Abstract
Aim: We investigated methylation of DNA repetitive sequences (LINE-1 and BAGE) in peripheral blood leukocytes from first-episode schizophrenia (FES) patients and healthy controls (HCs) with respect to childhood adversities. Materials & methods: Patients were divided into two subgroups based on the history of childhood trauma – FES(+) and FES(-) subjects. The majority of HCs had a negative history of childhood trauma – HCs(-) subjects. Results: FES(+) patients had significantly lower LINE-1 methylation in comparison with FES(-) patients or HC(-) subjects. Emotional abuse and total trauma score predicted lower LINE-1 methylation in FES patients, while general trauma score was associated with lower BAGE methylation in HCs. Conclusion: Childhood adversities might be associated with global DNA hypomethylation in adult FES patients.
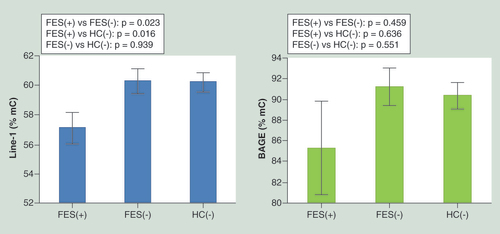
Mean values are presented and error bars refer to 95% CL.
FES(+): First-episode schizophrenia patients with childhood trauma; FES(-): First-episode schizophrenia patients without childhood trauma; HC(-): Healthy controls without childhood trauma.
The history of childhood trauma is highly prevalent among patients with schizophrenia-spectrum disorders and correlates with higher severity of psychotic symptoms, poorer clinical and functional outcomes [Citation1–4]. A recent meta-analysis of retrospective studies evaluating self-reported childhood adversities in psychosis estimated the prevalence of sexual, physical and emotional abuse in childhood at 26, 39 and 34%, respectively [Citation5]. Another meta-analysis revealed that the history of childhood trauma increases the risk of psychosis over two-times regardless of a study design [Citation6]. Interestingly, it has been found that childhood trauma might be associated with more prominent dysregulation of biological correlates that are involved in the etiology of psychosis. Indeed, there are studies showing lower brain-derived neurotrophic factor (BDNF) levels [Citation7], higher levels of pro-inflammatory cytokines [Citation8], higher hypothalamic–pituitary–adrenal (HPA) axis activation [Citation9,Citation10] in first-episode psychosis patients, who were exposed to traumatic events during childhood in comparison with those, who were not. Our group has also found that the history of childhood trauma might be associated with higher blood pressure and lipid disturbances in first-episode schizophrenia (FES) patients [Citation11]. Moreover, stress-related biological alterations in schizophrenia have been described in frame of useful conceptualization models including the Reactive Scope Model [Citation12], the Neural Diathesis-Stress Model [Citation13] or the Allostatic Load concept [Citation14]. These clinical findings and biological correlates of childhood adversities suggest that psychosis with positive history of childhood trauma might be a distinct psychiatric phenotype.
Convincing evidence indicates that early life stress may exert profound and sustained effects on epigenetic mechanisms, especially DNA methylation [Citation15]. Initially, studies of animal models have provided that maternal separation or early life adversities may influence DNA methylation of genes involved in HPA axis circuitry [Citation16]. Subsequently, this observation has provided grounds for epigenetic studies on post-traumatic stress disorder (PTSD) patients. These studies have associated PTSD or exposure to early trauma with aberrant DNA methylation of several genes involved in HPA axis regulation [Citation17–20], immune response [Citation21,Citation22], serotonergic transmission [Citation23], neuronal plasticity [Citation24], CNS development and immune tolerance induction pathways [Citation25].
Accumulating evidence indicates differential DNA methylation patterns of genes involved in dopaminergic and serotonergic transmission as well as neurodevelopmental processes in schizophrenia patients [Citation26]. Aberrant epigenetic regulation might be also an early phenomenon in schizophrenia patients as it has been also confirmed in drug-naive first-episode patients [Citation27–30]. Interestingly, it has been shown that epigenetic dysregulation in schizophrenia might be systemic since concordant patterns of DNA methylation have been observed in the brain and peripheral tissues [Citation30–34]. Although the majority of epigenetic studies look at gene-specific methylation patterns, global DNA hypomethylation has also been found in peripheral blood leukocytes of schizophrenia patients [Citation35,Citation36].
Although it has been widely reported that stressful life events including childhood trauma may influence epigenetic programming, there are no studies investigating this interaction in schizophrenia patients. A recent study by Rusiecki et al. [Citation37] indicated that different methylation patterns of repetitive DNA sequences including LINE-1 and Alu sequence might be involved in resilience and susceptibility to PTSD. Interestingly, upregulation of LINE-1 and Alu sequences in response to stress has been reported in horses and various human cell lines [Citation38,Citation39]. These findings suggest that increase in LINE-1 and Alu sequences might be an evolutionarily conserved mechanism of stress response. Indeed, repetitive DNA elements are abundant noncoding sequences that are considered as relics of previous transposition events [Citation40]. It has been found that LINE-1 transposition may play a role in differentiation of neurons during brain development [Citation41]. There is evidence that LINE-1 is the only retrotransposon that has autonomous retrotansposition activities in neural rat progenitor cells from the hippocampus, human fetal brain and human embryonic stem cells [Citation42,Citation43]. Additionally, LINE-1 retrotransposition has been identified during adult neurogenesis in the hippocampus [Citation44]. Importantly, Bundo et al. [Citation45] revealed increased LINE-1 retrotransposition in neurons from prefrontal cortex of patients with schizophrenia, especially in genes involved in synaptic functions. Authors confirmed these results in induced pluripotent stem cells from patients with 22q11 deletion syndrome [Citation45]. They also found a trend toward statistical significance for increased LINE-1 retrotransposition in patients with major depression and bipolar disorder [Citation45]. Hyperactive LINE-1 retrotransposition has been also documented in other neuropsychiatric phenotypes including Rett syndrome [Citation41] and ataxia telangiectasia [Citation46]. These findings suggest that aberrant LINE-1 retrotransposition plays an important role in a broader spectrum of developmental neuropsychiatric disorders. Less is known about B-melanoma antigen (BAGE) sequences, which are hypermethylated in normal tissues and hypomethylated in cancers [Citation47]. To date, these sequences have been mapped to chromosomes 9, 13, 14, 15, 18, 21 and 22 [Citation48]. Importantly, repetitive DNA sequences comprise approximately 50% of the human genome and thus might be perceived as surrogate measures of global DNA methylation [Citation49]. The aims of this study were: to compare DNA methylation levels of two repetitive sequences – LINE-1 and BAGE between FES patients and healthy controls and to investigate the relationship between childhood trauma and methylation levels of LINE-1 and BAGE.
Materials & methods
Subjects
We recruited 48 FES patients and 48 healthy controls matched for age, gender and BMI as well as serum levels of homocysteine (Hcy), vitamin B12 and folate since these variables have been associated with DNA methylation [Citation50–53]. A diagnosis of schizophrenia was based on DSM-IV and ICD-10 criteria and validated using the Operational Criteria for Psychotic Illness (OPCRIT) checklist [Citation54]. Psychopathology on the day of recruitment was assessed using the Positive and Negative Syndrome Scale (PANSS) [Citation55]. There were following exclusion criteria: mental retardation and/or general brain disorder, supplementation of folic acid or B vitamins, positive urine screening for illicit drugs (cannabis, amphetamine, opiates and ecstasy), drug and/or alcohol use disorder during 1 year prior to the onset of psychotic symptoms, severe somatic comorbidities and the use of medications altering one-carbon metabolic cycle (e.g., methotrexate, anticonvulsants, theophylline, proton pump inhibitors, statins, fibrates, antihypertensive or antidiabetic drugs). Cigarette smoking was assessed using the Fagerström test for nicotine dependence (FTND) and the pack-year index. On the day of recruitment, patients were treated using amisulpride (two patients), haloperidol (seven patients), olanzapine (16 patients) and risperidone (12 patients) in monotherapy. Average chlorpromazine equivalent and treatment duration were 126.04 ± 99.99 mg/day and 5.37 ± 4.63 days, respectively. There were 11 drug-naive patients. Agitation and hostility were managed using temporary injections of benzodiazepines and haloperidol. The study was approved by the local ethics committee and all participants gave an informed consent for participation in the study.
Assessment of childhood trauma
Assessment of childhood trauma was performed using the Early Trauma Inventory Self Report – Short Form (ETISR-SF) [Citation56]. Importantly, ETISR-SF is a 27-item self-administered questionnaire used for assessment of general trauma, physical punishment, emotional and sexual abuse that may have occurred under the age of 18 years. This measure has good reliability and internal consistency across various populations [Citation57–59]. There are two necessary criteria for recognizing early trauma: the subject experienced emotions of intense fear, horror or helplessness and the subject had an out-of-body experience or felt like being in a dream. If the subject experienced two or more traumas, he or she was asked to choose the traumatic event that had the greatest impact on his or her life. Based on ETISR-SF, FES patients were divided into those with and without childhood trauma – FES(+) and FES(-) patients. The majority of HC patients (46 subjects) had no history of childhood trauma – HC(-) subgroup.
Biochemical parameters
Venous blood samples were obtained between 7.30 and 8.30 a.m. after at least 10 h overnight fasting. All samples were centrifuged about 10 a.m. The same time interval between blood collection and centrifugation was maintained for all FES patients and healthy controls. Serum level of Hcy was measured using a chemiluminescence method in an Immulite 2000 analyzer (Siemens, Germany). Electrochemiluminescence method was used to measure serum folate and vitamin B12 levels on a Cobas 6000 analyzer (Roche, Switzerland).
DNA methylation of LINE-1 & BAGE sequences
Methylation of LINE-1 and BAGE sequences was assessed using the combined bisulphite restriction assay (COBRA) comprising sodium bisulphite treatment followed by PCR, restriction digestion and quantitation [Citation60]. Briefly, bisulphite treatment of 1 μg genomic DNA obtained from whole blood was carried out using the EpiTect kit (Qiagen). COBRA primers and PCR conditions were designed as described previously [Citation47,Citation61]. Both groups (FES patients and healthy controls) were equally distributed across COBRA reactions. Equal amounts of DNA served as an input for PCR. The PCR product of LINE-1 amplification represents several thousand genomic loci, while the BAGE assay allows for amplification of 11 BAGE loci. The PCR products were subsequently digested with 5U of the appropriate enzymes (HinfI for LINE-1 and MboI for BAGE). Two CpG sites per one locus are digested by HinfI, while one or two CpG sites per one locus are digested by MboI. The products of digestion were then run on a 3% agarose gel stained with SyBr Green, and the band intensities were measured by MultiDoc-It Imaging System (UVP) using VisionWorksLS software (UVP). Methylation of LINE-1 and BAGE were calculated as the percent concentration of 5-methylcytosine (%mC) [Citation47,Citation61].
Statistics
The Kolmogorov–Smirnov test with Lilliefors correction was used to test for the normality of data distribution. The majority of data had non-normal distribution (general trauma score, physical punishment score, emotional and sexual abuse score, total trauma score, Fagerström test score, pack-year index score, negative symptoms score, general psychopathology score, serum level of Hcy, vitamin B12 and folate as well as BAGE methylation levels) and were compared between FES patients and healthy controls using the Mann–Whitney U test. The comparison of normally distributed data (age, BMI, positive symptoms score, methylation level of LINE-1 sequence) between FES patients and healthy controls was performed using t-test. The same procedures were used to compare FES(+) and FES(-) patients with HC(-). The differences in the distribution of categorical variables between FES patients and HC were tested using χ2 test (gender and education). Gender and education differences between FES(+), FES(-) and HC(-) patients were tested using the Fisher’s test. Analysis of covariance (ANCOVA) was conducted to compare LINE-1 and BAGE methylation levels between FES(+), FES(-) and HC(-) with the following covariates: age, gender, BMI, cigarette smoking measures (FTND and pack-year index), one-carbon metabolism parameters (Hcy, folate and vitamin B12). Linear regression analysis with LINE-1 and BAGE as dependent variables and different types of traumatic events, total trauma score, age, gender and BMI as independent variables was performed separately for FES patients and HC. To isolate the medication effect, chlorpromazine equivalent was used as independent variable in linear regression analysis performed in FES patients. To avoid the problem of multicolinearity in the regression analysis, different types of traumatic events and total trauma score were analyzed separately. Statistical analysis was performed using the STATISTICA 10 software. Differences were considered as statistically significant if the p-value was less than 0.05.
Results
General characteristics of FES patients and healthy controls was shown in . Both groups did not differ significantly in terms of age, gender, education, BMI, cigarette smoking as well as serum levels of Hcy, vitamin B12 and folate. FES patients had significantly higher scores of general trauma (2.94 ± 1.14 vs 1.92 ± 1.35, p < 0.001), physical punishment (2.21 ± 1.09 vs 1.31 ± 1.27, p < 0.001), emotional abuse (2.08 ± 1.51 vs 0.98 ± 1.31, p < 0.001) and total trauma score (7.52 ± 3.18 vs 4.23 ± 3.33, p < 0.001) in comparison with HC. The history of childhood trauma was present in 18 FES patients and two HC (p < 0.001). Notably, FES patients and HC did not differ significantly with respect to methylation levels of BAGE and LINE-1. However, FES(+) patients had significantly lower LINE-1 methylation in comparison with FES(-) patients (57.10 ± 4.51% vs 60.28 ± 4.55%, p = 0.023) or HC(-) (57.10 ± 4.51% vs 60.20 ± 4.51, p = 0.016) (). Simultaneously, there was no significant difference in LINE-1 methylation between FES(-) patients and HC(-) (60.28 ± 4.55 vs 60.20 ± 4.51%, p = 0.939). These differences remained significant after controlling for age, gender, BMI, cigarette smoking measures (FTND and pack-year index) and serum levels of Hcy, folate and vitamin B12 (F = 5.372, p = 0.023) (). Differences in BAGE methylation between FES(+), FES(-) and HC(-) did not reach statistical significance (). There were no significant differences between FES(+) and FES(-) patients in terms of the number of subjects with positive family history of schizophrenia (16.67 vs 30.00%, p = 0.493), chlorpromazine equivalent (147.22 ± 81.30 vs 113.33 ± 109.02 mg/day, p = 0.113), the number of drug-naive subjects (16.67 vs 26.67%, p = 0.499), as well as the number of patients treated with olanzapine, risperidone and haloperidol (57.14/21.43/21.43% vs 38.09/42.86/19.05%, p = 0.403). There were also no significant correlations between both LINE-1 and BAGE methylation levels and PANSS scores of positive, negative and general psychopathology symptoms. Similarly, there were no significant correlations between serum levels of Hcy, folate and vitamin B12 and LINE-1 and BAGE methylation (data not shown).
& present the prediction of LINE-1 and B melanoma antigen methylation by childhood trauma in FES patients and HC. The linear regression model with age, gender, BMI and chlorpromazine equivalent as covariates revealed that higher emotional abuse score and higher total trauma score predicted lower LINE-1 methylation in FES patients (β = -0.33, t = -2.07, p = 0.045 and β = - 0.33, t = -2.15, p = 0.037, respectively). None of ETISR-SF scores predicted LINE-1 methylation level in HC. No association was found between BAGE methylation and ETISR-SF scores in FES patients. However, higher general trauma score predicted lower BAGE methylation (β = -0.34, t = -2.30, p = 0.021) and there were trends toward significant associations between BAGE methylation level and physical punishment score (β = -0.29, t = -1.82, p = 0.075) and total trauma score (β = -0.28, t = -1.93, p = 0.059) in HC.
Discussion
The major findings from our study indicate that the history of childhood trauma might be related to lower DNA methylation of LINE-1 sequence in peripheral blood leukocytes from FES patients. In addition, we found that emotional abuse and total trauma score, as assessed by means of ETISR-SF might be associated with LINE-1 hypomethylation. Moreover, general trauma score predicted lower BAGE methylation in HC. There were also trends toward significant associations between physical punishment scores or total trauma score and BAGE hypomethylation in HC. To the best of our knowledge, this is the first study investigating the effects of childhood trauma on DNA methylation in FES patients. Therefore, we are able to refer our results only to studies exploring stress-related epigenetic alterations in other clinical populations. To date, there are two studies suggesting that changes in global methylation of peripheral blood leukocytes may influence susceptibility or resilience to PTSD. The longitudinal study by Sipahi et al. [Citation62] revealed that DNA methylation of DNMT1 increased following trauma in PTSD cases but not controls. Additionally, DNA methylation of DNMT3A and DNMT3B genes increased after exposure to trauma in both PTSD cases and controls. Only in PTSD cases, pretrauma DNA methylation of DNMT3B gene was lower and overlapped with the activity of putative transcription factor binding sites explored by bioinformatic analysis. These results suggest that traumatic events might be related to global DNA hypomethylation via epigenetic silencing of genes encoding enzymes that are responsible for DNA methylation. A recent study investigating changes in methylation of repetitive DNA sequences in US military service members deployed to Afghanistan or Iraq also supports our results [Citation37]. Authors found that LINE-1 was hypermethylated in controls post- versus predeployment and hypomethylated in PTSD cases versus controls postdeployment. Alu sequence was hypermethylated in PTSD cases versus controls pre-deployment. These results indicate that DNA methylation patterns of LINE-1 and Alu might be considered as resilience or vulnerability factors for PTSD development.
Functional impact of DNA hypomethylation in FES patients as a consequence of childhood trauma remains unclear since it was not investigated in our study. However, several lines of evidence indicate that DNA hypomethylation might be responsible for genomic instability [Citation63]. In addition, it has been shown that chronic stress may lead to telomere shortening and thus to genomic instability [Citation64]. Finally, there are studies indicating that schizophrenia might be linked to genomic instability [Citation65]. In this regard, we may hypothesize that childhood trauma may induce global hypomethylation leading to genomic instability observed in schizophrenia patients. However, studies investigating functional consequences of global DNA methylation are required to elucidate this hypothesis.
In this study we failed to find the association of global DNA methylation, as measured by methylation level of LINE-1 and BAGE sequences, with schizophrenia. In addition, there was no significant correlation between both LINE-1 and BAGE methylation and serum levels of Hcy, folate and vitamin B12. Previous studies investigating global DNA methylation patterns have provided mixed results. Bromberg et al. [Citation66] did not find either a significant difference in DNA methylation of peripheral blood leukocytes between schizophrenia patients and healthy controls or a correlation between Hcy level and global DNA methylation. The same group excluded the effects of Hcy on global methylation in lymphocytes or the frontal cortex from mice [Citation67]. However, it has been shown that Hcy level might be associated with higher site-specific DNA methylation patterns in peripheral blood leukocytes from schizophrenia patients [Citation68]. There are also studies showing global hypomethylation in peripheral blood leukocytes of schizophrenia patients [Citation35,Citation69]. Shimabukuro et al. [Citation36] found that this relationship might be specific only for males with schizophrenia. However, in light of different methods used for assessment of global DNA methylation and the lack of inclusion of FES patients in previous studies, it is hard to discuss these discrepancies.
Our study has some limitations that should be addressed. First, our sample size was not large. However, to date there are only two studies investigating DNA methylation in patients with first-episode psychosis [Citation27,Citation28]. One of them had similar sample size [Citation28] and another one was even smaller [Citation27]. Additionally, it should be noted that FES patients and healthy controls were matched for possible confounding factors including, in other words, age, gender and BMI. Another point is that the majority of patients were not drug-naïve and antipsychotic treatment might have altered our results since the effects of antipsychotics on DNA methylation have been reported [Citation28,Citation35,Citation70,Citation71]. However, the linear regression analysis excluded the effects of short-term exposure to antipsychotics on LINE-1 methylation, as well as FES(+) and FES(-) patients did not differ significantly in terms of types of antipsychotics used. Furthermore, initial antipsychotic treatment was necessary to optimize the reliability of self-reports and interviews. Importantly, it might be argued that the reliability of self-reports of childhood trauma might be limited in patients with first psychotic presentation due to a potential confounding effect of delusional beliefs or detachment from the reality associated with psychosis. It is particularly relevant with respect to the ETISR-SF question regarding an out-of-body experience, which is a basis for classifying childhood adversities as traumatic experiences. However, it is important to note that we found that LINE-1 methylation was also predicted by the number of childhood adversities regardless of their perception as traumatic events. This controversial issue was addressed in the Aetiology and Ethnicity of Schizophrenia and Other Psychoses (ÆSOP) study, which used Childhood Experience of Care and Abuse questionnaire (CECA.Q) for assessment of childhood trauma [Citation72]. Authors revealed that self-reports of childhood trauma remain stable over a long period of time and are not influenced by psychopathological manifestation. In our study, we did not document the reports of third parties. However, documenting childhood trauma through interviews of family members is difficult and not feasible in most research settings, and the validity of their reports is questionable since they might have been involved directly or indirectly in the abuse [Citation56]. It should also be noted that we did not assess a dietary intake; however, serum levels of vitamin B12 and folate might be perceived as surrogate measures of dietary habits and have not been found to influence LINE-1 methylation. Finally, the lack of inclusion of HC with the history of childhood trauma does not enable to evaluate the specificity of LINE-1 hypomethylation in FES(+) subjects as the consequence of childhood adversities.
Conclusion & future perspective
In summary, our results indicate that early-life traumatic events may decrease global DNA methylation, as measured by LINE-1 methylation levels, in FES patients. The effects of childhood adversities on LINE-1 methylation cannot be attributed to potential confounding factors associated with antipsychotic treatment, one-carbon metabolism parameters or dietary intake. Since FES(+) and FES(-) patients did not differ significantly in terms of positive family history of schizophrenia, it is unlikely that familial environment serves as a mediating factor in the relationship between early-life stress and LINE-1 methylation. Given that LINE-1 methylation serves as a surrogate measure of global DNA methylation, it is warranted to use more accurate and specific techniques for assessment of global DNA methylation, for example, luminometric methylation assay (LUMA) [Citation73,Citation74] in studies on epigenetic underpinnings of childhood trauma in FES patients. Future studies should address limitations of this study and explore putative functional markers of hypomethylation induced by traumatic events in schizophrenia patients. It should be also investigated as to whether the association between childhood adversities and LINE-1 methylation appears regardless of a psychiatric diagnosis. Finally, it might be recommended that measures of traumatic events and psychosocial stress should be routinely included in epigenetic studies on schizophrenia patients.
Table 1. General characteristics of first-episode schizophrenia patients and healthy controls.
Table 2. Differences in LINE-1 methylation between first-episode schizophrenia patients and healthy controls with respect to the history of childhood trauma after co-varying for age, gender, cigarette smoking measures and one-carbon metabolism parameters.
Table 3. Prediction of LINE-1 methylation by childhood trauma.
Table 4. Prediction of BAGE methylation by childhood trauma.
The history of childhood trauma is highly prevalent in patients with psychotic disorders and might be associated with more prominent dysregulation of biological correlates that are involved in the etiology of psychosis.
Accumulating evidence indicates that early-life adversities may exert profound and sustained effects on DNA methylation. However, the association between the history of childhood trauma and DNA methylation in first-episode schizophrenia (FES) patients has not been tested so far.
In this study, we assessed methylation of two repetitive DNA sequences (LINE-1 and BAGE) in peripheral blood leukocytes from FES patients and healthy controls (HCs) with respect to childhood adversities.
Assessment of childhood trauma was performed using the Early Trauma Inventory Self-Report – Short Form (ETISR-SF). Based on positive and negative history of childhood trauma, FES patients were divided into two subgroups – FES(+) and FES(-) patients. The majority of HCs had a negative history of childhood trauma – HCs(-).
There were no significant differences in methylation of both sequences between FES patients and HCs.
FES(+) patients had significantly lower LINE-1 methylation in comparison with FES(-) patients (57.10 ± 4.51% vs 60.28 ± 4.55%, p = 0.023) or HCs(-) (57.10 ± 4.51% vs 60.20 ± 4.51; p = 0.016).
The difference in LINE-1 methylation between FES(-) patients and HCs(-) was not significant (p = 0.939).
The effect of childhood trauma on LINE-1 methylation was significant after controlling for potential confounders (F = 5.372; p = 0.023).
Linear regression analysis revealed that emotional abuse (β = -0.33, t = -2.07; p = 0.045) and total trauma score (β = -0.33, t = -2.15; p = 0.037) predicted lower LINE-1 methylation in FES patients, while general trauma score was associated with lower BAGE methylation in HCs (β = -0.34, t = -2.40; p = 0.021).
Our results indicate that the history of childhood trauma might be associated with lower global DNA methylation in FES patients.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Acknowledgements
The authors are deeply grateful to all patients and healthy controls participating in this study.
Financial & competing interests disclosure
This work was supported by the research grant awarded by National Science Centre (decision number: DEC-2011/03/N/NZ5/00248). B Misiak is supported by the START scholarship provided by the Foundation for Polish Science. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Gil A , GamaCS , De JesusDR , LobatoMI , ZimmerM , Belmonte-De-AbreuP . The association of child abuse and neglect with adult disability in schizophrenia and the prominent role of physical neglect . Child Abuse Negl.33 ( 9 ), 618 – 624 ( 2009 ).
- Boyette LL , Van DamD , MeijerCet al. Personality compensates for impaired quality of life and social functioning in patients with psychotic disorders who experienced traumatic events . Schizophr. Bull.40 ( 6 ), 1356 – 1365 ( 2014 ).
- Aas M , DazzanP , MondelliV , MelleI , MurrayRM , ParianteCM . A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation . Front. Psychiatry4 , 182 ( 2014 ).
- Muenzenmaier KH , SeixasAA , SchneebergerAR , CastilleDM , BattagliaJ , LinkBG . Cumulative effects of stressful childhood experiences on delusions and hallucinations . J. Trauma Dissociation16 ( 4 ), 442 – 462 ( 2015 ).
- Bonoldi I , SimeoneE , RocchettiMet al. Prevalence of self-reported childhood abuse in psychosis: a meta-analysis of retrospective studies . Psychiatry Res.210 ( 1 ), 8 – 15 ( 2013 ).
- Varese F , SmeetsF , DrukkerMet al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies . Schizophr. Bull.38 ( 4 ), 661 – 671 ( 2012 ).
- Theleritis C , FisherHL , ShaferIet al. Brain derived Neurotropic Factor (BDNF) is associated with childhood abuse but not cognitive domains in first episode psychosis . Schizophr. Res.159 ( 1 ), 56 – 61 ( 2014 ).
- Dennison U , MckernanD , CryanJ , DinanT . Schizophrenia patients with a history of childhood trauma have a pro-inflammatory phenotype . Psychol. Med.42 ( 9 ), 1865 – 1871 ( 2012 ).
- Mondelli V , DazzanP , HepgulNet al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment . Schizophr. Res.116 ( 2–3 ), 234 – 242 ( 2010 ).
- Aas M , DazzanP , MondelliVet al. Abnormal cortisol awakening response predicts worse cognitive function in patients with first-episode psychosis . Psychol. Med.41 ( 3 ), 463 – 476 ( 2011 ).
- Misiak B , KiejnaA , FrydeckaD . The history of childhood trauma is associated with lipid disturbances and blood pressure in adult first-episode schizophrenia patients . Gen. Hosp. Psychiatry37 ( 4 ), 365 – 367 ( 2015 ).
- Romero LM , DickensMJ , CyrNE . The Reactive Scope Model – a new model integrating homeostasis, allostasis, and stress . Horm. Behav.55 ( 3 ), 375 – 389 ( 2009 ).
- Walker EF , DiforioD . Schizophrenia: a neural diathesis-stress model . Psychol. Rev.104 ( 4 ), 667 – 685 ( 1997 ).
- Misiak B , FrydeckaD , ZawadzkiM , KrefftM , KiejnaA . Refining and integrating schizophrenia pathophysiology – relevance of the allostatic load concept . Neurosci. Biobehav. Rev.45 , 183 – 201 ( 2014 ).
- Mcgowan PO . Epigenomic mechanisms of early adversity and HPA dysfunction: considerations for PTSD research . Front. Psychiatry4 , 110 ( 2013 ).
- Maccari S , KrugersHJ , Morley-FletcherS , SzyfM , BruntonPJ . The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations . J. Neuroendocrinol.26 ( 10 ), 707 – 723 ( 2014 ).
- Vukojevic V , KolassaIT , FastenrathMet al. Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and post-traumatic stress disorder risk in genocide survivors . J. Neurosci.34 ( 31 ), 10274 – 10284 ( 2014 ).
- Yehuda R , DaskalakisNP , LehrnerAet al. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring . Am. J. Psychiatry171 ( 8 ), 872 – 880 ( 2014 ).
- Labonte B , AzoulayN , YerkoV , TureckiG , BrunetA . Epigenetic modulation of glucocorticoid receptors in posttraumatic stress disorder . Transl. Psychiatry4 , e368 ( 2014 ).
- Klengel T , MehtaD , AnackerCet al. Allele-specific FKBP5 DNA demethylation mediates gene–childhood trauma interactions . Nat. Neurosci.16 ( 1 ), 33 – 41 ( 2013 ).
- Smith AK , ConneelyKN , KilaruVet al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder . Am. J. Med. Genet. B Neuropsychiatr. Genet.156B ( 6 ), 700 – 708 ( 2011 ).
- Uddin M , AielloAE , WildmanDEet al. Epigenetic and immune function profiles associated with posttraumatic stress disorder . Proc. Natl Acad. Sci. USA107 ( 20 ), 9470 – 9475 ( 2010 ).
- Koenen KC , UddinM , ChangSCet al. SLC6A4 methylation modifies the effect of the number of traumatic events on risk for posttraumatic stress disorder . Depress. Anxiety28 ( 8 ), 639 – 647 ( 2011 ).
- Labonte B , SudermanM , MaussionGet al. Genome-wide epigenetic regulation by early-life trauma . Arch. Gen. Psychiatry69 ( 7 ), 722 – 731 ( 2012 ).
- Mehta D , KlengelT , ConneelyKNet al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder . Proc. Natl Acad. Sci. USA110 ( 20 ), 8302 – 8307 ( 2013 ).
- Dempster E , VianaJ , PidsleyR , MillJ . Epigenetic studies of schizophrenia: progress, predicaments, and promises for the future . Schizophr. Bull.39 ( 1 ), 11 – 16 ( 2013 ).
- Nishioka M , BundoM , KoikeSet al. Comprehensive DNA methylation analysis of peripheral blood cells derived from patients with first-episode schizophrenia . J. Hum. Genet.58 ( 2 ), 91 – 97 ( 2013 ).
- Ota VK , NotoC , GadelhaAet al. Changes in gene expression and methylation in the blood of patients with first-episode psychosis . Schizophr. Res.159 ( 2–3 ), 358 – 364 ( 2014 ).
- Tang H , DaltonCF , SrisawatU , ZhangZJ , ReynoldsGP . Methylation at a transcription factor-binding site on the 5-HT1A receptor gene correlates with negative symptom treatment response in first episode schizophrenia . Int. J. Neuropsychopharmacol.17 ( 4 ), 645 – 649 ( 2014 ).
- Abdolmaleky HM , NohesaraS , GhadirivasfiMet al. DNA hypermethylation of serotonin transporter gene promoter in drug naive patients with schizophrenia . Schizophr. Res.152 ( 2–3 ), 373 – 380 ( 2014 ).
- Wockner LF , NobleEP , LawfordBRet al. Genome-wide DNA methylation analysis of human brain tissue from schizophrenia patients . Transl. Psychiatry4 , e339 ( 2014 ).
- Auta J , SmithRC , DongEet al. DNA-methylation gene network dysregulation in peripheral blood lymphocytes of schizophrenia patients . Schizophr. Res.150 ( 1 ), 312 – 318 ( 2013 ).
- Murphy BC , O’ReillyRL , SinghSM . Site-specific cytosine methylation in S-COMT promoter in 31 brain regions with implications for studies involving schizophrenia . Am. J. Med. Genet. B Neuropsychiatr. Genet.133B ( 1 ), 37 – 42 ( 2005 ).
- Nohesara S , GhadirivasfiM , MostafaviSet al. DNA hypomethylation of MB-COMT promoter in the DNA derived from saliva in schizophrenia and bipolar disorder . J. Psychiatr. Res.45 ( 11 ), 1432 – 1438 ( 2011 ).
- Melas PA , RogdakiM , OsbyU , SchallingM , LavebrattC , EkstromTJ . Epigenetic aberrations in leukocytes of patients with schizophrenia: association of global DNA methylation with antipsychotic drug treatment and disease onset . FASEB J.26 ( 6 ), 2712 – 2718 ( 2012 ).
- Shimabukuro M , SasakiT , ImamuraAet al. Global hypomethylation of peripheral leukocyte DNA in male patients with schizophrenia: a potential link between epigenetics and schizophrenia . J. Psychiatr. Res.41 ( 12 ), 1042 – 1046 ( 2007 ).
- Rusiecki JA , ChenL , SrikantanVet al. DNA methylation in repetitive elements and post-traumatic stress disorder: a case-control study of US military service members . Epigenomics4 ( 1 ), 29 – 40 ( 2012 ).
- Capomaccio S , Verini-SuppliziA , GallaGet al. Transcription of LINE-derived sequences in exercise-induced stress in horses . Anim. Genet.41 ( 2 Suppl. ), 23 – 27 ( 2010 ).
- Li TH , SchmidCW . Differential stress induction of individual Alu loci: implications for transcription and retrotransposition . Gene276 ( 1–2 ), 135 – 141 ( 2001 ).
- Darby MM , SabunciyanS . Repetitive elements and epigenetic marks in behavior and psychiatric disease . Adv. Genet.86 , 185 – 252 ( 2014 ).
- Muotri AR , MarchettoMC , CoufalNGet al. L1 retrotransposition in neurons is modulated by MeCP2 . Nature468 ( 7322 ), 443 – 446 ( 2010 ).
- Muotri AR , ChuVT , MarchettoMC , DengW , MoranJV , GageFH . Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition . Nature435 ( 7044 ), 903 – 910 ( 2005 ).
- Coufal NG , Garcia-PerezJL , PengGEet al. L1 retrotransposition in human neural progenitor cells . Nature460 ( 7259 ), 1127 – 1131 ( 2009 ).
- Muotri AR , ZhaoC , MarchettoMC , GageFH . Environmental influence on L1 retrotransposons in the adult hippocampus . Hippocampus19 ( 10 ), 1002 – 1007 ( 2009 ).
- Bundo M , ToyoshimaM , OkadaYet al. Increased l1 retrotransposition in the neuronal genome in schizophrenia . Neuron81 ( 2 ), 306 – 313 ( 2014 ).
- Coufal NG , Garcia-PerezJL , PengGEet al. Ataxia telangiectasia mutated (ATM) modulates long interspersed element-1 (L1) retrotransposition in human neural stem cells . Proc. Natl Acad. Sci. USA108 ( 51 ), 20382 – 20387 ( 2011 ).
- Grunau C , SanchezC , EhrlichMet al. Frequent DNA hypomethylation of human juxtacentromeric BAGE loci in cancer . Genes Chromosomes Cancer43 ( 1 ), 11 – 24 ( 2005 ).
- Ruault M , Van Der BruggenP , BrunME , BoyleS , RoizesG , De SarioA . New BAGE (B melanoma antigen) genes mapping to the juxtacentromeric regions of human chromosomes 13 and 21 have a cancer/testis expression profile . Eur. J. Hum. Genet.10 ( 12 ), 833 – 840 ( 2002 ).
- Ehrlich M . DNA methylation in cancer: too much, but also too little . Oncogene21 ( 35 ), 5400 – 5413 ( 2002 ).
- Boks MP , DerksEM , WeisenbergerDJet al. The relationship of DNA methylation with age, gender and genotype in twins and healthy controls . PLoS ONE4 ( 8 ), e6767 ( 2009 ).
- Liu J , MorganM , HutchisonK , CalhounVD . A study of the influence of sex on genome wide methylation . PLoS ONE5 ( 4 ), e10028 ( 2010 ).
- Dick KJ , NelsonCP , TsaprouniLet al. DNA methylation and body-mass index: a genome-wide analysis . Lancet383 ( 9933 ), 1990 – 1998 ( 2014 ).
- Moustafa AA , HewediDH , EissaAM , FrydeckaD , MisiakB . Homocysteine levels in schizophrenia and affective disorders-focus on cognition . Front. Behav. Neurosci.8 , 343 ( 2014 ).
- Mcguffin P , FarmerA , HarveyI . A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system . Arch. Gen. Psychiatry48 ( 8 ), 764 – 770 ( 1991 ).
- Kay SR , FiszbeinA , OplerLA . The positive and negative syndrome scale (PANSS) for schizophrenia . Schizophr. Bull.13 ( 2 ), 261 – 276 ( 1987 ).
- Bremner JD , VermettenE , MazureCM . Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory . Depress. Anxiety12 ( 1 ), 1 – 12 ( 2000 ).
- Jeon JR , LeeEH , LeeSWet al. The early trauma inventory self report-short form: psychometric properties of the korean version . Psychiatry Investig.9 ( 3 ), 229 – 235 ( 2012 ).
- Plaza A , TorresA , Martin-SantosRet al. Validation and test-retest reliability of Early Trauma Inventory in Spanish postpartum women . J. Nerv. Ment. Dis.199 ( 4 ), 280 – 285 ( 2011 ).
- Osorio FL , SalumGA , DonadonMF , Forni-Dos-SantosL , LoureiroSR , CrippaJA . Psychometrics properties of early trauma inventory self report – short form (ETISR-SR) for the Brazilian context . PLoS ONE8 ( 10 ), e76337 ( 2013 ).
- Xiong Z , LairdPW . COBRA: a sensitive and quantitative DNA methylation assay . Nucl. Acids Res.25 ( 12 ), 2532 – 2534 ( 1997 ).
- Yang AS , EstecioMR , DoshiK , KondoY , TajaraEH , IssaJP . A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements . Nucl. Acids Res.32 ( 3 ), e38 ( 2004 ).
- Sipahi L , WildmanDE , AielloAEet al. Longitudinal epigenetic variation of DNA methyltransferase genes is associated with vulnerability to post-traumatic stress disorder . Psychol. Med.44 ( 15 ), 3165 – 3179 ( 2014 ).
- Eden A , GaudetF , WaghmareA , JaenischR . Chromosomal instability and tumors promoted by DNA hypomethylation . Science300 ( 5618 ), 455 ( 2003 ).
- Shalev I , EntringerS , WadhwaPDet al. Stress and telomere biology: a lifespan perspective . Psychoneuroendocrinology38 ( 9 ), 1835 – 1842 ( 2013 ).
- Smith CL , BoltonA , NguyenG . Genomic and epigenomic instability, fragile sites, schizophrenia and autism . Curr. Genomics11 ( 6 ), 447 – 469 ( 2010 ).
- Bromberg A , LevineJ , NemetzB , BelmakerRH , AgamG . No association between global leukocyte DNA methylation and homocysteine levels in schizophrenia patients . Schizophr. Res.101 ( 1–3 ), 50 – 57 ( 2008 ).
- Bromberg A , LevineJ , BelmakerR , AgamG . Hyperhomocysteinemia does not affect global DNA methylation and nicotinamide N-methyltransferase expression in mice . J. Psychopharmacol.25 ( 7 ), 976 – 981 ( 2011 ).
- Kinoshita M , NumataS , TajimaA , ShimoderaS , ImotoI , OhmoriT . Plasma total homocysteine is associated with DNA methylation in patients with schizophrenia . Epigenetics8 ( 6 ), 584 – 590 ( 2013 ).
- Bonsch D , WunschelM , LenzB , JanssenG , WeisbrodM , SauerH . Methylation matters? Decreased methylation status of genomic DNA in the blood of schizophrenic twins . Psychiatry Res.198 ( 3 ), 533 – 537 ( 2012 ).
- Melka MG , LauferBI , McdonaldPet al. The effects of olanzapine on genome-wide DNA methylation in the hippocampus and cerebellum . Clin. Epigenetics6 ( 1 ), 1 ( 2014 ).
- Misiak B , FrydeckaD , PiotrowskiP , KiejnaA . The multidimensional nature of metabolic syndrome in schizophrenia: lessons from studies of one-carbon metabolism and DNA methylation . Epigenomics5 ( 3 ), 317 – 329 ( 2013 ).
- Fisher HL , CraigTK , FearonPet al. Reliability and comparability of psychosis patients’ retrospective reports of childhood abuse . Schizophr. Bull.37 ( 3 ), 546 – 553 ( 2011 ).
- Karimi M , JohanssonS , StachDet al. LUMA (LUminometric Methylation Assay) - a high throughput method to the analysis of genomic DNA methylation . Exp. Cell Res.312 ( 11 ), 1989 – 1995 ( 2006 ).
- Karimi M , JohanssonS , EkstromTJ . Using LUMA: a luminometric-based assay for global DNA-methylation . Epigenetics1 ( 1 ), 45 – 48 ( 2006 ).
