Abstract
The ability of environmental exposures to induce phenotypic change across multiple generations of offspring has gathered an enormous amount of interest in recent years. There are by now many examples of nongenetic transgenerational effects of environmental exposures, covering a broad range of stressors. Available evidence indicates that epigenetic inheritance may mediate at least some of these transgenerational effects, but how environmental exposures induce changes to the epigenome of the germline is unknown. One possibility is that exposed somatic cells can communicate their exposures to the germline to induce a stable change. In this Perspective, we propose that extracellular vesicles shed by somatic cells represent a credible means by which environmental experience could effect a transmissible epigenetic change in the germline, leading to the inheritance of acquired traits.
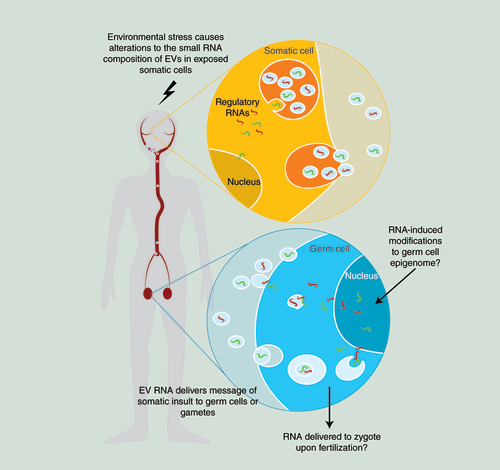
Somatic cells alter the regulatory RNA composition of EVs in response to an environmental stressors. Somatically derived EVs can passage through the circulation and may reach the germ line. Given the correct complement of surface antigens, somatic EVs may fuse with germ cells to deliver the somatic RNA message. RNA delivered to germ cells by somatic EVs may alter the germ cell epigenome, or alternatively be delivered to the egg at fertilization and interfere with molecular events in early embryogenesis.
EV: Extracellular vesicle.
“Hypotheses…are like the feelers which the short-sighted snail stretches forth on its darkened path, testing this way and that, and withdrawing them and altering its route as soon as they come across any obstacle; just as an unyielding fact may show that we are on a wrong road.”
The ‘impermeable’ Weismann barrier
More than 120 years ago, August Weismann articulated a theory of heredity in which he proposed that heritable information in multicellular organisms resides exclusively in an immortal germ-plasm [Citation1]. Central to this theory was the idea that information can travel in one direction only: from the germ-plasm (germ cells) to the soma, and not in reverse. Unsurprisingly, Weismann strongly rejected Lamarck’s thinking on the inheritance of acquired characters, and concluded that the existence of ‘unnecessary new forces’ would be required to explain such phenomena [Citation2]. While Darwin himself had not ruled out a place for such a phenomenon in his evolutionary theory [Citation3], Weismann’s views quickly gained general acceptance, and the principle of the impenetrable Weismann barrier separating germline and soma became a cornerstone of modern genetics. The concept has remained largely unchallenged in animal biology.
The Weismann barrier is a theoretical barrier – there is no structure that separates germ cells from surrounding somatic cells, in any organism. In fact, we now understand that Weismann’s core principle is moot in the plant kingdom: the gametes contained within pollen grains and ovules can be derived from mature somatic cells [Citation4]. This explains why plants sometimes inherit the mutations that occur in somatic tissues of the parent plant. It may also explain why pure epigenetic inheritance is more commonly observed in plants than in animals [Citation5]. Unlike plants, animals segregate a dedicated germline very early in development; the imperviousness of the Weismann barrier in metazoans has remained an accepted if empirically untested principle for more than a century (although not unchallenged [Citation6]). The steadily growing evidence for germline transmission of life experience suggests that Weismann’s barrier may not be entirely impermeable.
Intergenerational transmission of environmental effects
Environmental stress (both intrinsic and extrinsic) can have effects on phenotype that persists beyond the initially exposed generation without inducing a genetic change. The range of factors that is reported to induce such intergenerational effects is remarkably broad; it includes environmental chemicals [Citation7–9] nutritional and experiential factors such as famine [Citation10] or undernutrition [Citation11], obesity [Citation12] and smoking [Citation13] as well as stress and psychological trauma [Citation14–17]. Increasing evidence points to an epigenetic basis for transgenerational effects [Citation18], but the mechanisms by which environmental exposures might affect the germline epigenome remain unknown.
One possibility is that environmental stressors interact directly with germ cells to alter the epigenome. Although the blood–testis barrier reduces the potential for xenobiotic agents to enter the germline, some small molecules can cross this barrier; electrolytes, very small polar molecules and some classes of lipophilic molecules have the highest transfer potential [Citation19]. But the barrier restricts the entry of many substances: even glucose transport is tightly controlled [Citation20]. Direct interaction of restricted or excluded substances with germ cells is unlikely.
For certain types of transgenerational effects – those induced by psychological stressors in particular – direct interaction of the stressor and the germline is difficult to envisage. In one recent report, male mice conditioned to fear a particular odorant transmitted the fear response to their odor-naive offspring [Citation17]. The fear response could be recapitulated in offspring derived through in vitro fertilization, indicating that the heritable information was transmitted solely via sperm. In this example, a direct interaction of the stressor with the germline seems improbable: seminiferous tubules lack innervation, and available evidence indicates that the relevant odorant receptor is not expressed in the testis [Citation21]. Thus, if these findings are as they seem, it appears likely that some signal of the odorant–fear association was transmitted from the brain to the developing sperm.
Noncoding RNA can mediate transgenerational effects
Another recent report of transgenerational effects also implicates sperm in the transmission of a conditioned stress response in mice, and goes further to suggest that sperm RNA is a mediator of the inherited signal [Citation22]. Noncoding RNAs are known to direct epigenetic states in the soma and the germline [Citation23] and they have been implicated in transgenerational epigenetic inheritance in several systems [Citation24–27]. Most recently, studies in the model worm Caenorhabditis elegans clearly demonstrate soma-to-germline transfer of small noncoding RNA (sncRNA). In this study, a synthetic siRNA produced exclusively in neurons could silence a cognate reporter gene expressed in the germline [Citation28]. Furthermore, the germline silencing was manifested in multiple generations after withdrawal of the initiating neuronal RNA signal. This indicates that a stable alteration has been induced in the germline, a clear demonstration of the permeability of the Weissman barrier in a metazoan.
But how might RNA travel from neurons to germ cells? In the worm example above, the RNA transfer was dependent on the RNA transporter SID-1 [Citation28]; one way in which SID-1 can passage RNA is via vesicular shuttling [Citation29]. RNA-containing vesicles exist in all animals, and are produced constitutively by most, if not all, cell types. We propose that RNA-containing vesicles may provide a conduit by which environmental stressors can exert effects on the germline without directly contacting germ cells ().
Extracellular vesicles: tiny intercellular messengers
Extracellular vesicles (EVs) are tiny (40 nm to 1 µm) lipid-enclosed vesicles, produced by most cells, and found in all biological fluids (blood, urine, cerebrospinal fluid, semen, amniotic fluid and breast milk, among others). They are also secreted by cells into culture media. EVs are produced either by direct budding of the cell membrane, or internal to the cell within multivesicular bodies that subsequently fuse with the plasma membrane for EV release [Citation30–32]. Biological fluids usually contain a heterogeneous population of EVs, presumably contributed by multiple cell types and varying biogeneses; nevertheless all EVs, irrespective of their cell of origin or mode of release, contain a complex cargo of lipid, protein and nucleic acid.
Remarkably, the molecular cargo of EVs is not necessarily representative of the parent cell’s cytosol: specific proteins and nucleic acid species appear to be ‘selectively packaged’ into EVs [Citation33–38]. This is most obvious with RNA: EV RNA is usually devoid of ribosomal RNA, and instead is highly enriched for particular mRNAs and sncRNAs. The well-studied miRNAs are present (but not necessarily prominent), as are a variety of poorly characterized sncRNA species: vault RNAs and their derivatives, pseudogene-derived sncRNA, tRNA derivatives and retrotransposon-derived sncRNA [Citation33,Citation38–39]. In some cases, relatively abundant RNA species in EVs are virtually undetectable in the parent cell RNA [Citation33].
Once thought to be simply garbage disposal units of the cell [Citation40], it is now clear that EVs and their associated cargo represent a form of intercellular communication. It has been known for some time that EVs can trigger cellular responses by receptor-mediated binding to target cells, triggering intercellular signaling cascades [Citation41]. More recently it has become clear that EVs can fuse with, or be endocytosed by, target cells [Citation42]. This results in deposition of their molecular cargo within the recipient’s cytosol, which can change cell physiology. A plethora of studies demonstrate such changes; for a comprehensive review see [Citation43]. EVs can have a range of effects on target cells both in vitro and in vivo: tumor-derived EVs are implicated in tumor invasion, promotion of angiogenesis and metastasis [Citation44,Citation45]; EVs from cardiac progenitor cells can stimulate cardiomyocyte regeneration of the infarcted heart [Citation46]; EVs carry pathogenic prion proteins to promote neurodegeneration [Citation47]; embryonic stem cell EVs can even reprogram committed progenitors back to pluripotency [Citation34].
While EVs from many sources may be encountered by a given cell, there is selectivity to EV uptake: the specificity of EV–cell interactions is likely conferred by the complement of surface ligands on both EVs and the target cell [Citation48]. This receptor specificity was exploited in a landmark study highlighting the therapeutic potential of EVs for drug delivery across the blood–brain barrier (BBB) [Citation49]. In this study, EVs from murine dendritic cells were engineered to express a neural-specific surface peptide, and loaded with siRNAs targeting GAPDH. Infusion of these EVs into the tail vein of wild-type mice resulted in specific knockdown of GAPDH in neurons; no knockdown was observed in non-neuronal cells of the brain in glia or various distal tissues, including the liver [Citation49]. This experiment demonstrates that, given the correct surface markers, EVs derived from a distal cell type can traverse a physical barrier (the BBB) to modulate the behavior of another cell via sncRNA cargo.
EVs as messengers of somatic experience
EVs can cross the BBB – could they also cross the Weismann barrier to deliver information induced in somatic cells by environmental exposures? A limited amount of evidence indicates that EV contents – in particular, RNA – can be altered in response to environmental conditions. Exposure of cultured cardiomyocytes to growth factors known to be upregulated in heart failure causes widespread alterations in the mRNA content of EVs shed by these cells [Citation50]. Likewise, cultured endothelial cells exposed to hypoxia alter their EV RNA cargo [Citation51]. In vivo, circulating EVs exhibit miRNA expression profiles that differ in unstressed mice and mice exposed to a physical stress [Citation52]. In humans, the miRNA cargo within adipocyte-derived EVs differs depending on whether the subject is lean or obese [Citation53]. The effects of exogenous agents, such as xenobiotics, on the RNA composition of circulating EVs has not, to our knowledge, been tested, but if physiological stressors confer EV RNA changes, it is likely that nonphysiological stress will do so as well.
The idea that EVs may somehow be involved in mediating transgenerational transfer of information has been voiced by some others [Citation54–56]. While these ideas have never been tested experimentally, one recent study [Citation57] suggested that EVs may indeed deliver somatic RNA to the germline. In this study, mice xenografted with GFP-expressing human tumor cells also demonstrate GFP RNA in their sperm; GFP RNA was also found in crude EV preparations derived from plasma. Although these data are consistent with transport of GFP RNA to sperm via EVs, the use of tumor cells in this system leaves open the possibility that metastasis was responsible for the observation.
If EVs were to pass a message of somatic experience to the germline, this would require their interaction with developing germ cells or gametes. There is longstanding evidence for this: one of the very first observations of an EV–target cell interaction was between sperm and prostasomes (prostate-derived EVs that promote sperm motility and fertilization) [Citation58]. Sperm also encounter and interact with epididymis-derived EVs (epididymosomes) [Citation59–61] during their maturation: prostasomes and epididymosomes are rich in sncRNA [Citation62–65], which is likely to be delivered to the maturing or mature sperm along with the proteins known to be necessary for sperm maturation and fertilization. Likewise the oocyte encounters somatically derived EVs in follicular fluid prior to ovulation [Citation66–68], during transit though the oviduct [Citation69], and via the male ejaculate.
While EVs derived from somatic cells within the reproductive system probably have the greatest potential to breach the Weismann barrier, it is possible that EVs derived from distal somatic cells, such as neurons, also hold this potential. Studies in patients with brain tumors demonstrate that EVs can exit through the BBB and enter the circulation [Citation39,Citation70]; interrogation of proteome datasets from EVpedia [Citation71] reveals that EVs from multiple somatic origins, including blood, microglia and oligodendrocytes, express sperm surface proteins that could facilitate interactions with gametes. We propose that environmental stress alters the small RNA composition of EVs produced by distal somatic cells; these EVs then pass into the circulation and the somatic message is delivered to germ cells ().
Conclusion
EVs mediate RNA-based information exchange between cells and their idiosyncratic repertoires of surface receptors allow them to act locally or at distant sites. EVs can travel systemically and even cross physical barriers such as the blood–brain barrier. The intrinsic properties of EVs and their regulatory RNA cargo renders them potential conduits for environmental information transfer from the soma to the germline.
Future perspective
Research into extracellular vesicles is gaining tremendous momentum in both development and disease – there is almost no area of biology to which EVs do not bear some relevance. We are proposing EVs as a means by which information about environmental conditions can be transmitted to the germline. It may not be the only means, but it is a credible means that can be tested in the right model system.
The ‘impermeable’ Weismann barrier
The Weismann barrier is a theoretical barrier that disallows passage of information from the soma to the germline.
Evidence for transgenerational inheritance of life experiences suggests that this barrier may not be impenetrable.
Intergenerational transmission of environmental effects
Epigenetic inheritance is implicated in the multigenerational transmission of parentally induced phenotypes.
The mechanism by which parental environmental exposures may affect the germline epigenome is unknown.
Noncoding RNA can mediate transgenerational effects
Noncoding RNAs have been implicated in transgenerational epigenetic inheritance and can direct epigenetic states in the germline.
Soma-to-germline transfer of small RNAs has been demonstrated in Caenorhabditis elegans resulting in transgenerational inheritance.
RNA containing extracellular vesicles (EVs) may provide a conduit for somatic environmental stressors to influence the epigenome of germ cells.
EVs: tiny intercellular messengers
EVs are nanosized vesicles that contain a complex mix of selectively packaged lipid, protein and nucleic acid.
EVs are produced by most cells and found in all biological fluids.
EVs mediate intercellular communication; they can fuse with and deposit their cargo into target cells which can change cell physiology.
EVs derived from a one cell type can modulate the behavior of another cell via small RNA cargo.
EVs as messengers of somatic experience
RNA content of EVs can be altered in response to environmental exposures.
EVs have been shown to transfer cargo to germ cells.
EVs from somatic cells within the reproductive system have the greatest potential to breach the Weismann barrier but EVs derived from distal cells such as neurons also hold this potential.
Future perspective
We propose that EVs are a means by which information relating to somatic environmental conditions can be transmitted to the germline.
Financial & competing interests disclosure
JE Cropley and CM Suter are DECRA (DE120100723) and Future (FT120100097) Fellows of the Australian Research Council. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Weismann A . The Germ-Plasm: a Theory of Heredity. Translated by W. Newton Parker and Harriet Rönnfeldt . Scribner , NY, USA ( 1893 ).
- Winther RG . August Weismann on germ-plasm variation . J. Hist. Biol.34 ( 3 ), 517 – 555 ( 2001 ).
- Darwin C . The Variation of Animals and Plants Under Domestication . MurrayJ ( Ed. ). London, UK ( 1868 ).
- Alberts B , WilsonJH , HuntT . Molecular Biology of the Cell (5th Edition) . Garland Science , NY, USA ( 2008 ).
- Jablonka E , RazG . Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution . Q. Rev. Biol.84 ( 2 ), 131 – 176 ( 2009 ).
- Steele EJ , LindleyRA , BlandenRV . Lamarck’s Signature: How Retrogenes are Changing Darwin’s Natural Selection Paradigm . Allen-Unwin , Sydney, Australia ( 1998 ).
- Anway MD , CuppAS , UzumcuM , SkinnerMK . Epigenetic transgenerational actions of endocrine disruptors and male fertility . Science308 ( 5727 ), 1466 – 1469 ( 2005 ).
- Wolstenholme JT , EdwardsM , ShettySRet al. Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression . Endocrinology153 ( 8 ), 3828 – 3838 ( 2012 ).
- Salian S , DoshiT , VanageG . Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to bisphenol A . Life Sci.85 ( 1–2 ), 11 – 18 ( 2009 ).
- Heijmans BT , TobiEW , SteinADet al. Persistent epigenetic differences associated with prenatal exposure to famine in humans . Proc. Natl Acad. Sci. USA105 ( 44 ), 17046 – 17049 ( 2008 ).
- Jimenez-Chillaron JC , IsganaitisE , CharalambousMet al. Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice . Diabetes58 ( 2 ), 460 – 468 ( 2009 ).
- Ng SF , LinRC , LaybuttDR , BarresR , OwensJA , MorrisMJ . Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring . Nature467 ( 7318 ), 963 – 966 ( 2010 ).
- Pembrey ME , BygrenLO , KaatiGet al. Sex-specific, male-line transgenerational responses in humans . Eur. J. Hum. Genet.14 ( 2 ), 159 – 166 ( 2006 ).
- Matthews SG , PhillipsDI . Minireview: transgenerational inheritance of the stress response: a new frontier in stress research . Endocrinology151 ( 1 ), 7 – 13 ( 2010 ).
- Rosenheck R , FontanaA . Transgenerational effects of abusive violence on the children of Vietnam combat veterans . J. Trauma Stress11 ( 4 ), 731 – 742 ( 1998 ).
- Yehuda R , EngelSM , BrandSR , SecklJ , MarcusSM , BerkowitzGS . Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the world trade center attacks during pregnancy . J. Clin. Endocrinol. Metab.90 ( 7 ), 4115 – 4118 ( 2005 ).
- Dias BG , ResslerKJ . Parental olfactory experience influences behavior and neural structure in subsequent generations . Nat. Neurosci.17 ( 1 ), 89 – 96 ( 2014 ).
- Jirtle RL , SkinnerMK . Environmental epigenomics and disease susceptibility . Nat. Rev. Genet.8 ( 4 ), 253 – 262 ( 2007 ).
- Okumura K , LeeIP , DixonRL . Permeability of selected drugs and chemicals across the blood-testis barrier of the rat . J. Pharmacol. Exp. Ther.194 ( 1 ), 89 – 95 ( 1975 ).
- Turner TT , D’addarioDA , HowardsSS . [3H]3-O-methyl-D-glucose transport from blood into the lumina of the seminiferous and epididymal tubules in intact and vasectomized hamsters . J. Reprod. Fertil.60 ( 2 ), 285 – 289 ( 1980 ).
- Feldmesser E , OlenderT , KhenM , YanaiI , OphirR , LancetD . Widespread ectopic expression of olfactory receptor genes . BMC Genomics7 , 121 ( 2006 ).
- Gapp K , JawaidA , SarkiesPet al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice . Nat. Neurosci.17 ( 5 ), 667 – 669 ( 2014 ).
- Stuwe E , TothKF , AravinAA . Small but sturdy: small RNAs in cellular memory and epigenetics . Genes Dev.28 ( 5 ), 423 – 431 ( 2014 ).
- Ashe A , SapetschnigA , WeickEMet al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans . Cell150 ( 1 ), 88 – 99 ( 2012 ).
- Buckley BA , BurkhartKB , GuSGet al. A nuclear argonaute promotes multigenerational epigenetic inheritance and germline immortality . Nature489 ( 7416 ), 447 – 451 ( 2012 ).
- Rechavi O , Houri-Ze’eviL , AnavaSet al. Starvation-induced transgenerational inheritance of small RNAs in C. elegans . Cell158 ( 2 ), 277 – 287 ( 2014 ).
- Grentzinger T , ArmeniseC , BrunCet al. piRNA-mediated transgenerational inheritance of an acquired trait . Genome Res.22 ( 10 ), 1877 – 1888 ( 2012 ).
- Devanapally S , RavikumarS , JoseAM . Double-stranded RNA made in C. elegans neurons can enter the germline and cause transgenerational gene silencing . Proc. Natl Acad. Sci. USA112 ( 7 ), 2133 – 2138 ( 2015 ).
- Mcewan DL , WeismanAS , HunterCP . Uptake of extracellular double-stranded RNA by SID-2 . Mol. Cell47 ( 5 ), 746 – 754 ( 2012 ).
- Raposo G , StoorvogelW . Extracellular vesicles: exosomes, microvesicles, and friends . J. Cell Biol.200 ( 4 ), 373 – 383 ( 2013 ).
- Cocucci E , MeldolesiJ . Ectosomes and exosomes: shedding the confusion between extracellular vesicles . Trends Cell Biol.25 ( 6 ), 364 – 372 ( 2015 ).
- Colombo M , RaposoG , TheryC . Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles . Annu. Rev. Cell Dev. Biol.30 , 255 – 289 ( 2014 ).
- Li CC , EatonSA , YoungPEet al. Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cells . RNA Biology10 ( 8 ), 1333 – 1344 ( 2013 ).
- Ratajczak J , MiekusK , KuciaMet al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery . Leukemia20 ( 5 ), 847 – 856 ( 2006 ).
- Valadi H , EkstromK , BossiosA , SjostrandM , LeeJJ , LotvallJO . Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells . Nat. Cell Biol.9 ( 6 ), 654 – 659 ( 2007 ).
- Deregibus MC , CantaluppiV , CalogeroRet al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA . Blood110 ( 7 ), 2440 – 2448 ( 2007 ).
- Herrera MB , FonsatoV , GattiSet al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats . J. Cell. Mol. Med.14 ( 6B ), 1605 – 1618 ( 2010 ).
- Nolte-’t Hoen EN , BuermansHP , WaasdorpM , StoorvogelW , WaubenMH , T HoenPA . Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions . Nucleic Acids Res.40 ( 18 ), 9272 – 9285 ( 2012 ).
- Skog J , WurdingerT , Van RijnSet al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers . Nat. Cell Biol.10 ( 12 ), 1470 – 1476 ( 2008 ).
- Johnstone RM , AdamM , HammondJR , OrrL , TurbideC . Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) . J. Biol. Chem.262 ( 19 ), 9412 – 9420 ( 1987 ).
- Raposo G , NijmanHW , StoorvogelWet al. B lymphocytes secrete antigen-presenting vesicles . J. Exp. Med.183 ( 3 ), 1161 – 1172 ( 1996 ).
- Mulcahy LA , PinkRC , CarterDR . Routes and mechanisms of extracellular vesicle uptake . J. Extracell. Vesicles3 , 24641 ( 2014 ).
- Lee Y , El AndaloussiS , WoodMJ . Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy . Hum. Mol. Genet.21 ( R1 ), R125 – 134 ( 2012 ).
- Lee TH , D’astiE , MagnusN , Al-NedawiK , MeehanB , RakJ . Microvesicles as mediators of intercellular communication in cancer – the emerging science of cellular ‘debris’ . Semin. Immunopathol.33 ( 5 ), 455 – 467 ( 2011 ).
- Muralidharan-Chari V , ClancyJW , SedgwickA , D’Souza-SchoreyC . Microvesicles: mediators of extracellular communication during cancer progression . J. Cell Sci.123 ( Pt 10 ), 1603 – 1611 ( 2010 ).
- Lai RC , ArslanF , LeeMMet al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury . Stem Cell Res.4 ( 3 ), 214 – 222 ( 2010 ).
- Vella LJ , SharplesRA , LawsonVA , MastersCL , CappaiR , HillAF . Packaging of prions into exosomes is associated with a novel pathway of PRP processing . J. Pathol.211 ( 5 ), 582 – 590 ( 2007 ).
- Rana S , YueS , StadelD , ZollerM . Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection . Int. J. Biochem. Cell Biol.44 ( 9 ), 1574 – 1584 ( 2012 ).
- Alvarez-Erviti L , SeowY , YinH , BettsC , LakhalS , WoodMJ . Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes . Nat. Biotechnol.29 ( 4 ), 341 – 345 ( 2011 ).
- Genneback N , HellmanU , MalmLet al. Growth factor stimulation of cardiomyocytes induces changes in the transcriptional contents of secreted exosomes . J. Extracell. Vesicles2 , 2016 ( 2013 ).
- De Jong OG , VerhaarMC , ChenYet al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes . J. Extracell. Vesicles1 , 18396 ( 2012 ).
- Beninson LA , BrownPN , LoughridgeABet al. Acute stressor exposure modifies plasma exosome-associated heat shock protein 72 (hsp72) and microRNA (miR-142–5p and miR-203) . PLoS ONE9 ( 9 ), e108748 ( 2014 ).
- Ferrante SC , NadlerEP , PillaiDKet al. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease . Pediatr. Res.77 ( 3 ), 447 – 454 ( 2015 ).
- Barry G . Lamarckian evolution explains human brain evolution and psychiatric disorders . Front. Neurosci.7 , 224 ( 2013 ).
- Sharma A . Bioinformatic analysis revealing association of exosomal mRNAs and proteins in epigenetic inheritance . J. Theor. Biol.357 , 143 – 149 ( 2014 ).
- Smythies J , EdelsteinL , RamachandranV . Molecular mechanisms for the inheritance of acquired characteristics-exosomes, microRNA shuttling, fear and stress: Lamarck resurrected?Front. Genet.5 , 133 ( 2014 ).
- Cossetti C , LuginiL , AstrologoL , SaggioI , FaisS , SpadaforaC . Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes . PLoS ONE9 ( 7 ), e101629 ( 2014 ).
- Stegmayr B , RonquistG . Promotive effect on human sperm progressive motility by prostasomes . Urol. Res.10 ( 5 ), 253 – 257 ( 1982 ).
- Frenette G , SullivanR . Prostasome-like particles are involved in the transfer of p25b from the bovine epididymal fluid to the sperm surface . Mol. Reprod. Dev.59 ( 1 ), 115 – 121 ( 2001 ).
- Oh JS , HanC , ChoC . Adam7 is associated with epididymosomes and integrated into sperm plasma membrane . Mol. Cells28 ( 5 ), 441 – 446 ( 2009 ).
- Suryawanshi AR , KhanSA , JoshiCS , KholeVV . Epididymosome-mediated acquisition of mmsdh, an androgen-dependent and developmentally regulated epididymal sperm protein . J. Androl.33 ( 5 ), 963 – 974 ( 2012 ).
- Belleannee C , CalvoE , CaballeroJ , SullivanR . Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis . Biol. Reprod.89 ( 2 ), 30 ( 2013 ).
- Belleannee C , LegareC , CalvoE , ThimonV , SullivanR . microRNA signature is altered in both human epididymis and seminal microvesicles following vasectomy . Hum. Reprod.28 ( 6 ), 1455 – 1467 ( 2013 ).
- Li H , HuangS , GuoC , GuanH , XiongC . Cell-free seminal mRNA and microRNA exist in different forms . PLoS ONE7 ( 4 ), e34566 ( 2012 ).
- Vojtech L , WooS , HughesSet al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions . Nucleic Acids Res.42 ( 11 ), 7290 – 7304 ( 2014 ).
- Da Silveira JC , VeeramachaneniDN , WingerQA , CarnevaleEM , BoumaGJ . Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle . Biol. Reprod.86 ( 3 ), 71 ( 2012 ).
- Sang Q , YaoZ , WangHet al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo . J. Clin. Endocrinol. Metab.98 ( 7 ), 3068 – 3079 ( 2013 ).
- Sohel MM , HoelkerM , NoferestiSSet al. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence . PLoS ONE8 ( 11 ), e78505 ( 2013 ).
- Al-Dossary AA , StrehlerEE , Martin-DeleonPA . Expression and secretion of plasma membrane Ca2+ -ATPase 4a (PMCA4A) during murine estrus: association with oviductal exosomes and uptake in sperm . PLoS ONE8 ( 11 ), e80181 ( 2013 ).
- Noerholm M , BalajL , LimpergTet al. RNA expression patterns in serum microvesicles from patients with glioblastoma multiforme and controls . BMC Cancer12 , 22 ( 2012 ).
- Kim DK , LeeJ , KimSRet al. Evpedia: a community web portal for extracellular vesicles research . Bioinformatics31 ( 6 ), 933 – 939 ( 2015 ).
