al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state . Mol. Cell37 ( 4 ), 457 – 468 ( 2010 ).
- Mayer C , SchmitzKM , LiJ , GrummtI , SantoroR . Intergenic transcripts regulate the epigenetic state of rRNA genes . Mol. Cell22 ( 3 ), 351 – 361 ( 2006 ).
- Jeffery L , NakielnyS . Components of the DNA methylation system of chromatin control are RNA-binding proteins . J. Biol. Chem.279 ( 47 ), 49479 – 49487 ( 2004 ).
- Grewal SI , JiaS . Heterochromatin revisited . Nat. Rev. Genet.8 ( 1 ), 35 – 46 ( 2007 ).
- Brero A , EaswaranHP , NowakDet al. Methyl CpG-binding proteins induce large-scale chromatin reorganization during terminal differentiation . J. Cell Biol.169 ( 5 ), 733 – 743 ( 2005 ).
- Casas-Delucchi CS , BeckerA , BoliusJJ , CardosoMC . Targeted manipulation of heterochromatin rescues MeCP2 Rett mutants and re-establishes higher order chromatin organization . Nucleic Acids Res.40 ( 22 ), e176 ( 2012 ).
- Cedar H , BergmanY . Linking DNA methylation and histone modification: patterns and paradigms . Nat. Rev. Genet.10 ( 5 ), 295 – 304 ( 2009 ).
- Sakamoto Y , WatanabeS , IchimuraTet al. Overlapping roles of the methylated DNA-binding protein MBD1 and polycomb group proteins in transcriptional repression of HOXA genes and heterochromatin foci formation . J. Biol. Chem.282 ( 22 ), 16391 – 16400 ( 2007 ).
- Minkovsky A , SahakyanA , Rankin-GeeE , BonoraG , PatelS , PlathK . The MBD1-Atf7ip-Setdb1 pathway contributes to the maintenance of X chromosome inactivation . Epigenetics Chromatin7 , 12 ( 2014 ).
- Singleton MK , GonzalesML , LeungKNet al. MeCP2 is required for global heterochromatic and nucleolar changes during activity-dependent neuronal maturation . Neurobiol. Dis.43 ( 1 ), 190 – 200 ( 2011 ).
- Shahbazian M , YoungJ , Yuva-PaylorLet al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3 . Neuron35 ( 2 ), 243 – 254 ( 2002 ).
- Meehan RR , LewisJD , MckayS , KleinerEL , BirdAP . Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs . Cell58 ( 3 ), 499 – 507 ( 1989 ).
- Magdinier F , WolffeAP . Selective association of the methyl-CpG binding protein MBD2 with the silent p14/p16 locus in human neoplasia . Proc. Natl Acad. Sci. USA98 ( 9 ), 4990 – 4995 ( 2001 ).
- Lin X , NelsonWG . Methyl-CpG-binding domain protein-2 mediates transcriptional repression associated with hypermethylated GSTP1 CpG islands in MCF-7 breast cancer cells . Cancer Res.63 ( 2 ), 498 – 504 ( 2003 ).
- Zhao Q , RankG , TanYTet al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing . Nat. Struct. Mol. Biol.16 ( 3 ), 304 – 311 ( 2009 ).
- Di Lorenzo A , BedfordMT . Histone arginine methylation . FEBS Lett.585 ( 13 ), 2024 – 2031 ( 2011 ).
- Wang H , HuangZQ , XiaLet al. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor . Science293 ( 5531 ), 853 – 857 ( 2001 ).
- Pal S , VishwanathSN , Erdjument-BromageH , TempstP , SifS . Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor-suppressor genes . Mol. Cell. Biol.24 ( 21 ), 9630 – 9645 ( 2004 ).
- Becker PB , WorkmanJL . Nucleosome remodeling and epigenetics . Cold Spring Harb. Perspect. Biol.5 ( 9 ), a017905 ( 2013 ).
- Song JZ , StirzakerC , HarrisonJ , MelkiJR , ClarkSJ . Hypermethylation trigger of the glutathione-S-transferase gene (GSTP1) in prostate cancer cells . Oncogene21 ( 7 ), 1048 – 1061 ( 2002 ).
- Stirzaker C , SongJZ , DavidsonB , ClarkSJ . Transcriptional gene silencing promotes DNA hypermethylation through a sequential change in chromatin modifications in cancer cells . Cancer Res.64 ( 11 ), 3871 – 3877 ( 2004 ).
- Pombo A , DillonN . Three-dimensional genome architecture: players and mechanisms . Nat. Rev. Mol. Cell Biol.16 ( 4 ), 245 – 257 ( 2015 ).
- Georgel PT , Horowitz-SchererRA , AdkinsN , WoodcockCL , WadePA , HansenJC . Chromatin compaction by human MeCP2. Assembly of novel secondary chromatin structures in the absence of DNA methylation . J. Biol. Chem.278 ( 34 ), 32181 – 32188 ( 2003 ).
- Nikitina T , ShiX , GhoshRP , Horowitz-SchererRA , HansenJC , WoodcockCL . Multiple modes of interaction between the methylated DNA binding protein MeCP2 and chromatin . Mol. Cell. Biol.27 ( 3 ), 864 – 877 ( 2007 ).
- Stuss DP , CheemaM , NgMKet al. Impaired in vivo binding of MeCP2 to chromatin in the absence of its DNA methyl-binding domain . Nucleic Acids Res.41 ( 9 ), 4888 – 4900 ( 2013 ).
- Horike S , CaiS , MiyanoM , ChengJF , Kohwi-ShigematsuT . Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome . Nat. Genet.37 ( 1 ), 31 – 40 ( 2005 ).
- Nan X , HouJ , MacleanAet al. Interaction between chromatin proteins MECP2 and ATRX is disrupted by mutations that cause inherited mental retardation . Proc. Natl Acad. Sci. USA104 ( 8 ), 2709 – 2714 ( 2007 ).
- Rountree MR , BachmanKE , BaylinSB . DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci . Nat. Genet.25 ( 3 ), 269 – 277 ( 2000 ).
- Shibahara K , StillmanB . Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin . Cell96 ( 4 ), 575 – 585 ( 1999 ).
- Lu Y , LohYH , LiHet al. Alternative splicing of MBD2 supports self-renewal in human pluripotent stem cells . Cell Stem Cell15 ( 1 ), 92 – 101 ( 2014 ).
- Rais Y , ZviranA , GeulaSet al. Deterministic direct reprogramming of somatic cells to pluripotency . Nature502 ( 7469 ), 65 – 70 ( 2013 ).
- Shimoda N , HiroseK , KanetoRet al. No evidence for AID/MBD4-coupled DNA demethylation in zebrafish embryos . PLoS ONE9 ( 12 ), e114816 ( 2014 ).
- Cunha S , LinYC , GoossenEAet al. The RON receptor tyrosine kinase promotes metastasis by triggering MBD4-dependent DNA methylation reprogramming . Cell Rep.6 ( 1 ), 141 – 154 ( 2014 ).
- Pulukuri S , RaoJS . CpG island promoter methylation and silencing of 14–3–3σ gene expression in LNCaP and Tramp-C1 prostate cancer cell lines is associated with methyl-CpG-binding protein MBD2 . Oncogene25 ( 33 ), 4559 – 4572 ( 2006 ).
- Chatagnon A , BougelS , PerriaudL , LachuerJ , BenhattarJ , DanteR . Specific association between the methyl-CpG-binding domain protein 2 and the hypermethylated region of the human telomerase reverse transcriptase promoter in cancer cells . Carcinogenesis30 ( 1 ), 28 – 34 ( 2009 ).
- Lopez-Serra L , BallestarE , FragaMF , AlaminosM , SetienF , EstellerM . A profile of methyl-CpG binding domain protein occupancy of hypermethylated promoter CpG islands of tumor suppressor genes in human cancer . Cancer Res.66 ( 17 ), 8342 – 8346 ( 2006 ).
- Sansom OJ , BergerJ , BishopSM , HendrichB , BirdA , ClarkeAR . Deficiency of Mbd2 suppresses intestinal tumorigenesis . Nat. Genet.34 ( 2 ), 145 – 147 ( 2003 ).
- Hodge JC , MitchellE , PillalamarriVet al. Disruption of MBD5 contributes to a spectrum of psychopathology and neurodevelopmental abnormalities . Mol. Psychiatry19 ( 3 ), 368 – 379 ( 2014 ).
- Talkowski ME , MullegamaSV , RosenfeldJAet al. Assessment of 2q23.1 microdeletion syndrome implicates MBD5 as a single causal locus of intellectual disability, epilepsy, and autism spectrum disorder . Am. J. Hum. Genet.89 ( 4 ), 551 – 563 ( 2011 ).
- Williams SR , MullegamaSV , RosenfeldJAet al. Haploinsufficiency of MBD5 associated with a syndrome involving microcephaly, intellectual disabilities, severe speech impairment, and seizures . Eur. J. Hum. Genet.18 ( 4 ), 436 – 441 ( 2010 ).
- Cerami E , GaoJ , DogrusozUet al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data . Cancer Discov.2 ( 5 ), 401 – 404 ( 2012 ).
- Gao J , AksoyBA , DogrusozUet al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal . Sci. Signal.6 ( 269 ), pl1 ( 2013 ).
- Desai MA , WebbHD , SinananLMet al. An intrinsically disordered region of methyl-CpG binding domain protein 2 (MBD2) recruits the histone deacetylase core of the NuRD complex . Nucleic Acids Res.43 ( 6 ), 3100 – 3113 ( 2015 ).
- The Cancer Genome Atlas (TCGA) . http://cancergenome.nih.gov
- cBioPortal for Cancer Genomics . www.cbioportal.org/public-portal/
- cBioPortal for Cancer Genomics: MBD1 . http://bit.ly/15Opeso
- cBioPortal for Cancer Genomics: MBD2 . http://bit.ly/1zR4Jou
- cBioPortal for Cancer Genomics: MBD3 . http://bit.ly/15OplEn
- cBioPortal for Cancer Genomics: MBD4 . http://bit.ly/1zgXlSs
- cBioPortal for Cancer Genomics: MeCP2 . http://bit.ly/1vkwzth
- Miquel C , JacobS , GrandjouanSet al. Frequent alteration of DNA damage signalling and repair pathways in human colorectal cancers with microsatellite instability . Oncogene26 ( 40 ), 5919 – 5926 ( 2007 ).
- Khrapunov S , WarrenC , ChengH , BerkoER , GreallyJM , BrenowitzM . Unusual characteristics of the DNA binding domain of epigenetic regulatory protein MeCP2 determine its binding specificity . Biochemistry (Mosc.)53 ( 21 ), 3379 – 3391 ( 2014 ).
- Landau DA , ClementK , ZillerMJet al. Locally disordered methylation forms the basis of intratumor methylome variation in chronic lymphocytic leukemia . Cancer Cell26 ( 6 ), 813 – 825 ( 2014 ).
