Abstract
More than 56 million Americans have hypertriglyceridemia, including over 12 million statin-treated individuals. However, the contribution of elevated and high triglyceride levels to cardiovascular disease and death has not been extensively studied using real-world analyses. We review recent analyses of the Optum Research Database, which included patients aged ≥45 years with diabetes and/or atherosclerotic cardiovascular disease and on statin therapy. Triglyceride levels ≥150 and 200–499 mg/dl were significantly associated with a 25.8 and 34.9% increased relative risk of cardiovascular events, respectively, versus patients with triglyceride levels <150 mg/dl. In addition, hypertriglyceridemia predicted peripheral arterial revascularization, new heart failure diagnosis and new-onset renal disease. Increased triglyceride levels were also significantly associated with increased healthcare resource utilization and costs. Interventions such as icosapent ethyl reduce triglycerides and associated cardiovascular disease risk.
Lay abstract
More than 56 million Americans have very high triglyceride levels in their blood, including over 12 million patients taking statins. However, the contribution of elevated and high triglyceride levels to cardiovascular disease and death has not been extensively studied using real-world analyses. We review recent analyses of the Optum Research Database. Patients included in these analyses were men and women aged 45 years or older who had documented diabetes and/or atherosclerotic cardiovascular disease and were prescribed statins. Elevated triglyceride levels ≥150 mg/dl and high levels 200–499 mg/dl, respectively, were associated with a 25.8 and 34.9% increased relative risk of cardiovascular events compared with patients with normal triglyceride levels <150 mg/dl. In addition, high triglyceride levels were shown to be predictive of peripheral arterial revascularization, new heart failure diagnosis and new-onset renal disease. Increased triglyceride levels were also significantly associated with increased healthcare resource utilization and costs. Interventions such as icosapent ethyl reduce triglycerides and associated cardiovascular disease risk and may benefit patients with elevated or high triglyceride levels.
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality in the USA and around the world, accounting for approximately 650,000 deaths annually in the USA and 17.8 million worldwide [Citation1–3]. As many as 48% of adults aged ≥20 years have cardiovascular disease (CVD), accounting for 4.84 million hospital discharges and costing $351.2 billion annually [Citation4]. This high burden of disease persists despite the availability of effective primary and secondary prevention treatments such as statins [Citation5]. One possible source of this residual CVD risk is atherogenic lipids beyond LDL-cholesterol (LDL-C), including triglycerides (TGs) and TG-rich lipoproteins.
Elevated and high TG levels are associated with increased ASCVD event risk in patients with well-controlled LDL-C on statin therapy [Citation6]. TGs are present in plasma in very-low-density lipoprotein, very-low-density lipoprotein remnants and intermediate-density lipoprotein, all of which are proatherogenic, proinflammatory, induce endothelial dysfunction and present in atheromatous plaque () [Citation7–10]. Analyses of the National Health and Nutrition Examination Study (NHANES) database from 2007 to 2014 showed that as many as 56.9 million Americans have hypertriglyceridemia, defined as TG levels ≥150 mg/dl [Citation11]; 31.6% of patients treated with a statin have TG levels ≥150 mg/dl [Citation12]. A total of 12.3 million statin-treated patients have elevated TG levels (≥150 mg/dl) and 5.6 million have high TG levels (200–499 mg/dl) [Citation13]. Furthermore, hypertriglyceridemia is a key factor that increases the ASCVD event risks associated with Type 2 diabetes, metabolic syndrome and obesity [Citation14,Citation15].
Recently, there has been renewed interest in the contribution of TGs to CVD following publication of positive results of the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT). Icosapent ethyl 4 g/day reduced important initial and total ASCVD events by 25 and 30%, respectively, compared with placebo in patients with a history of ASCVD and/or diabetes [Citation16,Citation17]. Although the ASCVD risk associated with elevated and high TG levels are well recognized, the extent of the contribution to CVD outcomes in the general population and the associated burden on the healthcare system, as well as the potential impact of interventions like icosapent ethyl, have not been extensively studied. Healthcare administrative claims databases can provide important ‘real-world’ evidence into the impact of disease on clinical outcomes in the general population beyond the confines of a clinical trial. The purpose of this review is to summarize results from a series of analyses of the Optum Research Database that queried the role of elevated and high TG levels in development of a number of ASCVD-related conditions including major cardiovascular (CV) events, heart failure, renal disease and peripheral vascular disease in statin-treated patients with diabetes and/or a history of ASCVD, as well as the role of hypertriglyceridemia in health economics [Citation18–23].
The Optum Research Database
The Optum Research Database is an administrative claims database containing data from 160 million individuals and electronic health records for more than 80 million individuals nationally. As a result, robust, prospectively designed retrospective analyses representative of the US population can be performed.
Detailed descriptions of the inclusion and exclusion criteria and statistical methodology for the analyses reviewed here have been published previously [Citation18,Citation19]. Eligible patients for all of the analyses had one or more claims for statin therapy between 1 January 2010 and 31 December 2010 and had six or more months of baseline data prior to the index date (date of first statin claim during the identification period) and six or more months of follow-up (or until death). The follow-up period began on the index date and continued until the date of disenrollment from the plan, death, or the end of the study period on 31 March 2016. The base populations for these analyses included men and women aged 45 years or older who had documented diabetes and/or ASCVD and were prescribed statins. ASCVD was defined as acute coronary syndrome, myocardial infarction (MI), angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral artery disease. A propensity score analysis was used to generate a comparator population similar to the population with high TG, but with TG levels <150 mg/dl to control for possible confounding factors. This analysis matched patients based on age, sex, insurance type, region, baseline medical cost, LDL-C relative to the median, baseline use of lipid-lowering drugs and diagnosis of ASCVD, diabetes, stroke, hypertension, renal disease and peripheral artery disease [Citation18]. These variables were also included in pre-match multivariate analyses.
The primary end point for all of these analyses was the occurrence of major CV events, defined as a composite of CV-related death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina during the follow-up period. Secondary objectives included the quantification of healthcare costs and healthcare resource use. In addition, the effects of elevated and high TG levels on the risk of important ASCVD-related conditions such as heart failure diagnosis [Citation22], kidney disease [Citation21] and peripheral arterial revascularization [Citation23] were investigated.
These images depict a nonfasting or postprandial state with lipoprotein in plasma of both intestinal (chylomicrons and chylomicron remnants) and hepatic origin (VLDL, VLDL remnants = intermediate-density lipoproteins) and LDL. LDLs and remnants cross the endothelial layer of intima in a process dependent on blood pressure, lipoprotein size and lipoprotein concentration, possibly involving transcytosis.
Modified with permission from [Citation10].
![Figure 1. Elevated triglyceride-rich lipoproteins and atherosclerotic plaque initiation, plaque progression and plaque rupture causing myocardial infarction or ischemic stroke.These images depict a nonfasting or postprandial state with lipoprotein in plasma of both intestinal (chylomicrons and chylomicron remnants) and hepatic origin (VLDL, VLDL remnants = intermediate-density lipoproteins) and LDL. LDLs and remnants cross the endothelial layer of intima in a process dependent on blood pressure, lipoprotein size and lipoprotein concentration, possibly involving transcytosis.Modified with permission from [Citation10].](/cms/asset/302c47c0-896a-44cb-9186-923da969802a/ifca_a_12325954_f0001.jpg)
Study populations
Overall, more than 1.6 million statin-treated individuals were identified and more than 390,000 met additional inclusion criteria. A number of subpopulations were identified, including those with TG levels <150 mg/dl and HDL-cholesterol (HDL-C) >40 mg/dl (n = 32,506), TG levels ≥150 mg/dl (n = 27,471) and TG levels 200–499 mg/dl (n = 13,411) () [Citation21]. In addition, two propensity score-matched cohorts were generated: patients with TG levels <150 mg/dl and HDL-C levels >40 mg/dl versus either patients with TG levels ≥150 mg/dl (n = 23,181 in each group) or patients with TG levels 200–499 mg/dl (n = 10,990 in each group).
Baseline characteristics and patient demographics of the elevated-TG and high-TG cohorts and their corresponding comparator cohorts are summarized in [Citation18,Citation19,Citation21]. Overall mean age was approximately 62 years and slightly more than half of patients were male. At baseline, approximately 84% of patients had diabetes, 30% had ASCVD and 79% had hypertension. Overall, 83.7% of patients were taking statins only, 13.4% were taking a statin plus a fibrate, 1.9% were taking a statin plus an omega-3 fatty acid and 1.0% were taking a statin plus a fibrate and an omega-3 fatty acid [Citation24].
Table 1. Patient demographics, characteristics and baseline comorbidities.
(A) Elevated TG cohort and comparator. (B) High-TG cohort and comparator.
†Population used for patient characteristics and other analyses.
‡Population used for multivariate analyses.
HDL-C: High-density lipoprotein cholesterol; TG: Triglycerides.
Reproduced with permission from [Citation21].
![Figure 2. Identification of cohorts used for analyses. (A) Elevated TG cohort and comparator. (B) High-TG cohort and comparator. †Population used for patient characteristics and other analyses. ‡Population used for multivariate analyses.HDL-C: High-density lipoprotein cholesterol; TG: Triglycerides.Reproduced with permission from [Citation21].](/cms/asset/0aca07bd-7e4d-476c-99d6-227d2e24f8a5/ifca_a_12325954_f0002.jpg)
Hypertrigylceridemia is associated with increased risk of ASCVD events
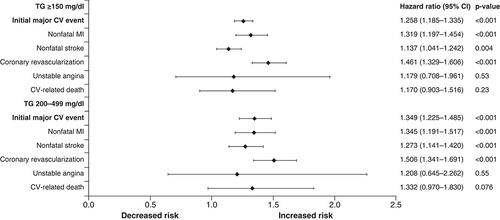
In multivariate analyses, there was a statistically significant 25.8% (hazard ratio [HR]: 1.26; 95% CI: 1.19–1.34; p < 0.001) higher risk of major ASCVD events including CV-related death in statin-treated patients with diabetes and/or ASCVD and TG levels ≥150 mg/dl compared with the prematched comparator cohort [Citation19]. This increased risk rose to 34.9% (HR: 1.35; 95% CI: 1.23–1.49; p < 0.001) for patients with TG levels 200–499 mg/dl [Citation18]. As shown in , elevated and high TG levels were also associated with a statistically significant increase in nonfatal MI, nonfatal stroke and coronary revascularization [Citation18,Citation19]. The increased risk of unstable angina and CV-related death did not reach statistical significance [Citation18,Citation19]. These trends remained significant in patients with TG levels >150 mg/dl and 200–499 mg/dl when non-HDL-C or HDL-C was added as a covariate to the model [Citation18].
Other statistically significant predictors of major CV events in the high-TG cohort (TG levels 200–499 mg/dl), after controlling for covariates, included ASCVD (HR: 2.30; 95% CI: 2.05–2.59; p < 0.001), diabetes (HR: 1.46; 95% CI: 1.26–1.69; p < 0.001) and male sex (HR: 1.36; 95% CI: 1.23–1.50; p < 0.001). Younger age had a protective effect. Similar results were seen in the cohort of patients with elevated TG levels (≥150 mg/dl) [Citation18,Citation19].
Covariates included TG cohort, age, sex, insurance coverage type, geographic region of enrollment, baseline clinical characteristics and baseline medication use. The composite end point included nonfatal MI, nonfatal stroke, coronary revascularization, unstable angina and CV-related death during the follow-up period.
CV: Cardiovascular; MI: Myocardial infarction; TG: Triglyceride.
Impact of hypertriglyceridemia on healthcare resource utilization
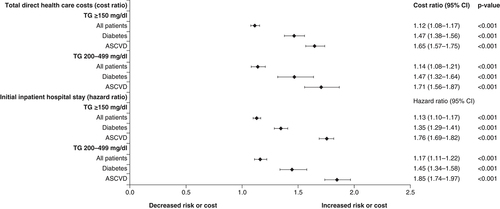
As a healthcare claims database, the Optum Research Database allows assessment of the healthcare resource utilization in this population. This is a critical step toward estimating the economic impact of any potential treatment directed at reducing TG levels and associated CV risk. shows the HR for the increase in total direct healthcare costs and risk of an initial inpatient hospital stay for patients with elevated TG levels (≥150 mg/dl) and high TG levels (200–499 mg/dl) versus those with TG levels <150 mg/dl and HDL-C >40 mg/dl [Citation18,Citation19]. The cost ratios for baseline diabetes and for baseline ASCVD and the risk of first hospitalization in the high-TG cohort were increased versus the comparator cohort. The increase in both direct healthcare costs and risk of initial inpatient hospitalization were also significantly increased in the high-TG cohort versus the comparator cohort (p < 0.001 for each). There was no significant difference in the proportion of patients with an ambulatory visit during the follow-up period in any cohort. However, a slightly higher, but statistically significant, percentage of patients with elevated and high TG levels made one or more emergency room visits versus the comparator group (55.7 vs 53.9%; p = 0.007 and 55.0 vs 53.7%; p = 0.005, respectively) [Citation18,Citation19].
For patients in the elevated-TG group, this increase in healthcare utilization represents an increase in mean monthly direct healthcare costs from $1270 to 1438 and for the high-TG group from $1279 to 1462. This translates to an increase in annual direct healthcare costs of $200 million and $220 million per 100,000 patients in the elevated- and high-TG populations, respectively. Being of younger age (particularly 45–54 years) had a protective effect in terms of both direct healthcare costs and risk of inpatient hospital stay [Citation18,Citation19].
Covariates included TG cohort, age, sex, insurance coverage type, geographic region of enrollment, baseline clinical characteristics and baseline medication use. The composite end point included nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, unstable angina and cardiovascular-related death during the follow-up period. The cost ratio is the relative healthcare cost in one group versus another.
ASCVD: Atherosclerotic cardiovascular disease; TG: Triglyceride.
Hypertrigylceridemia as a predictor of peripheral arterial revascularization
In addition to assessing the risk of hypertriglyceridemia on major CV events and CV-related death, the Optum Research Database was employed to assess the impact of high and elevated TG levels on a number of other ASCVD-related chronic conditions. These included the role of TGs on the need for peripheral arterial revascularization in patients ≥45 years of age with existing ASCVD and/or diabetes.
The risk of peripheral arterial revascularization was significantly higher among patients with elevated or high TG levels versus the comparator cohort [Citation23]. In Kaplan–Meier analyses, 4.9 versus 6.9% and 4.8 versus 7.3% in the comparator cohort compared with the elevated- and high-TG cohorts, respectively, had undergone peripheral arterial revascularization at 5 years. The HR for the increased risk of peripheral arterial revascularization was 1.37 (95% CI: 1.26–1.49; p < 0.001) and 1.49 (95% CI: 1.35–1.64; p < 0.001) in the elevated- and high-TG groups versus the comparator group, respectively.
Hypertriglyceridemia as a predictor of new heart failure diagnosis
There was a statistically significant 19% increase in the risk of a new heart failure diagnosis in the elevated-TG cohort (HR: 1.19; 95% CI: 1.13–1.25; p < 0.001) and a 24% increase in the high-TG cohort (HR: 1.24; 95% CI: 1.16–1.32; p < 0.001) compared with the respective comparator cohorts with TG levels <150 mg/dl [Citation22]. Kaplan–Meier analyses showed that 14.5 versus 16.6% and 14.2 versus 17.0% in the comparator cohort compared with the elevated- and high-TG cohorts, respectively, had received a new heart failure diagnosis by 5 years (p < 0.001 for each).
Hypertriglyceridemia as a predictor of new-onset renal disease
Chronic renal failure results in dysregulation of various key enzymes and receptors involved in lipoprotein metabolism, including TG-rich lipoproteins and is associated with dyslipidemia and increased risk of CV morbidity and mortality [Citation25–27]. In turn, dysregulation of lipid metabolism may contribute to progression of renal disease [Citation25]. It is therefore of interest to assess possible relationships between elevated TG levels and kidney disease at the population level.
A post hoc analysis of the rate of hospitalization for new-onset kidney disease (defined as hospitalization during follow-up in patients without kidney disease at baseline) was performed [Citation21]. At baseline, approximately 12% of patients in all cohorts had existing renal disease. The rate of hospitalization for new-onset kidney disease was increased by 31% in the elevated-TG cohort (HR: 1.31; 95% CI: 1.23–1.40; p < 0.001) and by 45% in the high-TG cohort (HR: 1.45; 95% CI: 1.34–1.57; p < 0.001) compared with the respective comparator groups.
A lower estimated glomerular filtration rate (eGFR) has been associated with dyslipidemia, CV events, hospitalization and death [Citation27,Citation28]. To further investigate the role of TGs on renal function, a second analysis was performed in which the effect of TGs on eGFR was performed in the cohort of patients with TG levels ≥150 mg/dl [Citation29]. The mean baseline eGFR in the elevated-TG and comparator cohorts, respectively, was 90.1 and 90.7 ml/min/1.73 m2. After more than 5 years of follow-up, the proportion of patients with eGFR <60 ml/min/1.73 m2, which corresponds to stage 3 chronic kidney disease, increased in both groups. There was no statistically significant difference between groups; the proportion of patients with eGFR <60 ml/min/1.73 m2 widened over time from 10.8% at 6 months to 18.4% at 6 years in the elevated-TG group and from 9.1 to 16.5%, respectively, in the comparator group. After 5 years, more patients in the comparator cohort (49.7%) had normal renal function compared with the elevated-TG cohort (44.9%); however, this difference was not statistically significant.
Statin persistence
Statin therapy is a critical component of treatment for both primary and secondary prevention of ASCVD [Citation5]. Consequently, a critical inclusion criterion for this retrospective analysis was that patients had been prescribed a statin at the start of the study period [Citation18,Citation19]. However, several studies have shown adherence and persistence with statin therapy to be low, negatively impacting CVD event risk and outcomes [Citation30,Citation31].
Analysis of the Optum Research Database showed that persistence with statin therapy was poor in patients with a history of diabetes and ASCVD and elevated or high TG levels [Citation20]. Persistence with statin therapy was measured as months to therapy discontinuation, inclusive of prescription fills on the index date; a switch to another statin was allowed. Discontinuation of statin was defined as a 30-day gap in therapy starting from the date when supply was exhausted. Using these definitions, the proportion of days covered during the first 6 months of treatment was 77 and 76% in the elevated- and high-TG cohorts, respectively. For the overall study period, the proportion of days covered was 68 and 67%, respectively. The time to treatment discontinuation was 10.4 months in the elevated-TG cohort and 10.1 months in the high-TG cohort. In terms of patients who discontinued statin treatment, approximately 56% of those in the elevated-TG cohort did so within the first 6 months. Using Kaplan–Meier analysis, the probability of remaining on index statin treatment after 1 year was 47% for both the elevated-TG and comparator cohorts. At 5 years, the proportion of patients on index statin therapy was reduced to 19%. There were no meaningful differences between the elevated- and high-TG cohorts and their comparator cohorts.
Statin persistence was worse among women than men (p < 0.001): after 5 years, 21.3% of men and 16.3% of women in the elevated-TG cohort remained on treatment. Only 13.5% of younger patients (45–54 years) in the elevated-TG cohort remained on index statin treatment after 5 years compared with 17.9% of patients aged 55 to 64 years and 22.9% of those aged ≥65 years (p < 0.001 for all differences). Although patients with ASCVD at baseline had better statin persistence than those with diabetes only, persistence at 5 years remained low at 20.3 versus 18.2% without ASCVD in the elevated-TG cohort (p = 0.001). Similar trends were seen for patients with a history of peripheral arterial revascularization, heart failure, peripheral artery disease and renal disease at baseline; patients without these risk factors at baseline had better, albeit still low, persistence at 5 years (comparisons were not statistically significant).
Discussion
Evidence from the Optum Research Database demonstrates that elevated and high TG levels in patients with Type 2 diabetes and/or ASCVD are associated with significantly worse CVD outcomes than matched patients with normal TG levels (i.e., <150 mg/dl) [Citation18,Citation19]. Statin-treated patients ≥45 years of age with diabetes and/or ASCVD were significantly more likely to experience an initial major CV event or CVD-related death, nonfatal MI, nonfatal stroke or coronary revascularization if they had TG levels ≥150 mg/dl or 200–499 mg/dl compared with normal TG levels after adjusting for confounding variables; the risk of unstable angina did not reach statistical significance in either cohort. Elevated and high TG levels were also significantly associated with an increased risk of renal disease, heart failure and peripheral revascularization.
The results of these analyses are in broad agreement with similar analyses recently published from Kaiser Permanente and the Veterans Affairs (VA) database of electronic health records [Citation32–34]. In the Kaiser Permanente database analysis, a similar statin-treated patient population ≥45 years of age with a charted diagnosis of MI, stroke, acute coronary syndrome, or peripheral arterial disease and LDL-C 40–100 mg/dl was followed for up to 6.5 years. Patients with TG levels 200–499 mg/dl were significantly more likely to suffer nonfatal MI (p = 0.045), coronary revascularization (p = 0.045), or peripheral revascularization (p = 0.006) versus those with TG levels <150 mg/dl; there was no significant difference for first composite outcome (all-cause mortality and first occurrence of a nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina), nonfatal stroke, unstable angina, aneurysm repair, or all-cause mortality. Overall, this high-TG cohort was found to be 10% more likely to experience a second primary composite outcome event (the first composite outcome plus peripheral revascularization and aneurysm repair) versus those with TG levels <150 mg/dl (p = 0.041) [Citation32]. The subset of patients in the Kaiser Permanente study with diabetes and TG levels 200–499 mg/dl had a significantly increased risk of nonfatal MI, nonfatal stroke and coronary revascularization, but not unstable angina, similar to the results discussed here [Citation35].
A 2010–2015 analysis of the VA electronic health records examined over 430,000 veterans on statins, identifying over 130,000 with elevated TG levels [Citation33]. CV event (nonfatal MI, stroke, unstable angina and coronary revascularization) rate ratio adjusted for HDL-C levels was 1.19 (95% CI: 1.16–1.22; p < 0.001) for patients with elevated TG levels versus normal TG levels [Citation33]. Therefore, patients with elevated TG levels showed a significant increase in CV events, independent of HDL-C levels and well-controlled LDL-C levels. Another analysis of the same VA database stratified statin-controlled patients into three groups of CV risk and found that elevated TG levels were associated with increased CV events regardless of CV event history and diabetes status [Citation34].
The Kaiser Permanente database study also found a significant increase in inpatient admission (p < 0.001), inpatient days (p = 0.038), emergency room visits (p < 0.001) and pharmaceutical dispenses (p < 0.001) in patients with high TG levels compared with those with TG levels <150 mg/dl [Citation36]. As with the results from the Optum Research Database, this translated into significantly increased annualized healthcare costs, including total costs (increase of $964; p = 0.006), emergency room visits ($70; p = 0.031), hospital ambulatory care ($199; p = 0.032), total ambulatory care ($327; p = 0.035) and pharmaceutical dispenses ($185; p = 0.012). For the 2702 patients with high TG levels included in the analysis, this amounted to $2.6 million/year. The Optum Research Database results show that this increase in healthcare utilization and costs extends to those with elevated TG levels (≥150 mg/dl) as well as those with high TG levels (200–499 mg/dl) [Citation18,Citation19]. Given that as many as 56.9 million US adults have TG levels ≥150 mg/dl, including 12.3 million on statins [Citation13], the potential cost savings associated with reducing CVD event risk related to elevated TG levels are substantial.
The recently published REDUCE-IT study showed that icosapent ethyl 4 g/day significantly reduced CVD event risk in statin-treated adults aged ≥45 years with established CVD or ≥50 years of age with diabetes and at least one additional CV risk factor. Patients in this study had a baseline median TG level of 216 mg/dl and median LDL-C level of 75 mg/dl. In that study, icosapent ethyl was associated with a 25% reduction in a composite end point of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina (HR: 0.75; 95% CI: 0.68–0.83; p < 0.001; A) [Citation16]. Icosapent ethyl was also associated with a significant decrease in 2nd, 3rd or ≥4th CV events (B) [Citation37] and in first and total revascularization events [Citation38].
Our analyses of the Optum Research Database found that only approximately 20% of patients were still on statin therapy after 5 years, even in those with additional risk factors. This finding is in line with what has been previously reported [Citation39–43]. Shroufi and Powles further estimated that an improvement in adherence from 50 to 75% could cut the number of deaths related to CVD by 200% [Citation44]. Adding medications to the CVD treatment regimen may make adherence even more challenging. Further patient education to improve medication adherence may be warranted. However, it is also possible that the definitions of persistence used for the Optum Research Database underestimated statin persistence, as it measured only whether prescriptions were filled rather than actual usage; it also only measured persistence with the index class of treatment and did not account for the possibility of patients switching to an alternative class of lipid-lowering agent [Citation20].
Real-world evidence has shown that hypertriglyceridemia is associated with an increased risk of CVD events and increased healthcare resource utilization and costs in patients ≥45 years of age with diabetes and/or ASCVD. Results from the REDUCE-IT clinical outcomes study have shown that the prescription eicosapentaenoic acid product icosapent ethyl 4 g/day can reduce CVD event risk in these patients by 25%. In more recent analyses, icosapent ethyl has been shown to reduce the risk of revascularization, serving as the first non-LDL-C intervention in a major randomized trial in which statin-treated patients underwent fewer coronary artery bypass grafting surgeries [Citation38]. As a result, icosapent ethyl has now been included in medical guidelines as a key element of contemporary medical therapy along with statin therapy to reduce ASCVD risk [Citation45–47]. Broad use of icosapent ethyl in patients with elevated and high TG levels with a history of diabetes and/or ASCVD has the potential to significantly impact management of CVD, resulting in important reductions in healthcare resource utilization and costs.
(A) Cumulative incidence of cardiovascular events in REDUCE-IT. Kaplan–Meier event curves for the primary efficacy composite end point of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina in the icosapent ethyl group and the placebo group, in a time-to-event analysis of the REDUCE-IT study. (B) Distribution of first and subsequent primary composite events in the reduced dataset for patients randomized 1:1 to icosapent ethyl versus placebo in REDUCE-IT. HRs and 95% CIs for between-treatment group comparisons were generated using Li-Lagakos-modified Wei-Lin-Weissfeld method for the first, second and third event categories. RR and 95% CI for between-group comparisons used a negative binomial model for additional events beyond first, second and third occurrences (i.e., fourth event or more) and overall treatment comparison. Analyses are based on a reduced dataset accounting for statistical handling of multiple end points occurring in a single calendar day by counting as a single event.
CV: Cardiovascular; HR: Hazard ratio; MI: Myocardial infarction; RR: Rate ratio.
(A) Reprinted with permission from [Citation16] © Massachusetts Medical Society (2019).
(B) Reproduced with permission from [Citation37].
![Figure 5. REDUCE-IT results. (A) Cumulative incidence of cardiovascular events in REDUCE-IT. Kaplan–Meier event curves for the primary efficacy composite end point of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina in the icosapent ethyl group and the placebo group, in a time-to-event analysis of the REDUCE-IT study. (B) Distribution of first and subsequent primary composite events in the reduced dataset for patients randomized 1:1 to icosapent ethyl versus placebo in REDUCE-IT. HRs and 95% CIs for between-treatment group comparisons were generated using Li-Lagakos-modified Wei-Lin-Weissfeld method for the first, second and third event categories. RR and 95% CI for between-group comparisons used a negative binomial model for additional events beyond first, second and third occurrences (i.e., fourth event or more) and overall treatment comparison. Analyses are based on a reduced dataset accounting for statistical handling of multiple end points occurring in a single calendar day by counting as a single event.CV: Cardiovascular; HR: Hazard ratio; MI: Myocardial infarction; RR: Rate ratio. (A) Reprinted with permission from [Citation16] © Massachusetts Medical Society (2019). (B) Reproduced with permission from [Citation37].](/cms/asset/eb651e87-d9d4-425d-b00e-99a2f5c8d4e8/ifca_a_12325954_f0005.jpg)
Conclusion
These analyses of the Optum Research Database demonstrate that elevated and high TG levels are independent risk factors for ASCVD and CV death. As a result, increased TG levels are associated with higher healthcare utilization and costs. Interventions, such as icosapent ethyl, that have been shown to improve CVD outcomes in statin-treated patients with elevated TG levels should be considered in this large population.
Future perspective
Our investigations of the Optum Research Database provide real-world support for the conclusion that hypertriglyceridemia is a statistically significant risk factor for ASCVD even after adjusting for both non-HDL-C and HDL-C. These analyses also demonstrated that hypertriglyceridemia correlates with increased risk for heart failure and chronic kidney disease. Much is yet to be learned about how TGs potentiate atherosclerosis, heart failure and chronic kidney disease. Elevated TG levels correlate with endothelial dysfunction, augmented systemic inflammatory tone and a pro-oxidative state. There is mounting evidence that excess fatty acids can be toxic to endothelial cells and cardiac myocytes. Specific pathways of toxicity require greater study. Whether TGs are toxic to renal glomeruli, mesangial cells, or podocytes remains to be determined. The impact of elevated TG levels on myocardium, arteries and the kidney will also have to be disentangled from the injurious effects potentiated by insulin resistance among patients with metabolic syndrome and/or diabetes.
The challenge will be selecting the next evidenced-based therapy additive to the ASCVD risk reduction achieved by statins, blood pressure and glucose control. This therapy would ideally be convenient for patients, safe and be easily prescribed/administered by primary care and specialists. Most importantly, this therapy should demonstrate a dramatic reduction in ASCVD risk and financial value. In patients with elevated TG levels and other high CV event risk factors, results from REDUCE-IT suggest that icosapent ethyl may offer benefits that meet these criteria.
Icosapent ethyl reduces ASCVD-related events but is also the first lipid-lowering therapy to significantly reduce CV mortality when used as an add-on to statin therapy. Historically, fibrates, EPA/DHA combination therapy, ezetimibe and the proprotein convertase/subtilisin kexin type 9 (PCSK9) monoclonal antibodies have all failed to demonstrate incremental reductions in mortality over and above that achieved with a statin. The results of the ongoing PROMINENT trial with pemafibrate will help to determine whether a fibrate drug provides any CV benefit over and above statin therapy in diabetic patients with hypertriglyceridemia and low HDL-C.
Clearly, elevated TG levels are a marker of heightened risk for ASCVD events and this risk is significantly attenuated by icosapent ethyl. It will be of interest to discern at which specific steps in atherogenesis/plaque instability, heart failure and chronic kidney disease icosapent ethyl is beneficial.
Hypertriglyceridemia is common
Over 12 million Americans on statin therapy have elevated (≥150 mg/dl) triglyceride (TG) levels.
The Optum Research Database
A propensity score analysis of the Optum Research Database has helped to define the role of elevated and high triglycerides in patients with diabetes and/or atherosclerotic cardiovascular disease (ASCVD).
Hypertrigylceridemia is associated with increased risk of ASCVD events
Elevated TG levels are significantly associated with increased ASCVD risk and healthcare utilization and costs, even in statin-treated patients.
TG levels were predictive of peripheral arterial revascularization, new heart failure diagnosis and new-onset renal disease.
Statin persistence is poor
After 5 years, the proportion of patients on index statin therapy was only 19%.
Interventions to reduce TG-associated risk
Implementation of evidence-based therapies proven to reduce ASCVD risk in patients with elevated TG levels and other ASCVD risk factors is needed.
Financial & competing interests disclosure
PP Toth serves on the speaker’s bureaus of Amarin Pharma, Inc., Amgen and Novo-Nordisk and serves as a consultant to Amarin Pharma, Inc., Amgen, Novo-Nordisk, Novartis and Resverlogic. M Hull was an employee of Optum at the time of these analyses. C Granowitz and S Philip are employees and stock shareholders of Amarin Pharma, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support was provided by R Shah, J Street, and J Wood, Peloton Advantage, LLC, an OPEN Health company and was funded by Amarin Pharma, Inc.
References
- Kochanek KD , MurphySL, XuJ, AriasE. Deaths: final data for 2017. Natl Vital Stat. Rep., 68(9), 1–75 (2019).
- Global Burden of Disease Study Investigators . Global, regional and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet, 392(10159), 1736–1788 (2018).
- Arnett DK , BlumenthalRS, AlbertMAet al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 140(11), e596–e646 (2019).
- Virani SS , AlonsoA, BenjaminEJet al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation, 141(9), e139–e596 (2020).
- Stone NJ , RobinsonJ, LichtensteinAHet al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 129(2 Suppl. 25), S1–S45 (2014).
- Miller M , CannonCP, MurphySA, QinJ, RayKK, BraunwaldE. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J. Am. Coll. Cardiol., 51(7), 724–730 (2008).
- Toth PP . Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc. Health Risk Manag., 12, 171–183 (2016).
- Rapp JH , LespineA, HamiltonRLet al. Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler. Thromb., 14(11), 1767–1774 (1994).
- Kugiyama K , DoiH, TakazoeKet al. Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation, 99(22), 2858–2860 (1999).
- Parhofer KG , ChapmanMJ, NordestgaardBG. Efficacy and safety of icosapent ethyl in hypertriglyceridaemia: a recap. Eur. Heart J. Suppl., 22(Suppl. J), J21–J33 (2020).
- Jellinger PS , HandelsmanY, RosenblitPDet al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr. Pract., 23(Suppl. 2), 1–87 (2017).
- Fan W , PhilipS, GranowitzC, TothPP, WongND. Prevalence of US adults with triglycerides ≥150 mg/dl: NHANES 2007–2014. Cardiol. Ther., 9(1), 207–213 (2020).
- Fan W , PhilipS, GranowitzC, TothP, WongN. Hypertriglyceridemia in statin-treated US adults: The National Health and Nutrition Examination Survey. J. Clin. Lipidol., 13, 100–108 (2019).
- Ganda OP , BhattDL, MasonRP, MillerM, BodenWE. Unmet need for adjunctive dyslipidemia therapy in hypertriglyderidemia management. J. Am. Coll. Cardiol., 72(3), 330–343 (2018).
- Heymsfield SB , WaddenTA. Mechanisms, pathophysiology and management of obesity. N. Engl. J. Med., 376(3), 254–266 (2017).
- Bhatt DL , StegG, MillerMet al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med., 380(1), 11–22 (2019).
- Bhatt DL , StegPG, MillerMet al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J. Am. Coll. Cardiol., 73(22), 2791–2802 (2019).
- Toth PP , GranowitzC, HullM, LiassouD and ersonA, PhilipS. High triglycerides are associated with increased cardiovascular events, medical costs and resource utilization: a real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J. Am. Heart Assoc., 7(15), e008740 (2018).
- Toth PP , PhilipS, HullM, GranowitzC. Association of elevated triglycerides with increased cardiovascular risk and direct costs in statin-treated patients. Mayo Clin. Proc., 94(9), 1670–1680 (2019).
- Toth PP , GranowitzC, HullM and ersonA, PhilipS. Long-term statin persistence is poor among high-risk patients with dyslipidemia: a real-world administrative claims analysis. Lipids Health Dis., 18, 175 (2019).
- Toth PP , PhilipS, HullM, GranowitzC. Elevated triglycerides (>/=150 mg/dl) and high triglycerides (200–499 mg/dl) are significant predictors of hospitalization for new-onset kidney disease: a real-world analysis of high-risk statin-treated patients. Cardiorenal Med., 9(6), 400–407 (2019).
- Toth PP , PhilipS, HullM, GranowitzC. Elevated triglycerides (≥150 mg/dl) and high triglycerides (200–499 mg/dl) are significant predictors of new heart failure diagnosis: a real-world analysis of high-risk statin-treated patients. Vasc. Health Risk Manage, 15, 533–538 (2019).
- Toth PP , PhilipS, HullM, GranowitzC. Hypertriglyceridemia is associated with an increased risk of peripheral arterial revascularization in high-risk statin-treated patients: a large administrative retrospective analysis. Clin. Cardiol., 42(10), 908–913 (2019).
- Toth P , GranowitzC, PhilipS, LiassouD, AndersonA, HullM. Baseline characteristics of a retrospective claims analysis of cardiovascular outcomes and health care resource utilization and costs in high-risk statin-treated patients with hypertriglyceridemia. J. Clin. Lipidol., 11(3), 797 (2017).
- Vaziri ND . Dyslipidemia of chronic renal failure: the nature, mechanisms and potential consequences. Am. J. Physiol. Renal Physiol., 290(2), F262–F272 (2006).
- Manjunath G , TighiouartH, IbrahimHet al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J. Am. Coll. Cardiol., 41(1), 47–55 (2003).
- Go AS , ChertowGM, FanD, McCullochCE, HsuCY. Chronic kidney disease and the risks of death, cardiovascular events and hospitalization. N. Engl. J. Med., 351(13), 1296–1305 (2004).
- Lin J , KhetarpalSA, TerembulaK, ReillyMP, WilsonFP. Relation of atherogenic lipoproteins with estimated glomerular filtration rate decline: a longitudinal study. BMC Nephrol., 16, 130 (2015).
- Toth P , GranowitzC, HullM, LiassouD and ersonA, PhilipS. Long-term renal function worsens in high cardiovascular risk patients with high triglycerides and well-controlled low-density lipoprotein cholesterol in a real-world analysis. J. Am. Coll. Cardiol., 71(Suppl. 11), A1445 (2018).
- Banach M , StulcT, DentR, TothPP. Statin non-adherence and residual cardiovascular risk: there is need for substantial improvement. Int. J. Cardiol., 225, 184–196 (2016).
- Toth PP , BanachM. Statins: then and now. Methodist Debakey Cardiovasc. J., 15(1), 23–31 (2019).
- Nichols GA , PhilipS, ReynoldsK, GranowitzCB, FazioS. Increased cardiovascular risk in hypertriglyceridemic patients with statin-controlled LDL cholesterol. J. Clin. Endocrinol. Metab., 103(8), 3019–3027 (2018).
- Leatherman S , FergusonR, WeirIet al. Increased residual cardiovascular risk in US veterans and monerately-elevated baseline triglycerides and well-controlled LCL-C levels on statins. J. Am. Coll. Cardiol., 73(Suppl. 91)), 1719 (2019).
- Leatherman S , FergusonRE, HauCet al. Residual cardiovascular risk in U.S. veterans with moderately-elevated baseline triglycerides across the cardiovascular risk spectrum. J. Am. Coll. Cardiol., 75(1 Suppl. 11), 1980 (2020).
- Nichols GA , PhilipS, ReynoldsK, GranowitzCB, FazioS. Increased residual cardiovascular risk in patients with diabetes and high vs. normal triglycerides despite statin-controlled LDL cholesterol. Diabetes Obes. Metab., 21(2), 366–371 (2019).
- Nichols GA , PhilipS, ReynoldsK, GranowitzCB, O'Keefe-RosettiM, FazioS. Comparison of medical care utilization and costs among patients with statin-controlled low-density lipoprotein cholesterol with versus without hypertriglyceridemia. Am. J. Cardiol., 122(7), 1128–1132 (2018).
- Bhatt DL , StegG, MillerMet al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J. Am. Coll. Cardiol., 73(22), 2791–2802 (2019).
- Peterson BE , BhattDL, StegGet al. Reduction of revascularization in patients with hypertriglyceridemia with icosapent ethyl: insights from REDUCE-IT REVASC [oral presentation]. Presented at: Annual Scientific Sessions of the Society for Cardiovascular Angiography and Interventions. ( virtual) (15 May 2020).
- Dorais M , ChirovskyD, AmbegaonkarBet al. Utilization patterns of extended-release niacin in Canada: analysis of an administrative claims database. Can. J. Cardiol., 26(7), e229–e235 (2010).
- Virani SS , AkeroydJM, NambiVet al. Estimation of eligibility for proprotein convertase subtilisin/kexin type 9 inhibitors and associated costs based on the FOURIER trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk): insights from the Department of Veterans Affairs. Circulation, 135(25), 2572–2574 (2017).
- Colantonio LD , HuangL, MondaKLet al. Adherence to high-intensity statins following a myocardial infarction hospitalization among Medicare beneficiaries. JAMA Cardiol., 2(8), 890–895 (2017).
- Deshpande S , QuekRG, ForbesCAet al. A systematic review to assess adherence and persistence with statins. Curr. Med. Res. Opin., 33(4), 769–778 (2017).
- Ofori-Asenso R , IlomakiJ, TaceyMet al. Patterns of statin use and long-term adherence and persistence among older adults with diabetes. J. Diabetes, 10(9), 699–707 (2018).
- Shroufi A , PowlesJW. Adherence and chemoprevention in major cardiovascular disease: a simulation study of the benefits of additional use of statins. J. Epidemiol. Community Health, 64(2), 109–113 (2010).
- American Diabetes Association . Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care, 43(Suppl. 1), S111–S134 (2020).
- Orringer CE , JacobsonTA, MakiKC. National Lipid Association Scientific Statement on the use of icosapent ethyl in statin-treated patients with elevated triglycerides and high or very-high ASCVD risk. J. Clin. Lipidol., 13(6), 860–872 (2019).
- Mach F , BaigentC, CatapanoALet al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J., 41(1), 111–188 (2020).
