Abstract
Aim: The Micra™ Transcatheter Pacing System is a leadless pacemaker that has been introduced recently. We share our experience in a low volume center and the use of right ventricular angiography (RVA) during implantation. Materials & methods: Patients underwent Micra implantation and RVA was performed to predetermine the implant site.Results: Nine patients underwent Micra implantation. The most common indication was atrial fibrillation with bradycardia. The device was implanted at apical-septum in seven and mid-septum in two. The procedure time ranged from 30 to 100 min and fluoroscopic time 4–18 min. Pacing parameters remained stable after 1-month follow-up. Conclusion: The Micra implantation technique can be easily learnt. RVA was helpful in selecting an appropriate site for the Micra implant.
The generator and leads are integral parts of a pacing system. Potential complications include pneumothorax/hemothorax after subclavian puncture; pocket hematoma, erosion or infection; vein stenosis or occlusion; injury to tricuspid valve or endocarditis; and lead complications due to connection issues, fracture or malfunction [Citation1–4]. Cardiac perforations have also been reported [Citation5]. The FOLLOW-PACE study [Citation4] reported an early complication rate of 12.4% and late complication rate of 9.2%, indicating the need for a new approach to reducing complications. Recently, data from a real-world registry from Denmark showed a 9.5% complication rate at 6 months [Citation6]. Following device implantation, infection rates range from 0 to 16.4% [Citation7] and lead related complications are reported in up 5.5% of patients at 2 months [Citation1]. Device-related infection occurs in about 1% of cases in high-volume facilities, majority occurring within first 3 months after the procedure [Citation8,Citation9]. Factors which could contribute to the higher complication rate include on-going miniaturization of leads, greater complexity of devices and increasing number of leads, especially for system revision procedures. Better lead design, careful surgical handling and use of antibiotics have reduced the rate of these complications, yet they exist in appreciable numbers.
Therefore, attempts to eliminate leads and implant devices directly inside the heart led to development of devices containing both battery and pacing electrodes within a small capsular structure to be implanted in the right ventricle (RV) [Citation10–13]. One such leadless pacemaker (LP), the Micra™ Transcatheter Pacing System (TPS) was launched for clinical use after demonstrating good safety and efficacy results from Micra TPS Global Clinical Trial [Citation14]. The cardiac center in Brunei Darussalam is a low-volume center catering for a population of 400,000 people. We adopted this new technology very early on after its launch in South East Asia. Data of experience from low-volume centers adopting this new technology are missing. We wish to share our early experience with this new technology, in particular, the use of RV angiography (RVA) to assist in the placement of the pacing system to potentially reduce the risk of complications.
Materials & methods
The Micra TPS is a miniaturized single-chamber pacing system that is delivered to the RV through a 27F catheter via the femoral vein. At our center, this procedure was done under general or local anesthesia. Preprocedure antibiotics were given and warfarin was continued if the International normalized ratio less than 2.5. Informed consent for the procedure was obtained for all patients. This study complies with the principles of the Declaration of Helsinki.
Micra TPS implant
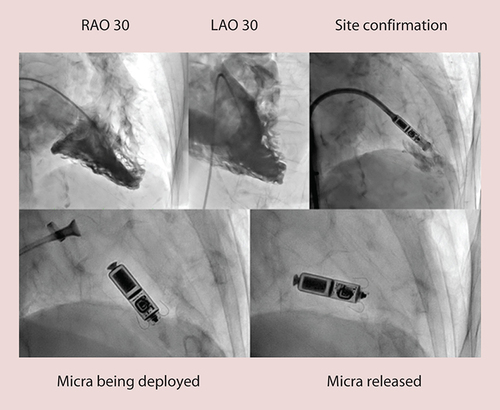
The implant procedure described earlier [Citation8] was followed except we performed RVA in right anterior oblique (RAO) and left anterior obloique (LAO) 30° in all patients at the beginning of the procedure by placing a pigtail catheter in RV apex and injecting 50–70 ml of contrast to record a cine-loop with a biplane angiography system (). The most appropriate site, with good myocardial thickness and trabeculation, was then road mapped for reference during implant. We used both serial dilatation from 10-12-14-16-18F and scalpel dissection at the entry site. Flexion of the sheath was released upon entry into the RV but in some cases it was maintained in order to reach the appropriate site. The rest of the procedure for the delivery of the Micra pacemaker including buckling of sheath, a tug test to check for adequate fixation, checking for pacing parameters, releasing the device from its tether and final removal of Micra TPS delivery system, remained same as described earlier [Citation8]. A Proglide vascular closure device and/or Z sutures were used in all patients.
Results
Since the release of the Micra TPS in the Asia-Pacific Region in 2015, as one of the first countries where the device was rolled out, we implanted nine such pacemakers during our initial experience. Mean patient age was 73 years, with the oldest being 84 years. Six patients were females and five males. All patients had a class I or II indication and provided informed consent. Six of these had persistent atrial fibrillation (AF) with symptomatic bradycardia or tachy–brady syndrome, two had a lack of venous access (hemodialysis from one side and thrombosed vessels on the other side in one patient and subclavian vein thrombosis due to old pacemaker leads and venous stenosis on the other side in another patient), and one with Down’s syndrome, who did not want any wound on the chest. RVA was performed in all these patients. The first six implants were done under general anesthesia and the last three under local anesthesia with sedation. The venous access site was dissected with a blade to expand the entry in first six patients. In the last three patients, we used only a gradual dilatation technique, without using a scalpel at all. A vascular closure device [Citation15] was used in all patients and was effective in five patients and an additional Z suture was required in four patients.
Appropriate implant sites were identified by RVA as those with good myocardial thickness and trabeculation, avoiding thin myocardium. These were the RV apical-septum in seven and mid-septum in two patients (). In two patients, we had to maintain the catheter bend until the device reached the desired site, rather than release it just after crossing the tricuspid valve, as described in the earlier trial [Citation8].
Table 1. Characteristics of patients with Micra Transcatheter Pacing System implants.
All the nine implants were successful, with a single attempt at deployment in seven patients and two attempts in two patients. Pacing parameters in all patients were good at implant and remained stable at the time of discharge (). Follow-up at 1-month showed no significant difference in R wave or pacing threshold. Average procedure time (starting from venous access until its closure) was 66 min, ranging from 30 to 100 min with Standard Deviation (SD): 21.06). Average fluoroscopy time was 8.9 min (SD: 3.99, range 4.06–18.33 min).
There were no complications whatsoever. All patients were discharged the next day except Patient 4 who remained hospitalized because of his other co-morbidities. This patient had ischemic cardiomyopathy and frequent ventricular tachycardia (VT) along with sinus node disease leading to severe bradycardia. The upper arm venous access on the left was used for hemodialysis and the subclavian vein was thrombosed on the right side. Hence a trans-venous implantable cardioverter-defibrillator (ICD) could not be implanted and the subcutaneous ICD was not available at our center. Therefore an LP was considered as an alternative to deal with the brady-arrhythmia. After 2 weeks of the implant, this patient had another episode VT/ventricular fibrillation that was cardioverted/defibrillated three-times with DC shocks (maximum @360 J Biphasic). Postshock, the pacemaker remained in situ and pacing parameters did not change. One patient with the Micra TPS underwent radiotherapy due to metastatic cancer of the rectum. He tolerated a total dose of 50.4 grays (Gy) in 28 fractions well, without complications. Pacing parameters remained stable during and after the radiotherapy. The device was turned off during the time of radiotherapy.
Discussion
Pacemakers are associated with pocket infection and lead complications [Citation1–9]. LPs aim to address these concerns. The Micra TPS is a single chamber pacemaker implanted inside the RV through the transfemoral venous route via a 27F catheter and anchored in the RV with tines [Citation14]. It does not have leads and eliminates the need for a pectoral pocket. It is MRI conditional (1.5 and 3 Tesla) [Citation16]. It gained Conformité Européene (CE) marking in April 2015 after good safety and efficacy outcomes were reported in the first 140 implants [Citation14]. In a global clinical trial, 725 patients from 56 centers in 19 countries were implanted, adding further experience with this device [Citation17]. This reported more than 50% reduction in major complication rates, with a 99.2% procedural success rate. There are, however, no reports of adoption of this new technology from small centers. Since its commercial release in South East Asia, we are one of the first few countries to implant the Micra TPS. We found that the learning curve to implant Micra TPS is short and easy to learn. Therefore, this technology can be easily adopted, even by smaller low-volume centers like ours. We have performed nine implants, with outcomes matching those in earlier trials [Citation14,Citation17]. Our successful deployment at first attempt stands at 77.8% compared with 58.6% in the Micra Transcatheter Pacing Study [Citation14] in which 37.4% had an additional 1–4 redeployments and 4.3% had five or more (one patient required as many as 18 deployments). They also had to use a second device in two patients due to inadequate electrical measurements.
We performed RVA in RAO and LAO views in all patients and preselected the site at the RV septum with adequate thickness and trabeculation of the myocardium, avoiding areas with thin myocardium. While this is not yet mandatory, we believe that preselection of a suitable site helped us achieve better deployment rates. Seven out of the nine devices (78%) were implanted at the RV apical-septum compared with 76.4% in the previous trial [Citation14]. Only in two patients, it was implanted at RV mid-septum. In one patient, the mid-septal region was preselected because of inadequate trabeculation in the apical-septal region where the myocardial wall was also thin, and in the other due to inadequate pacing parameters. It is important to note that fluoroscopy time was less in our series, in spite of adding RVA to our procedure. Average fluoroscopy time was 8.9 min (SD: 3.99, range 4.06–18.33 min), which is lower than in the Micra Transcatheter Pacing Study (average 9 min, SD: 7) [Citation14]. Reduction in fluoroscopy time may perhaps be due to a reduced number of redeployments in our study. The one patient where the fluoroscopy time was 18 min required second redeployment due to inadequate pacing parameters, further suggesting the greater the number of redeployments, the greater the fluoroscopy time. It is interesting to note that during the initial introduction of the technology, operators were trained at a special training lab with a hands-on simulator, cadaveric deployment and in vivo large animal deployment. Subsequently, operators were trained and proctored locally by the operators who had undergone training after a median of two cases [Citation18]. No difference in complication rates was observed with either training method. However, routine RVA is not recommended during either form of training, rather contrast is injected through the delivery sheath prior to deployment to confirm a septal position. This only provides a limited view of the RV anatomy and we feel that RVA can help improve the understanding of the anatomy for safe deployment. Of note, the primary operator for these procedures (S Johar) underwent training in the special training lab in the manner described above.
In our limited experience, we deviated from the recommended standard maneuver in two patients. Rather than releasing the catheter-bend upon entry into the RV, we maintained it in order to reach the appropriate site. Once the site was reached, adequate force with visible buckling of the catheter was important in achieving adequate fixing. Case 5 required redeployment due to inadequate force applied at deployment. This was the first case for the other operator.
Implants were done under general (first six) and local anesthesia (last three). In the Micra TPS trial, the majority of implants (96.3%) were done under local anesthesia [Citation14]. We decided to use general anesthesia to begin with and later switched to local anesthesia. We did not encounter any issues in doing so. The procedures went smoothly, without complications and procedure time remained the same ().
Generally, it is recommended to dissect the tissue at the puncture site to widen the entry for the large 27F sheath. We stopped using sharp dissection after the first six cases and instead used gradual dilatation by using 10F, 12F, 14F, 16F and 18F dilators before introducing the Micra TPS sheath. This resulted in less bleeding from the puncture site. We adopted this technique from our extra-corporeal membrane oxygenation experience as a means to prevent puncture site bleeding. As most of these patients were on anticoagulation at the time of the procedure and/or received heparin during the procedure it seems appropriate to avoid sharp dissections. In one patient, we implanted the device from the left femoral vein and did not face any issues. The procedure was as straightforward as from right.
Of interest, Case 4 required multiple DC shocks for ventricular arrhythmias, following Micra implantation without experiencing any significant change in pacing parameters. External DC cardioversion in the presence of an Micra system has been reported previously for AF with up to a 360J external biphasic shock [Citation19] without any changes in device function. In addition, shock therapy was successfully delivered for VT by a subcutaneous ICD in the presence of a Micra system, again without any changes in device function [Citation20]. These reports, together with our case, suggest that shock therapy, whether delivered externally or by an ICD, is safe in the presence of an Micra system.
In the Micra TPS safety trial, 30 adverse events occurred in 26 patients, including pericardial effusion in one who had repositioning done 18-times, dysrhythmias in few and groin events like hemorrhage, hematoma, pseudoaneurysm in some. There were no deaths or reoperations [Citation14]. In our limited experience with nine patients, we did not encounter any serious complications. There were no perforations, pericardial effusions or any vascular complications. There were no deaths and the one patient that remained hospitalized was due to other co-morbidities.
One of our patients underwent radiotherapy for metastatic rectal cancer. There has been one previous report of radiotherapy being delivered in the presence of an Micra device for a mediastinal mass without any adverse events [Citation21]. In contrast to that report, the Micra device was outside the radiotherapy field in our patient. This adds to the safety data for radiotherapy in patients with LPs. There are specific recommendations for safe radiotherapy delivery [Citation22] in the presence of an Micra device. Of note, for conventional devices, a 3% rate of device malfunctions may be expected during radiotherapy delivery [Citation23].
Limitations
This is a small series to make any definite recommendations. However, data from small centers are lacking. This study is an attempt to fill that gap. Deviation from standard implant procedure requires proper validation.
Conclusion
Implantation of Micra TPS can be easily learnt and safely adapted by new centers. The learning curve is quick and complications related to the procedure are minimal. In our experience, RVA in RAO and LAO views helped deployment success. The Micra TPS sustained DC shocks in one and radiotherapy in another patient without adverse effects.
Generator and leads are integral parts of a pacing system. Pocket infection and lead-related complications are encountered not infrequently. The greater complexity of modern devices and miniaturization of leads may make them more prone to malfunction.
Leadless intracardiac pacemakers like the Micra™ Transcatheter Pacing System (TPS) eliminate the complications associated with the pocket and leads. This device in inserted into the right ventricle via the femoral venous access.
Leadless pacemaker implantation is easy to learn and the technology can be easily adopted by small centers like ours.
This study is from a low-volume center catering for a population of 400,000 people only. We adopted this new technology very early on after its launch in South East Asia.
We adopted the standard implant procedure but in addition performed right ventricle angiography in RAO and LAO 30° projections in all patients at the beginning of the procedure. This helped us predetermine the most appropriate implant site, with good myocardial thickness and trabeculation.
There was no problem performing the procedure under general or local anesthesia, or from the left or right groin.
In some cases we also used gradual dilatation of the venous access rather than blunt dissection with scalpel in order to reduce bleeding.
We used the Proglide vascular closure system in all patients with good hemostasis.
Nine patients underwent Micra TPS implant with good outcome and no complications whatsoever. All patients had class I or II indication. Average age was 73 years and the oldest was 84 years.
Procedure-related indices like procedure time, fluoroscopy time and deployment attempts match those reported in bigger trials. Pacing parameters remained stable at discharge and 1-month follow-up.
In one patient, Micra TPS withstood anchoring and stability after repeated DC shocks were given to treat ventricular tachycardia.
Another patient underwent radiotherapy for rectal cancer and the Micra TPS remained unaffected.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Aggarwal RK , ConnellyDT, RaySG, BallJ, CharlesRG . Early complications of permanent pacemaker implantation: no difference between dual and single chamber systems . Br. Heart J.1 ( 73 ), 571 – 575 ( 1995 ).
- van Eck JWM , van HemelNM, ZuithofPet al. Incidence and predictors of in-hospital events after first implantation of pacemakers . Europace9 ( 6 ), 884 – 889 ( 2007 ).
- Ellenbogen KA , HellkampAS, WilkoffBLet al. Complications arising after implantation of DDD pacemakers: the MOST experience . Am. J. Cardiol.92 ( 9 ), 740 – 741 ( 2003 ).
- Udo EO , ZuithoffNP, Van HemelNMet al. Incidence and predictors of short-and long-term complications in pacemaker therapy: the FOLLOWPACE study . Heart Rhythm9, 728 – 735 ( 2012 ).
- Mahapatra S , BybeeKA, BunchTJet al. Incidence and predictors of cardiac perforation after permanent pacemaker placement . Heart Rhythm2 ( 9 ), 907 – 911 ( 2005 ).
- Kirkfeldt RE , JohansenJB, NohrEA, JorgensenOD, NielsenJC . Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark . Eur. Heart J.35 ( 18 ), 1186 – 1194 ( 2014 ).
- Polyzos KA , KonstanteliasAA, FalagasME . Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis . Europace17 ( 5 ), 767 – 777 ( 2015 ).
- Nery PB , FernandesR, NairGMet al. Device-related infection among patients with pacemakers and implanted defibrillators: incidence, risk factors and consequences . J. Cardiovasc. Electrophysiol.21 ( 7 ), 786 – 790 ( 2010 ).
- Johansen JB , JorgensenOD, MollerM, ArnsboP, MortensenPT, NielsenJC . Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients . Eur. Heart J.32 ( 8 ), 991 – 998 ( 2011 ).
- Auricchio A , DelnoyPP, RegoliF, SeifertM, MarkouT, ButterC, for collaborative study group . First-in-man implantation of leadless ultrasound-based cardiac stimulation pacing system: novel endocardia; left ventricular resynchronization therapy in heart failure patients . Europace15 ( 8 ), 1191 – 1197 ( 2013 ).
- Vardas P , PolitopoulosC, ManiosE, TsagarakisC . A miniature pacemaker introduced intravenously and implanted endocardially: preliminary findings from an experimental study . Eur. J. Card. Pacing Electrophysiol.1, 27 – 30 ( 1991 ).
- Spickler JW , RasorNS, KezdiP, MisraSN, RobinsKE, LeBoeufC . Totally self-contained intracardiac pacemaker . J. Electrocardiol.3 ( 3–4 ), 325 – 313 ( 1970 ).
- Reddy VY , KnopsRE, SperzelJet al. Permanent leadless cardiac pacing: results of LEADLESS trial . Circulation129 ( 4 ), 1466 – 1471 ( 2014 ).
- Ritter P , DurayGZ, SteinwenderCet al. Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study . Eur. Heart J.36 ( 37 ), 2510 – 2519 ( 2015 ).
- Geis NA , PlegerST, ChorianopoulosE, MüllerOJ, KatusHA, BekeredjianR . Feasibility and clinical benefit of a suture-mediated closure device for femoral vein access after percutaneous edge-to-edge mitral valve repair . EuroIntervention10 ( 11 ), 1346 – 1353 ( 2015 ).
- Soejima K , EdmonsonJ, EllingsonML, HerbergB, WiklundC, ZhaoJ . Safety evaluation of a leadless transcatheter pacemaker for magnetic resonance imaging use . Heart Rhythm13 ( 10 ), 2056 – 2063 ( 2016 ).
- Reynolds D , DurayGZ, OmarRet al. A leadless intracardiac transcatheter pacing system . N. Engl. J. Med.374 ( 6 ), 533 – 541 ( 2016 ).
- El-Chami M , KowalRC, SoejimaKet al. Impact of operator experience and training strategy on procedural outcomes with leadless pacing: insights from the Micra Transcatheter Pacing Study . Pacing Clin. Electrophysiol.40 ( 7 ), 834 – 842 ( 2017 ).
- Filipovic K , BellmannB, LükerJ, StevenD, SultanA . External electrical cardioversion of persistent atrial fibrillation in a patient with a Micra™ Transcatheter Pacing System . Indian Pacing Electrophysiol. J.18 ( 1 ), 44 – 46 ( 2018 ).
- Ahmed FZ , CunningtonC, MotwaniM, ZaidiAM . Totally leadless dual-device implantation for combined spontaneous ventricular tachycardia defibrillation and pacemaker function: a first report . Can. J. Cardiol.33 ( 8 ), 1066 ( 2017 ).
- Martínez-Sande JL , García-SearaJ, Rodríguez-MañeroM . Radiotherapy in a leadless pacemaker . Europace20 ( 1 ), 81 ( 2018 ).
- Lee JZ , MulpuruSK, ShenWK . Leadless pacemaker: performance and complications . Trends Cardiovasc. Med.28 ( 2 ), 130 – 141 ( 2018 ).
- Zaremba T , JakobsenAR, S⊘gaardM, Th⊘gersenAM, RiahiS . Radiotherapy in patients with pacemakers and implantable cardioverter defibrillators: a literature review . Europace18 ( 4 ), 479 – 91 ( 2015 ).