Abstract
ST-elevation myocardial infarction treatment in the modern era has focused on minimizing time of ischemia by reducing door-to-balloon time to limit infarct size and improve survival. Although there have been significant improvements in minimizing time to coronary reperfusion, the incidence of heart failure following a myocardial infarction has remained high. Preclinical studies have shown that unloading the left ventricle for 30 min prior to coronary reperfusion can reduce infarct size and promote myocardial recovery. The DTU-STEMI randomized prospective trial will test the hypothesis that left ventricular unloading for at least 30 min prior to coronary reperfusion will improve infarct size and heart failure-related events as compared with the current standard of care.
Lay abstract Improvements in the treatment of heart attacks over the years have focused on rapidly opening the blocked vessel to limit the amount of heart muscle damage. Although there have been significant improvements in minimizing the time to treatment using various options from medications to balloons and stents, there continues to be a high incidence of heart failure following a heart attack with larger heart attacks leading to more heart failure. Recent studies in animal models have shown that unloading the work of the heart with a temporary heart pump can decrease the size of the heart attack and improve heart muscle recovery. The door-to-unload research program continues to investigate the treatment strategy of unloading the heart for at least 30 min prior to opening the blocked vessel to improve patient outcomes.
Despite great advancements in treatment, coronary artery disease and acute myocardial infarction (AMI) remain among the leading causes of morbidity and mortality worldwide. While great strides have been made in the reduction of mortality associated with AMI, this has been accompanied by an expanding heart failure population due to AMI-induced cardiomyopathy. The last several decades have seen the care of ST-elevation MI (STEMI) patients focused upon the reduction of door-to-balloon (DTB) times. This focus on DTB was based upon the promise of reduced infarct size and improved survival with reducing the time of myocardial ischemia. Nonetheless, despite major successes, the incidence of postAMI heart failure has remained high, and for every 5% increase in myocardial infarct size, there is a 20% increased risk for heart failure hospitalization within 1 year [Citation1].
A growing body of preclinical data over the past two decades has shown that mechanical unloading of the left ventricle (LV) before reperfusion therapy can reduce infarct size and reperfusion injury. Preclinical data have shown that LV unloading with delaying reperfusion by 30 min triggers a cardioprotective signaling cascade reducing infarct size and promoting recovery after AMI. These preclinical data and early clinical experience are the foundation of the STEMI-DTU clinical trial program, which is based on the concept that a percutaneously placed transvalvular axial flow pump (Impella CP®, Abiomed, MA, USA) can reduce LV pressure and volume, thereby reducing LV myocardial oxygen demand. There is a significant association between LV systolic wall stress and LV oxygen demand, with peak systolic wall stress identified as one of the primary factors associated with myocardial oxygen demand and, consequently, LV performance [Citation2]. More recently, an association has been identified between elevated LV end diastolic pressure and mortality in acute coronary syndromes [Citation3]. While early reperfusion aims to reoxygenate myocardium at risk, some have posited that it may be more effective to first pursue a strategy of reducing myocardial oxygen demand by means of LV unloading [Citation4,Citation5]. Direct mechanical unloading with the Impella pump consists of continuous aspiration of blood from the LV that is then pumped into the ascending aorta for systemic circulation. Several studies have confirmed that LV unloading with Impella significantly reduces end-diastolic and/or peak LV wall stresses [Citation6–8]. Current indications for the Impella CP are cardiogenic shock and high-risk percutaneous coronary intervention (PCI). At present, the Impella CP is not indicated in STEMI without cardiogenic shock. Of note, immediate reperfusion (IR) is the current standard of care for STEMI patients and delays in reperfusion are not recommended.
The ‘Door to Unloading with IMPELLA CP System in Acute Myocardial Infarction – Safety and Feasibility Study (DTU)’ was undertaken as a pilot trial to determine the safety and feasibility of LV unloading and delayed reperfusion (DR) in patients with STEMI and without cardiogenic shock [Citation9]. The DTU-STEMI pilot trial was a multicenter, prospective, randomized trial that enrolled 50 patients with anterior STEMI to either LV unloading with the Impella CP and IR or DR after 30 min of unloading. The primary safety outcome was a composite of major adverse cardiovascular and cerebrovascular events (MACCE) at 30 days. Assessment of infarct size by cardiac MRI was used to assess efficacy of the unloading strategy. The pilot trial concluded that 30 min of LV unloading with the Impella CP followed by coronary reperfusion is feasible in patients with STEMI. Importantly, there were no prohibitive safety signals to preclude an adequately powered pivotal trial of unloading before reperfusion compared with standard of care with IR. The favorable results of the pilot trial paved the way for the currently enrolling Primary Unloading and Delayed Reperfusion in ST-Elevation Myocardial Infarction: STEMI-DTU trial.
The STEMI-DTU pivotal trial (clinicaltrials.gov no. NCT03947619) will test the hypothesis that primary LV unloading for 30 min followed by coronary reperfusion may reduce STEMI-induced myocardial damage compared with the standard of care (primary PCI) in qualifying patients with anterior STEMI without cardiogenic shock.
Background & rationale
AMI remains a common cause of morbidity and mortality in the USA, with approximately 550,000 new attacks and 200,000 recurrent attacks annually [Citation10]. Despite advances in reducing DTB times to <90 min, mortality in the first year is particularly high in patients experiencing anterior MI. In patients undergoing PCI alone, mortality ranges from 5.2 to 6.0% at 6 and 12 months, as shown in the CRISP-AMI and CIRCUS trials, respectively [Citation11,Citation12]. In the large SWEDEHEART registry reporting >100,000 patients from 1996 to 2014 with STEMI, 1-year mortality remained as high as 14.1% [Citation13]. In addition, pooled patient-level analysis from ten randomized primary PCI trials demonstrated that for every 5% increase in myocardial infarct size, there is a 19% elevated risk for all-cause mortality and a 20% elevated risk for heart failure hospitalization at 1 year [Citation1]. This relationship between infarct size and all-cause mortality and/or heart failure hospitalization was stronger with anterior MI, with authors identifying a 7.4× elevated risk for all-cause mortality or heart failure hospitalization within 1 year for those patients with large left anterior descending (LAD) infarct (>17.9%) compared with 2.2× elevated risk for patients with large non-LAD infarct [Citation1]. In a Canadian population-based study of 7733 elderly patients with AMI, Ezekowitz et al. found that 76% of those who survived AMI hospitalization developed heart failure in the following 5 years [Citation14].
Reperfusion injury
For decades, the primary metric by which successful management of AMI was measured was a DTB time of 90 min or less. However, further reduction in DTB times has not been found to meaningfully improve early mortality. A large CathPCI registry analysis found that median DTB time improvement of 83 to 67 min from 2005 to 2009 was not associated with improved in-hospital mortality rates [Citation15]. In recent years, more practitioners have begun to discuss the persistent need to improve performance on other metrics beyond DTB times and reducing ischemic injury – such as reduction in reperfusion injury – in order to further improve STEMI survival outcomes [Citation16–19]. As flat mortality rates despite improving DTB times have identified, a successful STEMI treatment strategy must involve consideration of more granular metrics as relates to reperfusion timing and efficacy. The focus cannot remain solely on minimizing ischemic injury, but must also consider reperfusion injury.
With nearly a quarter of patients that survive initial STEMI developing heart failure necessitating rehospitalization within 1 year [Citation11], identifying safe and effective strategies that limit the final infarct size and reduce the likelihood that one ischemic event progresses to heart failure is the current challenge facing clinicians. A proven approach to inhibit reperfusion injury, the paradox by which reperfusion to the ischemic myocardium causes more cell death than ischemia itself, remains elusive. Cardioprotective strategies to mitigate reperfusion injury have largely focused on pharmacologic interventions, though additional strategies such as ischemic pre and postconditioning, pressure-controlled intermittent coronary sinus occlusion and LV unloading have been proposed. LV unloading has been associated with multiple mechanisms proposed to be responsible for promoting cardioprotection in the setting of acute coronary ischemia. One important mechanism to highlight is an improvement in coronary perfusion by reducing LV wall stress. The concept of functional or gentle reperfusion whereby LV unloading improves collateral flow to the ischemic myocardium has been shown to be an important mechanism to limit infarct size in canine models [Citation20–23]. In sum, cardioprotective strategies, their mechanisms and the amelioration of reperfusion injury, remain an area of great scientific and clinical interest.
However, to date, promising results achieved in animal models have not been reliably replicated in human studies [Citation24]. This may be due in part to patient comorbidities inhibiting optimal performance of certain strategies [Citation24,Citation25]. Perhaps more importantly, there is now a greater understanding of the complex pathophysiology of ischemic reperfusion injury, which involves several systems contributing to oxidative stress on the cell as well as several distinct pathways to cell death [Citation26,Citation27]. The mechanistic underpinnings of the manifold processes contributing to reperfusion injury is beyond the scope of this report and have been reported previously [Citation25]. In brief, there is now agreement that a successful therapeutic strategy for reperfusion injury is unlikely to be one that targets a single pathway or system; rather, a synergistic cardioprotective strategy is necessary in order to mitigate reperfusion injury [Citation21,Citation25,Citation28].
LV remodeling
Adverse LV remodeling is more common following a large, transmural AMI, which can significantly alter the ventricular structure including both infarcted and noninfarcted regions [Citation29,Citation30]. In the last several years, numerous studies have identified infarct size as the most significant predictor for adverse LV remodeling [Citation31–33]. In a small German study, Lund et al. found that infarct size was the most significant predictor for adverse remodeling, with risk increasing by 2.8× for every 10% increase in infarct size, and infarct size of 24% identified as a predictive threshold for remodeling, with 92% sensitivity and 93% specificity [Citation31]. Similarly, Masci et al. found that, on multivariate analysis, MI size as a percentage of LV area was the sole significant predictor of adverse LV remodeling, with a 1.1× elevated risk for every percentage increase (p < 0.001) [Citation32]. Springeling et al. also found that infarct mass was the only significant predictor for progressive LV dilatation on multivariate analysis [Citation33].
In an American College of Cardiology Expert Analysis on the causes and prevention of ventricular remodeling in the setting of AMI, Abouzaki et al. theorized that the mechanism of action of the Impella – unloading the LV while providing enhanced perfusion – should encourage improved remodeling [Citation34]. However, the authors note that while this approach has shown promise in preclinical studies, it has not yet been affirmed in human studies and the invasiveness of the technique should limit its use to those with cardiogenic shock during MI. There is an ongoing need to assess the approach of LV unloading in clinical studies as a means to improve STEMI outcomes, and to confirm its safety and efficacy in AMI patients, with or without cardiogenic shock.
Preclinical studies
LV unloading in acute ischemia is not a novel concept. Medical literature dating back to the early 1960s has shown the benefit of mechanical LV unloading during ischemia, reducing the oxygen utilization and LV pressures using left heart bypass [Citation35]. Two decades later, with the improvement of cardiovascular surgery techniques, Allen et al. demonstrated a 32% reduction in infarct size with LV unloading using cardiopulmonary bypass, cardioplegia and LV venting [Citation36]. However, the application of left heart bypass at the time of acute MI remains challenging and is associated with considerable morbidity [Citation37]. Therefore, the concept of mechanical unloading in the setting of acute MI remained essentially dormant until the emergence of miniature transvalvular axial-flow pumps in the 1990s, which were found to be effective in reducing LV wall stress, stroke work and myocardial oxygen demand while maintaining mean arterial pressure, without the need for surgery [Citation4].
Preclinical studies evaluating LV unloading during coronary occlusion with Impella technology have focused on the timing and duration of LV unloading, as well as the level of support needed. summarizes important preclinical studies in large animal models [Citation23,Citation37–43]. Meyns et al. showed that support before reperfusion is crucial to achieve substantial reduction in infarct size [Citation37]. Moreover, this seminal study also identified that full support is required to achieve substantial reduction infarct size. These findings were recently confirmed by Ko et al. in a different animal model [Citation23].
Table 1. Preclinical studies assessing left ventricle unloading with Impella prior to reperfusion.
In a porcine model, Kapur et al. established that 30 min of LV unloading prior to coronary reperfusion achieves the best myocardial salvage [Citation38]. In addition, the authors identified that delaying coronary reperfusion activates endogenous protective signaling pathways which limit reperfusion injury and thereby myocardial loss [Citation38]. The mechanisms underlying the cardioprotective relationship of primary unloading with infarct size were further explored in a preclinical study of a porcine infarct model [Citation39]. Male Yorkshire swine was subjected to 90 min of mid-LAD occlusion followed by either IR (primary reperfusion group) or 30 min of unloading prior to reperfusion (primary unloading group). Primary unloading limited apoptosis in the infarct zone by both increasing the expression of genes associated with cellular respiration and mitochondrial integrity and reducing the activity levels of proteases known to degrade the cardioprotective cytokine, stromal-derived factor-1a. At 28 days post-AMI, the primary unloading group exhibited reduced LV scar size, improved cardiac function, and reduced expression of biomarkers associated with heart failure and maladaptive remodeling compared with the primary reperfusion group. A similar experiment performed by Sun et al. using surgically implanted Impella 5.0 pumps showed comparable findings [Citation40]. Finally, a recent analysis by Swain and Kapur further identified that LV unloading with an Impella CP reduces mitochondrial damage associated with reperfusion injury [Citation22]. By protecting mitochondrial function, heart muscle cells are more likely to recover function over time [Citation40].
The degree of LV support needed to achieve optimal unloading with and reduce infarct size was explored by Saku et al. [Citation41]. In this well-designed study, the authors found that infarct percent size showed greatest reduction in canines receiving full LV unloading with the Impella CP, compared with partial or no unloading. However, translating early unloading prior to coronary intervention into clinical practice is particularly challenging as it not only requires excellent logistical support but also defies the ‘time is muscle’ paradigm that has been engrained in the interventional communities for decades [Citation44,Citation45].
DTU-STEMI pilot trial
The Door to Unloading with IMPELLA CP System in Acute Myocardial Infarction – Safety and Feasibility Study (DTU) was the first exploratory human study testing the safety and feasibility of LV unloading and DR as a method to reduce infarct size in patients with STEMI (without cardiogenic shock). The trial was conducted at 14 US centers and randomly assigned 50 patients presenting with their first STEMI to either mechanical LV unloading with the Impella CP with IR, or DR after 30 min of unloading [Citation9]. The ST-segment sum in the precordial lead v1–v4 was used to assess the infarct size prior to randomization. Infarct size was evaluated by cardiac magnet resonance with area at risk similar to the pathology assessment in the preclinical studies (). The primary safety outcome was a composite of MACCE at 30 days.
Findings from this study suggest for the first time that LV unloading using the Impella CP device with a 30-min delay before reperfusion is feasible within a relatively short DTB time. The DTU-STEMI pilot trial did not identify any prohibitive safety signals that would preclude a larger pivotal study of LV unloading before reperfusion. In demonstrating the safety of DR, the DTU-STEMI study also identified a significant area for future research, with the possibility of pursuing adjunct pharmacologic strategies during the unloading period prior to reperfusion (which may ultimately identify the type of synergistic, multitarget strategy necessary to effectively mitigate reperfusion injury). MACCE at 30 days were not significantly different between the unloading with DR and IR groups, with MACCE occurring in three patients (12%; 95% CI: 2.6–31.2%) and two patients (8%; 95% CI: 1.0–26.0%), respectively (p > 0.99). These rates are in line with those generally seen in contemporary trials of anterior MI populations [Citation12,Citation46,Citation47].
Although the pilot trial was not designed to show efficacy, favorable signals in infarct size reduction were detected. In particular, subgroup analysis identified those with large anterior infarct with ST-segment sum >6 mm as showing the greatest benefit [Citation48]. Another thought-provoking finding was that the infarct size did not increase in the DR arm when plotted against the ST-segment sum. The authors further found that the duration of LV unloading before reperfusion showed an inverse relationship with infarct size [Citation48]. The strength of the correlation between unloading to reperfusion time and infarct size was found to be significant (p = 0.01), with an R value of -0.51. Based on results from this pilot trial, an appropriately powered pivotal trial comparing LV unloading and DR with the current standard of care (IR without unloading) was initiated.
Future perspective
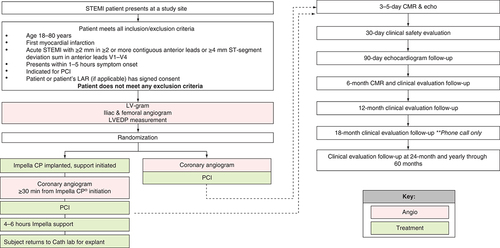
With safety and feasibility established via preclinical studies and a human pilot trial, the next logical step in the door-to-unload research program is a pivotal trial. Indeed, this trial is underway and currently enrolling. The STEMI-DTU pivotal trial is a prospective, multicenter, randomized control trial involving up to 60 centers in the USA, as well as additional international sites, that aims to investigate the potential benefit of mechanical LV unloading with the Impella CP system prior to PCI in patients with anterior STEMI. Patients will be randomized into either a control arm receiving the current standard of care, IR (DTB), or an investigational arm with Impella CP placement and mechanical LV unloading for at least 30 min prior to reperfusion (door-to-unload; ).
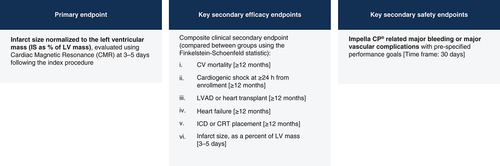
This study will include patients aged 18–80 years presenting within 1–5 h of chest pain onset, along with ST-segment elevation of ≥2 mm in two or more contiguous anterior leads or with ≥4 mm total ST-segment elevation sum in the anterior leads v1 through v4. Subjects with prior MI, prior coronary artery bypass grafting, cardiogenic shock, unwitnessed cardiac arrest or ≥30 min of cardiopulmonary resuscitation, contraindication to or inability for Impella CP insertion, and inability to undergo cardiac MRI will be excluded (). The estimated enrollment will be 688 subjects meeting the listed criteria. All subjects will be equally randomized to either the control or experimental arms. Two roll-in subjects will be treated at each site (one each randomized to the control and experimental arms) for investigators to familiarize and adapt to the study protocol. Roll-in subjects will not be included in the patient population for final analysis. The primary and secondary end points are shown in .
Table 2. Full inclusion and exclusion criteria for the STEMI-DTU pivotal trial.
The study enrolled its first patients on 12 December 2019. Enrollment continued until 20 March 2020 when the COVID-19 pandemic began to significantly impact clinical research at the investigational sites, and the decision was made to pause enrollment for the trial. Enrollment was restarted on 6 July 2020. The original estimated completion date for data collection of the primary outcome measure is October 2023. The estimated study end date is October 2027. Due to the pause in enrollment from COVID-19, these dates will likely be delayed as another major viral surge has occurred worldwide as of the time of writing (November 2020). Further information on the trial can be found at clinicaltrials.gov (trial no. NCT03947619).
Conclusion
The STEMI-DTU pivotal trial is a landmark trial that has the potential to impact the growing epidemic of post-AMI heart failure and improve outcomes for STEMI patients around the world. It has the potential to shift the paradigm of IR to one of LV unloading followed by reperfusion, to protect myocardium and impact the downstream consequences of impaired LV function over time. In this trial, we have the opportunity for the first time to test the preclinical data in a randomized controlled trial and translate these findings from the preclinical to the clinical patient arena.
Background
Early, effective reperfusion therapy, from thrombolytic therapies to angioplasty and stenting has defined ST-elevation myocardial infarction (STEMI) care for >30 years, reducing the morbidity and mortality from this devastating event. However, STEMI remains a leading cause of morbidity and mortality worldwide and contributes to the growing population of patients with heart failure requiring ongoing therapies and hospital readmissions.
Preclinical testing has demonstrated that compared with reperfusion therapy alone, mechanically unloading the left ventricle (LV) with the Impella CP® for 30 min prior to coronary reperfusion reduces infarct size following STEMI.
The DTU-STEMI pilot trial was the first study assessing the safety and feasibility of LV unloading with the Impella CP and delayed reperfusion as a method to reduce infarct size in patients with anterior STEMI without cardiogenic shock.
The randomized pilot trial demonstrated both the safety and feasibility of this approach, allowing the forthcoming STEMI-DTU pivotal trial.
DTU-STEMI trial
The DTU-STEMI pivotal trial will enroll up to 668 patients (plus two roll-in subjects per site) at up to 60 investigational sites in the USA, with additional international sites.
Patients will be randomized to either the global standard of care (immediate reperfusion) or ≥30 min of LV unloading prior to reperfusion.
The primary end point is infarct size as a percent of LV mass, as measured by cardiac magnet resonance. Patients with be followed through 5 years, with secondary efficacy outcomes assessed when all subjects have at least 12-months’ follow-up.
Conclusion
LV unloading to preserve LV myocardial function as an initial strategy in the treatment of patients with high-risk STEMI is a potentially paradigm changing concept. This approach brings hope for a future where door-to-unloading takes precedence over reperfusion in appropriate patients, offering the promise of reducing the short- and long-term consequences of left ventricular dysfunction and chronic heart failure.
This work also represents a great example of translational research bringing basic and preclinical science to the clinical arena for the benefit of patients.
Acknowledgments
The authors would like to thank D Bentley, Abiomed, for providing medical editing services and C Preston, Spectrum Health, for technical editing services.
Financial & competing interests disclosure
KH Schuleri and AK Chakrabarti are full-time employees of Abiomed and own Abiomed stock and stock options. WW O'Neill is a consultant for Abiomed. NK Kapur receives institutional research funding from Abbott, Abiomed, Boston Scientific, Getinge, LivaNova and MDStart. NK Kapur also receives consulting/speaking honoraria from Abbott, Abiomed, Boston Scientific, Getinge, LivaNova, Medtronic, Edwards, Zoll, MDStart and Precardia. DH Wohns receives institutional research and educational funding as well as consulting/speaking honoraria from Abiomed. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Stone GW , SelkerHP, ThieleHet al. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J. Am. Coll. Cardiol., 67(14), 1674–1683 (2016).
- Strauer BE . Myocardial oxygen consumption in chronic heart disease: role of wall stress, hypertrophy and coronary reserve. Am. J. Cardiol., 44(4), 730–740 (1979).
- Leistner DM , DietrichS, ErbayAet al. Association of left ventricular end-diastolic pressure with mortality in patients undergoing percutaneous coronary intervention for acute coronary syndromes. Catheter Cardiovasc. Interv., 96(4), E439–E446 (2020).
- Uriel N , SayerG, AnnamalaiS, KapurNK, BurkhoffD. Mechanical unloading in heart failure. J. Am. Coll. Cardiol., 72(5), 569–580 (2018).
- Heusch G , RassafT. Left ventricular unloading in myocardial infarction. J. Am. Coll. Cardiol., 76(6), 700–702 (2020).
- Remmelink M , SjauwKD, HenriquesJPet al. Effects of mechanical left ventricular unloading by Impella on left ventricular dynamics in high-risk and primary percutaneous coronary intervention patients. Catheter Cardiovasc. Interv., 75(2), 187–194 (2010).
- Watanabe S , FishK, KovacicJCet al. Left ventricular unloading using an Impella CP improves coronary flow and infarct zone perfusion in ischemic heart failure. J. Am. Heart Assoc., 7(6), e006462 (2018).
- Kapur NK , ParuchuriV, Urbano-MoralesJAet al. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation, 128(4), 328–336 (2013).
- Kapur NK , AlkhouliMA, DemartiniTJet al. Unloading the left ventricle before reperfusion in patients with anterior ST-segment-elevation myocardial infarction. Circulation, 139(3), 337–346 (2019).
- Writing Group M , MozaffarianD, BenjaminEJet al.Heart Disease and Stroke Statistics-2016 update: a report from the American Heart Association. Circulation, 133(4), e38–360 (2016).
- Cung TT , MorelO, CaylaGet al. Cyclosporine before PCI in patients with acute myocardial infarction. N. Engl. J. Med., 373(11), 1021–1031 (2015).
- Patel MR , SmallingRW, ThieleHet al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA, 306(12), 1329–1337 (2011).
- Szummer K , WallentinL, LindhagenLet al. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995–2014. Eur. Heart J., 38(41), 3056–3065 (2017).
- Ezekowitz JA , KaulP, BakalJA, ArmstrongPW, WelshRC, McalisterFA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J. Am. Coll. Cardiol., 53(1), 13–20 (2009).
- Menees DS , PetersonED, WangYet al. Door-to-balloon time and mortality among patients undergoing primary PCI. N. Engl. J. Med., 369(10), 901–909 (2013).
- Bagai A , DangasGD, StoneGW, GrangerCB. Reperfusion strategies in acute coronary syndromes. Circ. Res., 114(12), 1918–1928 (2014).
- Allencherril J , AlamM, LevineGet al. Do we need potent intravenous antiplatelet inhibition at the time of reperfusion during ST-segment elevation myocardial infarction? J. Cardiovasc. Pharmacol. Ther., 24(3), 215–224 (2019).
- Huang MH , LohPH, TanHC, PohKK. Reducing reperfusion injury during percutaneous coronary intervention. Singapore Med. J., 60(12), 608–609 (2019).
- Ibanez B , HeuschG, OvizeM, VanDe Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol., 65(14), 1454–1471 (2015).
- Achour H , BoccalandroF, FelliPet al. Mechanical left ventricular unloading prior to reperfusion reduces infarct size in a canine infarction model. Catheter Cardiovasc. Interv., 64(2), 182–192 (2005).
- Heusch G . Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol., 17(12), 773–789 (2020).
- Kapur NK , ReyeltL, SwainLet al. Mechanical left ventricular unloading to reduce infarct size during acute myocardial infarction: insight from preclinical and clinical studies. J. Cardiovasc. Transl. Res., 12(2), 87–94 (2019).
- Ko B , DrakosSG, IbrahimHet al. Percutaneous mechanical unloading simultaneously with reperfusion induces increased myocardial salvage in experimental acute myocardial infarction. Circ. Heart Fail., 13(1), e005893 (2020).
- Xia Z , LiH, IrwinMG. Myocardial ischaemia reperfusion injury: the challenge of translating ischaemic and anaesthetic protection from animal models to humans. Br. J. Anaesth., 117(Suppl. 2), ii44–ii62 (2016).
- Davidson SM , FerdinandyP, AndreadouIet al. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J. Am. Coll. Cardiol., 73(1), 89–99 (2019).
- Kalogeris T , BainesCP, KrenzM, KorthuisRJ. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol., 298, 229–317 (2012).
- Wu MY , YiangGT, LiaoWTet al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol. Biochem., 46(4), 1650–1667 (2018).
- Heusch G . Critical issues for the translation of cardioprotection. Circ. Res., 120(9), 1477–1486 (2017).
- Pfeffer MA , BraunwaldE. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation, 81(4), 1161–1172 (1990).
- Heusch G , LibbyP, GershBet al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet, 383(9932), 1933–1943 (2014).
- Lund GK , StorkA, MuellerleileKet al. Prediction of left ventricular remodeling and analysis of infarct resorption in patients with reperfused myocardial infarcts by using contrast-enhanced MR imaging. Radiology, 245(1), 95–102 (2007).
- Masci PG , GanameJ, FranconeMet al. Relationship between location and size of myocardial infarction and their reciprocal influences on post-infarction left ventricular remodelling. Eur. Heart J., 32(13), 1640–1648 (2011).
- Springeling T , KirschbaumSW, RossiAet al. Late cardiac remodeling after primary percutaneous coronary intervention-five-year cardiac magnetic resonance imaging follow-up. Circ. J., 77(1), 81–88 (2013).
- Abouzaki N , AbbateA. Causes and prevention of ventricular remodeling after MI: expert analysis. J. Am.Coll. Cardiol. (2016). https://www.acc.org/latest-in-cardiology/articles/2016/07/21/07/28/causes-and-prevention-of-ventricular-remodeling-after-mi
- Dennis C , HallDP, MorenoJR, SenningA. Reduction of the oxygen utilization of the heart by left heart bypass. Circ. Res., 10, 298–305 (1962).
- Allen BS , OkamotoF, BuckbergGD, BugyiH, LeafJ. Reperfusion conditions: critical importance of total ventricular decompression during regional reperfusion. J. Thorac. Cardiovasc. Surg., 92(3 Pt 2), 605–612 (1986).
- Meyns B , StolinskiJ, LeunensV, VerbekenE, FlamengW. Left ventricular support by catheter-mounted axial flow pump reduces infarct size. J. Am. Coll. Cardiol., 41(7), 1087–1095 (2003).
- Kapur NK , ParuchuriV, QiaoXet al. TCT-244 the Impella to balloon (ITB) strategy limits infarct size and improves survival in acute myocardial infarction complicated by cardiogenic shock: a bench to bedside study. J. Am. Coll. Cardiol., 66(Suppl. 15), B95–B96 (2015).
- Esposito ML , ZhangY, QiaoXet al. Left ventricular unloading before reperfusion promotes functional recovery after acute myocardial infarction. J. Am. Coll. Cardiol., 72(5), 501–514 (2018).
- Sun X , LiJ, ZhaoWet al. Early assistance with left ventricular assist device limits left ventricular remodeling after acute myocardial infarction in a swine model. Artif. Organs, 40(3), 243–251 (2016).
- Saku K , KakinoT, ArimuraTet al. Left ventricular mechanical unloading by total support of Impella in myocardial infarction reduces infarct size, preserves left ventricular function, and prevents subsequent heart failure in dogs. Circ. Heart Fail., 11(5), e004397 (2018).
- Briceno N , AnnamalaiSK, ReyeltLet al. Left ventricular unloading increases the coronary collateral flow index before reperfusion and reduces infarct size in a swine model of acute myocardial infarction. J. Am. Heart Assoc., 8(22), e013586 (2019).
- Kapur NK , QiaoX, ParuchuriVet al. Mechanical pre-conditioning with acute circulatory support before reperfusion limits infarct size in acute myocardial infarction. JACC Heart Fail., 3(11), 873–882 (2015).
- De Luca G , Van'THof AW, DeBoer MJet al. Time-to-treatment significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute myocardial infarction treated by primary angioplasty. Eur. Heart J., 25(12), 1009–1013 (2004).
- Gibson CM , DeLemos JA, AntmanEM. Group TS. Time is muscle in primary PCI: the strength of the evidence grows. Eur. Heart J., 25(12), 1001–1002 (2004).
- Egred M , BagnallA, SpyridopoulosIet al. Effect of Pressure-controlled intermittent Coronary Sinus Occlusion (PiCSO) on infarct size in anterior STEMI: PiCSO in ACS study. Int. J. Cardiol. Heart Vasc., 28, 100526 (2020).
- Ibanez B , MacayaC, Sanchez-BruneteVet al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation, 128(14), 1495–1503 (2013).
- Swain L , ReyeltL, BhaveSet al. Transvalvular ventricular unloading before reperfusion in acute myocardial infarction. J. Am. Coll. Cardiol., 76(6), 684–699 (2020).