Abstract
The SARS-CoV-2 pandemic put an unprecedented strain on modern societies and healthcare systems. A significantly higher incidence of invasive fungal co-infections was noted compared with the pre-COVID-19 era, adding new diagnostic and therapeutic challenges in the critical care setting. In the current narrative review, we focus on invasive mold infections caused by Aspergillus and Mucor species in critically ill COVID-19 patients. We discuss up-to-date information on the incidence, pathogenesis, diagnosis and treatment of these mold-COVID-19 co-infections, as well as recommendations on preventive and prophylactic interventions. Traditional risk factors were often not recognized in COVID-19-associated aspergillosis and mucormycosis, highlighting the role of other determinant risk factors. The associated patient outcomes were worse compared with COVID-19 patients without mold co-infection.
Aspergillosis
Incidence: prevalence
The incidence of COVID-19-associated pulmonary aspergillosis varies in the literature, mainly due to different diagnostic methods and definitions used, but is quite high.
Risk factors: pathogenesis
Most patients with CAPA lack the traditional host factors. The prolonged use of high doses of corticosteroids and anti-interleukin-6 agents may be implicated in the pathogenesis of CAPA.
Diagnosis
The diagnosis of CAPA is challenging as bronchocopy is in most cases necessary to discriminate infection from colonization. Serum biomarkers have low sensitivity and CT findings overlap with severe COVID-19 pneumonia. The newly defined diagnostic criteria for CAPA are based on compatible clinical symptoms, radiological findings and microbiology testing.
Treatment
First-line treatment for Aspergillus is voriconazole and isavuconazole, while triazole resistance is an emerging problem, which necessitates susceptibility testing.
Prevention & prophylaxis
CAPA prophylaxis with antifungals is not generally recommended, apart from patients with prolonged neutropenia, GVHD, secondary prophylaxis. Despite reports of effective application of systematic and/or inhaled antifungal CAPA prophylaxis in the critical care setting, further, larger studies are needed to clarify whether and which sub-groups might benefit from this approach.
Outcomes
Higher mortality has been reported in patients with CAPA, although the attributable mortality of the fungal coinfection has not yet been accurately assessed.
Mucormycosis
Incidence
The presentation of COVID-19-associated mucormycosis differs between Western countries and areas like India. In India a much higher incidence has been reported, and rhino-orbital cerebral mucormycosis is the most common form. In Western countries, mucormycosis is a rare complication of severe COVID-19, and the pulmonary form is more common.
Pathogenesis: risk factors
In India, the main risk factor is uncontrolled diabetes mellitus, while in Western countries it is usually associated with underlying immunosuppression.
Diagnosis
Mycology testing is essential, as the visualization of the characteristic hyphae confirms the diagnosis. Regarding imaging, it is challenging to distinguish whether ground-glass opacities or consolidations are due to mucormycosis or due to COVID-19 pneumonia/ARDS itself in deteriorating severely ill patients with COVID-19. Nevertheless, several lung nodules or the presence of pulmonary cavities should prompt a thorough search for invasive fungal coinfection. CT and MRI imaging contribute to disease staging.
Treatment
CAM treatment includes the management of the underlying predisposing condition, empirical initiation of liposomal amphotericin-B and surgical resection of necrotic tissues.
Prevention & prophylaxis
Mucormycosis prophylaxis in COVID-19 patients is only recommended in neutropenic patients and GVHD.
Outcomes
Mortality is higher in the pulmonary form of CAM.
The COVID-19 crisis has put an unprecedented strain on modern societies and healthcare systems through the toll of massive hospitalizations and deaths. Many patients were cared for by limited, exhausted and, often, not specialized healthcare workers in overcrowded hospital departments and, sometimes, outside the hospital (sports arenas, for example) [Citation1,Citation2]. Meanwhile, medical supplies were scarce, including personal protective equipment, an absolute cornerstone when it comes to healthcare safety [Citation3]. Under these circumstances, the infection control programs have had a prolonged hiatus. Moreover, COVID-19, as well as the corticosteroids and the immunomodulatory treatment, may predispose to co-infections with non-viral pathogens, such as bacteria and fungi [Citation4,Citation5]. Indeed, systematic corticosteroid administration has been universally used against the hyperinflammatory syndrome induced by SARS-CoV-2 [Citation6]. In addition, tocilizumab, a humanized monoclonal antibody against the IL-6 receptor acting as an immunomodulator minimizes the development of the pro-inflammatory process and might facilitate fungal infections [Citation6].
Aspergillus and Candida species are the fungi that cause most of the COVID-19 fungal co-infections, followed by Mucor species [Citation7]. In the current review we will focus on invasive fungal infections caused by molds, and we will present and critically discuss up-to-date information on the epidemiology, pathogenesis, diagnosis, treatment and prevention/prophylaxis of fungal infections caused by Aspergillus and Mucor species in critically ill COVID-19 patients.
Aspergillosis
Members of the genus Aspergillus are airborne fungi presenting a worldwide distribution that can cause a large spectrum of clinical presentations after being inhaled. With lungs as entry point, Aspergillus can affect any organ, including brain, heart and liver. Allergic bronchopulmonary aspergillosis, Aspergillus bronchitis, chronic pulmonary aspergillosis and community-acquired Aspergillus pneumonia are some of the possible presentations of the disease [Citation8]. Formerly considered a threat for patients with severe immunocompromised states, the incidence of invasive aspergillosis in patients without the traditional (classic) risk factors of immunosuppression has been steadily increasing, including critically ill patients [Citation9]. Moreover, invasive aspergillosis is now considered a well-known complication of severe viral pneumonia [Citation9]. A mortality rate higher than 50% was observed in critically ill patients with influenza-associated pulmonary aspergillosis (IAPA), which was predisposed by immunosuppressive drugs, malignancy, diabetes or corticosteroids [Citation10,Citation11]. Early after the start of the SARS-CoV-2 pandemic, fungal co-infections and especially those with Aspergillus species were reported [Citation12]. At the same time, attention was drawn to the difficulties of diagnosis, high associated mortality and continuous vigilance necessary for the optimal management of invasive fungal co-infections during COVID-19 infection [Citation13]. A new disease was, therefore, recognized and coined the term COVID-19-associated pulmonary aspergillosis (CAPA), in other words, pulmonary infection with Aspergillus spexies in COVID-19 patients. Aspergillus species involved in patients with SARS-CoV-2 patients are quite various. Among them, Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Aspergillus citronotterreus, Aspergillus lentelus, Aspergillus versicolor, Aspergillus terreus and Aspergillus calidoustus have been reported as causes of CAPA [Citation14]. However, most cases of invasive aspergillosis are associated with A. fumigatus; the second most common species is A. flavus, while A. terreus has been increasingly reported.
Incidence & prevalence
The incidence and prevalence of CAPA greatly differs between studies [Citation15-21]. This wide range of reported CAPA rates may reflect variability in several factors, including risk conditions, local epidemiology, surveillance practices, diagnostic methods and definitions used in the different studies [Citation15]. Interestingly, discrepancies in geographical distribution may reflect differences in the burden of environmental exposure or in genetic predisposition [Citation7,Citation22]. In addition, not all diagnostic procedures, either imaging (computerized tomography) or laboratory (fungal biomarkers), might have been available in all hospitals, in a pandemic setting. Bronchoscopy, which is regularly performed in non-COVID-19 patients, frequently had been avoided in critically ill patients with the disease, especially in the first waves, although special techniques to minimize exposure to the virus have been proposed, and the liberal use of so-called aerosol-generating procedures seem to have no impact on COVID-19 acquisition risk among healthcare-workers when PPE is applied appropriately [Citation23,Citation24]. Difficulty in interpretation of the diagnostic results might also be another contributing factor. Not only it is hard to discriminate colonization from infection, but also, the use of different diagnostics in various centers impedes comparison between reports. Lack of homogeneity of several studies, for example, inclusion of intubated and non-intubated patients in the same study further contributes to the difficulty of interpretation of CAPA incidence and prevalence. An important issue which might explain the differences in the incidence of CAPA is the period studied. During the third wave, a single-center prospective screening study revealed CAPA frequency of 20.1% (12.2% probable, 7.9% possible) [Citation25]. Given that in later waves the use of steroids and IL-6 inhibitors increased significantly, higher frequencies of mold infections might be ascribed to this fact [Citation26].
Based on these data, CAPA incidence in mechanically ventilated COVID-19 patients, ranges from 1.6 to 38% [Citation7]. A meta-analysis conducted by Kariyawasam et al. reported CAPA prevalence of 9.3% in patients with COVID-19, with a wide range from 0.0 to 33% [Citation15]. In ICU patients the prevalence was 10% (95% CI: 8–13%), while in those under mechanical ventilation 11% (95% CI: 9–15%) [Citation15]. With the uniform application of the 2020 ECMM/ISHAM consensus definitions, the reported probable CAPA prevalence dropped to 10.7% [Citation19]. In the latter study, CAPA prevalence was higher in older patients and in patients receiving invasive respiratory support [Citation19]. (For more details on the epidemiology of COVID-19-associated invasive fungal co-infections, visual representation of the timelines and relevant maps, we refer the reader to reference [Citation7]).
Risk factors & pathogenesis
The vast majority of CAPA patients lack the traditional host risk factors for aspergillosis such as, neutropenia, hematologic malignancy, bone marrow or solid organ transplantation and acute graft-versus-host disease (GVHD). Airborne contamination may contribute to the disease. Moreover, the widespread use of negative pressure for protection from COVID-19 may have increased the risk of fungal infection due to the air pulled from the environment [Citation27].
Several factors have been considered culprits for CAPA development. The lung epithelial damage caused by SARS-CoV-2, along with the immune dysregulation, are considered as predisposing factors for CAPA. Also, some of the drugs used for the treatment of COVID-19, in other words, azithromycin, corticosteroids and anti-interleukin-6 (anti-IL-6) agents have been implicated in CAPA pathogenesis. Azithromycin dosing over 1500 mg has been proposed as a risk factor in earlier publications [Citation28]. Possible mechanisms explaining its role included promoting Aspergillus colonization and down-regulation of neutrophil oxidative burst which is a system barrier against aspergillosis [Citation29,Citation30]. The use of corticosteroids is another factor that might be contributing to the Aspergillus invasion of the lung. Corticosteroids may enhance Aspergillus‘ growth by several mechanisms, including impairment of neutrophil function. During the first waves of the pandemic, the use of corticosteroids has been associated with CAPA [Citation16,Citation18]. Nevertheless, according to newer guidelines, corticosteroids are a part of standard treatment and newer publications do not support these findings [Citation19,Citation31]. In a systematic review with meta-analysis involving eight cohort studies, Chong et al. found that only prolonged corticosteroid treatment and older age were risk factors for CAPA [Citation32]. Meanwhile, Kim et al. found that cumulative dexamethasone above 60 mg, over the first 10 hospital days, was independently associated with CAPA [Citation33]. In a multicenter retrospective study conducted in Dutch ICUs, older age, male sex and higher APACHE IV on admission were associated with increased risk for CAPA in the multivariate analysis, while treatment with remdesivir was associated with decreased risk [Citation34]. In a French multicenter study, independent risk factors for CAPA development were age older than 62 (OR: 2.34; 95% CI: 1.39–3.92), dexamethasone and anti-IL-6 treatment (OR: 2.71; 95% CI: 1.12–6.56) and duration of mechanical ventilation >14 days (OR: 2.16; 95% CI: 1.14–4.09) [Citation35]. Tocilizumab, is an IL-6 receptor antagonist which, according to a large study, significantly improves survival as well as other clinical outcomes [Citation36]. However, the use of tocilizumab may predispose to CAPA [Citation19].
Disturbances of immune regulation caused by COVID-19, such as high pro-inflammatory and anti-inflammatory cytokine levels affecting T-helper-2 responses in patients with severe disease, may contribute to Aspergillus superinfection [Citation37]. The up-regulation of IL-10 may also inhibit macrophage activity [Citation38]. Early involvement of IL-1 also promotes a favorable medium for Aspergillus to colonize the lungs, thus the risk for CAPA is higher. Mucociliary clearance dysfunction due to COVID-associated damage of the epithelial lining of the respiratory system might facilitate the attachment of Aspergillus spores and bronchial mucosa invasion [Citation38,Citation39]. A recent report examined bio-banked BAL and transbronchial biopsy samples from respiratory-supported patients with influenza, IAPA, COVID-19 and CAPA. The authors have found that CAPA was associated with disrupted epithelial barrier, lower phagocytic ability and downregulated interferon-gamma signaling than COVID-19 alone individuals [Citation40].
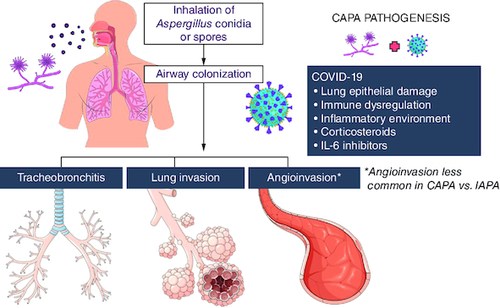
The summary of CAPA pathogenesis is depicted in .
Figure 1. COVID-19-associated pulmonary aspergillosis pathogenesis.
Invasive pulmonary aspergillosis is an opportunistic infection occurring in vulnerable patients after inhalation of conidia or spores of Aspergillus spp. SARS-CoV-2 pneumonia and ARDS create favorable conditions for Aspergillus growth and tissue invasion, causing epithelial lung damage, create an inflammatory environment, and dysregulating the immune response and clearance of the fungus, while corticosteroids and IL-6 inhibitors, used for severe COVID-19 treatment, also play a role in CAPA development.
Diagnosis
The median (interquantile range) number of days from COVID-19-associated ICU admission until CAPA diagnosis was 8 (4–14) [Citation35]. Interestingly, in a recent report, CAPA or aspergillus tracheobronchitis was diagnosed after a median of 20.5 (5–33) days after ICU admission [Citation41]. It should be noted though that most studies relied on diagnostic work-up performed when a patient was deteriorating, so the timing of onset remains to be defined more accurately.
The diagnosis of invasive aspergillosis in critically ill patients without any of the ‘classic’ host risk factors is a well-known challenge due to several factors, including the absence of symptoms in a sedated patient, the difficulty/risks in transporting a severely unstable patient for a CT scan, the non-specific radiologic findings of aspergillosis in immunocompetent patients or findings similar with those of the primary lung disease, the unavailability/contraindication of lung biopsy [Citation9,Citation12]. However, the diagnosis of CAPA is even more challenging because invasive pulmonary aspergillosis (IPA) and COVID-19 present overlapping symptoms and signs from the lungs, without any specific differential clinical characteristics [Citation12,Citation20,Citation42]. CAPA might be misdiagnosed or missed, because there are no uniform criteria for diagnosis in an intensive care unit [Citation12,Citation42]. On the other hand, CAPA might be overdiagnosed if colonization or Aspergillus tracheobronchitis is mistakenly classified as CAPA.
Bronchoscopy with performance of BAL is a tool of particular importance for the diagnosis of IPA; bronchoscopy enables direct visualization of the trachea and the main bronchi (biopsies and brush can be taken as needed), while BAL samples microscopy with optical brighteners provides rapid validated results, and when the culture results are available, helps in their interpretation [Citation12]. The general-purpose medium that is commonly used for the detection of Aspergillus is Sabouraud dextrose agar supplemented with chloramphenicol to prevent contamination from bacteria that may be present and cycloheximide to reduce the frequency of environmental fungal growth. Apart from BAL microscopy and cultures, galactomannan (GM; BAL GM index >1), Aspergillus PCR and azole resistance markers detection on BAL fluid have been also quite well validated [Citation12,Citation43-46]. Combination of GM and Aspergillus DNA PCR in BAL samples seems to increase the diagnostic specificity for IPA and it is strongly recommended [Citation45]. It should be noted that, although well validated, not even BAL samples test can reliably distinguish colonization from disease [Citation12]. For rapid detection of Aspergillus antigen, lateral flow device (LFD) or ELISA are also used but its marginally recommended to diagnose IPA and moderately to exclude it [Citation12]. Guidelines initially suggested avoiding routine diagnostic bronchoscopy, due to the risk of aerosol, but it is recommended in patients with negative nasopharyngeal cultures and when BAL sampling might change the clinical management of the patient, provided that all the necessary protections are followed [Citation12,Citation47]. Non-bronchoscopic lavage is an alternative method applied in COVID-19 patients to minimize the risk of virus exposure. It includes the blind application of 10–20 ml saline which is recovered by aspiration via a closed aspiration system [Citation48]. However, the thresholds for biomarkers from non-bronchoscopic sampling have not been validated. Such sampling might be performed as screening and confirmed with GM BAL testing.
Considering all the above, samples should be taken for direct microscopy and culture, GM, and PCR Aspergillus in cases of patients with a high risk of CAPA. In the case of isolation of Aspergillus species and after the identification, susceptibility testing should follow and be interpreted according to the EUCAST fungal breakpoints v10.0 [Citation49]. Finally, it should be emphasized that serum biomarkers have low sensitivity in CAPA patients; sensitivity of serum GM 0–40% and of serum (1–3)-Beta-D-glucan (BDG) 0–50%, while BDG is a panfungal marker not specific for aspergillosis. The low sensitivity of serum BDG has been attributed to the low angioinvasion in CAPA patients compared with neutropenic patients with aspergillosis [Citation12]. Moreover, the percentage of angioinvasion in CAPA is even lower than IAPA (~20% vs 60%, respectively).
With regards to imaging, the lung computed tomography (CT) scan is not specific for CAPA patients: the typical signs present in immunocompromised patients with the classic risk factors, in other words, halo sign, the air-crescent, nodules and cavitating lesions may be absent or maybe due to the COVID-19 itself or due to other co-infection or other lung pathology [Citation12,Citation47]. However, despite the lack of specificity, it may trigger a diagnostic work-up for CAPA, if new findings are revealed compared with the baseline CT on ICU admission in a deteriorating patient. For further details on CT imaging of CAPA, we refer the reader to the meta-analysis of Hong et al.; this meta-analysis corroborated previous studies that CAPA can frequently present without the common CT abnormalities of invasive aspergillosis, and reported also that, if abnormalities exist in chest CT, CAPA might often accompany bronchial abnormalities [Citation50].
In terms of invasive aspergillosis criteria, the revised European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium criteria (EORTC/MSGERC) 2020 are not particularly helpful for intubated patients if they are not immunocompromised, since the ‘host factor’ is absent [Citation51,Citation52]. The difficulty of lung biopsy, the lack of symptoms in a critically ill sedated patient, the difficulty of radiological confirmation in a patient whose situation does not allow the transportation for a CT scan, and the unspecific radiological findings are some of the reasons that made these criteria not easily applicable for the critically ill intubated patient. During the COVID-19 pandemic, several studies have been published, using different criteria previously developed for invasive aspergillosis or for IAPA that were not specific for CAPA; these criteria do not differ regarding proven aspergillosis but vary regarding the probable, possible and putative case definitions, in other words, differences related to the presence of host factors, imaging, diagnostic samples and mycological test (it is beyond the scope of this review to detail) [Citation12,Citation17,Citation53,Citation54].
In the meantime, consensus criteria have been developed and proposed for diagnosis of aspergillosis co-infection in patients with COVID-19. These definitions are the result of a combination of several diagnostic criteria (). The 2020 ECMM/ISHAM consensus criteria were developed by experts in the field, considering the diagnostic difficulties of invasive aspergillosis in COVID-19 patients, and adding a quantitative criterion for non-BAL specimens [Citation20]. Another case definition aiming specifically at CAPA patients was developed by Lahmer et al. [Citation55]. Finally, Hashim et al. quite recently suggested another diagnostic approach which significantly differs from other criteria [Citation56]. The presence of clinical and host risk factors together with CT findings (such as nodules, cavities or reverse halo sign) define ‘suspected’ CAPA, the additional finding of a fungal biomarker, (such as GM in serum, BAL or telescopic BAL) upgrades the diagnosis to ‘possible’, while the direct identification of the fungus by microscopy or PCR, defines the ‘probable’ disease [Citation56]. Further validation of the above-mentioned definitions is needed.
Table 1. Main case definitions for invasive pulmonary aspergillosis used in COVID-19 patients.
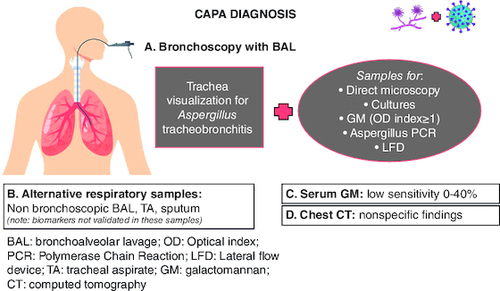
In , different criteria suggested for CAPA definition are presented. Diagnostic techniques for CAPA depicted in .
Figure 2. COVID-19-associated aspergillosis diagnosis.
CAPA diagnosis is challenging, as non-immunocompromised critically ill patients lack the classical host risk factors. Bronchoscopy with BAL is the most reliable diagnostic tool to obtain lower respiratory tract samples for direct microscopy, cultures, PCR, GM, LFD and discriminate colonization from invasive pulmonary aspergillosis (IPA). Imaging findings of IPA may overlap with those of COVID-19 pneumonia and typical signs, such as the halo and air crescent sign, are not commonly seen in CAPA.
Treatment
According to international guidelines, Aspergillus first-line treatment includes the triazoles voriconazole and isavuconazole [Citation57,Citation58]. Intravenous voriconazole might prolong the Q-T interval of the electrocardiogram (ECG), especially if other drugs with the same adverse effect potential are administered. The possibility of drug–drug interactions and subtherapeutic levels are also of concern. Therefore, voriconazole monitoring is deemed necessary to avoid drug toxicities [Citation59]. Furthermore, patients with COVID-19 disease might present impaired renal function. Voriconazole, in its intravenous form, might as well adversely affect renal function because of the potential toxic effects of the accumulation of the solvent vehicle sulphobutyletherbeta-cyclodextrin sodium. However, isavuconazole may be a better treatment option as it lacks nephrotoxicity and presents fewer drug–drug interactions. Of note, hydroxychloroquine and azithromycin, two drugs that might have had an interaction with azoles, are no longer used for COVID-19 treatment [Citation60,Citation61].
In a 5-year retrospective study, Lestrade et al. report a non-negligible percentage of triazole-resistant Aspergillus infections with untoward consequences on mortality [Citation62].
A matter of concern is the emergence of multi-triazole resistance which emphasizes the importance of antifungal susceptibility testing. Genotyping testing is another way to trace the mutations associated with triazole resistance (TR34/L98H and TR46/Y121F/T289A). In cases of resistance, the combination of voriconazole and echinocandin or switching to liposomal amphotericin B is suggested [Citation60]. To which extent CAPA treatment benefits patients' outcomes is not clear. The occurrence of aspergillosis may reflect a very debilitated physical condition with an anticipated harsh prognosis, already before CAPA onset. Nevertheless, upon diagnosis or suspicion of Aspergillus species infection, specific treatment should be initiated.
Prevention & prophylaxis
For prevention, an interesting approach is the use of preventive measures against Aspergillus species infection, such as room and workspace cleaning, ICU insulation from renovation work and the use of HEPA filters for air cleaning [Citation12,Citation61,Citation63]. Further environmental measures in the healthcare setting may include microfluidic chips' use for detecting bacteria, fungi and viruses and bipolar ionization systems for infection control [Citation64,Citation65].
CAPA prophylaxis with antifungals is not generally recommended, apart from cases of patients with classic risk factors for immunosuppression, such as prolonged neutropenia, graph-versus-host disease (GVHD), secondary prophylaxis, etc. [Citation12]. However, there are reports of effective application of systematic and/or inhaled antifungals as prophylaxis against CAPA in the critical care setting. Rutsaert et al. applied several measures for controlling the spread of the mold after rapid growth of Aspergillus species in their patients. These measures included not only the installment of high-efficiency particulate air filters (HEPA) for air cleaning but, also the initiation of prophylactic nebulization of 12.5 mg of liposomal amphotericin B [Citation61]. The prophylactic use of posaconazole was also studied in COVID-19 patients [Citation66]. Hatzl et al. conducted an observational study on active antifungal prophylaxis (98% with posaconazole) in critically ill patients with COVID-19 and showed a >90% reduction in the relative risk of CAPA development in the group that received prophylaxis versus the non-prophylaxis group (incidence of 1.4 vs 17.5%, respectively; p = 0.002); however, no significant difference in 30-day mortality was observed [Citation66]. This result is in contrast with that of POSA-FLU study, a randomized, open-label, proof-of-concept trial that reported no significant decrease in the incidence of IAPA with a 7-day IV posaconazole prophylaxis [Citation67]. This can be, at least partially explained, by the fact that IAPA had a quite earlier onset (71% were diagnosed within 48 h from ICU admission) compared with CAPA onset in the study of Hatzl et al. (6 days median onset from ICU admission) [Citation66,Citation67]. Another single-center study that used inhaled liposomal amphotericin B twice weekly as prophylaxis in mechanically ventilated patients with COVID-19 reported a decrease in the incidence of CAPA/Aspergillus tracheobronchitis (RR: 0.15, 95% CI: 0.05–0.48, p < 0.001) and colonization (RR: 0.28, 95% CI: 0.10–0.81, p = 0.017), while no adverse events (e.g., bronchospasm) were observed [Citation68]. Finally, the use of inhaled amphotericin lipid complex had contributed successfully tackling an aspergillosis outbreak in COVID-19 patients [Citation69]. Unfortunately, a phase III study of isavuconazole for CAPA prophylaxis was terminated due to participant challenges (ClinicalTrials.gov Identifier: NCT04707703). Further, larger studies are needed to clarify whether and for which subgroups of COVID-19 patients, CAPA prophylaxis with antifungals would be of benefit.
Outcomes
A systematic meta-analysis of studies comparing the clinical characteristics and outcomes of CAPA, reported that COVID-19 patients with CAPA compared with those that did not develop invasive aspergillosis had a higher severity of illness, an earlier admission to the ICU from COVID-19 onset and a higher overall in-hospital mortality (42.6 vs 26.5%, odds ratio 3.39) [Citation32]. Another systematic review reported a mortality rate of 59.2% for CAPA patients [Citation15]. In a multicenter, multinational study of COVID-19 ICU patients, the development of CAPA was found to be an independent risk factor, strongly associated with mortality (HR: 2.14; 95% CI: 1.59–2.87, p < 0.001) [Citation19]. Interestingly, Bartoletti et al. reported that the odds of death of CAPA patients within a 30-day period from ICU admission increased 1.44-fold (1.08–1.94; p = 0.014) for each point increase in the initial GM index of BAL (adjusted for age, SOFA, renal replacement therapy) [Citation16]. Last, in a multinational observational study by the European Confederation of Medical Mycology, a twofold increased risk of ICU mortality was noted in patients with CAPA, compared with patients without CAPA (71 vs 43%). Although CAPA was an independent negative prognostic variable, after adjusting for other predictors of survival, the authors underline that randomized controlled trials are needed to search for a causal relation [Citation19].
Therefore, it should be highlighted that the results should be interpreted with caution as variable diagnostic criteria and mortality end points have been used in different studies. Although mortality is indeed increased among critically ill COVID-19 patients that fulfil CAPA criteria, the attributable mortality of the fungal co-infection has not yet been accurately assessed.
Mucormycosis
Introduction
Mucormycosis (MCM) is a rare, rapidly progressive angio-invasive, opportunistic infection [Citation70,Citation71]. The Mucor genus includes many species that are ubiquitous in the soil of all climates. Their spores can be sampled in the dust and air of either the community or the hospital locations [Citation72]. Mucor spores are mainly transmitted through inhalation into the nares and invade the maxillary sinuses, the eye orbits, and the nearby cerebrum. Thus, the most common form of mucormycosis is rhino-orbito-cerebral disease (ROC-MCM) [Citation73]. Less often, the fungus can reach the lungs or can be transmitted into the GI tract via ingestion or directly inoculated into non-intact skin and cause skin and soft tissue infection [Citation70].
Disease presentation contrasts between Western countries and areas like India (the country with the highest disease prevalence), Iran, Mexico, the Middle East, and North Africa: In the first group of countries, the main risk factor is underlying hematologic malignancy while in the second group diabetes mellitus is the most common underlying disease. In the first case, the prevailing clinical expression is lung disease, while in the second, rhino-orbito-cerebral disease prevails [Citation74]. Factors associated with the highest MCM risk in COVID-19 patients were the presence of obesity, anosmia, new-onset diabetes, muscle aches and nasal discharge according to artificial intelligence (AI) modeling results [Citation75].
Incidence
The reported COVID-19 incidence varies widely. A significant number of studies on COVID-19-associated mucormycosis (CAM) originate from India. The incidence of ROC-MCM in the general COVID-19 population was about 0.1%, in Maharashtra state, India, that is 80-fold higher than the global estimate [Citation76]. The incidence of pulmonary MCM is unknown in the setting of severe COVID-19-associated respiratory failure, which precludes biopsy and, sometimes, bronchoalveolar lavage. A very recent systematic literature review (till July 2022) on COVID-19-associated MCM (CAM), identified 88 studies (13 prospective) with 8727 patients included, the vast majority (96.1%) from India [Citation77]. However, only 12 studies reported CAM incidence [Citation77]. The incidence in Indian studies ranged from 0.27 to 3.36% for hospitalized patients with confirmed COVID-19 infection, while in studies specifically of patients with kidney transplantation, a considerably higher incidence was reported, ranging from 4.4 to 10.8% [Citation77]. It should be highlighted that in Europe the reported incidence was significantly lower, ranging from 0.0036 to 0.67% [Citation77].
Risk factors & pathogenesis
Regarding MCM pathogenesis, hyperglycemia and diabetic ketoacidosis can impair phagocytosis, which is critical for fungal elimination. Additionally, Mucor species grow more by the increased availability of iron, particularly in the presence of acidosis [Citation70,Citation71]. CAM epidemic stormed India, a country with relatively high MCM prevalence and elevated rate of uncontrolled diabetes mellitus (DM). Apart from the COVID-19 stress response, the virus itself might alter glucose metabolism and cause DM and diabetic ketoacidosis (DKA) [Citation78]. COVID-19 is often a hyperferrinemic disease due to the action of proinflammatory cytokines, notably IL-6. Elevated ferritin levels are associated with high intracellular iron concentrations, which predispose to MCM [Citation79]. However, the serum iron and total iron-binding capacity levels of CAM individuals were lower, the latter independently, compared with the non-MCM-COVID-19 controls. Meanwhile, ferritin levels and glucocorticosteroid treatment status did not differ between the groups [Citation80].
COVID-19 treatment with steroids dysregulates DM and, if prolonged, might predispose patients to invasive disease through impaired macrophage function [Citation81]. New-onset DM can be the only risk factor for MCM, without steroid or immunosuppressive treatment [Citation82]. Moreover, in the COVID-19 setting, immunosuppression and protracted broad-spectrum antimicrobial chemotherapy may facilitate the development of this infection [Citation73]. In a large, retrospective, observational multicenter study from India (2826 patients with COVID-19-associated ROC-MCM), 87% received corticosteroids (21% for more than 10 days), 87% needed oxygen support for the COVID-19 infection and 78% had underlying DM [Citation83]. Another Indian multicenter retrospective cohort study examined specifically COVID-19 infection in kidney transplant recipients, found that most individuals were on triple chronic immunosuppressive regimen and were receiving chronic steroid treatment, while, notably, none of them presented DKA [Citation84]. On the other hand, in a French retrospective, multicenter study, the reported risk factors were hematologic malignancy or transplantation (41%), pre-existing immunosuppressive medication or DM (41%), dexamethasone or tocilizumab administration and DKA [Citation84].
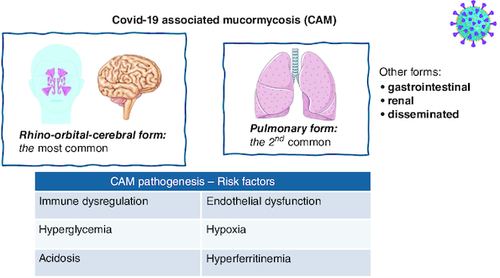
Summary of pathogenesis/risk factors depicted in .
Figure 3. COVID-19-associated mucormycosis presentation and pathogenesis.
The most common form is the rhino-orbital-cerebral, followed by the pulmonary form. SARS-CoV-2 infection enhances Mucor growth and contributes to tissue invasion in multiple ways: causing immune dysregulation and endothelial dysfunction, and creating a hypoxic, acidotic environment.
Diagnosis
The mean (±SD) day from initial COVID-19 diagnosis until MCM presentation was 17.4 days (Q1: 14.4, Q3: 21.8, IQR: 7.5 days) [Citation77]. Regarding ROC-MCM, the clinical symptoms include headache, fever and muscle aches while the nasal discharge might be black or bloody. The patient may also complain of impaired vision or sensorimotor function. The clinical findings in ROC-MCM may include gingival abscesses, mobile teeth, black/gray discoloration of the oral mucosa or even the palatal bone. Cheek or eyelid edema and proptosis may also occur [Citation73]. Endoscopy is indispensable to diagnose sinusitis due to MCM [Citation74]. Pulmonary MCM can coinfect and complicate the course of mechanically ventilated COVID-19 patients. It is challenging to distinguish from imaging studies whether lesions, such as ground-glass opacities or consolidations, are due to mucormycosis or COVID-19 pneumonia itself or acute respiratory distress syndrome (ARDS) lesions, or due to drug side effects. However, several lung nodules or the presence of pulmonary cavities necessitate a prompt, thorough search for invasive fungal infection. According to the revised and updated definitions of the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium, probable MCM diagnosis requires a host factor and a mycological diagnosis [Citation85]. Interestingly, a retrospective study from India reported than in more than half of CAM cases (59.1%), the diagnosis was made post-discharge and the patients had to be readmitted. Most (91.8%) cases were ROC-MCM, while the rest 8.2% presented pulmonary disease [Citation84].
Danion et al. conducted a retrospective, nationwide study in France, where they defined an MCM case if it had been confirmed by PCR and had occurred within three months following the viral diagnosis. COVID-19-associated-aspergillosis coexisted in five out of 17 cases, while pulmonary MCM occurred in nine patients. Less common MCM forms included gastrointestinal, disseminated, or ROC-MCM. All but one was treated in the ICU. Mucor species were mostly isolated or identified in the serum or BAL [Citation86]. A rare, daunting, complication of pulmonary MCM is massive hemoptysis from pulmonary artery pseudoaneurysm; clinicians dealing with COVID-19 patients with such complication should include PM-MCM in their differential diagnosis [Citation87].
In the very recent systematic literature review by Almyroudi et al., the vast majority of CAM was ROC-MCM, followed by pulmonary MCM (1.2%, with one-third of the cases from Europe), cutaneous (0.13%) and gastrointestinal tract (0.06%), while in 0.13% it was disseminated [Citation77].
Mycological diagnosis
Potassium hydroxide smear of nasal swabs or scrapings, or lower respiratory secretions could rapidly identify Mucor hyphae, which are ribbon-like, with variable width (6–16 μm), pauci- or non-septate with right-sided branching, and the angle of hyphal branching can vary from 45 to 90° [Citation87,Citation88]. Endoscopic nasal/paranasal or transbronchial biopsy samples that display these characteristic hyphae confirm the diagnosis. To confirm the diagnosis of Mucor, tissue samples should be stained with hematoxylineosin (HE), PAS, or GMS [Citation89]. Mucor thrive on most common bacterial (e.g., sheep blood agar, chocolate agar) and fungal culture media (e.g., Sabouraud dextrose agar and potato dextrose agar) with growing at 37 °C forming fluffy white, grey, or brownish colonies that quickly fill the petri dish in 1–7 days.
PCR-based methods can be used to detect Mucor DNA in fresh or paraffin-embedded tissue samples, with the internal transcribed spacer DNA region being the most widely sequenced and the preferred method for species identification [Citation90]. PCR-based methods on blood/serum samples, although seem promising and could provide rapid results, they need further clinical validation [Citation82]. Species identification and antifungal susceptibility is recommended for Mucor [Citation88]. Some studies found that MALDI-TOF provides a rapid and reliable method for species-level identification of most pathogenic Mucor species for humans, but mainly there are in-house databases and further validation is needed [Citation91,Citation92].
Finally, the physicians treating COVID-19 patients should always bear in mind that repeated negative galactomannan, (1,3)-Beta-D-glucan and Aspergillus PCR tests in a patient with strong suspicion of invasive fungal disease may suggest MCM.
Imaging
Imaging studies, such as CT and MRI, apart from contributing to MCM diagnosis, facilitate the assessment of disease extension [Citation77]. If ROC-MCM is suspected, head computerized tomography may show nasal maxillary and other paranasal sinus or orbital involvement, while the bones of the above structures and the skull base may be eroded [Citation77,Citation93]. Magnetic resonance imaging maps the tissues involved and is required for surgical planning [Citation94]. The scan qualifies three disease stages: stage I disease is restricted to nasal and paranasal tissues, stage II extends into the eye orbits, the hard palates, and the mouth, whereas in stage III, it expands into the cranial cavity [Citation94]. Non-contrast-enhancing sino-atrial mucosa and peri-antral fat inflammation were the most frequently found early signs in MRI, while orbital extension (stages II and III) was found in 86% of COVID-19 patients with ROC-MCM [Citation94]. MR angiographic images' analysis revealed occlusion of the third part of the internal maxillary artery in almost one third of the cases, ophthalmic artery thrombosis in 14%, and narrowing and irregularity of the internal carotid artery in 16% [Citation94]. Notably, the stage was positively correlated with the glycosylated hemoglobin concentrations [Citation95]. Regarding lung CAM, the CT findings more commonly encountered were consolidation, cavitation and ground-glass opacities, while the reverse halo sign occurred less often. Pulmonary CAM was less frequently associated with DM and was more often associated with mechanical ventilation compared with non-pulmonary CAM [Citation96]. In another recent CAM study, lung CT mostly showed consolidations (83%) and less often cavitations (33%), while reverse halo sign (8%) or nodules (6%) were rare [Citation86]. It should be noted though that, although infrequent, the presence of a thick-walled pulmonary lesion, several nodules, a reverse halo sign in lung CT increases the likelihood of pulmonary mucormycosis and should alert the clinicians to actively search for pulmonary PMC-MCM, and, also, to differentiate from CAPA that may present similar signs [Citation88].
Treatment
The severity of the infection and the complexity of treatment warrant awareness of the initially subtle and non-specific symptoms and signs and the design of a diagnostic strategy that includes tissue or secretion sampling and imaging. The predisposing factors, in other words, uncontrolled DM and DKA, steroid or immunosuppressive treatment aggravate MCM and should be managed accordingly. Early empirical treatment might be an option as often patients died before diagnosis [Citation86].
Surgical resection of necrotic and infected tissues until clear margins are reached contributes to better survival. Localized lung disease can be better controlled with pneumonectomy or lobectomy [Citation75,Citation88].
Initiation of empirical liposomal amphotericin-B without delay can improve survival. The regular daily dosing is 5–10 mg/kg of liposomal amphotericin B, while the higher dose is recommended for treating patients with a solid organ transplant or extension of MCM into the brain. Alternatively, isavuconazole and posaconazole can be given as rescue therapy or as first-line in cases with pre-existing renal failure, at a dose of 200 mg thrice daily for the first two days of treatment, followed by 200 mg once daily onward [Citation75,Citation77,Citation88].
Follow-up comprises of clinical evaluation and weekly imaging studies. If MCM is controlled, the initial duration of intravenous treatment lasts for 3–6 weeks, with the potential switch to oral isavuconazole or posaconazole for another 3–6 months. In case of uncontrolled MCM, debridement of involved tissues should be repeated, and the liposomal amphotericin dose increased up to the maximum of 10 mg/kg daily, while combination antifungal treatment is discouraged [Citation74,Citation87]. Despite the paucity of data regarding its use, intraorbital amphotericin B might increase the likelihood of eye and vision salvage [Citation82,Citation96].
Prevention & prophylaxis
The MCM prevention measures that have been suggested for patients with COVID-19 include the control of the major risk factors for MCM, in other words, comorbidities, particularly careful DM management to avoidance of hyperglycemia and ketoacidosis, and the judicious use of corticosteroids and other immunosuppressants/immunomodulators, along with meticulous application of hygiene measures [Citation97,Citation98].
Regarding MCM prophylaxis in COVID-19 patients, the international guidelines recommend posaconazole only in neutropenic patients and in graft-versus-host disease [Citation85].
Outcomes
Reported MCM mortality in COVID-19 has a very wide range [Citation75,Citation77,Citation81,Citation86,Citation98]. It is noteworthy that, although overall survival is severely compromised by the development of MCM, there is an important difference in mortality between ROC- and pulmonary-MCM coinfection in the setting of COVID-19 disease, with estimated median mortality 21.4% (IQR 31.9%) and 80% (IQR 50%) [Citation78]. In terms of mortality related to geographical distribution, median MCM mortality of COVID-19 patients was 18.2%, 53.8% and 38.8% in India, in Europe and in the rest of the world, respectively [Citation77]. As in COVID-19-associated aspergillosis, attributable mortality remains to be defined.
Conclusion
Diagnosing and treating CAPA represents a great challenge for the medical community. To avoid delayed diagnosis and, at the same time, avoid antifungal overuse, the diagnostic approaches need to be tailored to the particularities of the COVID-19 critically ill patients that lack the classic ‘host’ factors and develop invasive aspergillosis without the classical clinical and imaging features. Efforts should focus on earlier diagnosis to minimize both morbidity and mortality. Uncontrolled DM, corticosteroid use, immunosuppression due to hematologic malignancy, and kidney transplantation are the most frequent risk factors for CAM, with ROC-MCM being the most frequent form followed by pulmonary MCM. Physicians should be aware of the geographical variability of CAM characteristics and promptly recognize even the initial subtle presentation of the disease. CAM has a high mortality rate and on suspicion, immediate initiation of treatment in parallel with the diagnostic procedures, is more than crucial.
Future perspective
Diagnostic methods should evolve to facilitate quicker diagnosis of invasive aspergillosis, more accurate confirmation of infection, and discrimination from colonization in critically ill patients with COVID-19. Aspergillus plasma cell free DNA PCR has been proposed as an alternative to serum GM, with higher sensitivity for the non-invasive diagnosis of aspergillosis [Citation99]. On the other hand, the high-affinity Fe3+ chelators siderophores produced by Aspergillus species (and, also, by other fungi species), have shown very promising sensitivities and specificities as diagnostic tools for aspergillosis (serum, urine, BAL and in imaging, conjugated with radioisotopes) [Citation100]. However, for both the above emerging and promising diagnostic tools, larger multicenter studies are warranted to confirm their diagnostic performances.
The emergence of triazole resistant Aspergillus species is an important concern and makes essential the development of new antifungals and new management strategies to both achieve effective empirical treatment of invasive aspergillosis and tackle resistance development and spread.
Preventive measures and extended management optimization/control programs for diabetes mellitus are necessary, especially in developing/emerging countries, such as India, that recorded an extremely high incidence of mucormycosis during the COVID-19 pandemic.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Cag Y, Erdem H, Gormez A et al. Anxiety among front-line health-care workers supporting patients with COVID-19: a global survey. Gen Hosp. Psychiatry 68, 90–96 (2021).
- Bekker Mortensen C, Zachodnik J, FjordbakCaspersen S, Geisler A. Healthcare professionals’ experiences during the initial stage of the COVID-19 pandemic in the intensive care unit: a qualitative study. Intensive Crit. Care Nurs. 68, 103130 (2021).
- Jansson M, Liao X, Rello J. Strengthening ICU health security for a coronavirus epidemic. Intensive Crit. Care Nurs. 57, 102812 (2021).
- Blot S, Ruppé E, Harbarth S et al. Healthcare-associated infections in adult intensive care unit patients: changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensive Crit. Care Nurs. 70, 103227 (2022).
- Conway Morris A, Kohler K, De Corte T et al. coinfection and ICU-acquired infection in COVID-19 ICU patients: a secondary analysis of the UNITE-COVID data set. Crit. Care 26, 236 (2022).
- Kuchi Bhotla H, Balasubramanian B, Meyyazhagan A et al. Opportunistic mycoses in COVID-19 patients/survivors: epidemic inside a pandemic. J. Infect. Public Health 14(11), 1720–1726 (2021).
- Hoenigl M, Seidel D, Sprute R et al. COVID-19-associated fungal infections. Nat. Microbiol. 7, 1127–1140 (2022).
- Denning DW, Chakrabarti A. Pulmonary and sinus fungal diseases in non-immunocompromised patients. Lancet Infect. Dis. 17(11), e357–e366 (2017).
- Blot S, Rello J, Koulenti D. Diagnosing invasive pulmonary aspergillosis in ICU patients: putting the puzzle together. Curr. Opin. Crit. Care 25(5), 430–437 (2019).
- Dewi IMW, Janssen NAF, Rosati D et al. Invasive pulmonary aspergillosis associated with viral pneumonitis. Curr. Opin. Microbiol. 62, 21–27 (2021).
- Vanderbeke L, Spriet I, Breynaert C, Rijnders BJA, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr. Opin. Infect Dis. 31(6), 471–480 (2018).
- Verweij PE, Bruggemann RJM, Azoulay E et al. Taskforce report on the diagnosis and clinical management of COVID-19-associated pulmonary aspergillosis. Intensive Care Med. 47(8), 819–834 (2021).
- Gangneux J-P, Bougnoux M-E, Dannaoui E, Cornet M, Zahar JR. Invasive fungal diseases during COVID-19: we should be prepared. J. Mycol. Med. 30(2), 100971 (2020).
- Iqbal A, Ramzan M, Akhtar A, Ahtesham A, Aslam S, Khalid J. COVID-associated pulmonary aspergillosis and its related outcomes: a single-center prospective observational study. Cureus 13(8), e16982 (2021).
- Kariyawasam RM, Dingle TC, Kula BE, Vandermeer B, Sligl WI, Schwartz IS. Defining COVID-19-associated pulmonary aspergillosis: systematic review and meta-analysis. Clin. Microbiol. Infect. 28(7), 920–927 (2022).
- Bartoletti M, Pascale R, Cricca M et al. PREDICO study group. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin. Infect. Dis. 73(11), e3606–e3614 (2021).
- Bretagne S, Sitbon K, Botterel F et al. COVID-19-associated pulmonary aspergillosis, fungemia, and pneumocystosis in the intensive care unit: a retrospective multicenter observational cohort during the first french pandemic wave. Microbiol. Spectr. 9(2), e0113821 (2021).
- White PL, Dhillon R, Cordey A et al. A national strategy to diagnose coronavirus disease 2019-associated invasive fungal disease in the intensive care unit. Clin. Infect. Dis. 73(7), e1634–e1644 (2021).
- Prattes J, Wauters J, Giacobbe DR et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patientsa multinational observational study by the European Confederation of Medical Mycology. Clin. Microbiol. Infect. 28(4), 580–587 (2022).
- Koehler P, Bassetti M, Chakrabarti A et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 21(6), e149–e162 (2021).
- Paramythiotou E, Dimopoulos G, Koliakos N et al. Epidemiology and incidence of COVID-19-associated pulmonary aspergillosis (CAPA) in a Greek Tertiary Care Academic Reference Hospital. Infect Dis. Ther. 10(3), 1779–1792 (2021).
- Wasylyshyn AI, Wasylyshyn GR, Linder KA, Miceli MH. COVID-19-associated pulmonary aspergillosis at an academic medical center in the midwestern United States. Mycopathologia 186(4), 499–505 (2021).
- Koehler P, Cornely OA, Kochanek M. Bronchoscopy safety precautions for diagnosing COVID-19 associated pulmonary aspergillosis-a simulation study. Mycoses 64(1), 55–59 (2021).
- Lormans P, Blot S, Amerlinck S, Devriendt Y, Dumoulin A. COVID-19 acquisition risk among ICU nursing staff with patient-driven use of aerosol-generating respiratory procedures and optimal use of personal protective equipment. Intensive Crit. Care Nurs. 63, 1029 (2021).
- Er B, Er AG, Gülmez D et al. A screening study for COVID-19-associated pulmonary aspergillosis in critically ill patients during the third wave of the pandemic. Mycoses 65(7), 724–732 (2022).
- Armstrong-James D, Youngs J, Bicanic T et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur. Resp. J. 56(4), 2002554 (2020).
- Dellière S, Dudoignon E, Fodil S et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin. Microbiol. Infect. 27(5), 790; e1–e5 (2020).
- Culic O, Erakovic V, Cepelak I et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur. J. Pharmacol. 450, 277; e89 (2002).
- Latge J-P, Chamilos G. Aspergillus fumigatus and aspergillosis in 2019. Clin. Microbiol. Rev. 33, 310; e75 (2019).
- Dickson RP, Morris A. Macrolides, inflammation and the lung microbiome: untangling the web of causality. Thorax 72, 10; e2 (2017).
- Nuffield Department of Population Health. RECOVERY: randomised Evaluation of COVID-19 Therapy. Available at: https://www.recoverytrial.net ( Accessed April 2024).
- Chong WH, Saha BK, Neu KP. Comparing the clinical characteristics and outcomes of COVID-19-associate pulmonary aspergillosis (CAPA): a systematic review and meta-analysis. Infection 50(1), 43–56 (2022).
- Kim S-H, Hong JY, Bae S et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis in critically ill patients: a nationwide, multicenter, retrospective cohort study. J. Korean Med. Sci. 37(18), e134 (2022).
- van Grootveld R, van der Beek MT, Janssen NAF et al. CAPA2.0 study group. Incidence, risk factors and pre-emptive screening for COVID-19 associated pulmonary aspergillosis in an era of immunomodulant therapy. J. Crit. Care 76, 154272 (2023).
- Gangneux J-P, Dannaoui E, Fekkar A et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir. Med. 10(2), 180–190 (2022).
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 397(10285), 1637–1645 (2021).
- Zia M, Goli M. Predisposing factors of important invasive fungal coinfections in COVID-19 patients: a review article. J. Int. Med. Res. 49(9), 3000605211043413 (2021).
- Apostolopoulou A, Garrigos ZE, Vijayvargiya P, Hope Lerner A, Farmakiotis D. Invasive pulmonary aspergillosis in patients with SARS-CoV-2 infection: a systematic review of the literature. Diagnostics (Basel) 10(10), 807 (2020).
- Bulpa P, Duplaquet F, Dimopoulos G, Vogelaers D, Blot S. Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease exacerbations. Semin Respir. Crit. Care Med. 41(6), 851–861 (2020).
- Feys S, Gonçalves SM, Khan M et al. Lung epithelial and myeloid innate immunity in influenza-associated or COVID-19-associated pulmonary aspergillosis: an observational study. Lancet Respir. Med. 10(12), 1147–1159 S2213-2600(22)00259-4 (2022).
- Koukaki E, Rovina N, Tzannis K et al. Fungal infections in the ICU during the COVID-19 era: descriptive and comparative analysis of 178 patients. J. Fungi. (Basel) 8(8), 881 (2022).
- Mohamed A, Rogers TR, Talento AF. COVID-19 associated invasive pulmonary aspergillosis: diagnostic and therapeutic challenges. J. Fungi. (Basel) 6(3), 115 (2020).
- Cuenca-Estrella M, Kett DH, Wauters J. Defining standards of CARE for invasive fungal diseases in the ICU. J. Antimicrob. Chemother. 74(Suppl. 2), ii9–ii15 (2019).
- Dai Z, Cai M, Yao Y et al. Comparing the diagnostic value of bronchoalveolar lavage fluid galac-tomannan, serum galactomannanan, and serum 1,3-β-d-glucan in non-neutropenic respiratory disease patients with invasive pulmonary aspergillosis. Medicine (Baltimore) 100(14), e25233 (2021).
- Mikulska M, Furfaro E, Dettori S et al. Aspergillus-PCR in bronchoalveolar lavage – diagnostic accuracy for invasive pulmonary aspergillosis in critically ill patients. Mycoses 65(4), 411–418 (2022).
- Schroeder M, Simon M, Katchanov J et al. Does galactomannan testing increase the diagnostic accuracy for IPA in the ICU? A prospective observational study. Crit. Care 20, 139 (2016).
- Wahidi MM, Shojaee S, Lamb CR et al. The use of bronchoscopy during the coronavirus disease 2019 pandemic: CHEST/AABIP Guideline and Expert Panel Report. Chest 158, 1268–1281 (2020).
- Rouzé A, Martin-Loeches I, Nseir S. COVID-19-associated pulmonary aspergillosis: an underdiagnosed or overtreated infection? Curr. Opin. Crit. Care 28(5), 470–479 (2022).
- European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs for antifungal agents. ( Accessed April 2024). ( Version 10.0). https://www.eucast.org
- Hong W, White PL, Backx M et al. CT findings of COVID-19-associated pulmonary aspergillosis: a systematic review and individual patient data analysis. Clin. Imaging 90, 11–18 (2022).
- De Pauw B, Walsh TJ, Donnelly JP et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46, 1813–1821 (2008).
- Bassetti M, Azoulay E, Kullberg BJ et al. EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the intensive care unit working group. Clin. Infect. Dis. 72(Suppl. 2), S121–S127 (2021).
- Blot SI, Taccone FS, Van den Abeele AM et al. AspICU Study Investigators. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 186, 56–64 (2012).
- Verweij PE, Rijnders BJA, Brüggemann RJM et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 46(8), 1524–1535 (2020).
- Lahmer T, Kriescher S, Herner A et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: results from the prospective Asp COVID-19 study. PLOS ONE 16(3), e0238825 (2021).
- Hashim Z, Neyaz Z, Marak RSK, Nath A, Nityanand S, Tripathy NK. Practice guidelines for the diagnosis of COVID-19-associated pulmonary aspergillosis in an intensive care setting. J. Intensive Care Med. 37(8), 985–997 (2022).
- Ullmann AJ, Aguado JM, Arikan-Akdagli S et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 24(Suppl. 1), e1–e38 (2018).
- Patterson TF, Thompson GR, Denning DW et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 63, e1–e60 (2016).
- Levine M, Chandrasekar PH. Adverse effects of voriconazole: over a decade of use. Clin. Transplant. 30(11), 1377–1386 (2016).
- Verweij PE, Ananda-Rajah M, Andes D et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist. Updat. 21–22, 30–40 (2015).
- Rutsaert L, Steinfort N, Van Hunsel T et al. COVID-19-associated invasive pulmonary aspergillosis. Ann. Intensive Care 10(1), 71 (2020).
- Lestrade PP, Bentvelsen RG, Schauwvlieghe AFAD et al. Voriconazole resistance and mortality in invasive aspergillosis: a multicenter retrospective cohort study. Clin. Infect. Dis. 68, 1463–1471 (2019).
- Abdul Salam ZH, Karlin RB, Ling ML, Yang KS. The impact of portable high-efficiency particulate air filters on the incidence of invasive aspergillosis in a large acute tertiary-care hospital. Am. J. Infect. Control 38(4), e1–e7 (2010).
- Wang J, Yang L, Wang H, Wang L. Application of microfluidic chips in the detection of airborne microorganisms. Micromachines (Basel) 13(10), 1576 (2022).
- Kanesaka I, Katsuse AK, Takahashi H, Kobayashi I. Evaluation of a bipolar ionization device in inactivation of antimicrobial-resistant bacteria, yeast, Aspergillus spp. and human coronavirus. J. Hosp. Infect. 126, 16–20 (2022).
- Hatzl S, Reisinger AC, Posch F et al. Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: an observational study. Crit. Care 25, 335 (2021).
- Vanderbeke L, Jansen NAF, Bergmans DCJJ et al. Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): a randomised, open-label, proof-of-concept trial. Intensive Care Med. 47(6), 674–686 (2021).
- Van Ackerbroeck S, Rutsaert L, Roelant E, Dillen K, Wauters J, Van Regenmortel N. Inhaled liposomal amphotericin B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit. Care 25(1), 298 (2021).
- Soriano MC, Narvaez-Chavez G, Lopez-Olivencia M, Fortún J, de Pablo R. Inhaled amphotericin B lipid complex for prophylaxis against COVID-19-associated invasive aspergillosis. Intensive Care Med. 48(3), 360–361 (2022).
- Reid G, Lynch JP, Fishbein MC, Clark NM. Mucormycosis. Semin Respir. Crit. Care Med. 41(1), 99–114 (2020).
- Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin. Infect. Dis. 54(Suppl. 1), S16–S22 (2012).
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin. Microb. Rev. 13(2), 236–301 (2000).
- Arthanari KK, Annamalai S, Thangavelu A, Palanivelu C, Suresh G, Anbuselvan S. Mucormycosis during coronavirus disease pandemic: a diagnosis we cannot afford to miss. J. Pharm. Bioallied. Sci. 13(Suppl. 2), S1769–S1771 (2021).
- Cornely OA, Alastruey-Izquierdo A, Arenz D et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 19(12), e405–e421 (2019).
- Syed-Abdul S, Babu AS, Bellamkonda RS et al. Using artificial intelligence-based models to predict the risk of mucormycosis among COVID-19 survivors: an experience from a public hospital in India. J. Infect. 84(3), 351–354 (2021).
- Dravid A, Kashiva R, Khan Z et al. Epidemiology, clinical presentation and management of COVID-19 associated mucormycosis: a single center experience from Pune, Western India. Mycoses 65(5), 526–540 (2022).
- Almyroudi MP, Akinosoglou K, Rello J, Blot S, Dimopoulos G. Clinical phenotypes of COVID-19 associated mucormycosis (CAM): a comprehensive review. Diagnostics (Basel) 12(12), 3092 (2022).
- Rubino F, Amiel SA, Zimmet P et al. New-onset diabetes in covid-19. N. Engl. J. Med. 383(8), 789–790 (2020).
- Perricone C, Bartoloni E, Bursi R et al. COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol. Res. 68, 213–224 (2020).
- Kumar HM, Sharma P, Rudramurthy SM et al. Serum iron indices in COVID-19-associated mucormycosis: a case-control study. Mycoses 65(1), 120–127 (2022).
- Jeong W, Keighley C, Wolfe R et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 25(1), 26–34 (2019).
- Nair AG, Adulkar NG, D'Cunha L et al. Rhino-orbital mucormycosis following COVID-19 in previously non-diabetic, immunocompetent patients. Orbit 40(6), 499–504 (2021).
- Sen M, Honavar SG, Bansal R et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India – Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J. Ophthalmol. 69(7), 1670–1692 (2021).
- Meshram HS, Kute VB, Yadav DK et al. Impact of COVID-19-associated mucormycosis in kidney transplant recipients: a multicenter cohort study. Transplant Direct 8(1), e1255 (2021).
- Donnelly JP, Chen SC, Kauffman CA et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin. Infect. Dis. 71, 1367–1376 (2020).
- Danion F, Letscher-Bru V, Guitard J et al. Coronavirus disease 2019-associated mucormycosis in France: a rare but deadly complication. Open Forum Infect Dis. 9(2), ofab566 (2021).
- Pruthi H, Muthu V, Bhujade H et al. Pulmonary artery pseudoaneurysm in COVID-19-associated pulmonary mucormycosis: case series and systematic review of the literature. Mycopathologia 22, 1–7 (2021).
- Philip AC, Madan P, Sharma S, Das S. Utility of MGG and Papanicolaou stained smears in the detection of mucormycosis in nasal swab/scraping/biopsy samples of COVID 19 patients. Diagn. Cytopathol. 50(3), 93–98 (2022).
- Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med. Mycol. 56, S93 (2018).
- Skiada A, Pavleas I, Dragari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J. Fungi. (Basel) 6(4), 265 (2020).
- Dadwal SS, Kontoyiannis DP. Recent advances in molecular diagnosis of mucormycosis. Expert Rev. Mol. Diagn. 18, 845–854 (2018).
- Schwarz P, Guedouar H, Laouiti F, Grenouillet F, Dannaoui E. Identification of mucorales by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Fungi. 5, 56 (2019).
- Rudramurthy SM, Hoenigl M, Meis JD et al. ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries. Mycoses 64(9), 1028–1037 (2021).
- Yadav T, Tiwari S, Gupta A et al. Magnetic resonance imaging in coronavirus disease – 2019 associated rhino-orbital-cerebral mucormycosis (CA-ROCM) – imaging analysis of 50 consecutive patients. Curr. Probl. Diagn. Radiol. 51(1), 112–120 (2022).
- Garg M, Prabhakar N, Muthu V et al. CT findings of COVID-19-associated pulmonary mucormycosis: a case series and literature review. Radiology 302(1), 214–217 (2022).
- Ashraf DC, Idowu OO, Hirabayashi KE et al. Outcomes of a modified treatment ladder algorithm using retrobulbar amphotericin b for invasive fungal rhino-orbital sinusitis. Am. J. Ophthalmol. 237, 299–309 S0002-9394(21)00319-316 (2021).
- Ish P, Ish S. Prevention of mucormycosis in COVID-19 – the need of the hour. Indian J. Ophthalmol. 69(7), 1969 (2021).
- Honavar SG. Code mucor: guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J. Ophthalmol. 69, 1361–1365 (2021).
- Mah J, Nicholas V, Tayyar R et al. Superior accuracy of aspergillus plasma cell-free DNA polymerase chain reaction over serum galactomannan for the diagnosis of invasive aspergillosis. Clin. Infect. Dis. 77(9), 1282–1290 (2023).
- Kriegl L, Havlicek V, Dichtl K, Egger M, Hoenigl M. Siderophores: a potential role as a diagnostic for invasive fungal disease. Curr. Opin. Infect Dis. 35(6), 485–492 (2022).
