Abstract
Recurrent vulvovaginal candidiasis (RVVC) has significant disease, financial and quality-of-life burdens, affects women from all strata of society worldwide, and lacks an approved therapeutic solution. Fluconazole emerged in 2004 as an antifungal for RVVC; it provides symptom control and has been accepted worldwide as a first-line treatment. Its limitations include the development of resistance and a high rate of vulvovaginal candidiasis recurrence after therapy cessation. There is now an improved treatment option on the horizon: oteseconazole – a novel, oral, selective fungal cytochrome P450 enzyme 51 inhibitor, designed to avoid off-target toxicities. In clinical studies to date, oteseconazole has demonstrated impressive efficacy, a positive tolerability profile and hope for a superior RVVC treatment option.
Lay abstract
Many women are affected by vaginal fungal infections, also called yeast infections. These infections can affect normal daily activities and have negative emotional and financial effects as well, especially for women who have yeast infections repeatedly. There is no US FDA-approved treatment for repeated yeast infections although the symptoms are often managed with a prescription antifungal medication, fluconazole. However, using fluconazole can have health risks, especially when it is used repeatedly over months or years. Another problem is that the yeasts that cause the infection can become resistant to the treatment. A new medication has been developed and tested in clinical studies and may soon provide women with an effective treatment option for repeated yeast infections that is also safer.
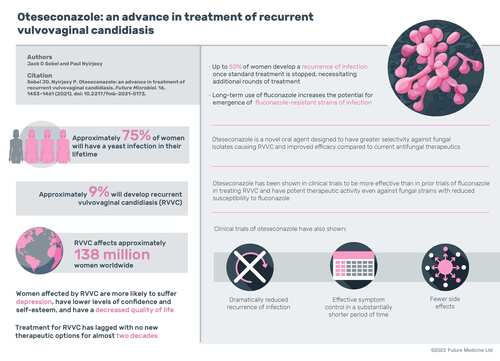
Approximately 75% of women develop vulvovaginal candidiasis (VVC) at least once in their lifetime, most commonly during the reproductive years; ∼9% of women develop repeat or recurrent VVC, or RVVC [Citation1]. A systematic review of global literature from 1985 to 2016 estimated that RVVC affects ∼138 million women worldwide, with a global annual prevalence of 3871 per 100,000 women [Citation2]. Risk factors for VVC include use of contraceptives, pregnancy, hormone replacement therapy and antibiotics, as well as sexual activity and diabetes [Citation3]. Susceptibility to RVVC likely has a genetic and immunological basis, although the pathogenesis is still poorly understood [Citation1,Citation4]; in many patients, no apparent underlying conditions or predisposing factors are apparent [Citation5].
RVVC symptoms range from moderate to severe, but almost invariably influence quality of life, increasing stress and decreasing self-esteem and confidence [Citation2]. Women affected by RVVC are more likely to suffer from clinical depression and have less satisfaction with their lives in general than women without RVVC [Citation3]. They often report that symptoms from RVVC make them feel ‘dirty’ and that partner relations become strained, as suspicions arise regarding the faithfulness of each partner [Citation2]. Qualitative interviews have elicited that women with RVVC report higher levels of anxiety and fear about social interactions and dating than women without RVVC [Citation3]. In addition to its effect on sexual relationships, mental health and quality of life, RVVC has a high economic burden. Women with RVVC have reported that the out-of-pocket costs for treatment, doctor visits and medication are one of the top burdens of the disease, along with discomfort and avoidance of sexual activity [Citation3].
RVVC is defined as ≥3 recurrences of symptomatic VVC within a 12-month period, with completely asymptomatic periods between acute VCC episodes [Citation5]. Currently, there is no US FDA-approved antifungal specifically indicated to treat RVVC [Citation1,Citation6]. The causative pathogen in ∼80–90% of cases of acute VVC is Candida albicans, a polymorphic opportunistic fungus [Citation7,Citation8]. Similarly, C. albicans is the cause of most cases of RVVC, and most cases caused by C. albicans are sensitive to fluconazole, which has been the standard treatment for VVC for the past 20 years [Citation1,Citation9]. Although less frequent, VVC and RVVC infections can also result from non-albicans Candida species, including Candida glabrata, Candida parapsilosis and Candida tropicalis, among other species [Citation8,Citation9].
Current treatment landscape
Azole antifungals are the primary treatment for RVVC recommended by clinical practice guidelines, including those published by the Infectious Diseases Society of America [Citation10] and the US CDC [Citation5]. Current US FDA-approved azoles inhibit the activity of fungal cytochrome P450 (CYP) enzyme 51 (CYP51) via competitive and reversible binding to the heme cofactor in the enzyme active site [Citation11]. CYP51 is essential for fungal growth and the formation and integrity of the fungal cell membrane; inhibition of CYP51 allows accumulation of fungal-toxic sterols [Citation12]. Although azoles are effective inhibitors of CYP51, they also inhibit a range of human CYP enzymes, resulting in safety issues, including drug–drug interactions (DDIs), visual disturbances and liver toxicity [Citation13]. The first successful RVVC treatment, daily oral ketoconazole, debuted in 1986 [Citation14], but is now rarely used to treat VVC because of the potential for hepatotoxicity. Following a landmark study in 2004 [Citation15], fluconazole emerged as the clinical guideline-recommended oral antifungal and has been used to treat VVC, including RVVC, for almost 20 years [Citation9]. In addition to fluconazole, topical azole antifungals are also widely prescribed to treat VVC; however, due to the 6-month duration of recommended maintenance treatment for RVVC, topicals are inconvenient for use in RVVC and are rarely prescribed in that population [Citation10].
The induction phase of the RVVC treatment regimen is 7–14 days of topical therapy or three doses of 150 mg of once-daily oral fluconazole every 72 h to treat acute symptoms [Citation5,Citation10]. For the maintenance phase, guidelines recommend 150 mg of fluconazole once weekly for at least 6 months. Fluconazole is effective in suppressing symptomatic recurrences while treatment is ongoing; however, within 6 months of treatment cessation, up to 50% of women have a recurrence of active symptoms, necessitating additional rounds of induction and maintenance treatment [Citation6,Citation15]. Repeated antifungal regimens of topical azoles and/or oral fluconazole can continue for years. Although most women report episodes of RVVC lasting 1–2 years, a substantial number struggle with recurrences lasting 4–5 years and, in some cases, even decades [Citation1]. Furthermore, in the 10–20% of women with C. glabrata or other non-albicans Candida species RVVC, suppressive therapy with azoles is not as effective as for C. albicans RVVC infections [Citation5].
In addition, long-term use of fluconazole increases the potential for emergence of azole-resistant strains of C. albicans and other Candida species [Citation13,Citation16]. These infections become challenging to treat because there are few therapeutic options [Citation6,Citation9]. The ubiquitous and sometimes prolonged use of fluconazole poses other clinical challenges, including multiple known DDIs with drugs such as tacrolimus and sirolimus, tolbutamide, and midazolam and triazolam [Citation13,Citation17]. Fluconazole carries a warning for use in women who are pregnant or may become pregnant because of off-target effects on human CYPs [Citation13], which is problematic in treating a disease that is common in reproductive-age women.
There is significant clinical need for a treatment that can greatly reduce recurrence of VVC infection and be used long term without adverse effects. The novel oral antifungal oteseconazole (VT-1161) was designed to have greater selectivity for fungal CYP51, with fewer adverse events (AEs) and improved efficacy compared with current VVC and RVVC antifungal therapeutics, and results of clinical studies have supported these design intents [Citation11,Citation18,Citation19]. Oteseconazole is expected to be the first US FDA-approved treatment indicated for RVVC.
Oteseconazole (VT-1161)
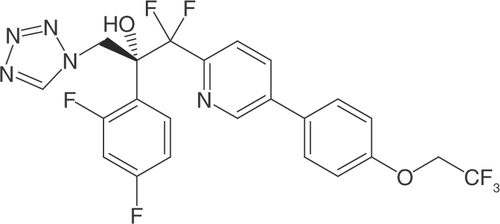
Oteseconazole is a novel oral agent. It is a white to off-white crystalline powder, chemically described as (R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(1H-tetrazol-1-yl)-1-(5-(4-(2,2,2-trifluoroethoxy)-phenyl)pyridin-2-yl)propan-2-ol () [Citation20]. Unlike previous azoles, which contain an imidazole or triazole moiety that binds the human cytochrome, oteseconazole has a tetrazole moiety (five-member ring of four nitrogen atoms and one carbon atom), with improved target selectivity due to the attenuated interaction between the metal-binding groups and the heme cofactor [Citation21]. This design produces the same or better selectivity for fungal CYP51 than can be seen with other azole drugs, with less interaction with off-target human CYPs, thus reducing the potential for safety issues [Citation22].
Unlike previous azoles, which contain an imidazole or triazole moiety that binds the human cytochrome P450 (CYP), oteseconazole has a tetrazole moiety (five-member ring of four nitrogen atoms and one carbon atom), with improved target selectivity, and does not bind to human CYP51. Oteseconazole strongly binds CYP51 of Candida species due to the attenuated interaction between the metal binding groups and the heme cofactor.
Oteseconazole binding with fungal CYP51 produces a strong type II difference spectrum, with a target affinity similar to that of clotrimazole, fluconazole, itraconazole and voriconazole [Citation13]. This combination of features may represent a significant advance in the treatment of RVVC [Citation13,Citation23].
Antifungal spectrum & targeted pathogenic fungi
Oteseconazole had potent activity against a broad range of Candida species when tested against a panel of clinical isolates of common yeast species that cause invasive infections, including isolates with reduced susceptibility to fluconazole. Oteseconazole had excellent activity against C. albicans and C. glabrata compared with fluconazole, as well as activity against less-common strains. For most species, oteseconazole was, on average, more than 40-fold more potent than fluconazole [Citation13,Citation20,Citation23].
Antifungal activity of oteseconazole against a panel of clinical isolates from patients with VVC was compared with the activity of fluconazole [Citation20]. Whereas the clinical isolates showed reduced susceptibility to fluconazole, oteseconazole had potent activity against most of the strains tested. Clinical isolates from a Phase IIa study (NCT01891331) [Citation19] investigating the efficacy of oteseconazole in acute VVC infection (described in the following clinical trials section) were also evaluated in comparison to fluconazole. Oteseconazole had potent activity against all strains obtained from VVC study participants, with MIC scores approximately 100-fold lower than for fluconazole and 64-fold lower than fluconazole for fluconazole-resistant C. glabrata strains [Citation19,Citation20]. Data on minimum inhibitory concentration (MIC) values and ranges are summarized in .
Table 1. Minimum inhibitory concentration.
Oteseconazole has shown in vitro antifungal activity against clinical isolates of Coccidioides immitis and Coccidioides posadasii [Citation24]. Activity of oteseconazole against Cryptococcus species was statistically significantly more potent than fluconazole against a panel of 79 Cryptococcus neoformans var grubii and 21 Cryptococcus gatti isolates [Citation23]. Oteseconazole has also been shown to be a potent inhibitor of dermatophytes. Testing against clinical isolates of Trichophyton rubrum and Trichophyton mentagrophytes showed that oteseconazole potency was similar to the potency of itraconazole, a US FDA-approved treatment for onychomycosis [Citation20].
Pharmacology
In vitro pharmacology studies of Candida were conducted to determine antifungal potency and selectivity of oteseconazole for fungal versus human CYP51, key CYP enzymes, noncytochrome metalloenzymes, and a broad panel of receptors, ion channels and transporters [Citation20]. In spectrophotometric assays, oteseconazole bound directly and with high potency to fungal CYP51 but weakly or not at all to the heme-iron of human CYP51 [Citation13]. Oteseconazole was shown to bind at least 2200-fold more tightly to fungal than to human CYP51; whereas itraconazole and voriconazole binding, respectively, were 2- and 92-fold greater for fungal versus human CYP51. Oteseconazole also inhibited enzymatic activity of C. albicans, but not human, CYP51. Half-maximal inhibitory concentration for fungal CYP51 was 1.5 μM, which was approximately half the concentration used in the assay of the enzyme. Human CYP51 was not inhibited by oteseconazole at a concentration of 50 μM, indicating much higher selectivity of oteseconazole for fungal CYP51, as determined in this sensitive spectrophotometric assay [Citation20].
In in vivo studies, oral oteseconazole significantly suppressed Candida burden in a mouse model of experimental acute vaginitis. Treatment of a murine model of VVC showed statistically significant efficacy versus placebo in reducing fungal burden at 1 and 4 days after treatment, including against fluconazole-resistant yeasts [Citation22]. Pharmacokinetics (PK) information from this murine model showed high oral bioavailability (73%) of oteseconazole, consistent with levels measured in vaginal tissue, as well as a long half-life of >48 h [Citation22].
In vitro plasma protein binding of oteseconazole was shown to be high – a concentration of 260 ng/ml was 97.6% protein-bound in a murine model and 99.5% in rat, 99.4% in dog and 99.5% in human plasma [Citation20]. Based on the limited interaction with human CYP51 and other CYP enzymes, high plasma binding level and low aqueous solubility of oteseconazole, no clinically significant DDIs are expected. Oteseconazole was well absorbed in animal models, with good oral bioavailability, and models all had a similarly long plasma half-life [Citation20]. In rats, plasma half-life ranged from 55 to 203 h after an oral dose of 5 mg/kg or repeat dosing for 26 weeks at 1.5 mg/kg, respectively. In dogs, the plasma half-life ranged from 56 to 872 h after an oral dose of 10 mg/kg or repeat dosing for 39 weeks at 17 mg/kg, respectively [Citation20]. When administered in a fasted state in dogs, the absolute oral bioavailability of the capsule formulation was approximately 40%, but when given in a fed state, it was up to 100%. Concentration of oteseconazole was shown to be higher in tissue than in blood, although elimination was similar for both. Oteseconazole was mainly excreted via feces and bile, with low levels of recovered radioactivity in urine.
Clinical trials
Phase I studies of the effect of oteseconazole in healthy adults have been completed [Citation20]. Summaries of the completed Phase I studies are presented in . A cross-study PK analysis was performed, incorporating data from eight of the Phase I studies; results included findings that drug clearance was not affected by age or sex, although there was an approximately linear relationship between participant weight and clearance. Clearance was affected by race, with non-white participants having 48% higher clearance, although the cause is unknown.
Table 2. Phase I trials of oteseconazole in healthy participants.
A Phase IIa proof-of-concept study of the tolerability, efficacy and PK of oteseconazole was conducted in patients with moderate to severe acute VVC [Citation19]. Healthy, nonpregnant female participants, aged 18–65 years, with a clinical diagnosis of symptomatic acute VVC were enrolled in the study (n = 65). The first portion of the study was a treatment period for current acute VVC episode, with random assignment to either fluconazole or oteseconazole. Dosages of oteseconazole were 300 mg daily for 3 days, 600 mg daily for 3 days, or 600 mg twice daily for 3 days. Fluconazole treatment was one dose of 150 mg, with administration matched to oteseconazole (once a day for 3 days) to maintain blinding.
The primary efficacy end point was therapeutic cure at day 28, defined as absence of signs and symptoms plus mycologic cure. Assessments for clinical recurrence and mycologic cure were continued for 5 months after study cessation [Citation19]. In the intent-to-treat (ITT) population (all randomized participants), the low-, mid- and high-dose oteseconazole groups and the fluconazole control group achieved effective therapeutic cure at rates of 64.3%, 75.0%, 78.6% and 66.7%, respectively. The differences between the three oteseconazole dose groups versus the fluconazole group were not statistically significant at day 28. Of note, the most substantial finding of the study was the rate of VVC symptom recurrence over the longer term. All participants who received oteseconazole demonstrated mycologic cure at 3 months and 6 months; in contrast, almost half of participants treated with fluconazole showed mycologic recurrence at 6 months. However, higher doses of fluconazole were not used in this data comparison, only the US FDA-approved dosage.
Based on these encouraging long-term effects, a Phase IIb (NCT02267382), multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study of treatment for RVCC was also conducted [Citation18]. Women aged 18–65 years (N = 254) with clinical diagnosis of symptomatic acute VVC and a history of RVVC, defined as ≥3 VVC episodes in the previous 12 months, were enrolled. The 1-week induction phase was open-label treatment of the patient’s current VVC episode, with a dose of 150-mg fluconazole orally every 72 h, times three doses. In the 48-week double-blind maintenance phase (n = 215), patients received one of four oteseconazole dosing regimens (42 participants in the 150-mg/12-week group; 43 participants in the 150-mg/24-week group; 43 participants in the 300-mg/12-week group; 41 participants in the 300-mg/24-week group) or placebo (46 participants) to obtain oteseconazole dose–response information as a second-line treatment and to gain further insight into the duration of oteseconazole treatment response.
The primary efficacy outcome was the proportion of participants in the ITT population with ≥1 culture-verified acute recurrence of VVC infection through the 48-week maintenance phase [Citation18]. Secondary outcomes included proportion of participants with ≥1 positive mycologic culture through weeks 24 and 48. Culture-verified recurrence in the oteseconazole groups and the placebo group occurred most often within the first 12 weeks. In the ITT population, seven of 169 (4.1%) participants in the oteseconazole treatment groups had a culture-verified episode of VVC versus 24 of 46 (52.2%) participants in the placebo group. The difference in proportion of recurrence was significant for each oteseconazole group versus placebo (p < 0.0001; ). As in the Phase IIa study, at 3-month and 6-month follow-up, all patients who received oteseconazole maintenance showed mycologic cure, whereas almost half of the patients who received placebo maintenance showed mycologic recurrence [Citation19]. Unfortunately, direct comparison with fluconazole was not evaluated in this study. Together, these studies show that oteseconazole was effective at treating acute and recurrent VVC [Citation18,Citation19]. In contrast to fluconazole maintenance, in which half of patients would be expected to recur after a prolonged regimen, patients treated with oteseconazole showed sustained treatment effect, continuing to demonstrate mycologic cure at 3-month and 6-month follow-up [Citation4,Citation6,Citation15,Citation18].
Table 3. Primary and secondary end points for the Phase IIb dose-ranging study, through week 48, in the intent-to-treat population.
In the Phase IIb study, plasma exposure of oteseconazole increased with the increasing dose and duration of treatment [Citation18]. Oteseconazole was well tolerated in all dosing groups; the most common treatment-emergent adverse events (TEAEs) occurring in >5% of patients were urinary tract infection, bacterial vaginosis, sinusitis, headache, upper respiratory tract infection and nausea (). Most TEAEs were judged by the investigator to be mild or moderate and unrelated to the study drug. There were no treatment-related changes in electrocardiography or urinalysis parameters. No serious AEs or TEAEs leading to discontinuation were reported. Data are pending for recently completed Phase III clinical studies (NCT03562156, NCT02267382, NCT01891331) evaluating oteseconazole treatment in girls and women (aged ≥12 years) with RVVC.
Table 4. Summary of treatment-emergent adverse events in the Phase IIb RVVC study safety population.
Potential indications & use
To define the potential role for oteseconazole in clinical practice, it is reasonable to review the current use of antifungal agents in the treatment of VVC. Oral fluconazole is used in a variety of dose regimens for acute uncomplicated VVC, as well as for the more challenging variety of complicated RVVC. Uncomplicated VVC refers to sporadic episodes of VVC of mild to moderate clinical disease in otherwise healthy women, and this category dominates the clinical picture. These episodes are generally caused by azole-susceptible Candida species, usually C. albicans. Treatment of VVC in nonpregnant women is dominated worldwide by administration of one or two doses of fluconazole, which is available as a generic, inexpensive prescription therapy. In this patient population, oteseconazole may offer a treatment option for patients with a history of fluconazole allergy or intolerance, in patients for whom polypharmacy or fluconazole DDIs may be a concern, or for patients with fluconazole-resistant Candida strains.
For women with RVVC, oteseconazole could likely be a first-line treatment consideration. In women with RVVC, a not inconsiderable population globally, treatment needs are currently unmet by available topical antifungal and oral azole agents, including fluconazole. It is highly inconvenient for women with RVVC to implement long-term topical antifungal use; therefore, that option is used infrequently, unless the specific Candida strain is more effectively targeted with a particular topical agent. Weekly fluconazole has emerged as a therapeutic success in the treatment of RVCC over the past ~20 years. The success resides in effectively controlling patients’ symptoms, as long and as often as regimens are rigorously followed (and strain resistance does not develop), but these regimens do not offer patients long-term prevention of disease recurrence.
The efficacy data for oteseconazole presented here indicate that effective symptom control can be achieved in substantially shorter treatment durations than with weekly fluconazole: treatment duration can be reduced by as much as 50%. The optimal duration of therapy remains to be determined, as well as length of protective period following cessation of oteseconazole; evidence from study follow-up durations of up to 1 year indicated the period is likely 12 months or longer. Moreover, efficacy in symptom control in these studies was accompanied by the even more impressive suppression of vaginal yeast recolonization. Although oteseconazole does not directly influence the likely genetic basis of RVVC, preventing vaginal recolonization for a prolonged period may be possible. The basis for this success includes PK factors and increased antifungal activity. The relative contributions of these two effects have yet to be determined.
Thus, if the promise of the Phase II studies is substantiated in not-yet-published Phase III studies, oteseconazole may replace fluconazole as the preferred therapy for women with RVVC caused by azole-sensitive C. albicans strains, provided treatment cost is reasonable and the AE profile does not change. This will be even more pertinent for women with RVVC resulting from infection with species that demonstrate reduced sensitivity to fluconazole, including both C. albicans and non-albicans Candida species. In particular, the treatment needs of women infected with C. glabrata are not currently met by the available azole agents, and published in vitro data suggest improved results for these patients with oteseconazole therapy. Similarly, women with medical conditions that compromise response to fluconazole, such as poorly controlled diabetes, may have substantial treatment benefits from oteseconazole. Phase III oteseconazole study results will provide further data to establish dosing and frequency, as well as confirm the efficacy and safety, of this novel agent in women with RVVC.
Conclusion
The in vivo and in vitro studies of oteseconazole to date demonstrate the potential for fewer AEs than are seen with previous-generation azole antifungals, as well as reduced DDIs and potential for hepatotoxicity. Oteseconazole has been shown to have potent activity against fungal clinical isolates of Candida species, including those with reduced susceptibility to standard azole drugs, and statistically significant efficacy in prevention of RVVC recurrence. The high degree of potency and selectivity of oteseconazole is superior to that of approved CYP51 inhibitors, and safety has been corroborated in multiple preclinical and Phase I and II studies. Oteseconazole has unique qualities that hold great promise to fill unmet treatment needs of patients with RVVC.
Future perspective
After two decades of empty antifungal drug pipeline, the arrivals of oteseconazole and similar agents are a major step forward but are not a comprehensive solution. Recognizing that it takes 10–15 years from drug discovery to treatment availability, it is unlikely that the next step forward will be yet another antifungal drug or, for that matter, agents that control the vaginal microbiome. Instead, progress is likely to emerge from previously unavailable sources. One foresees the likely availability of effective anti-inflammatory or anticytokine drugs that induce vaginal yeast tolerance. Let us not exclude subtle host genetic modification (CRISPR) methods to effectively prevent, rather than treat, vaginitis.
Recurrent vulvovaginal candidiasis is a major worldwide problem
Recurrent vulvovaginal candidiasis (RVVC) affects women from all strata of society, with multiple consequences.
Treatment for RVVC has lagged, with no new therapeutic options for almost two decades.
Adding to limited treatment options is the rapidly growing problem of RVVC caused by fluconazole-resistant vaginal yeast isolates that were previously limited to non-albicansCandida species, but are now seen frequently with Candida albicans.
Effective symptom control of RVVC with less recurrence & adverse effects may be achievable
Oteseconazole is a newly discovered, oral, selective fungal cytochrome P450 enzyme 51 inhibitor with enhanced potency against a broad spectrum of fungal pathogenic species, including fluconazole-resistant vaginal isolates.
Phase I and Phase II clinical study data demonstrate exciting therapeutic advantages, particularly in the field of RVVC.
Extensive safety data to date, together with a dramatic reduction in the long-term vulvovaginal candidiasis recurrence rate, point to an optimistic future for this new agent as clinical studies progress.
Infographic
Download PDF (2.8 MB)Acknowledgments
Susan A Leon and Tam Minh Nguyen-Cao of Claritas Scientific LLC provided medical writing services under the direction of the authors, and Ann D Bledsoe Bollert of Y-Axis Editorial provided editorial expertise.
Supplementary data
Financial & competing interests disclosure
Oteseconazole is under development by Mycovia Pharmaceuticals Inc. (Mycovia) for the treatment of recurrent vulvovaginal candidiasis. Mycovia was not involved in the development of this article. The authors worked independently from Mycovia. Jack D Sobel participated as a principal investigator of the Phase III VIOLET studies (NCT03562156 and NCT03561701) and has also served as a consultant for and/or has received research funding from Mycovia Pharmaceuticals and Scynexis. Paul Nyirjesy participated as a principal investigator of the VIOLET Study and has also served as a consultant for and/or has received research funding from Mycovia Pharmaceuticals, Scynexis Inc., and Hologic Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing services for this article were sponsored by Mycovia Pharmaceuticals Inc.
Additional information
Funding
References
- Sobel JD . Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol.214(1), 15–21 (2016).
- Denning DW , KnealeM, SobelJD, Rautemaa-RichardsonR. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect. Dis.18(11), e339–e347 (2018).
- Blostein F , Levin-SparenbergE, WagnerJ, FoxmanB. Recurrent vulvovaginal candidiasis. Ann. Epidemiol.27(9), 575–582.e3 (2017).
- Crouss T , SobelJD, SmithK, NyirjesyP. Long-term outcomes of women with recurrent vulvovaginal candidiasis after a course of maintenance antifungal therapy. J. Low. Genit. Tract Dis.22(4), 382–386 (2018).
- Sexually transmitted infections treatment guideline, 2021 (2021). www.cdc.gov/std/treatment-guidelines/candidiasis.htm
- Collins LM , MooreR, SobelJD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J. Low. Genit. Tract Dis.24(1), 48–52 (2020).
- Willems HME , AhmedSS, LiuJ, XuZ, PetersBM. Vulvovaginal candidiasis: a current understanding and burning questions. J. Fungi (Basel)6(1), 27 (2020).
- Azie N , AnguloD, DehnB, SobelJD. Oral ibrexafungerp: an investigational agent for the treatment of vulvovaginal candidiasis. Expert Opin. Investig. Drugs29(9), 893–900 (2020).
- Sobel JD , SobelR. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin. Pharmacother.19(9), 971–977 (2018).
- Pappas PG , KauffmanCA, AndesDRet al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis.62(4), e1–e50 (2016).
- Hoekstra WJ , GarveyEP, MooreWR, RaffertySW, YatesCM, SchotzingerRJ. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg. Med. Chem. Lett.24(15), 3455–3458 (2014).
- Yoshida Y . Cytochrome P450 of fungi: primary target for azole antifungal agents. Curr. Top. Med. Mycol.2, 388–418 (1988).
- Warrilow AG , HullCM, ParkerJEet al. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob. Agents Chemother.58(12), 7121–7127 (2014).
- Sobel JD . Recurrent vulvovaginal candidiasis. A prospective study of the efficacy of maintenance ketoconazole therapy. N. Engl. J. Med.315(23), 1455–1458 (1986).
- Sobel JD , WiesenfeldHC, MartensMet al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med.351(9), 876–883 (2004).
- Marchaim D , LemanekL, BheemreddyS, KayeKS, SobelJD. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet. Gynecol.120(6), 1407–1414 (2012).
- Bellmann R , SmuszkiewiczP. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection45(6), 737–779 (2017).
- Brand SR , DegenhardtTP, PersonKet al. A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of orally administered VT-1161 in the treatment of recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol.218(6), 624.e621–624.e629 (2018).
- Brand SR , SobelJD, NyirjesyP, GhannoumMA, SchotzingerRJ, DegenhardtTP. A randomized phase 2 study of VT-1161 for the treatment of acute vulvovaginal candidiasis. Clin. Infect. Dis.73(7), e1518–e1524 (2021).
- Data on File. Mycovia Pharmaceuticals Inc. (2021).
- Schell WA , JonesAM, GarveyEP, HoekstraWJ, SchotzingerRJ, AlexanderBD. Fungal CYP51 inhibitors VT-1161 and VT-1129 exhibit strong in vitro activity against Candida glabrata and C. krusei isolates clinically resistant to azole and echinocandin antifungal compounds. Antimicrob. Agents Chemother.61(3), e01817–16 (2017).
- Garvey EP , HoekstraWJ, SchotzingerRJ, SobelJD, LillyEA, FidelPL Jr. Efficacy of the clinical agent VT-1161 against fluconazole-sensitive and -resistant Candida albicans in a murine model of vaginal candidiasis. Antimicrob. Agents Chemother.59(9), 5567–5573 (2015).
- Fothergill AW , RinaldiMG, HoekstraWJet al. In vitro activity of two metalloenzyme inhibitors compared to caspofungin and fluconazole against a panel of 74 Candida spp. Presented at: Interscience Conference on Antimicrobial Agents and Chemotherapy.MA, USA (2010).
- Shubitz LF , TrinhHT, GalgianiJNet al. Evaluation of VT-1161 for treatment of coccidioidomycosis in murine infection models. Antimicrob. Agents Chemother.59(12), 7249–7254 (2015).